Abstract
Histamine is a biogenic amine performing pleiotropic effects in humans, involving tasks within the immune and neuroendocrine systems, neurotransmission, gastric secretion, cell life and death, and development. It is the product of the histidine decarboxylase activity, and its effects are mainly mediated through four different G-protein coupled receptors. Thus, histamine-related effects are the results of highly interconnected and tissue-specific signalling networks. Consequently, alterations in histamine-related factors could be an important part in the cause of multiple rare/orphan diseases. Bearing this hypothesis in mind, more than 25 rare diseases related to histamine physiopathology have been identified using a computationally assisted text mining approach. These newly integrated data will provide insight to elucidate the molecular causes of these rare diseases. The data can also help in devising new intervention strategies for personalized medicine for multiple rare diseases.
Keywords: histamine, histamine receptors, rare diseases, systems biology
Introduction: previous knowledge and hypothesis
Initial text mining approach
Histamine and inflammation/immune system-related rare RDs
Histamine and rare neurological disorders
Histamine and rare neuroinflammatory diseases
Histamine and rare neoplasias
Histamine and other RDs
Insights for new translational initiatives
Concluding remarks and proposed future actions
Introduction: previous knowledge and hypothesis
The biogenic amine histamine (Hia), 2-(1H-imidazol-4-yl) ethanamine, was discovered in 1910 by the winner of the 1936 Nobel Prize for Medicine, Sir Henry H. Dale [1]. It is the product of the alpha-decarboxylation of the proteinogenic amino acid histidine by the enzyme histidine decarboxylase (HDC, EC 4.1.1.22). In gram-positive bacteria, HDC activity is pyruvoyl-dependent, whereas in gram-negative bacteria and in animals, this is a pyridoxal 5-phosphate (PLP)-dependent enzyme [2, 3].
Only a reduced set of mammalian cells, known as Hia-producing cells (HPCs), expresses the HDC gene, namely, mast cells and other immune cells, gastric enterochromaffin-like cells (ECLCs) and histaminergic neurons. Newly synthesized Hia can be either stored in specialized granules, as it occurs in mast cells and basophils, or it can be released to the medium [4]. On its own, intracellular Hia can slow the G1/S progression of the cell cycle, as recently demonstrated in transfected human embryonic cells expressing active and inactive versions of HDC [5]. It can also act as a stress factor because it is degraded by amino oxidases [6]. Extracellular Hia can be taken up by organic cation transporters [7].
The major Hia-mediated effects are elicited through different signalling mechanisms dependent on target cell types [8]. In allergic reactions, Hia acts on vascular smooth muscle cells and endothelial cells, leading to vasodilation and increased vascular permeability. In the gastrointestinal system, Hia released by ECLCs act on parietal cells to stimulate H+, K+ ATPases, leading to acid gastric secretion. Hia is also a neurotransmitter in the central nervous system (CNS). It plays a key role in regulating the sleep–wake cycle, appetite, learning, memory and motor system [9, 10]. Thus, alterations in Hia metabolism are related to diverse human pathologies, such as anaphylaxis and other inflammatory conditions, peptic ulcer, neurological disorders and cancer progression [1].
There are four different Hia receptors, named H1R through H4R according to the order in which they were discovered. All four belong to class A family of G-protein coupled receptors (GPCRs). They trigger different signalling pathways that depend specifically on both the receptor and the cell type expressing it [11]. H1R couples Gαq/11 proteins, leading to phospholipase C activation, production of inositol phosphate and calcium mobilization. Although H1R has been traditionally related to the allergic response, it is also expressed in nerve cells, vascular smooth and endothelial cells among others, and takes part in neurotransmission and cellular adhesion respectively [9, 11]. H2R is expressed in many immune cell types, parietal cells and nerve cells. H2R mediates its effects by coupling GαS proteins, stimulating adenylate cyclase and increasing intracellular cAMP levels. Due to its broad localization, antagonists of this receptor have been used for treating gastric and neurological disorders, as well as in antitumour therapy [11, 12]. H3R, when located in the presynaptic membrane of histaminergic cells, regulates the production of Hia in the CNS; it also regulates the release of many neurotransmitters, acting as a presynaptic heteroceptor [12, 13]. H3R couples to Gαi/o proteins and inhibits cAMP accumulation. It has been demonstrated that several active isoforms of this receptor exist in humans, although their function remain unknown. H4R is expressed in certain immune cells of haematopoietic origin and in the CNS. Similar to H3R, H4R couples to Gαi/o proteins and inhibits cAMP formation. This receptor has been recently targeted as a key component of the immune response as well as being responsible for the mobilization of immune cells. Its discovery has generated new hypotheses about the possible roles of Hia in inflammatory and neurological diseases [9, 10, 14]. Owning to the plethora of processes in which it acts as a key element, Hia could be considered the most versatile biogenic amine, with many different and sometimes antagonistic roles in mammalian physiology.
The understanding of the symptoms associated with abnormal Hia synthesis and reception has progressed thanks to the generation and characterization of HDC and Hia receptors knock-out (KO) mice. The lack of Hia production has remarkable systemic effects, including those on immune cell differentiation and response, tumour progression, alteration of the sleep-wake cycle, bone loss and fertility [15–17]. The phenotypic characterization of KO mice lacking the different Hia receptors has been of great help in identifying specific effects mediated by these GPCRs [18]. Several of the phenotypic alterations found in human diseases are believed to be associated with Hia signalling, and they will be discussed later in the text. The search and development of new and more specific modulators of these proteins is an active area of research and development (R&D) initiatives [9, 19, 20].
