Abstract
Airway smooth muscle cells exhibit phenotype plasticity that underpins their ability to contribute both to acute bronchospasm and to the features of airway remodelling in chronic asthma. A feature of mature, contractile smooth muscle cells is the presence of abundant caveolae, plasma membrane invaginations that develop from the association of lipid rafts with caveolin-1, but the functional role of caveolae and caveolin-1 in smooth muscle phenotype plasticity is unknown. Here, we report a key role for caveolin-1 in promoting phenotype maturation of differentiated airway smooth muscle induced by transforming growth factor (TGF)-β1. As assessed by Western analysis and laser scanning cytometry, caveolin-1 protein expression was selectively enriched in contractile phenotype airway myocytes. Treatment with TGF-β1 induced profound increases in the contractile phenotype markers sm-α-actin and calponin in cells that also accumulated abundant caveolin-1; however, siRNA or shRNAi inhibition of caveolin-1 expression largely prevented the induction of these contractile phenotype marker proteins by TGF-β1. The failure by TGF-β1 to adequately induce the expression of these smooth muscle specific proteins was accompanied by a strongly impaired induction of eukaryotic initiation factor-4E binding protein(4E-BP)1 phosphorylation with caveolin-1 knockdown, indicating that caveolin-1 expression promotes TGF-β1 signalling associated with myocyte maturation and hypertrophy. Furthermore, we observed increased expression of caveolin-1 within the airway smooth muscle bundle of guinea pigs repeatedly challenged with allergen, which was associated with increased contractile protein expression, thus providing in vivo evidence linking caveolin-1 expression with accumulation of contractile phenotype myocytes. Collectively, we identify a new function for caveolin-1 in controlling smooth muscle phenotype; this mechanism could contribute to allergic asthma.
Keywords: phenotype plasticity, asthma, airway remodelling, caveolae, TGF-β1
Introduction
Smooth muscle cells regulate the diameter of hollow organs and are effectors of fibroproliferative remodelling in several disease states. In this regard, bronchial smooth muscle cells regulate airway diameter and play a key role in lung function and airway hyperresponsiveness in asthma [1]. Structural and phenotypic alterations in airway smooth muscle are frequently observed in asthma and include increased airway smooth muscle mass and increased abundance of contractile phenotype marker proteins. Phenotype plasticity of airway smooth muscle is regulated by external stimuli such as growth factors (e.g. transforming growth factor [TGF]-β1) and the extracellular matrix, that signal through a network of intracellular cascades to control the transcription and translation of smooth muscle-specific genes [2–4].
A feature of mature, contractile airway smooth muscle is the presence of abundant caveolae, plasma membrane invaginations that regulate a variety of physiological functions including myocyte contraction and cell proliferation [5–8]. Caveolae in smooth muscle are characterized by the presence of caveolin-1 and caveolin-2 [9] as well as the more recently described cavin family members including polymerase I and transcript release factor (PTRF)/cavin-1 [10, 11]. Caveolin-1 and cavin-1 interact at the plasma membrane which is required for caveola formation [10]. Caveolae exhibit a highly ordered linear array distribution on contractile smooth muscle cells; this because of indirect tethering to the actin cytoskeleton via direct binding of caveolin-1 to β-dystroglycan, which is also a contractile phenotype marker protein [12–15]. Increased caveolin-1 and caveolae abundance in mature myocytes likely maintains a mitotically quiescent phenotype, as disruption of caveolae, or caveolin-1 knockdown is sufficient to activate p42/p44 MAP kinase signalling and cell proliferation [6]. Caveolin-1 functionally represses myocyte proliferation via its caveolin scaffolding domain that binds to (receptor) tyrosine kinases (RTKs), such as epidermal growth factor receptor, platelet-derived growth factor receptor and Src [16–19]. The association of RTK with caveolae and caveolin-1 in airway smooth muscle is reversible, as mitogen stimulation induces the release of caveolin-1 from the activated RTK which facilitates RTK trafficking to non-caveolae membrane [6, 20]. Caveolin-1 thus plays an important role in maintaining airway myocyte quiescent, which is a hallmark of contractile phenotype cells.
The functional role of increased caveolae and caveolin-1 in contractile phenotype smooth muscle cells is not fully established. It may support molecular signalling associated with contraction, as for most smooth muscles, disruption of caveolae or caveolin-1 knockdown is sufficient to reduce agonist-induced Ca2+ responses and contraction [5, 7, 8, 21–28]. Indeed, airway smooth muscle contraction and Ca2+ signalling in response to the bronchoconstrictor agents methacholine, histamine and bradykinin requires intact caveolae, and the receptors and G proteins that regulate contraction in response to these agents are localized to caveolae [5, 7, 8]. In addition, a recent study indicates that the expression of dystrophin, β-dystroglycan, β-, γ- and ɛ-sarcoglycan, which localize to caveolae in airway smooth muscle, is tightly correlated with the expression of contractile phenotype markers and caveolin-1 [14, 15]. Although these studies collectively suggest a functional role for elevated caveolin-1 abundance in the contractile myocyte phenotype, the requirement for caveolin-1 in the acquisition of a contractile phenotype has not yet been explored.
Phenotype plasticity and heterogeneity of airway smooth muscle may contribute to asthma pathogenesis as asthmatic airway smooth muscle is characterized by increased expression of contractile proteins including sm-α-actin, myosin heavy chain (sm-MHC), SM-22 and myosin light chain kinase, and is associated with divergent cellular functions [29–35]. Alterations in airway smooth muscle mass and in contractile protein expression are also seen in animal models of asthma including the guinea pig, which is characterized by increased bronchial and bronchiolar smooth muscle mass and elevated expression of smooth muscle specific proteins such as sm-MHC. Although phenotype plasticity of airway smooth muscle involves regulation of caveolin-1 and caveolae expression in vitro, it is currently unknown whether regulation of caveolin-1 expression by airway smooth muscle is also a feature of airways disease and whether this may be linked with regulation of airway smooth muscle phenotype and function in the remodelled airway wall.
We suggested that the phenotype-dependent expression of caveolin-1 expression directly supports mycocyte maturation by regulating and facilitating cellular signalling involved in smooth muscle specific protein expression and that such a mechanism contributes to airway smooth muscle remodelling in asthma. Using human airway smooth muscle cell lines, and caveolin-1 knockdown strategies, we demonstrate that caveolin-1 is required for TGF-β1-induced myocyte maturation. Moreover, we confirm in vivo using allergen challenged guinea pigs that caveolin-1 expression is increased in the hypertrophied bronchial smooth muscle bundles.
Materials and methods
Cell culture
Canine and human airway smooth muscle cells were used for cell culture studies. Primary cultured canine airway myocytes were established from dissociated canine trachealis as we have described previously [36, 37]. Human bronchial smooth muscle cell lines immortalized by stable expression of human telomerase reverse transcriptase were prepared as described previously [6, 38]. The primary cultured human bronchial smooth muscle cells used to generate human telomerase reverse transcriptase airway smooth muscle cells were prepared from macroscopically healthy segments of second-to-fourth generation main bronchus obtained after lung resection surgery from patients with a diagnosis of adenocarcinoma. All procedures were approved by the Human Research Ethics Board (University of Manitoba, Winnipeg, MB, Canada).
Cells were grown to confluence using Dulbecco’s modified eagle medium (DMEM) supplemented with 10% foetal bovine serum, 50 U/ml streptomycin and 50 μg/ml penicillin. Cultures were maintained in a humidified incubator at 37°C/5% CO2 and media were changed every 2–3 days. For the induction of contractile phenotype myocytes serum-deprivation protocols were used (Fig. 1): confluent cultures of canine (passage number 0 or 1) or human airway smooth muscle cell (passages 10–25) were maintained in Ham’s F12 medium supplemented with 5 μg/ml insulin, 5 μg/ml transferrin and 5 ng/ml selenium (ITS) for 7–14 days. For the induction of contractile phenotype with TGF-β1 cultures were serum-deprived for 2 days in ITS-supplemented Hams F12 media then cells were switched to fresh media supplemented only with TGF-β1 (0.1–10 ng/ml), for 2, 4 or 7 days.
