Abstract
Skeletal muscle regeneration is severely compromised in the case of extended damage. The current challenge is to find factors capable of limiting muscle degeneration and/or potentiating the inherent regenerative program mediated by a specific type of myoblastic cells, the satellite cells. Recent studies from our groups and others have shown that the bioactive lipid, sphingosine 1-phosphate (S1P), promotes myoblast differentiation and exerts a trophic action on denervated skeletal muscle fibres. In the present study, we examined the effects of S1P on eccentric contraction (EC)-injured extensor digitorum longus muscle fibres and resident satellite cells. After EC, skeletal muscle showed evidence of structural and biochemical damage along with significant electrophysiological changes, i.e. reduced plasma membrane resistance and resting membrane potential and altered Na+ and Ca2+ current amplitude and kinetics. Treatment with exogenous S1P attenuated the EC-induced tissue damage, protecting skeletal muscle fibre from apoptosis, preserving satellite cell viability and affecting extracellular matrix remodelling, through the up-regulation of matrix metalloproteinase 9 (MMP-9) expression. S1P also promoted satellite cell renewal and differentiation in the damaged muscle. Notably, EC was associated with the activation of sphingosine kinase 1 (SphK1) and with increased endogenous S1P synthesis, further stressing the relevance of S1P in skeletal muscle protection and repair/regeneration. In line with this, the treatment with a selective SphK1 inhibitor during EC, caused an exacerbation of the muscle damage and attenuated MMP-9 expression. Together, these findings are in favour for a role of S1P in skeletal muscle healing and offer new clues for the identification of novel therapeutic approaches to counteract skeletal muscle damage and disease.
Keywords: eccentric contraction, muscle damage, myogenesis, satellite cells, sphingosine 1-phosphate, sphingosine kinase
Introduction
Several lines of evidence indicate that strenuous exercise, such as rigorous eccentric muscle contraction, may result in severe muscle wasting and functional deterioration [1, 2]. However, skeletal muscle tissue can regenerate via the activation of satellite cells [3, 4], a population of quiescent myogenic cells, localized between the basal lamina and plasma membrane of the muscle fibre, capable of proliferating and differentiating in response to muscle injury or disease [5]. These cells are characterized by the expression of the paired box transcription factor Pax7, which is required for their survival and functions upstream of the expression of the myogenic regulator factors (MRFs) Myf5, MyoD, Mrf4 and myogenin [6, 7]. During muscle repair/regeneration, a small subpopulation of satellite cells does not execute the myogenic program and reconstitute a compartment of quiescent ‘stem cell’ (self renew), able to respond to repeated muscle injury [8, 9]. It is generally assumed that the mere presence of satellite cells is not sufficient to ensure a rapid functional recovery of the injured muscle and that the release of appropriate factors and the establishment of suitable microenvironment are even more important in determining the effectiveness of the myogenic response [10–12]. However, in the case of extended damage, the propensity for an enhanced inflammatory response and the excessive fibroblast proliferation and connective tissue deposition may exceed the ability of stem cells to pursue the myogenic program and the muscle’s regenerative potential [10, 12]. Hence, the identification of factors and therapeutic interventions aiming at enhancing muscle regeneration and attenuating or preventing muscle damage and fibrosis would be very useful in order to preserve muscle functionality. Along this line, sphingosine 1-phosphate (S1P), a bioactive lipid present in a physiologic concentration in the plasma, exerts multiple biological functions, including the maintenance of skeletal muscle homeostasis, acting through S1P receptors [13, 14]. The inhibition of sphingosine kinase (SphK), the enzyme responsible for S1P formation, significantly reduces the amount of regenerating centrally nucleated myofibres in cardiotoxin-damaged muscle [15, 16]. Moreover, the administration of exogenous S1P has been shown to cause a dramatic reduction of muscle tension decline during fatigue [17] and attenuate the progress of denervation-induced muscle atrophy [18] suggesting that this bioactive sphingolipid may act as a myogenic trophic factor. In this context, studies from our group have demonstrated that S1P is capable to stimulate C2C12 myoblast differentiation and have identified the underlying cellular and molecular mechanisms [19–24]. Finally, there is evidence to support a role for the sphingolipid in the stimulation of extracellular matrix remodelling and in the regulation of matrix metalloproteinase 9 (MMP-9) expression in cardiomyocytes and skeletal myoblasts [25–28]. Interestingly, changes in extracellular matrix composition are considered as crucial steps for the restoration of the tissue architecture during skeletal muscle regeneration [29].
Based on these considerations, in the present study we further investigated the beneficial effects of S1P on skeletal muscle repair/regeneration, focusing our attention on the ability of this bioactive factor to protect the tissue against eccentric contraction (EC)-induced damage and exert its action on satellite cell function.
Materials and methods
The detailed version of materials and methods is reported in the supplemental file.
Animals and muscle injury model
Extensor digitorum longus (EDL) muscles were removed from anesthetized young adult Swiss mice (25–30 g) and subjected to forced EC in isometric condition, in the absence or presence of 1 μM S1P (EC+ S1P) or of compound II, a SphK1 inhibitor (EC + iSphK).
Morphological analysis of EDL skeletal muscle
Muscle tissue samples were fixed in buffered glutaraldehyde, post-fixed in phosphate-buffered OsO4 and routinely processed for light and transmission electron microscopy [30]. The severity of tissue damage was evaluated by counting the number of fibres showing key morphological signs of cell damage and evaluating the degree of injury in each fibre, using a semi-quantitative histological score, as reported previously for similar purposes [31]. The criteria used are reported in Table 1.
Table 1.
Scoring method of skeletal muscle fibre damage
| Score | Description | |
|---|---|---|
| 0 | Normal myocyte | |
| 1 | Slight | Mild intermyofibre oedema |
| Mild intramyofibrillar oedema | ||
| 2 | Moderate | Mild intramyofibrillar oedema |
| Cytoskeletal disarrangement | ||
| Focal sarcomere contraction | ||
| Mild nuclear chromatin clumping | ||
| 3 | Severe | Severe intramyofibrillar oedema |
| Severe myofibrillar disarrangement | ||
| Plasma membrane ruptures | ||
Lactate dehydrogenase (LDH) assay
LDH was assayed by oxidation of NADH with pyruvate [32].
Western blotting
Protein aliquots (10–30 μg) from EDL muscle tissue and satellite cell-enriched fraction lysates were separated by a SDS-PAGE and immunoblotted as described previously [21]. Specific primary antibodies anti-Bax, anti-Cytc, anti-SphK1, anti-cyclin D1, anti-phospho p44/42 mitogen-activated protein (MAP) kinase (MAPK) (Thr202/Tyr204) [p-extracellular-signal-regulated kinase 1/2 (p-ERK1/2)], anti-catalytic domain of MMP-9, anti β-actin were used and signal detected by peroxidase-conjugated secondary IgG1 using Enhanced Chemiluminescence (ECL) reagent. Densitometric analysis of the bands was performed with Imaging and Analysis Software by Bio-Rad (Hercules, CA, USA). For Bax expression determination, mitochondria fraction was prepared from muscle tissue lysates by centrifugation at 15,000 ×g for 10 min. and the precipitate used.
Caspase 3/7 activity
Caspase activity was measured in the lysates by determining the cleavage of the fluorogenic caspase 3/7 substrate acetyl Asp-Glu-Val-Asp 7-amino-4-methylcoumarin-derived caspase (Ac-DEVD-AMC) (50 mM final concentration, Ex/Em 354 nm/442 nm) in 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) pH 7.4, 1 mM ethylenediaminetetraacetic acid, 10 mM dithiothreitol, 100 mM NaCl, 10% glycerol. The extent of Ac-DEVD-AMC was measured as the increase in relative fluorescence units (RFU) resulting from the release of free fluorescent AMC.
