Abstract
Physical activity induces favourable changes of arterial gene expression and protein activity, although little is known about its effect in venous tissue. Although our understanding of the initiating molecular signals is still incomplete, increased expression of endothelial nitric oxide synthase (eNOS) is considered a key event. This study sought to investigate the effects of two different training protocols on the expression of eNOS and extracellular superoxide dismutase (ecSOD) in venous and lung tissue and to evaluate the underlying molecular mechanisms. C57Bl/6 mice underwent voluntary exercise or forced physical activity. Changes of vascular mRNA and protein levels and activity of eNOS, ecSOD and catalase were determined in aorta, heart, lung and vena cava. Both training protocols similarly increased relative heart weight and resulted in up-regulation of aortic and myocardial eNOS. In striking contrast, eNOS expression in vena cava and lung remained unchanged. Likewise, exercise up-regulated ecSOD in the aorta and in left ventricular tissue but remained unchanged in lung tissue. Catalase expression in lung tissue and vena cava of exercised mice exceeded that in aorta by 6.9- and 10-fold, respectively, suggesting a lack of stimulatory effects of hydrogen peroxide. In accordance, treatment of mice with the catalase inhibitor aminotriazole for 6 weeks resulted in significant up-regulation of eNOS and ecSOD in vena cava. These data suggest that physiological venous catalase activity prevents exercise-induced up-regulation of eNOS and ecSOD. Furthermore, therapeutic inhibition of vascular catalase might improve pulmonary rehabilitation.
Keywords: physical activity, catalase, endothelial nitric oxide synthase, pulmonary rehabilitation
Introduction
Physical activity has been shown to reduce cardiovascular morbidity and mortality and is an important non-pharmacological strategy to prevent and treat cardiovascular diseases [1, 2]. Different exercise protocols induce favourable changes of various cardiovascular functions such as reduction in heart rate and blood pressure, increase of maximal myocardial oxygen uptake, various metabolic modifications and physiologic adaptations such as angiogenesis and arteriogenesis involving skeletal muscle and cardiac muscle [3–6]. One of the key events in terms of vascular adaptations to exercise training appears to be an up-regulation of endothelial nitric oxide synthase (eNOS). This vascular adaptation was initially described in 1994 [7] and has been confirmed many times in many species including human beings [1]. Likewise, exercise was shown to increase vascular nitric oxide bioavailability [8–10]. Furthermore, we could demonstrate that induction of eNOS by exercise triggers induction of extracellular SOD expression [11]. The dependence of ecSOD up-regulation by vascular nitric oxide was recently confirmed using a transgenic animal and treatment with exogenous nitric oxide [12].
The mechanism of exercise-induced up-regulation of eNOS is multifactorial [1]. Experimental evidence suggest that activation of cSrc [13] and vascular hydrogen peroxide [14] are critically involved. Both conditions are closely related to increased shear stress suggesting that activation of shear stress regulatory cis-elements in the promoter of the eNOS gene play an important role [15]. However, our understanding of the signals initiating up-regulation of arterial eNOS is still incomplete.
All these published data rely on studies in arteries and richly arterialized tissue such as the left ventricular myocardium. In striking contrast, little is known about the effect of exercise in veins and/or low-pressure tissues such as the lung. In an attempt to understand the importance of exercise on venous vascular biology and the underlying mechanisms, we investigated whether exercise increases the expression of eNOS and ecSOD in venous tissue such as vena cava and lung. We used two strikingly different training protocols to further answer the question whether the type of exercise training, e.g. short-term high-intensity (forced physical activity) or long-term low-intensity (voluntary running), is important in this context.
Materials and methods
Permission for this study was provided and the experiments were performed according to the guidelines for the use of experimental animals as given by the Deutsches Tierschutzgesetz.
Animals
A total of 60 male C57BL/6 mice, 2–3 months of age were used. All mice were bred at the university’s animal facilities in a specified pathogen-free area and were singularized for 8 weeks prior exercise training. Sedentary mice and forced exercise mice lived individually in small cages (0.036 m2) for the whole experimental period of 12 weeks, voluntary running mice were transferred after 8 weeks of singularization in 0.036 m2 cages to larger cages and remained there for 4 weeks. These cages offered a floor space of 0.051 m2 and were supplied with running wheels as described below (Fig. 1A). Groups of sedentary mice, forced exercise mice and voluntary running mice (n= 8 each) were used for the initial investigation. Additional 20 male C57BL/6 mice underwent voluntary running to obtain further data in vena cava and 16 male C57BL/6 mice were treated with the catalase inhibitor aminotriazole (Sigma, Munich, Germany) for 6 weeks and 12 underwent voluntary running during the last 4 weeks. All mice were killed and the tissues were snap-frozen in liquid nitrogen.