Fig 1.

Preferential expression of caveolin-1 by contractile phenotype airway myocytes. (A) Human airway smooth muscle cell cultures were grown to 50% confluence in serum-enriched (10% FBS) DMEM (serum-fed condition) or grown to confluence and then serum deprived in Ham’s F12 supplemented with ITS (serum-deprived condition) for 7 days. Cell lysates were prepared and analysed by immunoblotting for the abundance of caveolin-1, sm-MHC and β-actin. Densitometric data shown are the means ± S.E. of four experiments. Differences between data were analysed by a two-tailed Student’s t-test for unpaired observations; *P < 0.05. (B) Primary human (left row) and canine (right row) tracheal smooth muscle cells were grown to confluence on cover slips and then serum-deprived for 14 days in Ham’s F12 supplemented with ITS. After fixation cells were dually immunolabelled for sm-MHC (red Cy3; upper row) and caveolin-1 (green FITC; lower row). For the images shown, nuclei of human cells were also labelled with the DNA dye H33342 (blue). Micrographs in each row are matched fields and arrows are drawn for orientation and to highlight elongate, contractile phenotype myocytes. (C) Left panel shows a typical image captured by laser scanning cytometry of sm-MHC staining (red) of in a 14-day serum deprived canine tracheal myocyte culture. The image is shown with an overlay of the phantom contour lattice (yellow circles) used to identify regions of interest in which integrated fluorescence of both sm-MHC and caveolin-1 (green) were determined. After determining background fluorescence each region was classified as either sm-MHC– (fluorescence equal to or less than background) or sm-MHC+ (fluorescence greater than background fluorescence). The right panel shows the results of measuring coincident caveolin-1 and sm-MHC staining in 1054 contour regions of interest in five scan fields for three different cell cultures. Differences in mean integrated fluorescence between sm-MHC+ and sm-MHC– groups were analysed by a two-tailed Student’s t-test for unpaired observations; *P < 0.001.
Western blot analysis
Protein content in samples was determined using the Bio-Rad protein assay with bovine serum albumin as a reference (Bio-Rad, Hercules, CA, USA). Equal amounts of protein from total protein lysates were subjected to electrophoresis, transferred to nitrocellulose membranes and analysed for the proteins of interest using specific primary and horseradish peroxidase (HRP)-conjugated secondary antibodies. Bands were subsequently visualized on film using enhanced chemiluminescence reagents and were quantified by densitometry using Totallab™ software (Nonlinear dynamics; Newcastle, UK). Expression of proteins of interest was normalized to the expression of β-actin.
Immunocytochemistry
Airway smooth muscle cells were plated onto pre-cleared glass cover slips in six-well culture dishes. Cells were fixed for 15 min. at 4°C in cytoskeletal buffer [10 mM 2-(N-morpholino)ethanesulfonic acid (MES), 150 mM NaCl, 5 mM glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), 5 mM MgCl2 and 5 mM glucose at pH 6.1] containing 3% paraformaldehyde. Cells were then permeabilized by incubation for 5 min. at 4°C in cytoskeletal buffer containing 3% paraformaldehyde and 0.3% Triton X-100. For immunofluorescence microscopy, fixed cells were first blocked for 2 hrs at room temperature in Cyto-Tris-buffered saline (TBS) buffer (20 mM Tris base, 154 mM NaCl, 20 mM EGTA and 20 mM MgCl2 at pH 7.2) containing 1% bovine serum albumin and 2% normal donkey serum. Incubation with primary antibodies occurred overnight at 4°C in Cyto-TBS containing 0.1% Tween 20 (Cyto-TBST). Incubation with FITC- or Cy5-conjugated secondary antibodies was for 2 hrs at room temperature in Cyto-TBST. Nuclei were stained with Hoechst 33342 (10 μg/ml). Fluorescence micrographs of stained cells were then obtained using an inverted Fluoview Confocal Microscope (Olympus, Markham, ON, Canada).
Laser scanning cytometry
For quantitative assessment of the of co-expression of caveolin-1 and sm-MHC of individual cells of serum deprived cultures, fluorescent labelling in dual-labelled cultures (as described in ‘Immunocytochemistry’) as measured using an inverted microscope-based iCys Research Laser Scanning Cytometer (CompuCyte Corp., Cambridge MA, USA). For analysis fluorescent images acquired with calibrated photomultiplier tubes were overlaid with a lattice of 1054 circular contours (regions of interest) that enable comprehensive sampling of the entire scanned fields; the diameter of each contour was 5 μM, thus was approximately equivalent to the minimum width of individual nuclei. From the contour lattice integrated fluorescence, after background adjustment, of both sm-MHC (red) and caveolin-1 (green) in each region of interest was determined. Coincident staining of caveolin-1 and sm-MHC in each contour was determined in at least five scan fields for three different cell cultures for analyses.
siRNA transfection
The small interfering RNA (siRNA) Generation Kit (Gene Therapy Systems, San Diego, CA, UDA) was used to prepare dicer-generated siRNA from human airway smooth muscle cDNA as we have described previously [38] with primers that amplified caveolin-1 (forward 5′-GTAGACTCGGAGGGACATC-3′; reverse 5′-GTCCGCATCAACTTGCAG-3′). Primer sequences also included the 52-bp T7 promoter sequence linkers (5′-GCGTAATACGACTCACTATAGGGAGA-target DNA-3′), which were incorporated into the DNA template PCR product to allow for in vitro transcription with the TurboScript T7 Transcription Kit (Gene Therapy Systems). Double-stranded RNA was generated using the TurboScript T7 RNA Transcription Kit and then diced into 21 bp fragments using recombinant human dicer enzyme following the manufacturer’s instructions (Gene Therapy Systems). Transfection of siRNA (1 μg/ml) was performed with Genesilencer reagent (Gene Therapy Systems) according to manufacturer’s instructions, and cells were maintained in serum-free Ham’s F12 thereafter. For negative control studies the transfection protocol was performed in the absence of siRNA.
In separate studies, caveolin-1 knockdown was achieved using a commercially available 21 bp, double-stranded siRNA targeted against a sequence between residues 529 and 589 of the caveolin-1 transcript (Qiagen, Mississauga, ON, Canada). Transfection to suppress caveolin-1 protein expression was carried out as we have previously described [6, 7, 38]. Cells were transfected using 2.5 μg/ml of siRNA in combination with 6 μl/μg siRNA of RNAiFect™ transfection reagent (Qiagen). For negative control studies the transfection protocol was performed in the absence of siRNA.
As a third strategy we used to constitutively silence caveolin-1, human airway smooth muscle were infected with lentivirus harbouring short hairpin RNA directed against caveolin-1 and after selection with puromycin, cell lines that stably express caveolin-1 short hairpin (sh)RNA were established as we have described [39]. shRNA containing constructs were from Open Biosystems distributed by the Manitoba Centre for Systems Biology and Proteomics at the University of Manitoba. The caveolin-1 shRNA construct (accession #NM_001172897), distributed in a bacterial culture of Escherichia coli (DH5α) included a ‘stem’ of 21 sense (GAGGGTCCTGTACAATCTCAT), antisense base pairs, and a 6 bp loop, cloned into the lentiviral vector pGIPZ. Individual colonies were grown up in Luria-Bertani (LB) broth with ampicillin (100 μg/ml), purified using a Qiagen Maxi-prep Kit. A vesicular stomatitis virus G(VSVG) pseudo-typed lentiviral vector was made using human embryonic kidney (HEK) 293T cells by calcium phosphate transfection of purified caveolin-1 shRNA plasmid, virus packaging vector (8.2Δvpr), and a VSVG plasmid as described previously [40]. Primary human airway myocytes were grown to 70% confluence and transduced at a multiplicity of infection (MOI) of 6, in the presence of 8 μg/ml polybrene, for 2 hrs. Excess viral particles were removed, and the transduced cells were cultured in fresh medium for 2 days. Cell cultures exhibiting stable expression of the shRNA (Cav-1silence) were selected by growing in culture media containing puromycin (4 μg/ml) for 3 weeks. For control cells (Cav-1scramble) a pGIPZ vector harbouring ‘scrambled’ non-coding shRNA was also prepared and used to generate lentivirus for transduction of the same primary human airway smooth muscle (HASM) cell lines that were used to generate caveolin-1-silenced stable cultures.