TUNEL assay
Nuclear DNA fragmentation was analysed by TUNEL (terminal deoxyribonucleotidyl transferase mediated dUTP nick end labelling) reaction in cryosectioned muscle samples. Detection of apoptosis was performed with a commercially available In Situ Cell Death Detection Kit according to the instructions provided by the manufacturer. TUNEL apoptotic index was then expressed as relative percentage of TUNEL+ nuclei on the total number of haematoxylin-stained nuclei evaluated in parallel sections.
Electrophysiology
Resting membrane potential (RMP) was recorded in current-clamp condition by a microelectrode inserted into a single fibre of a EDL isolated muscle bathed in Ringer–Krebs solution. The passive properties of the fibres (membrane resistance Rm and capacitance, Cm), L-type Ca2+ current (ICa) and Na+ current (INa) were evaluated using the double Vaseline-gap method, as described previously [33].
Isolation of satellite cell-enriched fractions from EDL muscle
A population of muscle-derived cells, mostly satellite cells, was purified by enzymatic dissociation and 40–70% Percoll gradient centrifugation from EDL muscles as described previously, with minor modifications [34]. For 3-[4,5-dimethyltiazol-2yl]-2,5 diphenyltetrazolium bromide (MTS) assay and S1P quantification, satellite cell-enriched fractions were cultured on collagen-coated dishes in growth medium for 24 hrs.
Cell viability by MTS assay
3-[4,5-dimethyltiazol-2yl]-2,5 diphenyltetrazolium bromide (5 mg/ml) was added to the satellite cell-enriched fractions plated and incubated for 24 hrs after isolation. Cell viability was determined by measuring the absorbance at 490 nm with a microplate spectrophotometer and expressed as percent of cell viability (OD treated/OD control) setting the control (undamaged muscle-derived satellite cells) as 100.
SphK activity
SphK activity was determined in control and EC-injured muscle by in vitro assay essentially as described previously [21]. Briefly, equal amounts of protein (20–60 μg) from muscle, were incubated in SphK1 and SphK2 specific assay buffer [35] in the presence of 20 μmol/l sphingosine dissolved in 5% Triton X-100 and 200 mmol/l ATP ([32P]ATP (3μCi/assay).
S1P quantification in satellite cell-enriched fractions
Sphingolipids were quantified in satellite cell-enriched fractions from control and damaged muscles incubated with serum-free medium and 2 μCi of [3H]sphingosine for 24 hrs at 37°C, essentially as reported previously [21, 36].
Confocal immunofluorescence
Cryostat EDL muscle sections (8 μm) were fixed in paraformaldehyde vapours, incubated with the following antibodies: mouse monoclonal anti-Pax7, rabbit polyclonal anti-MyoD and mouse monoclonal anti-myogenin and then immunorevealed with specific Alexa Fluor 488- or Alexa Fluor 568- conjugated IgG. Observations were performed under a confocal Leica TCS SP5 (5 SpectroPhotometer channels) microscope (Leica Microsystems, Mannheim, Germany) as reported previously [22].
Culture of satellite cells isolated from single myofibres
Living single myofibres of EDL muscles were isolated and individually cultured as described previously [37]. After 48 hrs culture, the myofibres were removed and the derived satellite cells were cultured in growth medium until reaching confluence and then shifted in differentiation medium [DM: DMEM, 2% horse serum (HS)]. Cells sprouting from each single myofibre were counted 24 hrs after the fibre removal. Myotubes were evaluated after 4 days satellite cell culture in DM.
Determination of tissue fibrosis by Van Gieson staining
Cryostat EDL muscle sections (8 μm) were fixed in paraformaldehyde vapours and stained with Van Gieson method for collagen [38].
Results
Exogenous S1P exerts protective effects during EC- induced muscle damage
We first evaluated the effects of EC on EDL skeletal muscle by morphological, biochemical and electrophysiological analyses. At light microscopic examination, skeletal muscle fibres subjected to EC showed extensive and severe morphological alterations (histological score of 2.7 ± .0.1, Table 1; Fig. 1A and B). At the ultrastructural level, the majority of the injured fibres displayed variable degree of myofibrillar disarrangement and Z-disk disruption, dilated cistaerne and tubules of sarcoplasmic reticulum, and swollen mitochondria with shortening and disappearance of cristae (Fig. 1C and D). Biochemical analysis revealed that LDH activity was significantly decreased of approximately 35% in EC-injured muscle compared to control (8.9 ± 0.9 versus 13.7 ± 1.2; mean ± S.E.M.; P < 0.05, n= 3). Moreover, we demonstrated a significant increase in the association of the pro-apoptotic protein, Bax, with mitochondrial fractions, and in the release of respiratory chain protein, Cytc, into the cytosolic fractions obtained from EC-damaged muscle compared to control (Fig. 2A). However, EC damage provoked only a slightly increase in caspase 3/7 activity (Fig. 2B), and a certain positivity to the TUNEL reaction. In particular, positive nuclei were found both in the myofibres and in cells located in the close vicinity, and represented an approximately 2% of the total nuclei (Fig. 2C). The electrophysiological assessment of the effects of EC performed on single muscle fibres showed the occurrence of significant changes in the sarcolemnic functionality (reduced plasma membrane resistance (RmCm) and resting membrane depolarization, Table 2), and in the myofibre excitability (reduced Na+ current amplitude (INa) and altered kinetic, Fig. 3A and C; Table 2). EC also affected the excitation-contraction coupling, reducing L-type-mediated Ca2+ current (ICa) and altering the channel kinetics (Fig. 3B and D; Table 2).
Fig 1.
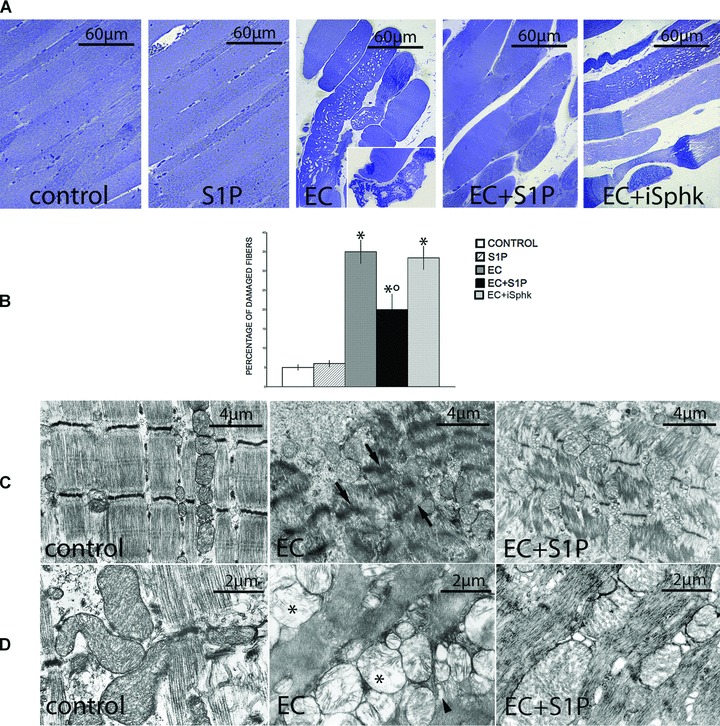
Protective effect of S1P against EC-induced structural and ultrastructural damage in EDL muscle fibre. (A) Light microscopy. Semi-thin sections from control, S1P-treated, EC-, EC + S1P- and EC + SphK1 inhibitor, compound II (iSphK)-injured skeletal muscles. EC-injured fibres showed substantial damage and some displayed signs of necrosis (inset). These structural abnormalities are attenuated in EC + S1P muscle. Note that inhibition of the endogenous S1P production by iSphK does not confer much protection against EC damage. (B) Quantitative analysis of the number of damaged fibres expressed as percentage on the total fibre number (mean ± S.E.M., One-way anova*P < 0.05 versus specific control; °P < 0.05 versus EC; n= 3). (C–D) Transmission electron microscopy. EC-damaged fibres display severe sarcomere disorganization and Z-disc disruption (arrows), together with swollen mitochondria with loss of organized cristae (asterisks) and dilated sarcoplasmic tubules (arrowheads). In the presence of S1P, these ultrastructural alterations appear strongly attenuated.