Fig 1.

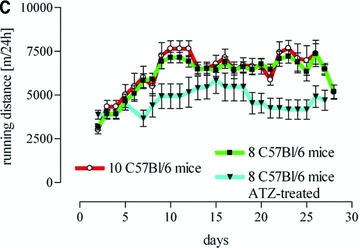
(A) Image of the forced exercise machine and (B) a representative cage equipped with running wheels as described previously and (C) activity of voluntary running mice expressed as daily running distance (m/24 hrs).
Exercise training
After a 5 day adaptation training program forced exercise mice were exercised 5 days a week for 30 min. at 0.25 m/sec. for 3 weeks in a self-build exercise treadmill especially designed for mice (Fig. 1B). During exercise, control sedentary mice were placed in small cages at the sides of the training apparatus to expose them to the same stress, noise and vibration. Voluntary running mice underwent training for 3 weeks in running wheels (Fig. 1B) especially designed to record the daily running distance for each mouse (Techniplast, Hohenpeissenberg, Germany). The adaptation to this exercise protocol lasted 1 week (Fig. 1C). Control mice were housed in cages of the same size but without a running wheel. Within 16 to 20 hrs after the last training mice were killed by inhalation of carbon dioxide and the heart weight, body weight, soleus weight and tibia length were determined (Tables 1 and 2).
Table 1.
Training efficacy as measured results from voluntary running mice compared to sedentary controls
| Measure | Sedentary | Voluntary running | P-value |
|---|---|---|---|
| HW (g) | 0.1391 ± 0.0029 | 0.1536 ± 0.0024 | 0.0013 |
| BW (g) | 31.50 ± 0.9443 | 28.61 ± 0.5552 | 0.0168 |
| SW (g) | 0.009567 ± 0.00039 | 0.01082 ± 0.00029 | 0.0365 |
| TL (cm) | 1.903 ± 0.03142 | 1.871 ± 0.03736 | n.s. |
| HW/TL (mg/cm) | 75.14 ± 1.96 | 83.60 ± 1.57 | 0.004 |
| SW/BW (mg/g) | 0.2982 ± 0.008 | 0.3756 ± 0.0094 | 0.0001 |
| SW/TL (mg/cm) | 5.157 ± 0.2 | 5.871 ± 0.16 | 0.0132 |
Values are means ± SE. BW, body weight; HW, heart weight; TL, tibia length; SW, soleus weight.
Table 2.
Measures of training efficacy in aminotriazole treated voluntary running mice C57Bl/6 mice compared to sedentary controls
| Measure | Sedentary | Voluntary running | P-value |
|---|---|---|---|
| HW (g) | 0.09180 ± 0.003443 | 0.1044 ± 0.003434 | 0.0210 |
| BW (g) | 26.57 ± 0.5615 | 25.76 ± 0.6854 | n.s. |
| SW (g) | 0.00926 ± 0.000305 | 0.01187 ± 0.000327 | <0.0001 |
| TL (cm) | 1.751 ± 0.02597 | 1.696 ± 0.02243 | n.s. |
| HW/BW (mg/g) | 3.456 ± 0.1129 | 4.058 ± 0.09696 | 0.0012 |
| HW/TL (mg/cm) | 52.55 ± 2.254 | 61.60 ± 1.874 | 0.0080 |
| SW/BW (mg/g) | 0.3484 ± 0.009816 | 0.4618 ± 0.01126 | <0.0001 |
| SW/TL (mg/cm) | 5.283 ± 0.1470 | 7.000 ± 0.1598 | <0.0001 |
Values are means ± SE. BW, body weight; HW, heart weight; TL, tibia length; SW, soleus weight.
Measurements of citrate synthase activity
Citrate synthase activity was measured in soleus muscle by the method of Kusnetzov et al.[16] as described previously [17]. The samples were measured at 412 nm at 30°C for 5 min. Determination of total protein in the homogenates was done following Bradford method [18] and citrate synthase activity was calculated in U/μg.
Determination of eNOS mRNA expression
Total RNA isolation and real-time measurements were performed as described previously [12]. Briefly, real-time PCR was performed with the TaqMan Gene Expression Assay (Applied Biosystems, Weiterstadt, Germany) for the eNOS gene and the endogenous control hypoxanthine guanine phosphoribosyl transferase 1.
The relative eNOS expression was determined using the ΔΔCt method. All real-time PCR experiments were carried out on an ABI PRISM 7500 Real PCR System (Applied Biosystems).