Guinea pig asthma model
Outbred, male, specified pathogen free Dunkin Hartley guinea pigs (Harlan, Heathfield, UK) weighing 250–300 g were sensitized to ovalbumin using Al(OH)3 as adjuvant. The animals were used experimentally 4 weeks later. All protocols described in this study were approved by the University of Groningen Committee for Animal Experimentation. Provocations were performed by inhalation of aerosolized solutions of ovalbumin (Sigma, St. Louis, MO, USA) or saline under conscious and unrestrained conditions, as described previously [41]. Allergen inhalations were discontinued when the first signs of respiratory distress were observed. No anti-histaminic was needed to prevent anaphylactic shock. Guinea pigs were challenged with either ovalbumin or saline once weekly, for 12 consecutive weeks, to induce airway remodelling as described previously [42, 43]. Twenty-four hours after the last challenge, guinea pigs were killed by experimental concussion, followed by rapid exsanguination. The lungs were immediately resected and kept on ice for further processing.
Histochemistry
Transverse cross-sections of the main bronchi in both right and left lung lobes were used for immunohistochemical analysis of caveolin-1. Sections were blocked using 0.3% hydrogen peroxide and stained using a polyclonal anti-caveolin-1 antibody (SantaCruz Biotechnology, Santa Cruz, CA, USA). The primary antibody was visualised using a HRP-linked secondary antibody and diaminobenzidine. Airways within sections were digitally photographed. All immunohistochemical measurements were carried out digitally using quantification software.
Materials
Cell culture media (DMEM and Ham’s F12), supplements (foetal bovine serum and ITS-A) and antibiotics (penicillin and streptomycin) were obtained from Invitrogen (Carlsbad, CA, USA). Caveolin-1 monoclonal antibody was from BD Biosciences (San Jose, CA, USA) and polyclonal antibody from SantaCruz Biotechnology. Fluorescein isothiocyanate (FITC)- and Cy5-conjugated secondary antibodies were from Jackson ImmunoResearch (West Grove, PA, USA). Recombinant human TGF-β1, β-actin primary antibody, sm-α-actin primary antibody, sm-MHC primary antibody, calponin primary antibody anti-rabbit and antimouse HRP conjugated secondary antibodies, were from Sigma-Aldrich (St. Louis, MO, USA). Anti-phospho-Thr37/46–4E-BP1 primary antibody and anti-4E-BP1 primary antibody were all from Cell Signaling Technology (Beverly, MA, USA). Enhanced chemiluminescence reagent was from Amersham (Oakville, ON, Canada).
Results
Caveolin-1 is preferentially expressed by contractile phenotype airway myocytes
We previously reported that proliferating human airway smooth muscle cells in culture express reduced levels of caveolin-1 protein [6]. We first confirmed the preferential expression of caveolin-1 in cell culture conditions that promote acquisition of a contractile phenotype that is characterized by enhanced expression of marker proteins such as sm-MHC, sm-α-actin and calponin [44]. Immunoblot analysis of caveolin-1 abundance in 7 day serum deprived human airway smooth muscle cultures revealed it was increased approximately 2-fold compared to myocytes in proliferating cultures (Fig. 1A). This paralleled the accumulation of sm-MHC in the same cultures, thus revealing correlation between the expression of contractile phenotype markers and caveolin-1 by human airway smooth muscle cells.
To more precisely assess caveolin-1 and sm-MHC expression in individual myocytes we employed serum-deprived primary cultured human airway and canine tracheal smooth muscle. Confocal microscopy of dually labelled human and canine myocyte cultures reveal divergent labelling of individual cells for sm-MHC, and this pattern appeared to be mimicked by that for caveolin-1 (Fig. 1B). To quantify the degree to which caveolin-1 was co-expressed in individual cells we next used laser scanning cytometry of 7 day serum-deprived canine tracheal myocyte cultures. We selected this culture system for analysis because we have shown it to be a unique and effective model to study myocyte phenotype switching using microscopy because it retains a monolayer organization and two disparate subpopulations develop that differ markedly in morphological and phenotypic features [36]. As confirmed in Figure 1B, a large fraction of cells exhibit a relatively small, flat oblong shape and are devoid of contractile proteins, whereas the remaining myocytes develop tracks of cells having an elongate hypertrophic morphology that are uniquely enriched in contractile phenotype markers, including sm-MHC and sm-α-actin [35]. Our laser scanning cytometry analysis clearly demonstrated caveolin-1 expression was selectively enriched in those myocytes that accumulated sm-MHC in serum free conditions, whereas in myocytes that were essentially sm-MHC– based on fluorescent staining, caveolin-1 was low and significantly less that in sm-MHC-rich cells (Fig. 1C). This confirms that abundant caveolin-1 expression is a specific feature of mature, contractile airway smooth muscle.
TGF-β1-induced contractile protein expression by airway smooth muscle requires caveolin-1
We next tested the functional significance of the increased expression of caveolin-1 by mature airway smooth muscle for the induction of contractile protein expression. To investigate this, we used the growth factor TGF-β1, which induces the expression of contractile phenotype marker proteins in primary human airway smooth muscle cells [45]. In addition, autocrine production of TGF-β1 has been reported to be responsible for the serum-deprivation induced phenotype maturation of airway smooth muscle cells in culture [46]. We first established the functional effects of TGF-β1 on contractile protein expression by the human airway smooth muscle cell lines. In these protocols, cells were grown to confluence and then serum-deprived in the absence or presence of TGF-β1 for 2, 4 or 7 days. As reported for primary myocytes, TGF-β1 induced a profound increase in the expression of the contractile phenotype markers sm-α-actin and calponin (Fig. 2). The induction of these marker proteins was clearly visible after 2 days of TGF-β1 exposure, although 4 and 7 days of exposure did further increase sm-α-actin and calponin expression (e.g. calponin expression induced by TGF-β1 on days 4 and 7 was 125 ± 19% and 124 ± 31% of that measured after 2 days of TGF-β1 exposure; Fig. 2A). Concentration response relationships of TGF-β1 after 2 days of treatment indicated that the induction of calponin and sm-α-actin expression was submaximal at a concentration of 1 ng/ml (Fig. 2B–D). Notably, TGF-β1 treatment did not augment spontaneously increased caveolin-1 expression during serum deprivation at any of the time-points or concentrations studied (Fig. 2E). Because 2 days of TGF-β1 treatment with 1 ng/ml was found to induce sub-maximal induction of contractile protein expression, this condition was used in all further experiments.
Fig 2.