Rare diseases (RDs) are pathologies characterized by a very low prevalence (less than two patients per 1000 inhabitants in Europe), although almost 6000 of these conditions have been described (http://www.orpha.net). As there are only a few patients affected by each of these diseases, pharmaceutical companies express a general lack of interest in investing in drug development for these conditions. In the case of Hia, the modulators of its action could not only be taken into consideration for the treatment of RDs, but they are also useful for the treatment of more common diseases, as noted above [1, 9, 10, 19–21]. Thus, a question arises: for how many of these RDs is Hia a part of the problem? Hia has been clearly related to several RDs, including the following: histidinaemia [22]; mastocytosis and basophilic leukaemias [23]; Zollinger-Ellison syndrome; rare gastric carcinomas [24]; and in the development of inflammatory bowel diseases [25], among others. In fact, modulators of the Hia receptors have been proposed or are actually being used as part of current treatments against these pathologies.
As stated recently by Roubertoux and de Vries [26], RDs may share several common pathophysiological mechanisms with other diseases. Therefore, a discovery in one of them can have direct relevance to many others, especially in the case of non-monogenic RDs involving mutations affecting the complex protein-protein interaction networks of signalling pathways. In the elegant work published by Goh et al. [27] on the human disease network (‘diseasome’), the authors were surprised by most disease-related genes being located in the periphery of the human interactome network. This means that these genes show poorer correlations between their expression patterns and those of the rest of the genes than expected from random, as they tend to be expressed only in a few tissues.
Hia-related genes have a lot of the most common properties of disease-related genes. Hia is a product of secondary metabolism, produced in a reduced set of cell types, and its effects are mainly elicited by different, tissue-specific signalling pathways. This fact should link this amine to multiple RDs involving cell-specific signalling mechanisms, as concluded from the diseasome analysis [27, 28]. However, a simple search for hits using the keywords ‘histamine & rare/orphan diseases’ in any bibliographic database (for example, Pubmed – http://www.ncbi.nlm.nih.gov/pubmed/), will only retrieve approximately 100 references, although there are many reasons to expect that Hia-related genes may be well represented and highly connected in a complete diseasome.
Systems Biology resources provide useful tools for producing new data through the integration of previous data fragments [29–31]. Hence, we have used a text mining approach to help us reveal the links between Hia-related factors and RDs, mainly inflammatory, neurological and neuroendocrine, and cardiovascular diseases, as well as rare tumours, for which consistent molecular/genetic together with physiological information is found.
Initial text mining approach
The data used to determine the relationship between Hia and RDs has been obtained using a biomedical literature mining approach and a subsequent experts' review. The first stage of the text mining analysis was completed with SciMiner [32], an online tool based on a dictionary- and rule-based biomedical literature mining analysis. A specific query was performed through SciMiner to identify PubMed Unique Identifiers (PMID) and genes associated with diseases and Hia. The specific query used Medical Subject Headings (MeSH) terms to ensure that the query will address the aims of our study. The MeSH terms belong to a controlled vocabulary following a hierarchical structure where more general terms include the specific ones [33]. This resource has been widely used to index journal articles in Medline, books and journal titles [33–35]. We have selected ‘Congenital, Hereditary, and Neonatal Diseases and abnormalities’ (MeSH ID: D009358) as the broader term associated with genetic or hereditary diseases; and ‘Histamine’ (MeSH ID: D006632) to link our query with the right molecular pathway.
From the literature mining analysis, we gathered 382 genes in 1419 PMIDs. These genes represent a global genetic background of diseases involving Hia. This is the global genetic background used to annotate genetic RDs associated with those genes using the information annotated in the Orphanet database. For each gene, we counted the number of papers that cited it and the total gene-RD co-occurrences. This method serves as an estimate of the contribution of each gene related to RDs to the results. As a result, we obtained 211 RDs related to 140 genes in the global genetic background. In addition, a functional enrichment analysis based on a Fisher's exact test was used to value more representative and statistically significant MeSH terms annotated within papers retrieved from the biomedical literature mining analysis.
However, to identify the most significant results among the collected data, we isolated genes and their associated RDs directly annotated to the ‘Histamine’ MeSH term. From this last search, we obtained 20 genes that were also present in the first broader search process. We prioritized them according to the number of cited papers and the total occurrence, and then we performed a manual curation of the results. In this review, we include results for which there is a clear relationship between one or more components in the Hia signalling network and the molecular and phenotypic aspects of a specific RD.
With the text mining tools described previously, we have been able to identify different types of interrelationships between genes involved in Hia metabolism and genes encoding for proteins directly correlated with the appearance and development of several RDs. Table 1 includes the list of these RDs, together with the Orphanet and OMIM (http://www.ncbi.nlm.nih.gov/omim) identification codes and at least, a representative reference.
Table 1.