Immunoblotting
Western blot analysis was performed as described previously [17], using commercially available monoclonal antibody directed against human eNOS (BD, Heidelberg, Germany), polyclonal antibody against rabbit Ser1177 (Santa Cruz, Heidelberg, Germany), polyclonal antibody against rabbit catalase (Calbiochem, Darmstadt, Germany) and goat ecSOD (R&D Systems, Wiesbaden-Nordenstadt, Germany). Blots were subsequently challenged with a horseradish peroxidase-conjugated antibody antimouse IgG (Biorad, Munich, Germany), anti-rabbit antibody (Calbiochem) or donkey anti-goat (Santa Cruz). Western blot signals of eNOS phosphorylation Ser1177 were related to total eNOS protein detected by co-incubation as described above. All blots were standardized to polyclonal anti-actin (Sigma). Blots were developed using ECL (Roche, Mannheim, Germany) and exposed to X-ray film and analysed by densitometry (Gel Doc 1000; Bio-Rad, Munich, Germany). Comparative quantitative evaluation was performed with signals appearing on the same blot only.
Measurements of catalase activity
Catalase activity was measured by the method of Cohen et al.[19] as described previously [14]. The measurements were performed with 100 ×g homogenates of the aorta and the lung isolated from untrained C57Bl/6 mice. The absorbance of residual KMnO4 obtained after KMnO4 reduction was measured spectrophotometrically at 480 nm wavelength.
Statistics
All data were analysed by standard computer program (GraphPad Prism PC software, version 3.03) and are expressed as mean ± S.E.M of n individual samples. Statistical comparisons between groups were performed by either Student’s t-tests or Newman–Keuls multiple comparisons test following one-way anova. P < 0.05 was considered statistically significant.
Results
Efficacy of physical activity
All forced exercised mice (5.149 ± 0.049 mg/g) and all voluntary running mice (5.250 ± 0.128 mg/g) showed a higher heart weight/body weight ratio than sedentary controls (4.706 ± 0.08 mg/g, P = 0.0007, Fig. 2A). There was a significant increase in citrate synthase activity in soleus muscle of forced exercise mice as compared to sedentary control (Fig. 2B). In contrast, citrate synthase activity did not change in voluntary running mice (Fig. 2B), although these mice ran a mean distance of 5.8 km/24 hrs (Fig. 1C). However, other measures for exercise efficacy such as the ratios heart weight/body weight, heart weight/tibia length, soleus weight/body weight and soleus weight/tibia length were increased in this group (Table 1) and in aminotriazole-treated voluntary running mice (Table 2).
Fig 2.
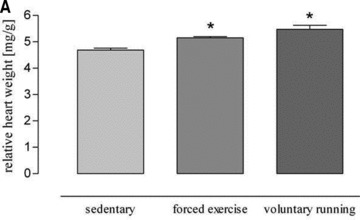
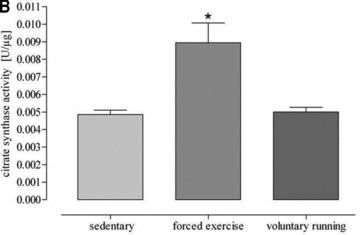
(A) Changes of heart weight body weight ratio induced by both exercise protocols. Forced physical activity and voluntary running induced a significant higher heart weight body weight ratio as compared to sedentary controls (*P= 0.0007 versus sedentary controls). (B) Citrate synthase activity of soleus muscle of sedentary and exercised mice (*P < 0.0001 versus sedentary controls).
Effect of physical activity on eNOS and ecSOD expression in mice
Both training protocols showed a significantly higher expression level of eNOS mRNA compared to both aortic tissue of sedentary mice and lung tissue of exercised mice (Fig. 3). In striking contrast, such changes did not occur in lung tissues (Fig. 3). Likewise, physical activity according to both training protocols significantly increased eNOS protein expression in aorta and heart but not in lung tissue and/or vena cava (Fig. 4). Finally, eNOS phosphorylation at Ser1177, which is an estimate for shear stress, was increased by both training protocols in the aorta, in lung tissue and in vena cava (Fig. 5). These data demonstrate that exercise activates eNOS-Ser1177 phosphorylation in venous tissue but initiation of the expression of eNOS does not occur.
Fig 3.