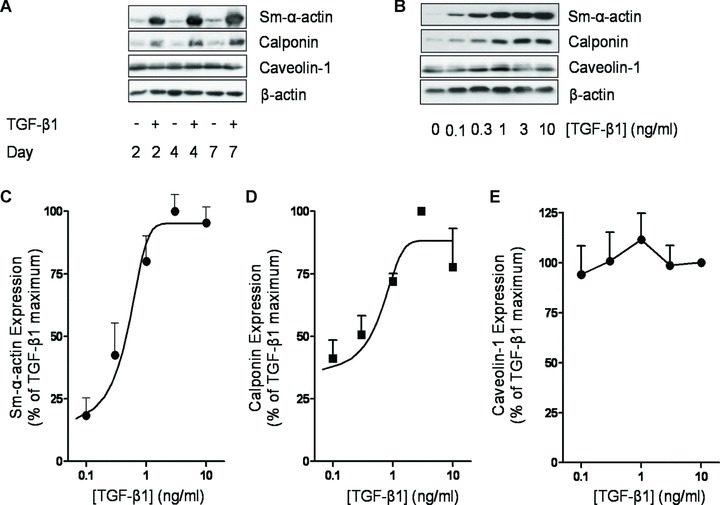
TGF-β1 induces contractile phenotype marker expression in airway smooth muscle. (A) Human airway smooth muscle cells were grown to confluence, serum-deprived for 2 days and subsequently treated for 2, 4 or 7 days in F-12 media with 10 ng/ml TGF-β1, as indicated. Cell lysates were then prepared and Western analysed for the expression of sm-α-actin, calponin, caveolin-1 or β-actin. (B) Human airway smooth muscle cells were grown to confluence, serum-deprived for 2 days in F-12 media, followed by 2 days treatment w increasing concentrations (0.1–10 ng/ml) of TGF-β1, as indicated. Cell lysates were then prepared and analysed by Western blot for the expression of sm-α-actin, calponin, caveolin-1 or β-actin. Expression of (C) sm-α-actin, (D) calponin and (E) caveolin-1 was also analysed by densitometry and normalized to the maximal response induced by TGF-β1, which was set at 100%. β-actin expression was used to correct for small differences in protein loading.
To investigate whether caveolin-1 expression by airway smooth muscle was required for contractile phenotype maturation induced by TGF-β1, we next employed an siRNA knockdown strategy to reduce caveolin-1 expression. Initial experiments indicated that caveolin-1 expression was clearly reduced by siRNA treatment 48 hrs after transfection with maximal reduction of ∼50% being reached at 72 hrs (Fig. 3A, [6, 7]). Notably, this reduction in caveolin-1 expression is quantitatively similar in magnitude to the opposing increase in caveolin-1 expression that we observe when proliferating myocytes are subjected to prolonged serum withdrawal (Fig. 1A). Indeed we previously reported that this level of caveolin-1 silencing is sufficient to promote spontaneous p42/p44 MAP kinase activation and cell proliferation of airway smooth muscle cells [6]. To utilize the window of caveolin-1 knockdown optimally, we transfected airway smooth muscle cells with caveolin-1 siRNA and 48 hrs thereafter stimulated them with TGF-β1. Cell lysates were harvested 48 hrs later (96 hrs after transfection). Three distinct knockdown strategies were employed: (1) a commercially available siRNA directed against a single sequence of the caveolin-1 transcript, (2) a dicer-generated siRNA mix, produced from a 537 bp sequence within the caveolin-1 transcript digested using recombinant dicer enzyme and (3) stable silencing using shRNAi delivered by lentivirus infection to generate Cav-1silence human airway smooth muscle cell lines. We confirmed that both the siRNA and shRNA strategies did not impair smooth muscle cell viability. In fact, cell proliferation and ERK activation were enhanced by the caveolin-1 knockdown ([6] and unpublished observations).
Fig 3.
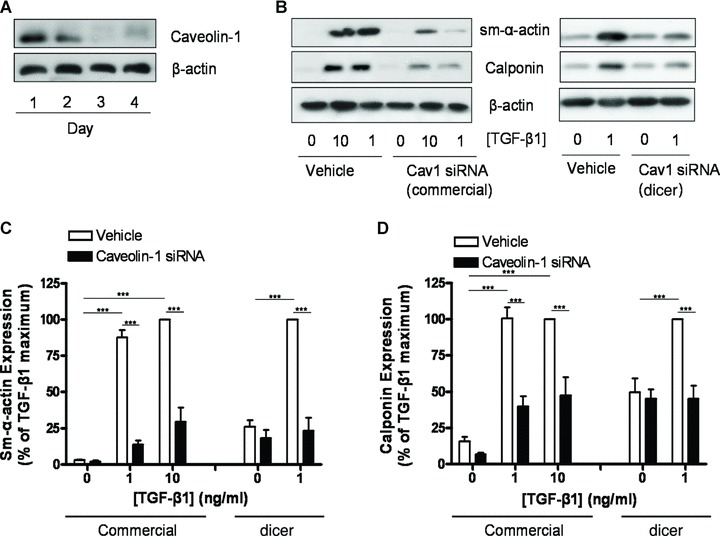
Caveolin-1 knockdown reduces TGF-β1 induced contractile phenotype marker expression. (A) Human airway smooth muscle cells were grown to 70–80% confluence and then serum-deprived in F-12 media. Concomitant with serum-deprivation, cells were transfected with an siRNA directed against caveolin-1 and maintained in culture for up to 4 days. Cell lysates were then prepared and analysed for caveolin-1 and β-actin content. (B) Human airway smooth muscle cells were grown to 70–80% confluence and then serum-deprived in F-12 media for 2 days. Concomitant with serum-deprivation, cells were transfected with either a commercially available siRNA directed against caveolin-1 or a dicer-generated siRNA mix directed against caveolin-1. Cells treated with transfection reagent alone served as controls (vehicle). Caveolin-1 knockdown was allowed to occur for 2 days in serum-free F-12 media, after which cells were treated with TGF-β1 (1 or 10 ng/ml, as indicated) for another 2 days. Cell lysates were then prepared and analysed for sm-α-actin, calponin and β-actin content. Expression of (C) sm-α-actin and (D) calponin was analysed by densitometry and normalized to the maximal response induced by TGF-β1, which was set at 100%. β-actin expression was used to correct for small differences in protein loading. Differences between data were analysed by two-way anova, with post hoc Bonferroni’s t-test for multiple comparisons. **P < 0.01; ***P < 0.001.
Both the commercially available siRNA and the dicer-generated siRNA mix strongly reduced the potential for TGF-β1 to induce contractile phenotype marker expression by airway smooth muscle (Fig. 3). The reduction in marker expression by the commercially available caveolin-1 siRNA was profound, particularly for sm-α-actin, and the reduction in marker expression induced by the dicer-generated siRNA was almost complete. These data indicate a requirement for increased expression of caveolin-1 in TGF-β1 induced contractile protein accumulation. Baseline expression of marker proteins also appeared to be reduced by the caveolin-1 siRNA in some conditions, but this effect did not reach statistical significance.
We next investigated whether reduced induction of contractile phenotype marker expression was associated with a change in TGF-β1 induced intracellular signalling. TGF-β1 activates phosphatidylinositol-3-kinase signalling, which leads to the phosphorylation of 4E-BP1. 4E-BP1 phosphorylation releases its inhibitory function to facilitate protein translation, which is required for airway smooth muscle hypertrophy and phenotype maturation induced by TGF-β1[37, 47, 48]. Indeed, TGF-β1 induced the phosphorylation of 4E-BP1 in a concentration dependent manner (Fig. 4A). Interestingly, caveolin-1 knockdown using either the commercially available or the dicer-generated siRNA strongly reduced, or even abrogated the capacity for TGF-β1 to induce 4E-BP1 phosphorylation, suggesting that this mechanism is in part responsible for the observed effects on calponin and sm-α-actin expression (Fig. 4B and C). These results were confirmed using the Cav-1silence human airway smooth muscle cell line that exhibits >60% reduced expression of caveolin-1 protein. Our findings show that constitutive suppression of caveolin-1 expression markedly limits capacity for TGF-β1-induction of sm-α-actin expression and 4E-BP1 phosphorylation (Fig. 4D).