Fig 2.
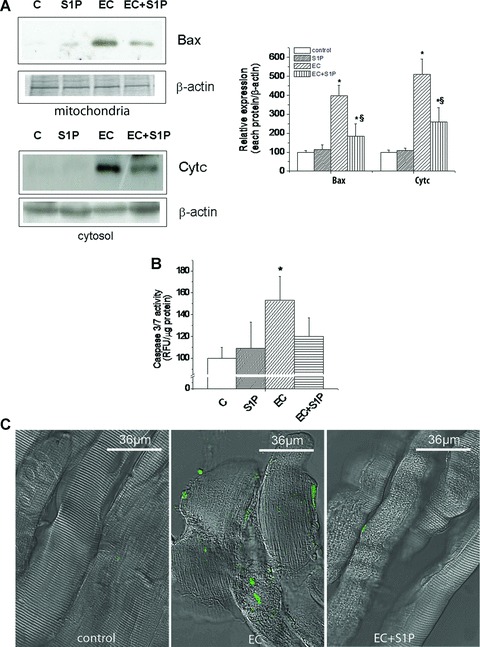
Effects of S1P treatment on EC-induced apoptosis in EDL muscle. (A) Western blotting analysis of pro-apoptotic protein, Bax and Cytochrome c (Cytc) expression. Protein aliquots (10–25 μg) corresponding to the 15,000 ×g fraction (mitochondria) and the cytosol obtained from control (C), S1P-treated, EC- and EC + S1P-injured muscle fibres were immunodetected by specific antibodies and revealed by enhanced chemiluminescence. The relative percentage of band intensity to control set as 100 (mean ± S.E.M.) and normalized to gel staining or β-actin is shown in the graphs. Student’s t-test, *P < 0.05 versus specific control; n= 3; §P < 0.05 versus EC, n= 3. (B) Determination of caspase 3/7 activity. The caspase 3/7 assay was performed in lysates obtained from the muscle samples as indicated in (A). RFU resulting from the release of free fluorescent caspase 3/7 substrate (Ac-DEVD-AMC) are reported as mean ± S.E.M. (Student’s t-test, *P < 0.05 versus specific control). (C) TUNEL assay. Cryostat sections from control, EC- and EC + S1P- injured muscles. Sparse apoptotic nuclei showing positive TUNEL labelling (green) are detected within the muscle fibres and the surrounding cells in EC injured muscles. Only scanty TUNEL+ cells can be seen in EC + S1P-injured muscles, whereas no apoptotic cells are detected in control muscles.
Table 2.
Specific plasma membrane resistance (RmCm), RMP, Boltzmann parameters of activation and inactivation of INa and ICa in control, S1P treated, EC, EC + S1P and EC + iSphK fibres
| Parameters | Control | 1S1P | EC | EC + S1P | EC + iSphK |
|---|---|---|---|---|---|
| RmCm (Ω.μF) | 8900 ± 700 | 9200 ± 650 | 6240 ± 500*** | 7750 ± 500** | 5700 ± 480§ |
| RMP (mV) | −75 ± 7 | −75 ± 6 | −55 ± 6** | −73 ± 7 | −50 ± 6 |
| INa | |||||
| INa (nA/pF) | 3.4 ± 0.2 | 3.5 ± 0.2 | 2.0 ± 0.2** | 2.7 ± 0.3* | 1.7 ± 0.2§ |
| Gm/Cm (mS/μF) | 157 ± 18 | 162 ± 17 | 77 ± 9 *** | 114 ± 13** | 68 ± 8§ |
| Va (mV) | −22±0.6 | −21 ± 0.7 | −17±1.1** | −20±0.8 | −16±1.2 |
| ka (mV) | 6.0 ± 0.5 | 6.0 ± 0.5 | 7.8 ± 0.7** | 7.0 ± 0.6* | 7.6 ± 0.6 |
| Vr (mV) | 53 ± 4 | 54 ± 5 | 43 ± 4** | 49 ± 5 | 38 ± 4§ |
| Vh (mV) | −60 ± 5 | −63 ± 5 | −72 ± 5** | −64 ± 6 | −76 ± 6 |
| kh (mV) | 6.6 ± 0.6 | 6.6 ± 0.5 | 8.5 ± 0.7** | 7.8 ± 0.6* | 8.6 ± 0.6 |
| Tp (msec.) | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.9 ± 0.1** | 0.6 ± 0.1 | 1.3 ± 0.2§ |
| ICa | |||||
| ICa (pA/pF) | 4.1 ± 2 | 4.4 ± 2 | 0.8 ± 0.07*** | 3.1 ± 1** | 0.4 ± 0.08§ |
| Gm/Cm (μS/μF) | 150 ± 16 | 155 ± 17 | 33 ± 7*** | 100 ± 18** | 26 ± 6§ |
| Va (mV) | −2.1 ± 1 | −11.2 ± 3*** | 12.2 ± 3*** | 3.0 ± 1* | 13 ± 2 |
| ka (mV) | 7.2 ± 0.4 | 7.1 ± 0.5 | 8.0 ± 0.4** | 7.4 ± 0.4 | 7.9 ± 0.4 |
| Vr (mV) | 76 ± 5 | 73 ± 6 | 67 ± 5** | 74 ± 6 | 60 ± 5§ |
| Vh (mV) | −53 ± 4 | −48 ± 3** | −63 ± 5** | −56 ± 5 | −65 ± 5 |
| Kh (mV) | 6.5 ± 0.5 | 6.4 | 8.3 ± 0.5** | 7.3 ± 0.6* | 8.4 ± 0.6 |
| Tp (msec.) | 150 ± 32 | 140 ± 35 | 250 ± 45** | 160 ± 34 | 299 ± 46§ |
In control fibres the presence of S1P shifts the voltage dependence values of Va and Vh parameters of ICa. EC injured fibres show significant changes of RmCm and RMP indexes of a leaky plasma-membrane as compared to controls. The increased intracellular [Na+] and [Ca2+] is confirmed by the negative voltage shift of Vr of both INa and ICa. Moreover, EC injured fibres show a reduction of the maximal INa and ICa peaks and related Gm; both are index of reduced channel functionality. The voltage dependence of INa and ICa activation and inactivation (Boltzmann functions) indicate significant changes of Va, ka, Vh and kh. Finally, the current kinetics appear altered: the maximal INa and ICa show an increased time to peak (Tp). All these alterations are strongly reduced in EC + S1P fibres. By contrast, in the presence of iSphK (EC + iSphK fibres), some electrophysiological parameters worsen compared with EC. *P < 0.05, **P < 0.01 and ***P < 0.001 versus control. §P < 0.05 versus EC. Data in each experimental condition were from 24 to 28 fibres (16 EDL; 14 mice).
Fig 3.