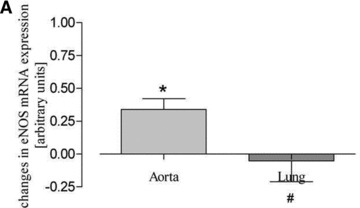
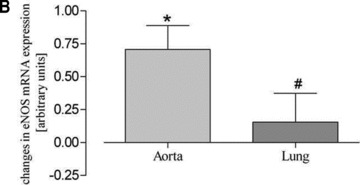
Real-time mRNA measurement of aortic and lung tissues. Aortic and lung eNOS mRNA expression after (A) forced physical activity and (B) voluntary running compared to sedentary controls (set to zero) (*P < 0.05 versus controls, #P < 0.05 versus aorta).
Fig 4.
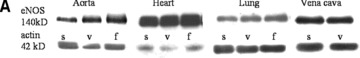
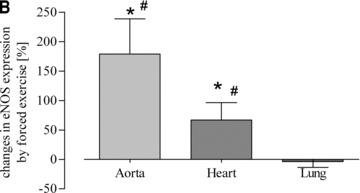
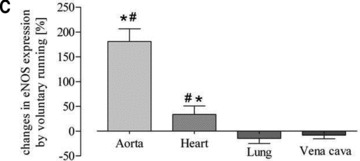
Quantitative evaluation of eNOS protein expression in the aorta, heart, lung and vena cava. (A) Representative Western blot of aorta, heart, lung and vein homogenates from sedentary controls (s), forced exercise (f) and voluntary running mice (v); (B) quantitative evaluation of Western blot signals following forced exercise (*P= 0.0012 versus control) and (C) voluntary running (*P < 0.0001 versus control). Sedentary controls (bars not shown) were set to zero (#P < 0.05 versus lung and vein).
Fig 5.
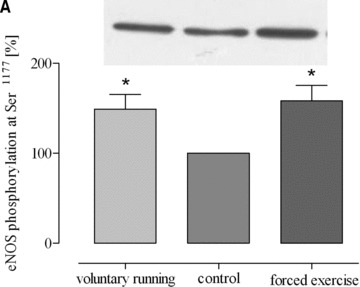
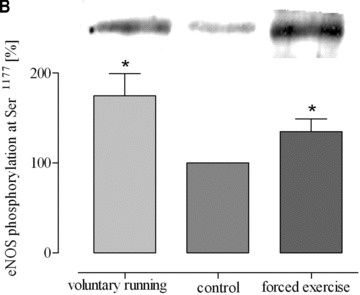
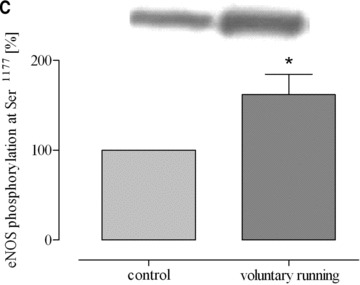
Phosphorylation of eNOS (eNOS-Ser1177) in response to exercise in (A) aorta (*P= 0.0326 versus control), (B) lung tissues (*P= 0.0113 versus control) and (C) vena cava (*P < 0.05 versus control). Blot signals for controls were set to 100%.
To further assess the functional activity of exercise-induced eNOS expression, eNOS-dependent induction of ecSOD was measured [11]. While a higher eNOS expression level was associated with increased ecSOD expression in aortic and heart tissue, there was no change detectable in lung tissue. This was true for both training protocols (Fig. 6). These data suggest that exercise, independently of its type, selectively increases eNOS and subsequently ecSOD expression in arterial but not in venous tissue.
Fig 6.
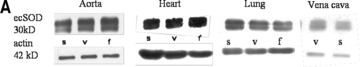
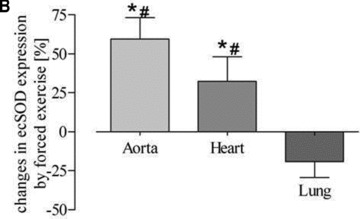
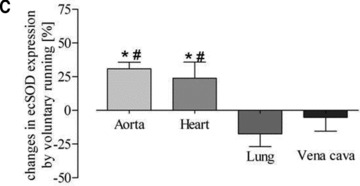
Quantitative evaluation of ecSOD protein expression in the aorta, heart, lung and vena cava. (A) Representative Western blot of aorta, heart, lung and vein homogenates from sedentary controls (s), forced exercise (f) and voluntary running mice (v); (B) quantitative evaluation of Western blot signals following forced exercise (*P= 0.0001 versus control) and (C) voluntary running (*P < 0.0001 versus control). Sedentary controls (bars not shown) were set to zero (#P < 0.05 versus lung and vein).