Rare diseases related to histamine signalling and/or metabolism
| Disease | Mutated gene/origin | Orphanet ID | Omim ID | Reference |
|---|---|---|---|---|
| Immune/Inflammatory RDs | ||||
| Psoriatic arthritis | HLA-C, NOD/CAR15, TNFA, LTA | 40050 | 607507 | — |
| Idiophatic aplastic anaemia | TERC, TERT, IFNG,NBS1, PRF1, SBDS | 88 | 609135 | [49, 50] |
| Familial cold autoinflammatory syndrome | NLPR3 | 47045 | 120100 | [39, 40] |
| Muckle-Wells syndrome | NLRP3 | 575 | 191900 | [39, 40] |
| Infantile neurologic cutaneous articular syndrome | NLRP3 | 1451 | 607115 | [39, 40] |
| Systemic juvenile idiopathic arthritis | IL6, MIF | 92 | 604302 | [42, 43, 46] |
| Crohn's disease | NOD2/CAR15, IL6 | 206 | 266600 | [53, 54] |
| Ulcerative colitis | Autoimmune | 771 | 266600 | [53, 54] |
| Neurological RDs | ||||
| Narcolepsy with cataplexy | HCRT | 2073 | 161400 | [63, 64] |
| Tourette's syndrome | HDC, SLITRK2 | 856 | 137580 | [65] |
| Hereditary essential tremor | DRD3, ETM2, ETM3 | 862 | 190300 | [75] |
| Myoclonic dystonia | DRD2, SGCE | 36899 | 159900 | [76, 77] |
| Neuroinflammatory RDs | ||||
| Myasthenia gravis | Autoimmune | 589 | 159400* | [83, 86] |
| Hereditary sensory and autonomic neuropathy, type IV | NTRK1 | 642 | 256800 | [85, 87] |
| Hereditary sensory and autonomic neuropathy, type V | NGFB | 64752 | 608654 | [85, 87] |
| Multiple sclerosis | HLA genes on 6p21, MS2, MS3, MS4 | 802 | 126200 | [89–91] |
| Rare neoplasias | ||||
| Acute myeloid leukaemia | Heterogeneous | 519 | 252270 | [97] |
| Zollinger-Ellison syndrome | Sporadic/associated to MEN1 mutation | 913 | 131100 | [99] |
| Mastocytosis | cKIT, TET2 | 98292 | 154800 | [23, 100–105] |
| Other RDs | ||||
| Vitamin D-dependent rickets type 2A | VDR | 437 | 277440 | [110] |
| Familial long QT syndrome | Potassium voltage-gated channels | 768 | 152427 | [112] |
| Brugada syndrome | Ionic voltage-gated channels | 130 | 601144 | [113] |
| von Willebrand disease | VWF | 166078 | 193400 | [114–117] |
| Metabolic syndrome | Heterogeneous | 68367 | – | [118] |
| Congenital adrenal hyperplasia | Heterogeneous | 418 | 145295 | [119] |
| Histidinaemia | HAL | 2157 | 235800 | [22, 122] |
The position of each disease in the table follows its order of appearance in the text. Under the ‘mutated gene/origin’ column, the reported causes of the different rare diseases are indicated.
Histamine and inflammation/immune system-related RDs
The immune response is a highly multifactorial process. Hence, it is almost impossible to accurately determine how many diseases are related to or are dependent on the immune/inflammatory response. Furthermore, there are multiple pathologies involving inflammation that are not traditionally treated as inflammatory diseases, so there is not a solid classification of these conditions. If we focus on RDs, there are approximately 200 RDs that are related somehow with the immune system, representing approximately 3.2% of the RDs indexed in the Orphanet database, although this value is most likely underestimated.
The proinflammatory effects exerted by Hia seem to be elicited mainly through H1R and H4R. H1R, expressed by endothelial cells and smooth muscle cells, mediates vasodilation and bronchoconstriction associated with multiple inflammatory responses. On the other hand, H4R is mainly expressed in cells of hematopoietic origin, in particular dendritic cells, mast cells, eosinophils, monocytes, basophils and T cells. H4R demonstrates a higher affinity for Hia compared with H1R, and it also promotes Ca++ mobilization and activates MAP kinase-dependent signalling pathway [9, 36]. H4R is therefore considered to be the coordinator of immune system communication in response to Hia.
Hia levels are increased in bronchoalveolar fluids extracted from allergic asthma patients, in the skin and plasma of patients with atopic dermatitis, in chronic urticaria biopsies, in both plasma and synovial fluid of patients with rheumatoid arthritis and in the plasma of patients with psoriatic arthritis. These observed phenomena occur because the antihistamines that are currently used in the clinic have little, if any, affinity for H4R. Thurmond et al. [9] suggest that there is a need to develop better H4R modulators to revisit the potential of antihistamines against inflammatory diseases, including the newly identified ones. Lots of different research groups are working towards achieving a complete biochemical and pharmacological characterization of this novel Hia receptor [10, 37, 38].
Cryopyrin-associated periodic syndrome (CAPS) comprises a group of inflammatory diseases associated with a mutation in the NLRP3 gene (NACHT, LRR and PYD domains-containing protein 3) that include the following: familial cold autoinflammatory syndrome, Muckle-Wells syndrome and neonatal-onset multisystem inflammatory disease, formerly known as chronic infantile neurological cutaneous articular syndrome. CAPS is characterized by a neurological condition, articular symptoms, a skin urticarial rash and chronic inflammation. The NLPR3 gene encodes for the protein cryopyrin, a component of the inflammasome, which is a multiprotein complex that mediates the innate immune response [39]. In the skin of CAPS patients, mast cells produce interleukin 1β (IL-1β) in a constitutive manner (Fig. 1A). This is in contrast with normal mast cells, which produce IL-1β only in response to proinflammatory stimuli. Increased IL-1β production has also been observed in monocyte-derived dendritic cells in CAPS patients [40]. According to the information provided by Orphanet, lots of anti-inflammatory drugs have failed in palliating the symptoms accompanying these syndromes. Inhibition of IL-1β production leads to a reduction of the inflammation observed in the patients. Recent results show that the H4R antagonist JNJ7777120 decreased the expression of IL-1β mRNA in murine dendritic cells [41]. The expression of many other immune mediators (IFN-γ, IL-6, IL-10, etc.) can also be modified by signalling activation or repression through H4R in these dendritic cells.
Fig 1.