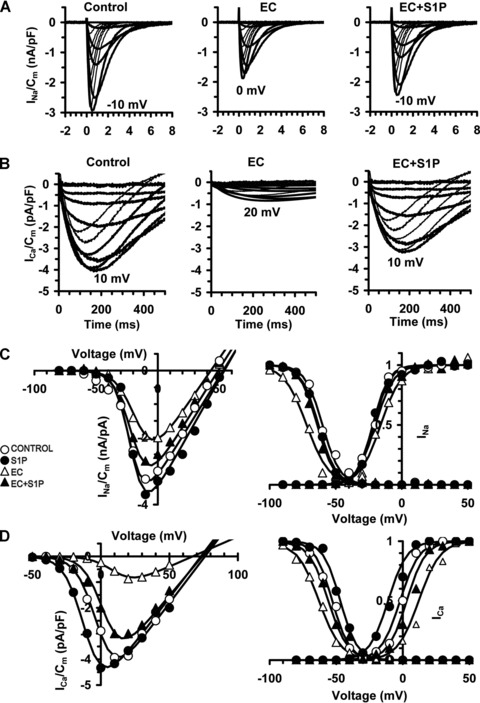
Protective effect of S1P against EC-induced electrophysiological changes in EDL muscle. Electrophysiological analyses of the functional properties of sarcolemma of control, S1P-treated, EC- and EC + S1P-injured muscle fibres evaluated by the double-Vaseline gap method in voltage-clamp. (A–B) Representative normalized Na+ current (INa) and L-type Ca2+current (ICa) traces. (C–D) Activation and inactivation Boltzmann curves for INa and ICa (Current to peak-V plots). RMP data and Boltzmann parameters are in Table 2.
When EC was performed in the presence of 1 μM S1P (EC + S1P), the observed structural and ultrastructural muscle alterations appeared strongly attenuated (Fig. 1). In fact, S1P caused a significant reduction in the number of damaged fibres, with an average decrease ranging from 20% to 30% and decreased the severity of myofibre damage, as shown by the significantly lower histological score (1.8 ± 0.2, Table 1) as compared to that of EC-injured muscle (Fig. 1A and B). By transmission electron microscopy (TEM) analyses, most of the muscle fibres exhibited moderate myofibrillar disarrangements and mitochondria alterations (Fig. 1C and D). Moreover, the treatment with S1P of EC muscle was capable of strongly attenuating the expression levels of the assayed apoptotic markers (Fig. 2A) and caspase 3/7 activation (Fig. 2B), reducing the percentage of apoptotic nuclei (approximately of 0.8.%; Fig. 2C) and improving the examined electrophysiological parameters (Fig. 3, Table 2). The treatment with S1P of undamaged EDL muscle did not cause any morphological, biochemical and electrophysiological modifications (Figs 1–3).
EC also affected satellite cell functionality; in fact, cell viability, evaluated performing MTS assay in satellite cell-enriched fractions declined drastically in the injured muscle (Fig. 4), suggesting that satellite cell metabolic activity could be impaired by muscle damage, as also previously reported [39–42]. Interestingly, satellite cell viability resulted significantly higher in EC + S1P muscle with respect to EC, thus extending the protective action of the sphingolipid in damaged skeletal muscle to the resident muscle stem cells (Fig. 4).
Fig 4.
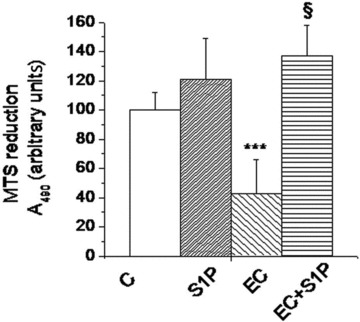
Effects of S1P on viability of satellite cell-enriched fractions from EC-injured EDL muscle. Cell viability was evaluated by MTS assay in satellite cell-enriched fractions 24 hrs after isolation from muscle in the indicated experimental conditions. Relative percentage to control set as 100 (mean ± S.E.M.) is shown. Student’s t-test, ***P < 0.01 versus control, n= 4; §P < 0.01 versus EC, n= 4.
All together, the above findings, indicated that EC caused muscle damage, consisting in a prevalence of necrotic over apoptotic cell death, and suggested that S1P exerted beneficial effects, protecting both the myofibres and adjacent satellite cells in these conditions.
EC induces endogenous SphK1 activation and S1P formation
We next searched whether EC caused the activation of the endogenous S1P synthesis in the injured muscle fibres, by evaluating the activity of two known SphK isoforms (SphK1 and SphK2), responsible for the production of the bioactive lipid in skeletal muscle [43]. As shown in Fig. 5A, the activity of SphK1, but not SphK2 isoform, measured by specific in vitro assay, was significantly higher in EC-injured muscle lysates as compared to control; however, enhanced SphK1 activity was not paralleled by the up-regulation of SphK1 protein expression (Fig. 5B), as judged by Western analysis. The levels of [3H]S1P formed in the [3H]sphingosine-labelled satellite cell-enriched fractions from EC-injured muscle were also increased (Fig. 5C), confirming the ability of EC to activate intracellular SphK1/S1P axis. Interestingly, the addition of exogenous S1P to damaged fibres further increased SphK1 activity (Fig. 5A), providing the first evidence that the activation of S1P receptor-mediated signalling could augment the endogenous S1P synthesis during EC and suggesting the intriguing hypothesis that the treatment with the sphingolipid could amplify the physiological role played by endogenous S1P in muscle protection. Consisting with these findings, we observed an exacerbation of some electrophysiological parameters (RmCm, Vr and Gm/Cm for both INa and ICa, Table 2) when EC was performed in the presence of compound II, a specific SphK1 inhibitor (iSphK). However, no detectable morphological differences could be observed between EC and EC + iSphK (Fig. 1A and B; histological score 2.5 ± 0.2, Table 1).
Fig 5.
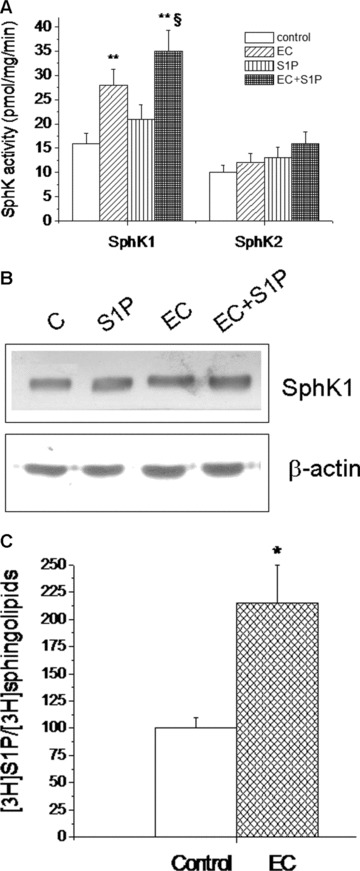
Effect of EC on SphK activity, enzyme expression and S1P formation in EDL muscle. (A) Tissue lysates (40–70 μg) from control, S1P-treated, EC- and EC + S1P-injured muscles were analysed for SphK1 and SphK2 activity. Data are mean ± S.E.M. (Student’s t-test, **P < 0.05 versus control, n= 3; §P < 0.05 versus EC, n= 3). (B) SphK1 expression. Protein aliquots (20 μg) of lysates from muscle samples as indicated in (A) were immunoblotted and detected by ECL. The relative percentage of band intensity to control arbitrarily normalized to 100 set (mean) is shown. S.E.M. (not shown) is less than 15%. C: control. (C) S1P formation in satellite cell-enriched fractions from Percoll gradient obtained from control and EC-injured fibres. [3H]S1P formation was determined in 2 μCi [3H]sphingosine-labelled cells after 24 hrs incubation. Lipids were extracted by adding chloroform-methanol, and samples from TLC separation evaluated for the radioactivity corresponding to [3H]S1P. Data are means ± S.E.M. (Student’s t-test; *P < 0.05; n= 3).