Effect of exercise on catalase expression
According to the results of our previous investigations [14, 20] we determined catalase protein expression levels in vena cava and lung in comparison to aorta and myocardium. In sedentary mice, we found a 4.5-fold higher catalase expression in vena cava compared to aorta and after voluntary running this difference increased to 10.5-fold (Fig. 7). Likewise, in sedentary mice we found a 2.9-fold higher catalase expression in lung tissue compared to aorta and after voluntary running this difference increased to 6.9-fold (Fig. 7). In accordance, catalase activity was higher in lung tissue than in aorta (Fig. 7D). These data suggest that high catalase activity in vena cava and lung tissue compared to aorta reduces the steady state level of venous hydrogen peroxide which in turn may impair exercise-induced up-regulation of eNOS expression.
Fig 7.

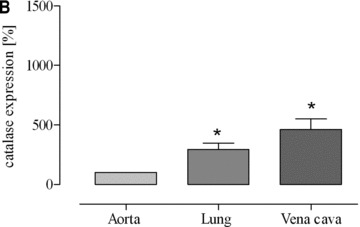
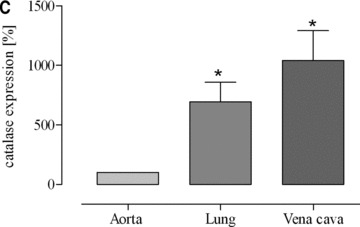
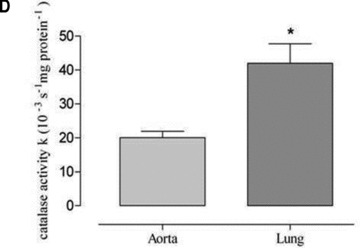
Expression of catalase protein as (A) representative Western blots of vena cava (V), lung (L) and aorta in sedentary (s) and voluntary running mice (e) and as quantitative evaluation in (B) sedentary mice (*P= 0.0066 versus aorta) and (C) voluntary running mice (*P= 0.0093 versus aorta). Catalase activity is shown in aorta and lung homogenates of sedentary mice (*P= 0.0026 versus aorta) (D). Catalase expression in aortic tissues of voluntary running mice compared to sedentary mice was not significantly changed and amounted to 119%± 24.81 (P = 0.4788).
Effect of aminotriazole treatment on exercise-induced eNOS expression
To challenge the hypothesis mentioned above, catalase was blocked by treating the mice with aminotriazole. During this treatment mice were subjected to voluntary running. Inhibition of catalase blunted the arterio-venous difference and resulted in a significant exercise-induced up-regulation of eNOS in vena cava and lung which equalized the effect in the aorta (Fig. 8B). Furthermore, up-regulation of eNOS in vena cava and lung tissue was associated with augmented ecSOD expression (Fig. 8C) indicating increased NO-bioavailability. These data suggest that physiological catalase activity in vena cava may prevent exercise-induced up-regulation of eNOS.
Fig 8.


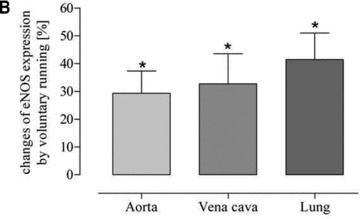
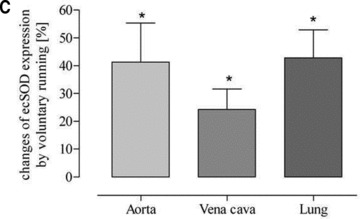
Effect of the catalase inhibitor aminotriazole on exercise-induced up-regulation of eNOS and ecSOD protein expression in voluntary running mice (A) as representative Western blots in isolated aorta (A), lung (L) and vena cava (V); (B and C) as quantitative evaluation (*P < 0.0001 and *P= 0.0021, respectively) compared to sedentary controls (set to zero).
Discussion
We intended to investigate the effects of two different training protocols on the expression of eNOS and ecSOD in venous and lung tissue and to evaluate the underlying molecular mechanisms. Our major new finding is that – in striking difference to arterial tissue – exercise had no effect on the expression of eNOS and ecSOD in venous tissue such as vena cava and lung. Furthermore, treatment with the catalase inhibitor aminotriazole resulted in up-regulation of eNOS in vena cava tissue. These data suggest that physiological venous catalase activity prevents exercise-induced up-regulation of eNOS and ecSOD.
Previous investigations including those from our lab have shown that physical activity increases the expression of arterial eNOS in animals and human beings [1]. These investigations have been performed almost exclusively in large conduit arteries such as aorta or coronary arteries and some data suggest that this effect does not occur in a similar manner throughout the arterial tree [21]. In striking contrast, little is known about the expression and activity of eNOS in low-pressure vasculature such as vena cava or the lung circulation. In one investigation there was a small increase of eNOS after 1 week of exercise in pulmonary arteries of miniature pigs [22] and another investigation showed similar effects after an acute bout of exercise in rats [23]. Thus, the lack of eNOS up-regulation in the venous circulation following exercise is a new observation.