Fig 4.

Caveolin-1 knockdown reduces TGF-β1 induced 4EBP-1 phosphorylation. (A) Human airway smooth muscle cells were grown to confluence and subsequently serum-deprived for 2 days in F-12 media in the absence or presence of increasing concentrations (0.1–10 ng/ml) of TGF-β1, as indicated. Cell lysates were then prepared and Western analysed for the expression of phospho-(Thr37/46)-4E-BP-1, total 4E-BP-1 and β-actin content. (B) Human airway smooth muscle cells were grown to 70–80% confluence and then serum-deprived in F-12 media for 2 days. Concomitant with serum-deprivation, cells were transfected with a dicer-generated siRNA mix directed against caveolin-1. Cells treated with transfection reagent alone served as controls (vehicle). Caveolin-1 knockdown was allowed to occur for 2 days in serum-free F-12 media, after which cells were treated with TGF-β1 (1 ng/ml) for another 2 days. Cell lysates were then prepared and analysed for phospho-(Thr37/46)-4E-BP-1, total 4E-BP-1 and β-actin content. (C) Human airway smooth muscle cells were grown to 70–80% confluence and then serum-deprived in F-12 media for 2 days. Cells were transfected with either a commercially available siRNA directed against caveolin-1 or a dicer-generated siRNA directed against caveolin-1. Cells treated with transfection reagent alone served as controls (vehicle). Caveolin-1 knockdown was allowed to occur for 2 days in serum-free F-12 media, after which cells were treated with TGF-β1 (1 or 10 ng/ml, as indicated) for another 2 days. Cell lysates were then prepared and analysed for phospho-(Thr37/46)-4E-BP-1, total 4E-BP-1 and β-actin content. Expression of phospho-(Thr37/46)-4E-BP-1 was analysed by densitometry and normalized to the maximal response induced by TGF-β1, which was set at 100%. β-actin expression was used to correct for small differences in protein loading. Differences between data were analysed by two-way anova, with post hoc Bonferroni’s t-test for multiple comparisons. *P < 0.05; **P < 0.01; ***P < 0.001. (D) Human airway smooth muscle cell lines (Cav-1silence) stably expressing shRNA against caveolin-1, and Cav-1scramble, which stably express a control, non-coding shRNA were treated for 48 hrs with TGF-β1 (1 ng/ml). Cell lysates were prepared and analysed for the proteins of interest using Western blotting.
Caveolin-1 expression is increased in smooth muscle after allergen challenge in guinea pigs
Our current results and published data [6–8] indicate a role for caveolin-1 in contractile function and contractile phenotype maturation of airway smooth muscle. Increased contraction of smooth muscle, and increased expression of contractile phenotype markers are characteristic for asthma, and can be mimicked in animal models of asthma [43]. Guinea pigs repeatedly challenged with ovalbumin develop airway hyperresponsiveness, airway smooth muscle thickening, increased pulmonary contractile protein expression and increased contractile capacity of airway smooth muscle [41]. Thus, to determine whether our in vitro findings were related to increased contractile airway smooth muscle mass in vivo, we determined caveolin-1 expression by airway smooth muscle using guinea pigs subjected to repeated allergen exposure. As demonstrated in Figure 5A and B, Western analysis of whole lung indicated that repeated allergen exposures increased caveolin-1 expression. The increase in caveolin-1 was due, in part, to a specific increase in its expression by the airway smooth muscle, as determined using quantitative immunohistochemistry (Fig. 6A and B). These data mimic these we have reported for contractile phenotype marker proteins [42, 43]. Thus, it appears that increased caveolin-1 expression is a feature of allergen-induced airway smooth muscle remodelling in this animal model.
Fig 5.

Caveolin-1 expression is increased in the lungs of allergen challenged guinea pigs. Ovalbumin sensitized guinea pigs were challenged using ovalbumin once a week for 12 weeks. Twenty-four hours after the last allergen exposure, lung tissue was collected for further analyses. (A) Whole lung expression of caveolin-1 was determined using Western blotting for caveolin-1 or β-actin to correct for small differences in protein loading. Densitometric analysis shown in (B) indicates increased caveolin-1 expression after allergen exposures. Densitometric data shown are the means ± S.E. of seven saline and six ovalbumin challenged animals. Differences between data were analysed by a two-tailed Student’s t-test for unpaired observations; *P < 0.05.
Fig 6.

Increased caveolin-1 expression in the airway smooth muscle bundle of allergen challenged guinea pigs. Ovalbumin sensitized guinea pigs were challenged using ovalbumin once a week for 12 weeks. Twenty-four hours after the last allergen exposure, lung tissue was collected for further analyses. (A) Airway smooth muscle expression of caveolin-1 was determined using immunohistochemical analysis for caveolin-1 which showed a clear positive staining within the airway smooth muscle bundle. Airway smooth muscle bundles in sections of saline challenged animals or ovalbumin challenged animals were then digitally photographed and caveolin-1 intensity was quantified. Densitometric data shown in (B) are the means ± S.E. of five saline and five ovalbumin challenged animals. Inflammatory cells within the muscle bundle were excluded from the quantification procedure. Differences between data were analysed by a two-tailed Student’s t-test for unpaired observations; *P < 0.05.
Discussion
This study indicates that caveolin-1 is a marker of the mature, contractile smooth muscle cell phenotype and is required for contractile protein expression induced by the growth factor TGF-β1. These findings are in line with previous studies indicating that caveolin-1 expression and caveolae number are highest in airway and vascular myocytes with a contractile phenotype [6, 49, 50]. Here we reveal that abundant expression of caveolin-1 promotes phenotype maturation by TGF-β1, as demonstrated by our experiments showing that silencing of caveolin-1 expression prevents TGF-β1-induced contractile protein accumulation. This effect was associated with a reduction in the capacity of TGF-β1 to induce the phosphorylation of 4E-BP1, a downstream effector of PI 3 kinase/Akt signalling that is required for contractile phenotype maturation and cellular hypertrophy [48]. Finally, we demonstrate that caveolin-1 expression is increased in the bronchial smooth muscle bundle of guinea pigs after repeated allergen exposures. Collectively, this indicates that caveolin-1 plays a key regulatory role in contractile phenotype maturation of airway smooth muscle. This mechanism may contribute to increased airway smooth muscle contractility associated with asthma, and more broadly in hyperconstrictive disorders of hollow organs.
It appears that caveolin-1 plays several key roles in smooth muscle contraction and contractile phenotype maturation, and is a determinant of functional characteristics of myocytes. First, as demonstrated in this study, increased caveolin-1 expression subserves acquisition of a mature, contractile phenotype that is characterized by increased expression of contractile and contraction regulatory proteins (e.g. sm-α-actin, calponin). Second, via its caveolin scaffolding domain, caveolin-1 plays a facilitating role in excitation contraction coupling by enhancing Ca2+ mobilization in response to contractile agonists [7, 8, 21, 22, 24, 25]. This facilitatory role is likely associated with the ability of caveolin-1 to recruit and organize Gq proteins, ion channels, Ca2+ regulatory molecules and signalling effectors such as phospholipase C in caveolae [51]. Third, via its WW domain, caveolin-1 connects to the dystrophin-dystroglycan complex, which is expressed by contractile myocytes, allowing interaction with the actin cytoskeleton [12–14]. This interaction could play a structural role, by supporting the formation of the characteristic Ω shape of caveolae, and a functional role by spatially organizing receptors and signalling effectors of contraction with intracellular Ca2+ stores in the sarcoplasmatic reticulum and mitochondria [12, 14, 52, 53]. In parallel, caveolin-1 prevents the activity of proliferative phenotype myocytes, as it represses the activity of RTKs and non-RTKs that induce myocyte proliferation and are sequestered in myocyte caveolae [6, 16, 19, 54]. This repressive role of caveolin-1 is reversed by RTK mitogens that induce caveolin-1 phosphorylation and trafficking of the activated RTK to non-caveolae membrane where activation of the p42/p44 MAP kinase cascade is achieved [6, 20]. The repressive role of caveolin-1 in myocyte proliferation is further illustrated by studies that show reduced expression of caveolin-1 is a feature of proliferating smooth muscle cells and that experimental siRNA knockdown of caveolin-1 is sufficient for the induction of smooth muscle cell proliferation and spontaneous p42/p44 MAP kinase activation [6, 20]. In contrast, ectopic overexpression of caveolin-1 silences mitogen responses and induces cell cycle arrest [54]. Collectively, the results of this study and previous studies indicate that caveolin-1 is not only a marker of myocyte phenotype but also a key determinant of the phenotypic state of the smooth muscle and in the functions that are associated with it.