Exogenous S1P promotes satellite cell renewal and differentiation during EC muscle damage
Next, we analysed the effects of S1P on the resident satellite cells, in an attempt to evaluate the contribution of the bioactive lipid to EDL muscle repair/regeneration after damage. It has been previously reported that upon activation, quiescent Pax7+ satellite cells rapidly express MyoD and proliferate as Pax7+/MyoD+ co-expressing cells, before adopting divergent fates and giving rise to two cell populations: one which down-regulates Pax7, maintains MyoD expression (Pax7−/MyoD+) and starts the myogenic program, and the other which down-regulates MyoD and maintains Pax7 (Pax7+/MyoD−) contributing to satellite cell reservoir [4, 9]. On this basis, confocal immunofluorescence analysis revealed that EC per se caused a significant increase of Pax7+/MyoD+ activated satellite cells (Fig. 6A). Notably, in the presence of S1P, the pool of activated Pax7+/MyoD+ cells appeared significantly reduced, while the number of quiescent Pax7+/MyoD−, committed progenitor Pax7−/MyoD+ and terminally differentiated myogenin+ cells were markedly increased (Fig. 6A), suggesting the ability of S1P to positively influence the self-renewal and differentiation potential of satellite cells after EC. As judged by the biochemical analysis, the expression of cyclin D1 and p-ERK1/2 were significantly increased in satellite cell-enriched fractions obtained from EC + S1P muscle compared to EC, underscoring the ability of the bioactive lipid to activate signalling pathways leading to satellite cell proliferation during EC (Fig. 6B).
Fig 6.
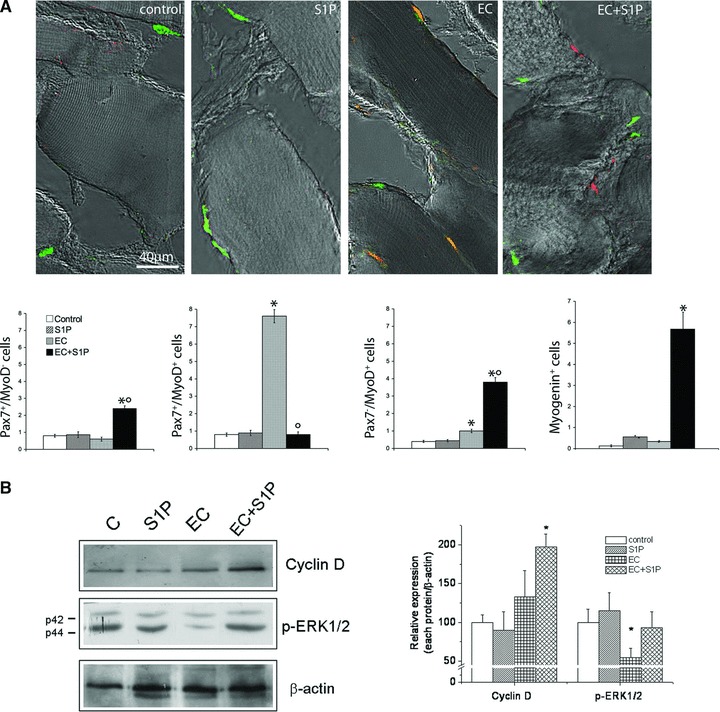
Effect of S1P on satellite cell renewal and differentiation. (A) Superimposed DIC and confocal immunofluorescence images of myofibres from control, S1P-treated, EC- and EC + S1P-injured EDL muscle immunostained for Pax7 (green) and MyoD (red). Cells co-expressing both the markers are stained in orange. In the histogram quantitative analysis of the number of Pax7+/MyoD−, Pax7+/MyoD+ and Pax7−/MyoD+ and myogenin+ satellite cells. Data are mean ± S.E.M. (One-way anova. *P < 0.05 versus control; °P < 0.05 versus EC; n= 3). (B) Western Blotting analysis of cyclin D1 and phospho p44/42 MAPK (p-ERK1/2) expression in satellite cells-enriched fractions. Relative percentage of band intensity to control set as 100 (mean ± S.E.M.) is shown in the graph. (Student’s t-test, *P < 0.05, n= 3).
Satellite cell activation and viability was also investigated in the cells sprouting from cultured isolated myofibres (Fig. 7). It was found that the number of viable muscle satellite cells derived from EC and EC + S1P muscle fibres was significantly higher (approximately 1.5- and 2-fold, respectively) compared to control (Fig. 7A and B). In agreement, the sprouting myoblasts from EC and EC + S1P-damaged fibres exhibited a higher tendency (approximately 2- and 3-fold, respectively) to fuse into multinucleated myotubes than those from control (Fig. 7C–F).
Fig 7.
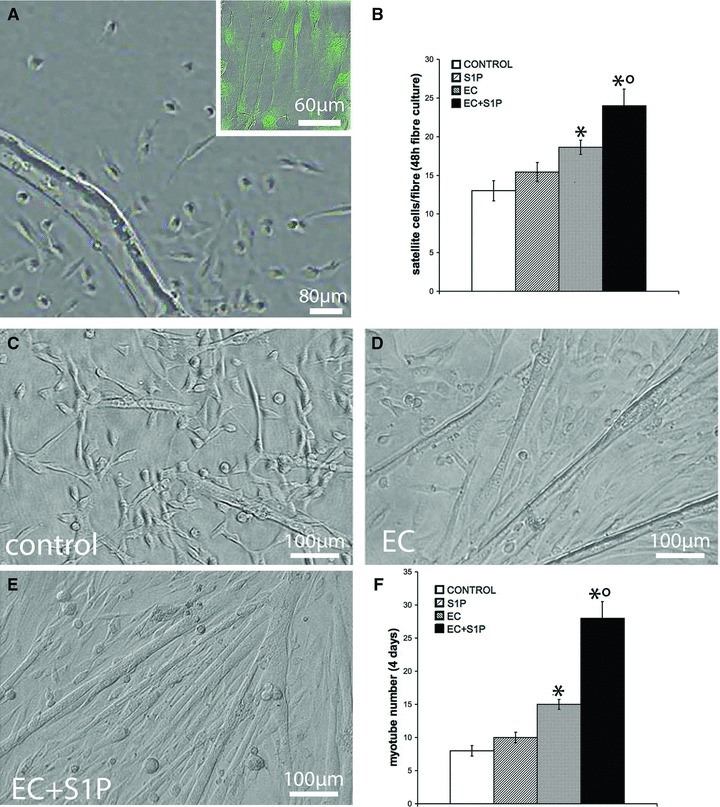
Proliferation and differentiation of satellite cell derived from single muscle fibres. Satellite cells were isolated from single fibres of control, S1P-treated, EC- and EC + S1P-injured EDL muscle and analysed for cell proliferation and differentiation. (A) Representative reverse-phase contrast microscopic image of a single fibre cultured for 48 hrs and sprouting satellite cells positive for Pax7 (inset). (B) Quantitative analyses of the number of sprouting cells evaluated 24 hrs after the fibre removal (One-way anova, *P < 0.05 versus control; °P < 0.05 versus EC; n= 3, 24 fibres in each experiment). (C–E) Representative reverse-phase contrast images of myotube formation. (F) Quantitative analyses of myotubes counted in the same samples of (B). *P < 0.05 versus control; °P < 0.05 versus EC; (One-way anova; n= 3).