It has been shown that exercise-induced up-regulation of eNOS is completely inhibited in mice with an endothelium-specific overexpression of catalase [14] suggesting that hydrogen peroxide appears to be an important mediator in this process. Several sources of hydrogen peroxide during exercise may be considered. First, exercise increases shear–stress which has been shown to activate endothelial NADPH oxidase and subsequent superoxide generation [24]. In accordance, Ashton et al. demonstrated that exercise induced an increase of a carbon-centred radical in plasma of human beings [25]. Secondly, exercise increases ATP production in skeletal and myocardial muscle during which the coenzyme Q radical can transfer its unpaired electron to molecular oxygen, thereby generating superoxide [26]. In contrast to superoxide itself, hydrogen peroxide generated from non-endothelial superoxide by SODs may reach endothelial cells by diffusion, since the molecule is uncharged. Of note, shear stress is a potent stimulus for the expression of Cu/ZnSOD [27] and exercise has been shown to up-regulate ecSOD in mice [11]. Thus, steady-state levels of hydrogen peroxide likely contribute to the regulation of eNOS expression induced by exercise.
Given this, we have comparatively measured catalase expression and found much more catalase protein in venous tissues such as vena cava and lung. This difference was more pronounced after training, because the expression of catalase increased in the lung, while catalase expression in the aorta and in the heart does not respond to exercise training with a change of catalase expression [14]. In addition, in vivo treatment of normal mice with the catalase inhibitor aminotriazole resulted in up-regulation of eNOS in vena cava and this finding strongly supports a crucial role of catalase in preventing exercise-induced up-regulation of eNOS in venous tissue. Aminotriazole has been shown to irreversibly inhibit human erythrocyte catalase activity in the presence of a constant source of hydrogen peroxide [28]. The daily dose of 666 mg/kg body weight which was used in this study is likely effective in vivo in mice. A previous study showed that this dose completely corrected hypotension in transgenic mice characterized by a 2.5-fold endothelium-specific overexpression of catalase [29].
Physical forces such as shear are important mediators of exercise-induced regulation of eNOS expression [30]. However, blood flow velocity, which strongly contributes to the magnitude of shear stress on endothelial cells in vivo, largely decreases from the aorta to the arterioles and is lowest in veins. Despite this, we could demonstrate that Ser1177 phosphorylation of eNOS in vena cava and lung strongly increases in response to exercise suggesting that increased shear is induced by exercise in these tissues. Nevertheless, this increase of the shear signal did not induce eNOS expression in venous tissue.
The up-regulation of eNOS mRNA and protein expression in arterial tissue in vivo is associated with increased eNOS activity. This has been demonstrated in animals and human beings, e.g. by increased generation of vascular cGMP [8] and by increased coronary flow [9]. In the present investigation we assessed eNOS activity by monitoring the expression of ecSOD as previously described [11]. While exercise-induced up-regulation of ecSOD expression was detected in arterial tissues, there was no ecSOD up-regulation in the lung. This effect is most likely not caused by an insensitivity of venous smooth muscle cells to up-regulate ecSOD in response to exercise, since up-regulation of lung ecSOD was observed in non-exercised trangenic mice carrying an endothelial-specific overexpression of eNOS [12]. Our new data suggest that exercise-induced eNOS expression following inhibition of catalase activity initiates ecSOD expression in venous tissue.
The lack of exercise-induced up-regulation of eNOS and ecSOD in the lung was observed with two different training protocols. Forced physical activity is a short-term high-intensity intervention which might be more efficient than endurance training as indicated by activation of skeletal muscle adaptations [31, 32]. Likewise, we repeatedly detected profound changes in the skeletal muscle such as an increase of citrate synthase activity [17]. In contrast, such changes were absent following voluntary running which is a long-term low intensity training protocol consisting of short bouts of 2–3 min. for a total of 100–120 bouts per night [33]. The absence of increased citrate synthase activity following voluntary training of mice has been observed by other investigators as well [34] and many aspects such as the total running distance and changes of heart weight/body weight ratio, soleus weight/body weight ratio and soleus weight/tibia length ratio closely matches previously reported results and prove the efficacy of this training protocol [33–35]. Despite these considerations, there was no effect of the training protocol on the observed changes of arterial and venous expression of eNOS and ecSOD.