Despite these findings caveolin-1 knockout mice do not lack expression of smooth muscle in the airways or in other organs and, although physiological differences from genetic controls in the lungs and cardiovascular system do exist, they do not suffer from major defects in smooth muscle contraction or intrinsic contractile regulation [23]. Species differences are not likely to account for these effects, as caveolin-1 induction in contractile phenotype myocytes is a feature observed in both human and canine airway smooth muscle cells in the current study, and is also seen in rat arterial smooth muscle [49, 50]. Apparently, smooth muscle differentiation during development is not affected in caveolin-1 knockout mice, which suggests that not all growth factors involved in smooth muscle differentiation and maturation require the presence of caveolin-1 for their effects on smooth muscle specific gene expression. A similar discrepancy exists for the role of caveolin-1 in calcium handling and contraction as disruption of caveolae or knockdown of caveolin-1 reduces muscarinic receptor mediated calcium mobilization in airway smooth muscle cells [7], whereas contraction of tracheal smooth muscle strips of caveolin-1 knockout mice in response to cholinergic stimulation is not reduced (Halayko et al., unpublished observations). It appears therefore that knockout mice functionally compensate for the lack of caveolin-1, or the role of caveolin-1 in smooth muscle phenotype and function in smooth muscle cells is highly species or context specific.
Our results indicate a requirement for caveolin-1 in TGF-β1 induced contractile protein accumulation in airway smooth muscle. This appears to contrast to previous studies that show caveolin-1 is an inhibitor of TGF-β1 signalling by inhibiting the activity of the TGF-β1 type I receptor and Smad2 signalling [55]. Also, caveolin-1 knockout mice have an elevated bronchoalveolar lavage fluid expression of TGF-β1, and have fibrotic lungs and airways [56]. However, although caveolin-1 inhibits certain aspects of TGF-β1 signalling, more recent studies indicate that regulation of TGF-β1 signalling by caveolin-1 is, in fact, pathway specific. As such, caveolin-1 inhibits TGF-β1 induced Smad3 phosphorylation in human dermal fibroblasts, but facilitates TGF-β1 induced signalling to the PI 3 kinase/Akt pathway in the same cells [57]. Moreover, although TGF-β1 induced Smad2/3 phosphorylation is maintained in primary rat mesangial cells treated with cholesterol depleting agents and in murine caveolin-1 knockout mesangial cells, signalling to RhoA in both cell systems is completely abrogated [58]. This illustrates that caveolin-1 plays key roles (both facilitating and repressive) in directing TGF-β1 signalling to specific intracellular pathways. Our results indicate that caveolin-1 is required for TGF-β1 induced signalling to 4E-BP1, a downstream target of the PI 3 kinase/Akt signalling pathway. Clearly, the role of caveolin-1 in TGF-β1 signalling is complex and future mechanistic studies are needed to clarify this differential signalling in more detail. The key role of caveolin-1 in TGF-β1 signalling suggests that aberrant expression of caveolin-1 protein may contribute to structural remodelling and disease pathogenesis. Indeed, a strong reduction of caveolin-1 expression has been observed in idiopathic pulmonary fibrosis, a progressive fibrotic disorder of the lung [59]. Caveolin-1 expression is particularly reduced within the fibrotic lesions, and appears to facilitate fibroblast proliferation. A reduction in the pulmonary expression of caveolin-1 is also observed in patients suffering from pulmonary hypertension, although interestingly, in this disease the aberrant caveolin-1 expression profile appears strongly cell type specific [60, 61]. Although caveolin-1 expression is reduced in the whole lung homogenate and within the plexiform vascular lesions, caveolin-1 abundance within the contractile smooth muscle layer is actually enhanced. Enhanced smooth muscle expression of caveolin-1 may contribute to increased vascular resistance by facilitating Ca2+ mobilization [61], and, as the results from the present study suggest, contractile protein expression by the myocyte.
Airway smooth muscle contributes to disease pathogenesis in obstructive airways diseases such as asthma and COPD [1]. Studies on the stereology, phenotype and function of airway smooth muscle in these diseases reveal an increased smooth muscle mass, particularly in asthma, that is accompanied by an increased contractile function of the muscle and, at least in asthma, by increased expression of contractile phenotype markers and increased Ca2+ mobilization [29, 30, 62–67]. We used a guinea pig model of asthma to determine caveolin-1 expression within the bronchial smooth muscle bundle after repeated allergen exposures. Remodelling of airway smooth muscle in this model is characterized by an increase in smooth muscle mass, myocyte sm-MHC expression and smooth muscle contractility [43]. Our results indicate that caveolin-1 expression by airway smooth muscle is indeed increased as a result of repeated allergen exposures, which may contribute to smooth muscle maturation as our in vitro studies using human airway smooth muscle indicate.
In conclusion, the results from our study indicate that caveolin-1 is a marker of the contractile airway smooth muscle phenotype that is necessary for contractile protein accumulation by TGF-β1. Caveolin-1 appears to function, in part, by directing signalling via PI3 kinase to 4E-BP1 phosphorylation which is essential for contractile phenotype marker expression. Increased airway smooth muscle expression of caveolin-1 was also a feature of allergen challenged guinea pigs. Collectively, we identify a novel function for caveolin-1 in regulating airway smooth muscle contractile phenotype maturation.