In the absence of the muscle damage, the addition of S1P to the isolated EDL muscle did not affect the proliferating progeny nor modify the number of committed satellite cells, indicating that the exogenous S1P per se, without any stress-mediated signals, was unable to affect satellite cell functions (Figs 6 and 7).
S1P affects extracellular matrix remodelling in EC-damaged muscle fibres
We finally evaluated the effects of S1P treatment on the remodelling of the extracellular matrix proteins. Staining of EDL muscle sections with Van Gieson, which stains collagen, revealed considerable reduced fibrosis in EC + S1P compared with EC (Fig. 8A). We also showed that the treatment with S1P up-regulated (approximately 2-fold) MMP-9 expression in EC-injured muscle (Fig. 8B). Interestingly, EC treatment by itself increased MMP-9 expression of approximately 2-fold above the control and this increase appeared significantly reduced when muscle fibres were incubated in the presence of SphK1 inhibitor (Fig. 8C), supporting the role of endogenous S1P and the activation of SphK1/S1P axis by EC in counteracting muscle tissue fibrosis.
Fig 8.
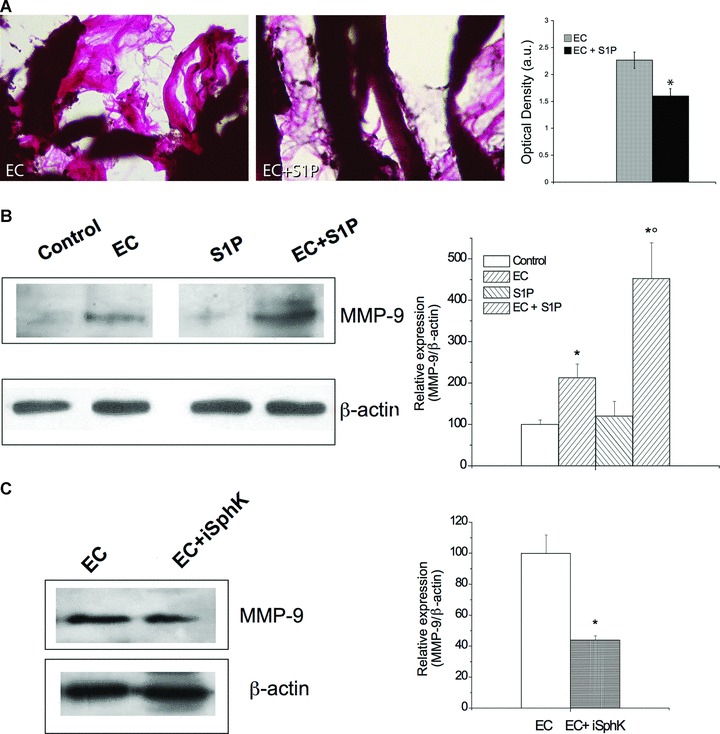
Effect of S1P on extracellular matrix remodeling and MMP-9 expression. (A) Light microscopy. Van Gieson stained muscle sections revealing collagen fibres (pink). In the histogram densitometric analysis of collagen staining intensity (Student’s t-test; *P < 0.05, n= 3). (B) Western blot analysis of MMP-9 protein expression in control, S1P-treated, EC- and EC + S1P-damaged muscle fibres. (C) MMP-9 protein expression in EC-treated cells in the absence and presence of SphK1 inhibitor (iSphK). Protein aliquots (30 μg) of tissue lysates were immunoblotted and detected by ECL. Band intensity was determined by densitometry and relative percentage to control arbitrarily normalized to 100 is shown in the graphs. (Student’s t-test; *P < 0.05 versus specific control, n= 3; °P < 0.05 versus EC).
Discussion
S1P is a well-described bioactive lipid mediator which has been shown to mediate a wide variety of fundamental biological processes, including cell growth, survival, migration and differentiation.
It is considered a physiological factor which is released in the extracellular milieu by activated platelets and by many cell types through a member of the ATP-binding cassette (ABC) transporter family [44, 45]. Membrane-associated SphK1, the enzyme responsible for S1P production, can act as an exoenzyme, as shown in myogenic C2C12 cells [43], and contribute to the autocrine/ paracrine action of the sphingoid molecule via the activation of specific S1P receptors [13, 14, 28].
Recent studies are beginning to highlight the importance of S1P in skeletal muscle homeostasis and disease, exerting a protective role against muscle atrophy and promoting myoblast differentiation and satellite cell activation [15, 16, 19–23, 46]. In this contest, the data reported here contribute to expand the list of the beneficial effects of S1P on muscle tissue, unrevealing a major role for this factor in the prevention and attenuation of EC-induced morphological, biochemical and electrophysiological skeletal muscle damage. We also found a strong up-regulation of intracellular S1P synthesis after EC, as demonstrated by the rise in SphK1 activity and S1P formation in skeletal muscle fibres and satellite cells, respectively, further supporting the protective role of S1P against skeletal muscle injury. Interestingly, S1P was able to augment SphK1 activity in response to damage, suggesting that the activation of endogenous SphK1/S1P axis could represent potential mechanisms of S1P muscle protection. Consistent with this assumption, some electrical parameters appeared exacerbated when endogenous S1P formation was prevented during EC. In view of our previous reports, it is also likely that S1P may afford cytoprotection of muscle cells through its ability to promote cortical F-actin organization and plasma membrane stiffening [47, 48] which could enhance the resistance in these cells to the stress caused by EC. This assumption is consistent with the data of other groups showing that eccentric exercise elicits a cytoskeletal remodelling response in human skeletal muscle in the attempt to limit the extent of the mechanical stress-induced damage [49, 50]. Moreover, as predicted by the tensegrity cell model [51], it also possible that actin cytoskeleton may transmit signals to the nucleus of the muscle cells through the activation of peculiar mechanical Ca2+ channels and biochemical processes leading to cell survival and protection [20, 22, 23, 52].
We also found that EC caused a significant reduction in satellite cell viability, in line with the growing evidence in the literature showing a loss of satellite cell population following extensive muscle damage. Interestingly, S1P preserved satellite cell viability. Although, we did not investigate the molecular aspects of satellite cell protection by S1P, it may be suggested that increased satellite cell viability is related to the ability of the sphingolipid to limit the damaged tissue, thus contributing to the recreation of a less prohibitive environment for satellite cell functions.
Satellite cell renewal and differentiation was also potentiated by the bioactive lipid.
Indeed, we reported here that the number of activated Pax7+/MyoD+ co-expressing cells significantly enhanced after damaged and that S1P forced this pool to enter cell cycle and subsequently adopt divergent fates; in fact, most of them differentiated, down-regulating Pax7 and maintaining MyoD expression (Pax7−/MyoD+) and expressing myogenin, while others regained a phenotype characteristic of quiescent satellite cells (Pax7+/MyoD−). Consisting with these data, we found that number of viable satellite cells sprouting from EC + S1P-injured muscles together with their tendency to form myotubes was increased as compared with those from EC muscles, in perfect agreement with the known myogenic action of S1P [15]. The observed inability of S1P per se to affect satellite cells of uninjured tissue is in line with the recent findings showing that the specific S1P receptor isoform, S1P2, is rapidly up-regulated in skeletal muscle undergoing regeneration, but it is absent in quiescent cells [46].
It is generally accepted that dismantling the extracellular matrix is essential for myoblast migration, differentiation and fusion in vivo[53]. Unfortunately, these events may be hindered in case of severe muscle wasting by the excessive deposition of collagen fibres in the form of a fibrotic scar which limits vascular perfusion of the injury site [54]. In such a view, it was particularly interesting to find out that S1P decreased fibrosis and up-regulated MMP-9 expression in the EC-injured muscle, inducing an additive effect in promoting collagen remodelling beyond the EC effect. This event was strongly reduced by incubation with SphK1 inhibitor, suggesting a critical contribution of SphK1/S1P axis in counteracting tissue fibrosis.