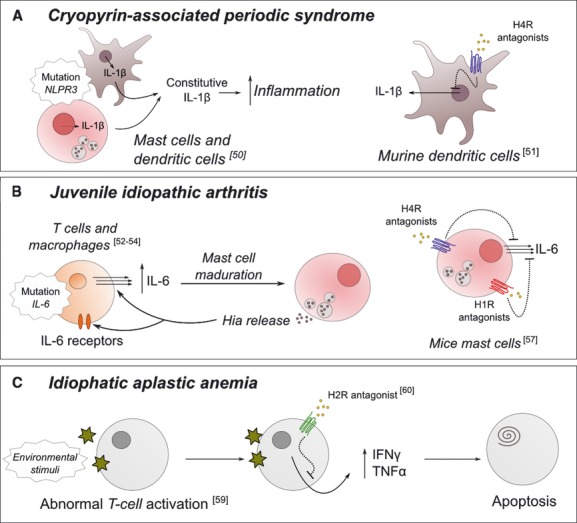
Schematic representation of the reported role of histamine-related factors in three of the inflammatory rare diseases mentioned in the text. For cryopyrin-associated periodic syndrome and juvenile idiopathic arthritis, the experimentally validated inhibitory mechanism in non-human cells is also depicted. Representative references are provided.
Systemic juvenile idiopathic arthritis (JIA) is a chronic inflammatory disease characterized by articular disorders and immunoregulatory abnormalities. Severe cases of systematic JIA result in macrophage activation syndrome, which presents an excessive activation and proliferation of T cells and macrophages. During the active phases of JIA, patients display an abnormally high rate of interleukin 6 (IL-6) production [42] (Fig. 1B), and these elevated IL-6 levels correlate with the daily fever peaks suffered by JIA patients. A polymorphism found in the promoter of IL-6 gene has been thought to be responsible for the overproduction of this cytokine [43]. IL-6 is a crucial cytokine for mast cell maturation and it upregulates Hia release rather than increasing its storage [44]; reciprocally, Hia stimulates both IL-6 production and expression of IL-6 receptors on different cell types, such as human lymphoid, monocytoid and hepatoma cell lines [45]. There are some reported cases in the literature regarding JIA patients who presented an increased number of mast cells in the gastric and duodenal mucosa or elevated levels of urinary Hia [46]. Desai and Thurmond have reported on Hia's role in regulating both IL-6 production and mRNA expression in mice mast cells via H4R activation [47]. At the same time, this production was shown to be blocked by two H4R antagonists, JNJ 7777120 and JNJ 28307474; partially blocked by H1R antagonists; and not blocked by H2R or H3R antagonists. Furthermore, there was no Hia-dependent IL-6 production in H4R-deficient mice. At the same time, H4R antagonists have already been used for in vivo tests, showing a reduction of IL-6 levels in asthma [48]. As far as we know, the potential usefulness of this relationship for preventing the development of JIA has not yet been explored.
There is also evidence for a role for H2R in regulating Hia synthesis and cytokine production in immune cells, and consequently, it may be implicated in immune abnormalities developed in certain RDs. For instance, the RD idiopathic aplastic anaemia is characterized by a disturbed immune system, usually associated with abnormally activated T lymphocytes, leading to high levels of suppressive cytokines, such as IFN-γ and TNF-α [49] (Fig. 1C). These cytokines prevent stem cells in the bone marrow from differentiating and can even induce stem cells to undergo apoptosis. H2R is expressed in T lymphocytes, and cimetidine, its antagonist, modulates the function of these cells by not fully characterized mechanisms. It has been demonstrated that treatment with cimetidine leads to a reduction in the production of both cytokines, IFN-γ and TNF-α, and partially reverses their haematopoietic suppressive effect in aplastic anaemia in mice [50]. Thus, the antagonist cimetidine could be an appropriate treatment for patients of this RD. Like H2R, H4R expression has also been found in several T cell types [36, 51]. For instance, it has been observed that in HDC KO mice, there is a drastic decrease in the production of IFN-γ by natural killer T cells. This is reversed by a single injection of Hia, and this process is mediated by H4R [52]. It would be of great interest to determine whether H4R-mediated IFN-γ production occurs in other T cell types, because the use of H4R antagonists could be useful for the modulation of cytokine overproduction in idiopathic aplastic anaemia patients.
Crohn's disease and ulcerative colitis are rare inflammatory bowel diseases in which Hia produced by mast cells plays an important role. Patients with these conditions show an increased level of Hia excreted in the urine, which correlates with the clinical manifestation of this disease [53]. It has been suggested that mediators released from Hia-expressing cells in the intestine could be responsible for the progression of these diseases. Hia activity through H1R mediates inflammatory effects, whereas H2R and H4R signalling trigger the production and secretion of immune mediators, such as cytokines. Furthermore, Hia is involved in the preferential activation of Th2 cells, which promote further inflammatory effects that can lead to the appearance of intestinal infections and tumours. Several authors have also proposed the clinical use of H4R antagonists as promising anti-inflammatory effectors [54].
All these findings together suggest a promising future for further development of new modulators of the Hia synthesis and signalling in the treatment of this group of RDs and other more prevalent inflammatory/immune diseases.
Histamine and rare neurological disorders
There are 1937 RDs related to some neurological abnormality. This is over 30% of all the RDs indexed to date. Over 60,000 neurons localized in the hypothalamic tuberomammillary nuclei are the major source of Hia produced in the brain [12, 55]. The histaminergic system is involved in the development of very different functions in the CNS (wakefulness, appetite control, learning and memory, stress, etc.) [56], and all these physiological functions are mediated through H1, H2 and H3 receptors [12]. H1R is located in most brain regions, and Hia exerts neuroendocrine, behavioural and nutritional control through it. H2R in the brain mediates postsynaptic functions of Hia related to cognition, nociception and immune function. Due to their colocalization in some regions of the brain, H1R and H2R could carry out synergistic effects [12]. H3R regulates the synthesis and release of Hia from histaminergic neurons through a negative feedback mechanism [57] and controls the release of some other neurotransmitters, such as the biogenic amines glutamate, gamma-aminobutyric acid and acetylcholine. The presence of H4R in different regions of the CNS has already been demonstrated, although its specific functions and protein-protein interactions are not yet fully elucidated [14]. The first studies in this field are currently being published [58].