Acknowledgments
This work was supported by a Marie Curie Outgoing International Fellowship from the European Community to R.G. (008823). Operating funds for this work (to A.J.H.) were supported by a grant from the Canadian Institutes of Health Research, and in part by the Canada Foundation for Innovation, and the Canada Research Chairs Program. A.J.H. holds a Canada Research Chair in Airway Cell and Molecular Biology.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.An SS, Bai TR, Bates JH, et al. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J. 2007;29:834–60. doi: 10.1183/09031936.00112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halayko AJ, Solway J. Molecular mechanisms of phenotypic plasticity in smooth muscle cells. J Appl Physiol. 2001;90:358–68. doi: 10.1152/jappl.2001.90.1.358. [DOI] [PubMed] [Google Scholar]
- 3.Halayko AJ, Tran T, Ji SY, et al. Airway smooth muscle phenotype and function: interactions with current asthma therapies. Curr Drug Targets. 2006;7:525–40. doi: 10.2174/138945006776818728. [DOI] [PubMed] [Google Scholar]
- 4.Halayko AJ, Tran T, Gosens R. Phenotype and functional plasticity of airway smooth muscle: role of caveolae and caveolins. Proc Am Thorac Soc. 2008;5:80–8. doi: 10.1513/pats.200705-057VS. [DOI] [PubMed] [Google Scholar]
- 5.Darby PJ, Kwan CY, Daniel EE. Caveolae from canine airway smooth muscle contain the necessary components for a role in Ca(2+) handling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1226–35. doi: 10.1152/ajplung.2000.279.6.L1226. [DOI] [PubMed] [Google Scholar]
- 6.Gosens R, Stelmack GL, Dueck G, et al. Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;291:L523–34. doi: 10.1152/ajplung.00013.2006. [DOI] [PubMed] [Google Scholar]
- 7.Gosens R, Stelmack GL, Dueck G, et al. Caveolae facilitate muscarinic receptor-mediated intracellular Ca2+ mobilization and contraction in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1406–18. doi: 10.1152/ajplung.00312.2007. [DOI] [PubMed] [Google Scholar]
- 8.Prakash YS, Thompson MA, Vaa B, et al. Caveolins and intracellular calcium regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1118–26. doi: 10.1152/ajplung.00136.2007. [DOI] [PubMed] [Google Scholar]
- 9.Gosens R, Mutawe M, Martin S, et al. Caveolae and caveolins in the respiratory system. Curr Mol Med. 2008;8:741–53. doi: 10.2174/156652408786733720. [DOI] [PubMed] [Google Scholar]
- 10.Hill MM, Bastiani M, Luetterforst R, et al. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–24. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastiani M, Liu L, Hill MM, et al. MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes. J Cell Biol. 2009;185:1259–73. doi: 10.1083/jcb.200903053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halayko AJ, Stelmack GL. The association of caveolae, actin, and the dystrophin-glycoprotein complex: a role in smooth muscle phenotype and function. Can J Physiol Pharmacol. 2005;83:877–91. doi: 10.1139/y05-107. [DOI] [PubMed] [Google Scholar]
- 13.Sotgia F, Lee JK, Das K, et al. Caveolin-3 directly interacts with the C-terminal tail of beta -dystroglycan. Identification of a central WW-like domain within caveolin family members. J Biol Chem. 2000;275:38048–58. doi: 10.1074/jbc.M005321200. [DOI] [PubMed] [Google Scholar]
- 14.Sharma P, Tran T, Stelmack GL, et al. Expression of the dystrophin-glycoprotein complex is a marker for human airway smooth muscle phenotype maturation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L57–68. doi: 10.1152/ajplung.00378.2007. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P, Ghavami S, Stelmack GL, et al. beta-Dystroglycan binds caveolin-1 in smooth muscle: a functional role in caveolae distribution and Ca2+ release. J Cell Sci. 2010;123:3061–70. doi: 10.1242/jcs.066712. [DOI] [PubMed] [Google Scholar]
- 16.Couet J, Sargiacomo M, Lisanti MP. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem. 1997;272:30429–38. doi: 10.1074/jbc.272.48.30429. [DOI] [PubMed] [Google Scholar]
- 17.Liu P, Ying Y, Ko YG, et al. Localization of platelet-derived growth factor-stimulated phosphorylation cascade to caveolae. J Biol Chem. 1996;271:10299–303. doi: 10.1074/jbc.271.17.10299. [DOI] [PubMed] [Google Scholar]
- 18.Rothberg KG, Heuser JE, Donzell WC, et al. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–82. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, Toya Y, Jensen RA, et al. Caveolin is an inhibitor of platelet-derived growth factor receptor signaling. Exp Cell Res. 1999;247:380–8. doi: 10.1006/excr.1998.4379. [DOI] [PubMed] [Google Scholar]
- 20.Gosens R, Dueck G, Gerthoffer WT, et al. p42/p44 MAP kinase activation is localized to caveolae-free membrane domains in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1163–72. doi: 10.1152/ajplung.00471.2006. [DOI] [PubMed] [Google Scholar]
- 21.Daniel EE, El-Yazbi A, Cho WJ. Caveolae and calcium handling, a review and a hypothesis. J Cell Mol Med. 2006;10:529–44. doi: 10.1111/j.1582-4934.2006.tb00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniel EE, Eteraf T, Sommer B, et al. The role of caveolae and caveolin 1 in calcium handling in pacing and contraction of mouse intestine. J Cell Mol Med. 2009;13:352–64. doi: 10.1111/j.1582-4934.2008.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drab M, Verkade P, Elger M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–52. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 24.Dreja K, Voldstedlund M, Vinten J, et al. Cholesterol depletion disrupts caveolae and differentially impairs agonist-induced arterial contraction. Arterioscler Thromb Vasc Biol. 2002;22:1267–72. doi: 10.1161/01.atv.0000023438.32585.a1. [DOI] [PubMed] [Google Scholar]
- 25.Je HD, Gallant C, Leavis PC, et al. Caveolin-1 regulates contractility in differentiated vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2004;286:H91–8. doi: 10.1152/ajpheart.00472.2003. [DOI] [PubMed] [Google Scholar]
- 26.Shakirova Y, Mori M, Ekman M, et al. Human urinary bladder smooth muscle is dependent on membrane cholesterol for cholinergic activation. Eur J Pharmacol. 2010;634:142–8. doi: 10.1016/j.ejphar.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Schlenz H, Kummer W, Jositsch G, et al. Muscarinic receptor-mediated bronchoconstriction is coupled to caveolae in murine airways. Am J Physiol Lung Cell Mol Physiol. 2009 doi: 10.1152/ajplung.00261.2009. 10.1152/ajplung.00261.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sommer B, Montano LM, Carbajal V, et al. Extraction of membrane cholesterol disrupts caveolae and impairs serotonergic (5-HT2A) and histaminergic (H1) responses in bovine airway smooth muscle: role of Rho-kinase. Can J Physiol Pharmacol. 2009;87:180–95. doi: 10.1139/y08-114. [DOI] [PubMed] [Google Scholar]
- 29.Benayoun L, Druilhe A, Dombret MC, et al. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–8. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 30.Ma X, Cheng Z, Kong H, et al. Changes in biophysical and biochemical properties of single bronchial smooth muscle cells from asthmatic subjects. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1181–9. doi: 10.1152/ajplung.00389.2001. [DOI] [PubMed] [Google Scholar]
- 31.Dekkers BG, Maarsingh H, Meurs H, et al. Airway structural components drive airway smooth muscle remodeling in asthma. Proc Am Thorac Soc. 2009;6:683–92. doi: 10.1513/pats.200907-056DP. [DOI] [PubMed] [Google Scholar]
- 32.Leguillette R, Laviolette M, Bergeron C, et al. Myosin, transgelin, and myosin light chain kinase: expression and function in asthma. Am J Respir Crit Care Med. 2008;179:194–204. doi: 10.1164/rccm.200609-1367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodruff PG, Dolganov GM, Ferrando RE, et al. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med. 2004;169:1001–6. doi: 10.1164/rccm.200311-1529OC. [DOI] [PubMed] [Google Scholar]