In conclusion, the results of the present study provide evidence that S1P is capable of protecting skeletal myofibres against muscle damage and influencing muscle repair/regeneration. The findings concerning the activation of SphK1/S1P axis in response to muscle damage further reinforce the importance of the endogenous bioactive sphingolipid in the promotion of skeletal muscle repair/regeneration and provide clues for the identification of novel targets for improving muscle healing after injury.
Acknowledgments
This work was supported by grants from Fondazione Cassa di Risparmio di Pistoia e Pescia to E.M., Fondazione Banche di Pistoia e Vignole to E.M., Ente Cassa di Risparmio di Firenze to L.F., S.Z.-O., Programma Vigoni to E.M., and from the Italian Ministry for Education, University and Research, Rome, Italy to E.M., L.F., C.S., F.F. and S.Z.-O. The authors are grateful to Prof. Carla Ghelardini (Dept. of Pharmacological Sciences, University of Florence, Italy) for kindly providing us the Swiss mice, Dr. Daniele Nosi (Dept. of Anatomy, Histology and Forensic Medicine, University of Florence) for his valuable contribution in confocal image acquisition and processing and to Dr. Giorgia Luciani (Dept. of Physiological Sciences, University of Florence) for her valuable contribution in electrophysiological experiments.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Supporting Information
References
- 1.Liao P, Zhou J, Ji LL, Zhang Y. Eccentric contraction induces inflammatory responses in rat skeletal muscle: role of tumor necrosis factor {alpha} Am J Physiol Regul Integr Comp Physiol. 2010;298:R599–607. doi: 10.1152/ajpregu.00480.2009. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki A, Aizawa T, Tomiya A, et al. Effect of resting interval for muscle regeneration in mice. Ups J Med Sci. 2007;112:175–81. doi: 10.3109/2000-1967-191. [DOI] [PubMed] [Google Scholar]
- 3.Chargè SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–38. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 4.Buckingham M, Montarras D. Skeletal muscle stem cells. Curr Opin Genet Dev. 2008;18:330–6. doi: 10.1016/j.gde.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–73. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Zammit PS, Relaix F, Nagata Y, et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006;119:1824–32. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]
- 7.Yablonka-Reuveni Z, Day K, Vine A, et al. Defining the transcriptional signature of skeletal muscle stem cells. J Anim Sci. 2008;86:E207–16. doi: 10.2527/jas.2007-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins CA, Olsen I, Zammit PS, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Zammit PS. All muscle satellite cells are equal, but are some more equal than others. J Cell Sci. 2008;121:2975–82. doi: 10.1242/jcs.019661. [DOI] [PubMed] [Google Scholar]
- 10.Carosio S, Berardinelli MG, Aucello M, et al. Impact of ageing on muscle cell regeneration. Ageing Res Rev. 2011;10:35–42. doi: 10.1016/j.arr.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Morgan JE, Zammit PS. Direct effects of the pathogenic mutation on satellite cell function in muscular dystrophy. Exp Cell Res. 2010;N316:3100–8. doi: 10.1016/j.yexcr.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Filippin LI, Moreira AJ, Marroni NP, et al. Nitric oxide and repair of skeletal muscle injury. Nitric Oxide. 2009;21:157–63. doi: 10.1016/j.niox.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Snider AJ, Alexa Orr Gandy K, Obeid LM. Sphingosine kinase: role in regulation of bioactive sphingolipid mediators in inflammation. Biochimie. 2010;92:707–15. doi: 10.1016/j.biochi.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verzijl D, Peters SL, Alewijnse AE. Sphingosine-1-phosphate receptors: zooming in on ligand-induced intracellular trafficking and its functional implications. Mol Cells. 2010;29:99–104. doi: 10.1007/s10059-010-0041-z. [DOI] [PubMed] [Google Scholar]
- 15.Nagata Y, Partridge TA, Matsuda R, et al. Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling. J Cell Biol. 2006;174:245–53. doi: 10.1083/jcb.200605028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagata Y, Kobayashi H, Umeda M, et al. Sphingomyelin levels in the plasma membrane correlate with the activation state of muscle satellite cells. J Histochem Cytochem. 2006;54:375–84. doi: 10.1369/jhc.5A6675.2006. [DOI] [PubMed] [Google Scholar]
- 17.Danieli-Betto D, Germinario E, Esposito A, et al. Sphingosine 1-phosphate protects mouse extensor digitorum longus skeletal muscle during fatigue. Am J Physiol Cell Physiol. 2005;288:C1367–73. doi: 10.1152/ajpcell.00246.2004. [DOI] [PubMed] [Google Scholar]
- 18.Zanin M, Germinario E, Dalla Libera L, et al. Trophic action of sphingosine 1-phosphate in denervated rat soleus muscle. Am J Physiol Cell Physiol. 2008;294:C36–46. doi: 10.1152/ajpcell.00164.2007. [DOI] [PubMed] [Google Scholar]
- 19.Squecco R, Sassoli C, Nuti F, et al. Sphingosine 1-phosphate induces myoblast differentiation through Cx43 protein expression: a role for a gap junction-dependent and -independent function. Mol Biol Cell. 2006;17:4896–910. doi: 10.1091/mbc.E06-03-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Formigli L, Meacci E, Sassoli C, et al. Cytoskeleton/stretch-activated ion channel interaction regulates myogenic differentiation of skeletal myoblasts. J Cell Physiol. 2007;211:296–306. doi: 10.1002/jcp.20936. [DOI] [PubMed] [Google Scholar]
- 21.Meacci E, Nuti F, Donati C, et al. Sphingosine kinase activity is required for myogenic differentiation of C2C12 myoblasts. J Cell Physiol. 2008;214:210–20. doi: 10.1002/jcp.21187. [DOI] [PubMed] [Google Scholar]
- 22.Formigli L, Sassoli C, Squecco R, et al. Regulation of transient receptor potential canonical channel 1 (TRPC1) by sphingosine 1-phosphate in C2C12 myoblasts and its relevance for a role of mechanotransduction in skeletal muscle differentiation. J Cell Sci. 2009;122:1322–33. doi: 10.1242/jcs.035402. [DOI] [PubMed] [Google Scholar]
- 23.Meacci E, Bini F, Sassoli C, et al. Functional interaction between TRPC1 channel and connexin-43 protein: a novel pathway underlying S1P action on skeletal myogenesis. Cell Mol Life Sci. 2010;67:4269–85. doi: 10.1007/s00018-010-0442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bencini C, Squecco R, Piperio C, et al. Effects of Sphingosine 1-phosphate on excitation-contraction coupling in mammalian skeletal muscle. J Mus Res Cell Mot. 2003;24:539–554. doi: 10.1023/b:jure.0000009898.02325.58. [DOI] [PubMed] [Google Scholar]
- 25.Bini F, Martinesi M, Formigli L, et al. Paracrine effects of transplanted Rlx-transfected-myoblasts on extracellular matrix remodeling of the post-infarcted heart. Ital J Biochem. –SIB 2007 Riccione, September 26–30, 2007. [Google Scholar]