During the sleep-wake cycle, higher levels of Hia have been reported when the organism is awake than when sleeping [59]. Wakefulness is mediated by Hia in conjunction with orexins and hypocretin [60, 61], and both systems are located in close proximity. Nevertheless, orexin neurons control motor-related functions, including posture, muscle tone and food intake, whereas the Hia system controls cognitive activities when the organism is awake [62]. Therefore, both systems are responsible for distinct, but complementary tasks during wakefulness. The autoimmune sleep disorder narcolepsy with cataplexy is caused by a loss of hypocretin-producing neurons in the brain [63]. In zebrafish, several studies of the interaction of the two systems have revealed that low levels of Hia, either by the use of HDC inhibitors or by the use of HDC KO fish, lead to a reduction in hypocretin neurons and this effect is mediated by signalling through H1R [61]. In humans, this phenomenon has been observed for years in the sedative effects exerted by first generation antihistamines that target H1R. Hia has also been connected to sleep regulation through H3R. In fact, several H3R antagonists are in clinical development not only for cognitive enhancement but also for the treatment of narcolepsy and cognitive deficits [64]. In Orphanet database, there are 29 diseases related to sleep-wake cycle abnormalities, and 79 genes are associated with these conditions. Due to its important role in wakefulness, we propose that Hia metabolism and signalling genes should be sequenced in patients with these RDs. This could contribute to the development of new personalized medicine treatments. We are confident that the proposed effort will reveal the molecular mechanisms involved in the development of these RDs.
The histaminergic system is also implicated in Tourette's syndrome, a developmental neurological disease characterized by chronic motor and vocal tics along with other psychiatric symptoms. In a recent study comprised of several members of the same family, it was observed that each family member presented a premature termination codon in the HDC gene at the position coding for residue 317 [65]. Our group have generated and validated the first structural model of the active conformation of mammalian HDC [2, 3]. In this truncated version, the enzyme lacks important residues involved in the formation of the binding pocket and the establishment of the proper catalytic environment, leading to a decrease in the production of Hia in the CNS. These symptoms can be mimicked in HDC KO mice, which demonstrate enhanced locomotor behaviour after treatment with dopamine agonists, such as metamphetamine (discussed below). A novel treatment for these motor-related symptoms requires an increase in histaminergic neurotransmission, achievable by the use of selective antagonists of the autoreceptor H3R [66]. Even when HDC activity does not seem to be the only cause of the motor tics in all Tourette's syndrome patients [65], the putative influence of distinct Hia-related elements in this pathology is an interesting open field of study.
Hia system is closely linked to dopamine metabolism and signalling in the brain (Fig. 2A). The histaminergic system enhances its activity via D2 receptor when dopamine agonists (L-dopa for example) are administrated [67]. Methamphetamine (MET) is an antipsychotic drug used by schizophrenia patients that stimulates locomotor activity and has been extensively used in the medical field to simulate psychosis and schizophrenia in animal models [68, 69]. MET stimulates the release of dopamine and Hia, the latter stimulated by endogenous dopamine acting on the D2 receptor. MET also stimulates HDC activity in localized parts of the CNS [70, 71]. When MET is administered, both hyperactive locomotion and an increase of Hia levels are observed. However, enhanced locomotor activity reaches its maximum peak just a short time after administration, correlated with the maximal dopamine concentrations. The enhanced motor activity then rapidly decreases, whereas the levels of Hia metabolites continue to increase for several hours. Hence, it has been proposed that the histaminergic system is involved in the inhibition of the response due to MET and this effect is mediated by dopamine and D2 receptor. This assumption was further corroborated with H3R antagonists that increase the release of Hia in the brain, leading to an overall decrease in the motor alterations exerted by MET [72]. This relationship has also been found in the early stages of life. The use of MET in prenatal and young mice has shown that Hia mediates the harmful effects that MET has on their developing brain, and the appearance of these effects can be avoided by treating them with the H3R agonist imepip [73]. The administration of MET results into abnormally high levels of Hia in the CNS during the first stages of mice development, resulting into long-term detrimental cognitive functions in the adult. In human children, the exposure to MET in the prenatal stage leads to the reduction of several brain structures and cognitive impairments related to attention, verbal and spatial memory [74].
Fig 2.
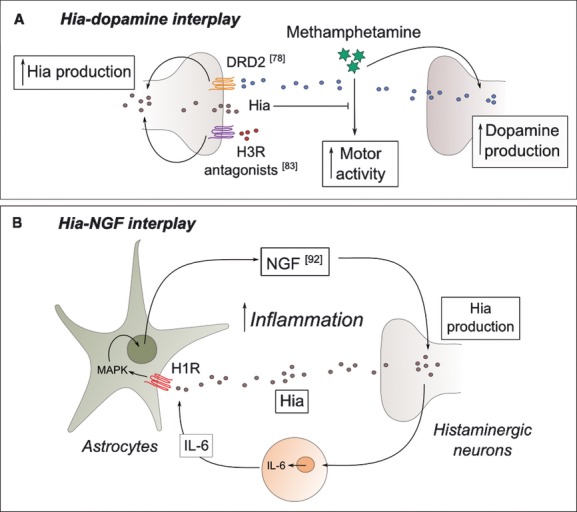
Schematic representation of the interplay between histamine-related factors and both dopamine and nerve growth factor (NGF). HDC, histidine decarboxylase; DRD2, dopamine receptor D2; MAPK, mitogen-activated protein kinase. Representative references are provided.