- 34.Halayko AJ, Rector E, Stephens NL. Airway smooth muscle cell proliferation: characterization of subpopulations by sensitivity to heparin inhibition. Am J Physiol. 1998;274:L17–25. doi: 10.1152/ajplung.1998.274.1.L17. [DOI] [PubMed] [Google Scholar]
- 35.Halayko AJ, Stelmack GL, Yamasaki A, et al. Distribution of phenotypically disparate myocyte subpopulations in airway smooth muscle. Can J Physiol Pharmacol. 2005;83:104–16. doi: 10.1139/y04-128. [DOI] [PubMed] [Google Scholar]
- 36.Halayko AJ, Camoretti-Mercado B, Forsythe SM, et al. Divergent differentiation paths in airway smooth muscle culture: induction of functionally contractile myocytes. Am J Physiol. 1999;276:L197–206. doi: 10.1152/ajplung.1999.276.1.L197. [DOI] [PubMed] [Google Scholar]
- 37.Halayko AJ, Kartha S, Stelmack GL, et al. Phophatidylinositol-3 kinase/mammalian target of rapamycin/p70S6K regulates contractile protein accumulation in airway myocyte differentiation. Am J Respir Cell Mol Biol. 2004;31:266–75. doi: 10.1165/rcmb.2003-0272OC. [DOI] [PubMed] [Google Scholar]
- 38.Tran T, Ens-Blackie K, Rector ES, et al. Laminin-binding integrin {alpha}7 is required for contractile phenotype expression by human airway myocyte. Am J Respir Cell Mol Biol. 2007;37:668–80. doi: 10.1165/rcmb.2007-0165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghavami S, Mutawe MM, Hauff K, et al. Statin-triggered cell death in primary human lung mesenchymal cells involves p53-PUMA and release of Smac and Omi but not cytochrome c. Biochim Biophys Acta. 2010;1803:452–67. doi: 10.1016/j.bbamcr.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Tran J, Kung SK. Lentiviral vectors mediate stable and efficient gene delivery into primary murine natural killer cells. Mol Ther. 2007;15:1331–9. doi: 10.1038/sj.mt.6300184. [DOI] [PubMed] [Google Scholar]
- 41.Meurs H, Santing RE, Remie R, et al. A guinea pig model of acute and chronic asthma using permanently instrumented and unrestrained animals. Nat Protoc. 2006;1:840–7. doi: 10.1038/nprot.2006.144. [DOI] [PubMed] [Google Scholar]
- 42.Bos IS, Gosens R, Zuidhof AB, et al. Inhibition of allergen-induced airway remodelling by tiotropium and budesonide: a comparison. Eur Respir J. 2007;30:653–61. doi: 10.1183/09031936.00004907. [DOI] [PubMed] [Google Scholar]
- 43.Gosens R, Bos IS, Zaagsma J, et al. Protective effects of tiotropium bromide in the progression of airway smooth muscle remodeling. Am J Respir Crit Care Med. 2005;171:1096–102. doi: 10.1164/rccm.200409-1249OC. [DOI] [PubMed] [Google Scholar]
- 44.Halayko AJ, Salari H, Ma X, et al. Markers of airway smooth muscle cell phenotype. Am J Physiol. 1996;270:L1040–51. doi: 10.1152/ajplung.1996.270.6.L1040. [DOI] [PubMed] [Google Scholar]
- 45.Goldsmith AM, Bentley JK, Zhou L, et al. Transforming growth factor-beta induces airway smooth muscle hypertrophy. Am J Respir Cell Mol Biol. 2006;34:247–54. doi: 10.1165/rcmb.2005-0166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gawaziuk JP, X, Sheikh F, et al. Transforming growth factor-beta as a differentiating factor for cultured smooth muscle cells. Eur Respir J. 2007;30:643–52. doi: 10.1183/09031936.00141805. [DOI] [PubMed] [Google Scholar]
- 47.Zhou L, Li J, Goldsmith AM, et al. Human bronchial smooth muscle cell lines show a hypertrophic phenotype typical of severe asthma. Am J Respir Crit Care Med. 2004;169:703–11. doi: 10.1164/rccm.200307-964OC. [DOI] [PubMed] [Google Scholar]
- 48.Zhou L, Goldsmith AM, Bentley JK, et al. 4E-binding protein phosphorylation and eukaryotic initiation factor-4E release are required for airway smooth muscle hypertrophy. Am J Respir Cell Mol Biol. 2005;33:195–202. doi: 10.1165/rcmb.2004-0411OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thyberg J, Roy J, Tran PK, et al. Expression of caveolae on the surface of rat arterial smooth muscle cells is dependent on the phenotypic state of the cells. Lab Invest. 1997;77:93–101. [PubMed] [Google Scholar]
- 50.Thyberg J. Differences in caveolae dynamics in vascular smooth muscle cells of different phenotypes. Lab Invest. 2000;80:915–29. doi: 10.1038/labinvest.3780095. [DOI] [PubMed] [Google Scholar]
- 51.Isshiki M, Anderson RG. Function of caveolae in Ca2+ entry and Ca2+-dependent signal transduction. Traffic. 2003;4:717–23. doi: 10.1034/j.1600-0854.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- 52.Gherghiceanu M, Popescu LM. Caveolar nanospaces in smooth muscle cells. J Cell Mol Med. 2006;10:519–28. doi: 10.1111/j.1582-4934.2006.tb00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Popescu LM, Gherghiceanu M, Mandache E, et al. Caveolae in smooth muscles: nanocontacts. J Cell Mol Med. 2006;10:960–90. doi: 10.1111/j.1582-4934.2006.tb00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peterson TE, Guicciardi ME, Gulati R, et al. Caveolin-1 can regulate vascular smooth muscle cell fate by switching platelet-derived growth factor signaling from a proliferative to an apoptotic pathway. Arterioscler Thromb Vasc Biol. 2003;23:1521–7. doi: 10.1161/01.ATV.0000081743.35125.05. [DOI] [PubMed] [Google Scholar]
- 55.Razani B, Zhang XL, Bitzer M, et al. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem. 2001;276:6727–38. doi: 10.1074/jbc.M008340200. [DOI] [PubMed] [Google Scholar]
- 56.Le Saux CJ, Teeters K, Miyasato SK, et al. Down-regulation of caveolin-1, an inhibitor of transforming growth factor-beta signaling, in acute allergen-induced airway remodeling. J Biol Chem. 2008;283:5760–8. doi: 10.1074/jbc.M701572200. [DOI] [PubMed] [Google Scholar]
- 57.Kim S, Lee Y, Seo JE, et al. Caveolin-1 increases basal and TGF-beta1-induced expression of type I procollagen through PI-3 kinase/Akt/mTOR pathway in human dermal fibroblasts. Cell Signal. 2008;20:1313–9. doi: 10.1016/j.cellsig.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 58.Peng F, Zhang B, Wu D, et al. TGF{beta}-Induced RhoA Activation and Fibronectin Production in Mesangial Cells Requires Caveolae. Am J Physiol Renal Physiol. 2008;295:F153–64. doi: 10.1152/ajprenal.00419.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang XM, Zhang Y, Kim HP, et al. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med. 2006;203:2895–906. doi: 10.1084/jem.20061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Achcar RO, Demura Y, Rai PR, et al. Loss of caveolin and heme oxygenase expression in severe pulmonary hypertension. Chest. 2006;129:696–705. doi: 10.1378/chest.129.3.696. [DOI] [PubMed] [Google Scholar]
- 61.Patel HH, Zhang S, Murray F, et al. Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J. 2007;21:2970–9. doi: 10.1096/fj.07-8424com. [DOI] [PubMed] [Google Scholar]
- 62.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 63.Jeffery PK. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:176–83. doi: 10.1513/pats.200402-009MS. [DOI] [PubMed] [Google Scholar]
- 64.Jiang H, Rao K, Halayko AJ, et al. Ragweed sensitization-induced increase of myosin light chain kinase content in canine airway smooth muscle. Am J Respir Cell Mol Biol. 1992;7:567–73. doi: 10.1165/ajrcmb/7.6.567. [DOI] [PubMed] [Google Scholar]
- 65.Jiang H, Rao K, Liu X, et al. Increased Ca2+ and myosin phosphorylation, but not calmodulin activity in sensitized airway smooth muscles. Am J Physiol. 1995;268:L739–46. doi: 10.1152/ajplung.1995.268.5.L739. [DOI] [PubMed] [Google Scholar]
- 66.Opazo Saez AM, Seow CY, Pare PD. Peripheral airway smooth muscle mechanics in obstructive airways disease. Am J Respir Crit Care Med. 2000;161:910–7. doi: 10.1164/ajrccm.161.3.9903138. [DOI] [PubMed] [Google Scholar]
- 67.Trian T, Benard G, Begueret H, et al. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J Exp Med. 2007;204:3173–81. doi: 10.1084/jem.20070956. [DOI] [PMC free article] [PubMed] [Google Scholar]