- 26.Inoue S, Nakazawa T, Cho A, et al. Regulation of arterial lesions in mice depends on differential smooth muscle cell migration: a role for sphingosine-1-phosphate receptors. J Vasc Surg. 2007;46:756–63. doi: 10.1016/j.jvs.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 27.Annabi B, Lachambre MP, Plouffe K, et al. Modulation of invasive properties of CD133+ glioblastoma stem cells: a role for MT1-MMP in bioactive lysophospholipid signaling. Mol Carcinog. 2009;48:910–9. doi: 10.1002/mc.20541. [DOI] [PubMed] [Google Scholar]
- 28.Leong WI, Saba JD. S1P metabolism in cancer and other pathological conditions. Biochimie. 2010;92:716–23. doi: 10.1016/j.biochi.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimowska M, Brzoska E, Swierczynska M, et al. Distinct patterns of MMP-9 and MMP-2 activity in slow and fast twitch skeletal muscle regeneration in vivo. Int J Dev Biol. 2008;52:307–14. doi: 10.1387/ijdb.072331mz. [DOI] [PubMed] [Google Scholar]
- 30.Formigli L, Francini F, Nistri S, et al. Skeletal myoblasts overexpressing relaxin improve differentiation and communication of primary murine cardiomyocyte cell cultures. J Mol Cell Cardiol. 2009;47:335–45. doi: 10.1016/j.yjmcc.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Bani D, Masini E, Bello MG, et al. Relaxin protects against myocardial injury caused by ischemia and reperfusion in rat heart. Am J Pathol. 1998;152:1367–76. [PMC free article] [PubMed] [Google Scholar]
- 32.Vassault A. Lactate dehydrogenase. UV-method with pyruvate and NADH. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Weinheim: Verlag Chemie; 1983. pp. 118–26. [Google Scholar]
- 33.Squecco R, Carraro U, Kern H, et al. A sub-population of rat muscle fibers maintains an assessable excitation-contraction coupling mechanism after long-standing denervation, despite lost contractility. J Neuropathol Exp Neurol. 2009;68:1256–68. doi: 10.1097/NEN.0b013e3181c18416. [DOI] [PubMed] [Google Scholar]
- 34.Dusterhöft S, Yablonka-Reuveni Z, Pette D. Characterization of myosin isoforms in satellite cell cultures from adult rat diaphragm, soleus and tibialis anterior muscles. Differentiation. 1990;45:185–91. doi: 10.1111/j.1432-0436.1990.tb00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Don AS, Martinez-Lamenca C, Webb WR, et al. Essential requirement for sphingosine kinase 2 in a sphingolipid apoptosis pathway activated by FTY720 analogues. J Biol Chem. 2007;282:15833–42. doi: 10.1074/jbc.M609124200. [DOI] [PubMed] [Google Scholar]
- 36.De Palma C, Meacci E, Perrotta C, et al. Endothelial nitric oxide synthase activation by tumor necrosis factor {alpha} through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors. A novel pathway relevant to the pathophysiology of endothelium. Arterioscler Thromb Vasc Biol. 2006;26:99–105. doi: 10.1161/01.ATV.0000194074.59584.42. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki K, Murtuza B, Heslop L, et al. Single fibers of skeletal muscle as a novel graft for cell transplantation to the heart. J Thorac Cardiovasc Surg. 2002;123:984–92. doi: 10.1067/mtc.2002.121507. [DOI] [PubMed] [Google Scholar]
- 38.Formigli L, Perna AM, Meacci E, et al. Paracrine effects of transplanted myoblasts and relaxin on post-infarction heart remodelling. J Cell Mol Med. 2007;11:1087–100. doi: 10.1111/j.1582-4934.2007.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jejurikar SS, Kuzon WM., Jr Satellite cell depletion in degenerative skeletal muscle. Apoptosis. 2003;8:573–8. doi: 10.1023/A:1026127307457. [DOI] [PubMed] [Google Scholar]
- 40.Cassano M, Biressi S, Finan A, et al. Magic-factor 1, a partial agonist of Met, induces muscle hypertrophy by protecting myogenic progenitors from apoptosis. PLoS ONE. 2008;3:e3223. doi: 10.1371/journal.pone.0003223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parise G, McKinnell IW, Rudnicki MA. Muscle satellite cell and atypical myogenic progenitor response following exercise. Muscle Nerve. 2008;37:611–9. doi: 10.1002/mus.20995. [DOI] [PubMed] [Google Scholar]
- 42.Li X, McFarland DC, Velleman SG. Transforming growth factor-beta1-induced satellite cell apoptosis in chickens is associated with beta1 integrin-mediated focal adhesion kinase activation. Poult Sci. 2009;88:1725–34. doi: 10.3382/ps.2008-00534. [DOI] [PubMed] [Google Scholar]
- 43.Meacci E, Cencetti F, Donati C, et al. Sphingosine kinase activity is required for sphingosine-mediated phospholipase D activation in C2C12 myoblasts. Biochem J. 2004;381:655–63. doi: 10.1042/BJ20031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim RH, Takabe K, Milstien S, Spiegel S. Export and functions of sphingosine-1-phosphate. Biochim Biophys Acta. 2009;1791:692–6. doi: 10.1016/j.bbalip.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takabe K, Kim RH, Allegood JC, et al. Estradiol induces export of sphingosine-1-phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem. 2010;85:10477–86. doi: 10.1074/jbc.M109.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danieli-Betto D, Peron S, Germinario E, et al. Sphingosine 1-phosphate signaling is involved in skeletal muscle regeneration. Am J Physiol Cell Physiol. 2010;298:C550–8. doi: 10.1152/ajpcell.00072.2009. [DOI] [PubMed] [Google Scholar]
- 47.Formigli L, Meacci E, Sassoli C, et al. Sphingosine 1-phosphate induces cytoskeletal reorganization in C2C12 myoblasts: physiological relevance for stress fibres in the modulation of ion current through stretch-activated channels. J Cell Sci. 2005;118:1161–71. doi: 10.1242/jcs.01695. [DOI] [PubMed] [Google Scholar]
- 48.Sbrana F, Sassoli C, Meacci E, et al. Role for stress fiber contraction in surface tension development and stretch-activated channel regulation in C2C12 myoblasts. Am J Physiol Cell Physiol. 2008;295:C160–72. doi: 10.1152/ajpcell.00014.2008. [DOI] [PubMed] [Google Scholar]
- 49.Thompson HS, Scordilis SP, Clarkson PM, Lohrer WA. A single bout of eccentric exercise increases HSP27 and HSC/HSP70 in human skeletal muscle. Acta Physiol Scand. 2001;171:187–93. doi: 10.1046/j.1365-201x.2001.00795.x. [DOI] [PubMed] [Google Scholar]
- 50.Féasson L, Stockholm D, Freyssenet D, et al. Molecular adaptations of neuromuscular disease-associated proteins in response to eccentric exercise in human skeletal muscle. J Physiol. 2002;543:297–306. doi: 10.1113/jphysiol.2002.018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gieni RS, Hendzel MJ. Mechanotransduction from the ECM to the genome: are the pieces now in place? J Cell Biochem. 2008;104:1964–87. doi: 10.1002/jcb.21364. [DOI] [PubMed] [Google Scholar]
- 52.McBride TA, Stockert BW, Gorin FA, et al. Stretch-activated ion channels contribute to membrane depolarization after eccentric contractions. J Appl Physiol. 2000;88:91–101. doi: 10.1152/jappl.2000.88.1.91. [DOI] [PubMed] [Google Scholar]
- 53.Pallafacchina G, François S, Regnault B, et al. An adult tissue-specific stem cell in its niche: a gene profiling analysis of in vivo quiescent and activated muscle satellite cells. Stem Cell Res. 2009;4:77–91. doi: 10.1016/j.scr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Wang W, Pan H, Murray K, et al. Matrix metalloproteinase-1 promotes muscle cell migration and differentiation. Am J Pathol. 2009;174:541–9. doi: 10.2353/ajpath.2009.080509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