Several RDs are characterized by malfunctions of the locomotor system, such as dystonia or ataxia. The D3 dopamine receptor has been traditionally associated to hereditary essential tremor [75]. However, controversial results have been published concerning the role of D2 dopamine receptor in the development of the RD myoclonic dystonia [76, 77]. Taking into account the data presented above, there is evidence suggesting that the histaminergic system may play an important role in the development and modulation of symptoms involving the motor system. There is experimental evidence of the physical interaction between dopamine D1 and D2 receptors and H3R when they are located in the postsynaptic membranes of certain brain cells [78, 79]. The regulation of the signal through these heteromers has been demonstrated to be significantly different from what occurs in the separated receptors. These physical interactions are excellent proof of the close relationship existing between dopaminergic and histaminergic neurotransmission systems.
In 2008, Ledesma and collaborators reported the linkage of a non-synonymous polymorphism in the enzyme histamine n-methyltransferase to an increased risk of developing essential tremor [80]. This enzyme is responsible for part of the Hia degradation, so alterations in its function may lead to unbalanced levels of Hia. Nevertheless, two independent groups have recently found opposing results to those of Ledesma and coworkers in patients with essential tremor, Parkinson's disease and Alzheimer's disease [81, 82]. It is possible that the alterations in different Hia-related elements could cause a phenotypic convergence in these pathologies, whose symptoms comprise both locomotor and/or cognition alterations.
Histamine and rare neuroinflammatory diseases
There are a significant number of neurological diseases that are also characterized by inflammatory symptoms; therefore, we are dedicating a separate section for the discussion of these diseases. In our text mining study, Hia has been linked to nerve growth factor (NGF), a homodimeric protein that acts as a mediator of inflammation in injured tissues, induces neuronal differentiation, promotes neuronal survival and prevents apoptosis in neurons of both central and peripheral nervous systems. It exerts its function through the neurotrophin tyrosine kinase receptor type A (TrKA) and the p75NTR receptor for neurotrophins [83–85]. Most inflammatory cells in humans express both TrKA and p75NTR receptors, and this expression is increased in certain inflammatory diseases, such as atopic dermatitis and myasthenia gravis (a rare autoimmune neuromuscular disorder) [83, 86]. Mutations in the genes encoding for TrKA and NGF have been linked to the rare neuropathy hereditary sensory and autonomic disease types IV and V respectively [85, 87]. These pathologies are characterized by loss of pain perception, mild mental retardation and immune deficiency. All these symptoms occur with varying severity. Whilst it has been demonstrated that TrKA expression is Hia-independent [88], this amine is a powerful stimulator of NGF production, and the main signalling pathway involved in its synthesis and secretion in the CNS includes H1R activation [45, 83] (Fig. 2B). Moreover, NGF enhances the production and release of Hia. The positive feedback loop between these two factors leads to a substantial increase in the inflammatory response, which promote more severe symptoms in many inflammatory diseases. To control this process, several H1R antagonists have been used in the treatment of skin inflammatory conditions [83]. Besides its direct stimulation of NGF production, Hia is able to indirectly stimulate it together with different cytokines, such as IL-6 [45].
Different types of sclerosis are classified as RDs, and multiple sclerosis (MS) being the most studied. In MS patients, the myelin sheaths around the axons of nerve cells within the brain and spinal cord are damaged, leading to demyelination. As a result, there is impaired signal transmission in the affected nerves, causing a wide variety of symptoms. Mast cells, the main Hia producers, are involved in the onset of this disease through the release of inflammatory intermediates and the activation of T cells at the blood-brain barrier [89]. In a recent review by Jadidi-Niaragh and collaborators [90], the importance of Hia and its receptors in MS is discussed in depth. According to these authors, Hia would play a key role in regulating the pathogenesis of MS by promoting the differentiation of the T helper cell population to Th2 subgroup instead of the Th1 subgroup, leading to less severe symptoms, and by acting on oligodendrocytes and the remyelination process. Furthermore, several H1R antagonists have been shown to ameliorate the symptoms of experimental MS models [90, 91]. Due to its localization in the brain, H4R has been the focus of studies intending to determine its function in neuroinflammation.
Histamine and rare neoplasias
Hia and several elements of its metabolism are also directly related with tumour progression and angiogenesis in different types of cancer, including haematologic, breast, pancreatic, melanoma and colon cancer, as recently reviewed by Medina and Rivera [92]. Of course, both, cancer origin (gene-disease relationships) and cell-specific signal transduction mechanisms elicited by Hia, are diverse and multifactorial. As a consequence of this diversity, both growth-promoting and antineoplastic effects have been described for Hia in tumour cells of different origins.
An inflammatory environment is crucial in the development of malignant neoplasias. Immature cells in the myeloid linage (iMCs) are essential to tumour establishment and progression since they are able to avoid the cytotoxic response exerted by T lymphocytes and by negatively regulating the immune response [93, 94]. Under cancerous conditions, iMCs are localized within tumours and in their periphery; they are unable to synthesize Hia due to a disruption in the HDC gene [95]. HDC KO mice are more susceptible to chemically induced skin and colon cancer, so Hia seems to be effective in restricting tumour growth. Furthermore, Hia has been shown to exert inhibitory function in the development of many other types of experimental cancer (see the references in [96]). Nowadays, a treatment combining IL-12 and Hia is used to prevent relapse in acute myeloid leukaemia by the activation of cytotoxic lymphocytes [97] and by blocking the generation of reactive oxygen species, and hence protecting NK and T cells from apoptosis, in patients with renal cell carcinoma [98].