Our data might be helpful to explain why pulmonary rehabilitation programs do not improve lung mechanics and gas exchange, while exercise improves arterial functions such as endothelium-dependent vasodilation and organ perfusion [9, 36]. In view of the significance of lung perfusion for blood arterialization, exercise-induced up-regulation of eNOS in the pulmonary circulation could theoretically improve pulmonary gas exchange. Furthermore, up-regulation of eNOS might reduce the transmural pressure of pulmonary blood vessels by shear-induced endothelium-dependent vasodilation and this might have a protective effect on the blood gas barrier [37]. According to our data, it might be possible to achieve exercise-induced up-regulation of eNOS in pulmonary vessels by therapeutic inhibition of vascular catalase. This would be accomplished by the expense of hypocatalasemia, though. Using such a pharmacological approach, i.e. a pill that works with exercise only [38], might be associated with some side effects which might accelerate cardiovascular disease by increasing vascular oxidative stress and accelerating atherosclerosis. Data from patients with acatalesemia suggest that a complete loss of catalase activity might be associated with a higher incidence of diabetes mellitus, high levels of blood lipids and hyperhomocysteinemia [39]. Furthermore, oral gangrene and ulceration (Takahara disease) might occur. However, in the rare cases of acatalesemia reported so far, only about half of the affected individuals have mild symptoms and the comparison of patients and their non-affected relatives showed no change in life-span, despite the increased risk of diabetes mellitus and arteriosclerosis [39].
Acknowledgments
This study was supported by the Forschungskommission of the Heinrich-Heine-Universitaet Duesseldorf (Projects 9772408 and 9772345).
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Kojda G, Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy. Cardiovasc Res. 2005;67:187–97. doi: 10.1016/j.cardiores.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 2.Gielen S, Schuler G, Hambrecht R. Exercise training in coronary artery disease and coronary vasomotion. Circulation. 2001;103:E1–6. doi: 10.1161/01.cir.103.1.e1. [DOI] [PubMed] [Google Scholar]
- 3.Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training. J Appl Physiol. 2004;97:1119–28. doi: 10.1152/japplphysiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- 4.Brown MD, Hudlicka O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: involvement of VEGF and metalloproteinases. Angiogenesis. 2003;6:1–14. doi: 10.1023/a:1025809808697. [DOI] [PubMed] [Google Scholar]
- 5.Green DJ, Maiorana A, O’Driscoll G, et al. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowles DK, Wamhoff BR. Coronary smooth muscle adaptation to exercise: does it play a role in cardioprotection? Acta Physiol Scand. 2003;178:117–21. doi: 10.1046/j.1365-201X.2003.01130.x. [DOI] [PubMed] [Google Scholar]
- 7.Sessa WC, Pritchard K, Seyedi N, et al. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994;74:349–53. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- 8.Kojda G, Cheng YC, Burchfield J, et al. Dysfunctional regulation of endothelial nitric oxide synthase (eNOS) expression in response to exercise in mice lacking one eNOS gene. Circulation. 2001;103:2839–44. doi: 10.1161/01.cir.103.23.2839. [DOI] [PubMed] [Google Scholar]
- 9.Hambrecht R, Wolf A, Gielen S, et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–60. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 10.Hambrecht R, Adams V, Erbs S, et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–8. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 11.Fukai T, Siegfried MR, Ushio-Fukai M, et al. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000;105:1631–9. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oppermann M, Balz V, Adams V, et al. Pharmacologic induction of vascular extracellular superoxide dismutase expression in-vivo. J Cell Mol Med. 2009;13:1271–8. doi: 10.1111/j.1582-4934.2008.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis ME, Cai H, McCann L, et al. Role of c-Src in regulation of endothelial nitric oxide synthase expression during exercise training. Am J Physiol Heart Circ Physiol. 2003;284:H1449–53. doi: 10.1152/ajpheart.00918.2002. [DOI] [PubMed] [Google Scholar]
- 14.Lauer N, Suvorava T, Rüther U, et al. Critical involvement of hydrogen peroxide in exercise-induced upregulation of endothelial NO-synthase. Cardiovasc Res. 2005;65:254–62. doi: 10.1016/j.cardiores.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 16.Kusnetzov A, Lassnig B, Gnaiger E. Laboratory protocol – citrate synthase, mitochondrial marker enzyme. Mitochondrial Physiology Network. 2003;8(14):1–7. http://www.oroboros.at. [Google Scholar]