Zollinger-Ellison syndrome is a severe disease in which gastric acid secretion is over five times the upper normal limit, arising from the presence of a neuroendocrine gastrinoma. Several H2R antagonists have been used to reduce this gastric acid hypersecretion [99].
Hia overproduction has also been associated with mastocytosis, a RD characterized by an abnormal accumulation of mast cells in different tissues [23, 100]. When released, the mediators of mast cells account for the majority of the symptoms associated with the disease and can even lead to death. Urine methylhistamine (a Hia metabolite) levels and HDC expression are considered markers in the diagnosis of this condition [101]. Nowadays, H1R and H2R antagonists are used in the treatment of skin and gastric ailments experienced by mastocytosis patients respectively [102]. Moreover, H1R antagonists terfenadine and loratadine have been shown to inhibit the in vitro proliferation of neoplastic mast cells and induce apoptosis in a dose-dependent manner [103]. The activating mutation D816V in the c-Kit/stem cell factor is found in most mastocytosis patients [104]. Although the structural changes in c-Kit caused by the mutation have recently been predicted [105], we are far from fully understanding the alterations in the signal transduction cascade that lead to Hia synthesis/storage deregulation in these cells.
Histamine and other RDs
In several studies, Hia has been shown to increase the number and activity of osteoclasts (the cells responsible for bone resorption) [106]. HDC KO mice, which virtually lack Hia, have an increased rate of bone formation, increased cortical bone thickness and a reduced rate of bone resorption [107]. On the one hand, significantly higher levels of Hia are found in patients with osteoporosis and mastocytosis [108]. Hence, it has been proposed that specific inhibitors of Hia production or signalling could be used to counter the effects observed in these conditions [108, 109].
Mutations in the vitamin D3 receptor (VDR) gene have been found to be the cause for the rare disease vitamin D resistant rickets, characterized by an increased rate of circulating vitamin D3 and physical defects, such as the aberrant mineralization of cartilage and bones. In normal osteogenesis, VDR acts as a transcription factor, and one of its downstream targets is H1R [110], whose levels are increased in cells overexpressing VDR. Pochampally and collaborators determined that mineralization was promoted via H1R, but not H2R, in cells expressing VDR. However, Hia was not able to significantly increase osteogenesis, although H1R antagonists are able to inhibit the differentiation of bone cells, suggesting the existence of unknown H1R interactions. Therefore, H1R-mediated signalling, independent of Hia, must be involved in bone construction/destruction equilibrium. However, there is no doubt that this is a complex problem that needs further molecular characterization.
On the other hand, Hia has also been found to be involved in several cardiovascular conditions. In heart, the response to Hia is mediated by H1R and H2R, which resemble the functions exerted by the adrenergic system receptors [111]. H1R antagonists are known to block HERG1 potassium channels, so they delay the action potential repolarization and prolong the QT syndrome [112]. Besides, the activation of H2R by Hia produces a positive ionotropic effect in the heart. There are several RDs characterized by a prolongation of the QT interval, syncope, arrhythmia and cardiac failure. A recent example in the literature shows that several H1 antihistamines have been found to deteriorate one of the symptoms associated with Brugada syndrome, a cardiac RD [113].
The von Willebrand factor (VWF) is a glycoprotein present in plasma that plays an essential role in platelet adhesion and thrombosis. A deficiency in the level and/or quality of VWF leads to the so-called von Willebrand disease, comprised of at least seven types, which are mainly characterized by different coagulation abnormalities [114]. VWF can be released either constitutively from endothelial cells or from storage granules, called Weibel-Palade bodies. VWF release is mediated by an increase in intracellular Ca++ induced by Hia through H1R [115, 116]. Dopamine, through its receptors D2, D3 and D4 can inhibit Hia-mediated VWF release, but this effect is not mediated by Ca++-dependent signalling; hence, the biochemical aspects of this inhibition still remain unknown [117]. This is an additional example of the complex network surrounding this biogenic amine.
Hia is also playing a key role in metabolic disorders by triggering the inflammatory response and by taking part in the modulation of the metabolism [118]. Fatty liver syndrome and its related severe condition, namely non-alcoholic steatohepatitis, is associated with obesity, insulin resistance and inflammation, among other symptoms. The implication of Hia in these syndromes, and more precisely, its action through H1 and H2 receptors, have been proven to be of great importance in the regulation of glucose and lipid metabolism.
Congenital adrenal hyperplasia comprised a group of syndromes involving abnormalities in the production of adrenal hormones, due to the deficiency of different enzymes of the cytochrome P450 superfamily [119]. Adult patients are often obese and present bone and metabolic abnormalities, as well as fertility problems. This cytochrome is the known target for multiple biogenic amines including Hia, which regulates the catalytic activity of these enzymes and cell function by binding them on specific sites [120]. Not only is Hia known to bind cytochrome P450 enzymes but also several H3R antagonists [121].
On the other hand, patients with histidinaemia, a benign disorder caused by a defect in the enzyme histidine ammonia-lyase, responsible for the conversion of histidine into ammonia, show an increased Hia metabolism [122]. Recently, it has also been observed that a mild chronic homocysteinemia leads to increased HDC expression and macrophage infiltration in mouse liver. These findings can be interesting for homocystinuria patients [123].
Insights for new translational initiatives
This review points out some connections between RD-related elements and Hia-metabolism/signalling related factors. This provides insights for deeper characterization of the underlying molecular mechanisms of these pathologies and for new intervention strategies. We are convinced that this approach will become progressively more informative as human interactomes and diseasomes [27, 28] are enriched and properly curated.