- 17.Suvorava T, Lauer N, Kojda G. Physical inactivity causes endothelial dysfunction in healthy young mice. J Am Coll Cardiol. 2004;44:1320–7. doi: 10.1016/j.jacc.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 19.Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970;34:30–8. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- 20.Suvorava T, Kumpf S, Rauch BH, et al. Hydrogen peroxide inhibits exercise-induced increase of circulating stem cells with endothelial progenitor capacity. Free Radic Res. 2010;44:199–207. doi: 10.3109/10715760903402898. [DOI] [PubMed] [Google Scholar]
- 21.Laughlin MH, Rubin LJ, Rush JW, et al. Short-term training enhances endothelium-dependent dilation of coronary arteries, not arterioles. J Appl Physiol. 2003;94:234–44. doi: 10.1152/japplphysiol.00246.2002. [DOI] [PubMed] [Google Scholar]
- 22.Johnson LR, Rush JW, Turk JR, et al. Short-term exercise training increases ACh-induced relaxation and eNOS protein in porcine pulmonary arteries. J Appl Physiol. 2001;90:1102–10. doi: 10.1152/jappl.2001.90.3.1102. [DOI] [PubMed] [Google Scholar]
- 23.Miyauchi T, Maeda S, Iemitsu M, et al. Exercise causes a tissue-specific change of NO production in the kidney and lung. J Appl Physiol. 2003;94:60–8. doi: 10.1152/japplphysiol.00269.2002. [DOI] [PubMed] [Google Scholar]
- 24.Laurindo FR, Pedro Mde A, Barbeiro HV, et al. Vascular free radical release: ex vivo and in vivo evidence for a flow-dependent endothelial mechanism. Circ Res. 1994;74:700–9. doi: 10.1161/01.res.74.4.700. [DOI] [PubMed] [Google Scholar]
- 25.Ashton T, Rowlands CC, Jones E, et al. Electron spin resonance spectroscopic detection of oxygen-centred radicals in human serum following exhaustive exercise. Eur J Appl Physiol Occup Physiol. 1998;77:498–502. doi: 10.1007/s004210050366. [DOI] [PubMed] [Google Scholar]
- 26.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 27.Inoue N, Ramasamy S, Fukai T, et al. Shear stress modulates expression of Cu/Zn superoxide dismutase in human aortic endothelial cells. Circ Res. 1996;79:32–7. doi: 10.1161/01.res.79.1.32. [DOI] [PubMed] [Google Scholar]
- 28.Ou P, Wolff SP. Aminoguanidine: a drug proposed for prophylaxis in diabetes inhibits catalase and generates hydrogen peroxide in vitro. Biochem Pharmacol. 1993;46:1139–44. doi: 10.1016/0006-2952(93)90461-5. [DOI] [PubMed] [Google Scholar]
- 29.Suvorava T, Lauer N, Kumpf S, et al. Endogenous vascular hydrogen peroxide regulates arteriolar tension in vivo. Circulation. 2005;112:2487–95. doi: 10.1161/CIRCULATIONAHA.105.543157. [DOI] [PubMed] [Google Scholar]
- 30.Uematsu M, Ohara Y, Navas JP, et al. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol Cell Physiol. 1995;269:C1371–8. doi: 10.1152/ajpcell.1995.269.6.C1371. [DOI] [PubMed] [Google Scholar]
- 31.Gibala MJ, Little JP, van Essen M, et al. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006;575:901–11. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibala MJ. Molecular responses to high-intensity interval exercise. Appl Physiol Nutr Metab. 2009;34:428–32. doi: 10.1139/H09-046. [DOI] [PubMed] [Google Scholar]
- 33.De Bono JP, Adlam D, Paterson DJ, et al. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol. 2006;290:R926–34. doi: 10.1152/ajpregu.00694.2005. [DOI] [PubMed] [Google Scholar]
- 34.Noble EG, Moraska A, Mazzeo RS, et al. Differential expression of stress proteins in rat myocardium after free wheel or treadmill run training. J Appl Physiol. 1999;86:1696–701. doi: 10.1152/jappl.1999.86.5.1696. [DOI] [PubMed] [Google Scholar]
- 35.Laufs U, Werner N, Link A, et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–6. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 36.Casaburi R, ZuWallack R. Pulmonary rehabilitation for management of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:1329–35. doi: 10.1056/NEJMct0804632. [DOI] [PubMed] [Google Scholar]
- 37.West JB. Comparative physiology of the pulmonary blood-gas barrier: the unique avian solution. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1625–34. doi: 10.1152/ajpregu.00459.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodyear LJ. The exercise pill–too good to be true? N Engl J Med. 2008;359:1842–4. doi: 10.1056/NEJMcibr0806723. [DOI] [PubMed] [Google Scholar]
- 39.Goth L, Rass P, Pay A. Catalase enzyme mutations and their association with diseases. Mol Diagn. 2004;8:141–9. doi: 10.1007/BF03260057. [DOI] [PubMed] [Google Scholar]