It is obvious that the recent discovery of H4R and its ubiquitous location, together with its key role in the regulation of the immune response, has forced researchers to revisit the previously collected data on the effects exerted by Hia [9]. Thus, new translational initiatives require a better molecular characterization of the H4R-mediated responses and the development of new H4R-specific ligands [20, 124].
The data presented in this review demonstrate a clear interplay (including physical interactions) between Hia and dopamine metabolism/signalling-related factors. Their implication in several rare neurological diseases, primarily those affecting motor functions, suggests that they will be key targets for pharmacological intervention [57].
Another possibility for therapeutic intervention in these Hia-related RDs might be to take advantage of the previously reported properties of natural compounds, some of them cheap and easy to bring to the clinical testing phase [125, 126]. In this type of compounds, tea polyphenols are one of the most noteworthy [127]. Epigallocatechin-3-gallate (EGCG), the most abundant polyphenol in green tea leaves, has been described as an antioxidant [128, 129], antiangiogenic [130], antiproliferative [131–133] and anti-inflammatory compound [134, 135]. In 2003, Rodríguez-Caso et al. demonstrated a direct inhibition of HDC activity by EGCG. In the last few years, knowledge about the physiological and molecular effects of EGCG has increased enormously, as has its demonstrated positive effects in the treatment of several diseases [136–138]. Some of the Hia–related genes mentioned in this review are known to be targets of EGCG. For example, Gundimeda et al. [139] demonstrated that EGCG potentiated NGF-induced neurite outgrowth. As EGCG can cross the blood-brain barrier, these authors propose that this compound can be a highly useful tool in the treatment of neuronal injuries. EGCG also inhibits IL-6 secretion [140], and the relationship of IL6 with Hia and NGF secretion has already been mentioned before. Consequently, the connection between EGCG and all these factors as well as their consequences on RDs deserves further attention as it may have translational potential. Another application for EGCG may be to use its potential as a preventive agent in combined therapies against skin inflammatory diseases, such as mastocytosis [23, 127]. EGCG has already been used in the treatment of non-rare skin inflammation [141–143].
Concluding remarks and proposed future actions
Here, we present evidence that suggests the involvement of different Hia-related factors in more than 25 RDs, most of them inflammatory and neurological diseases. The initial search for connection between Hia and RDs was performed with text mining tools, followed by a manual curation of the retrieved data. Our observations reveal several new points of discussion on each topic and suggest several valuable lines of action for the near future. They indicate a need for further R&D efforts to advance the topic, supporting the idea that research on RDs can efficiently progress towards translational applications by combining computational and experimental approaches (including high-throughput and post-genomic technologies among others) and clinical information.
Several RDs are associated with polymorphisms in Hia-related proteins. In our opinion, these data only represent the beginning of the potential knowledge on this topic. The characterization of the exome and transcriptome of RD patients should reveal further relationships among Hia-related gene polymorphisms and RDs, which can help advance the goal towards personalized therapies. Fortunately, KO animals have been developed for most of the Hia metabolism/signalling-related genes, and many experimental animal models also exist for many RDs. Collaboration between clinical and basic research groups could provide critical information about the systemic effects of alteration of these Hia-related factors. These efforts, together with high-throughput post-genomic technologies and biocomputational analysis (systems biology-associated technologies), will accelerate the process and will help patients afflicted with many different RDs.
Many modulators of Hia receptors have been described and used in therapies against a wide range of pathologies. As for the newest Hia receptor, H4R, it is crucial to discover its structure and its interacting partners in different cell types, taking into account the possibility that it generates dimers with other receptors (for example, dopamine receptors). This information can provide new methods for interfering with the relevant roles of H4R as coordinator of immune cell communication not only in RDs, but in the full diseasome. A European Science Foundation initiative (COST Action BM08/06) includes action towards these objectives.
A handicap in the development of novel drugs against RDs is the high cost of investment compared with the number of potential drug consumers. That will not be the case for Hia modulators, because they would have a wide range of applications in many emergent inflammatory, neurological and gastrointestinal diseases. In fact, as we still do not have a full view of the human diseasome, the information collected in this review leads us to suggest that Hia-related factors must be directly connected to, or at least take part in, the signalling networks of many different diseases.
Acknowledgments
This work was supported by the grants from the Ministerio de Ciencia e Innovación (MICINN), Spain (SAF2008-02522, cofunded by ERDF (EU), and SAF2011-26518); CVI-6585 and funds from group BIO-267 [Junta de Andalucía, cofunded by ERDF (EU)]. This work takes part in the activities of the COST Action BM0806 (ESF). CIBERER is an initiative of the Instituto de Salud Carlos III (Spain).
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Shahid M, Tripathi T, Khan R. Biological and pharmacological aspects of histamine receptors and their ligands. In: Shahid M KN, Khan RA, Tripathi T, et al., editors. Biomedical aspects of histamine: current perspectives. London: Springer; 2010. pp. 61–91. [Google Scholar]
- 2.Moya-Garcia AA, Medina MA, Sanchez-Jimenez F. Mammalian histidine decarboxylase: from structure to function. Bioessays. 2005;27:57–63. doi: 10.1002/bies.20174. [DOI] [PubMed] [Google Scholar]
- 3.Moya-Garcia AA, Ruiz-Pernia J, Marti S, et al. Analysis of the decarboxylation step in mammalian histidine decarboxylase. A computational study. J Biol Chem. 2008;283:12393–401. doi: 10.1074/jbc.M707434200. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Faroldi G, Rodriguez CE, Urdiales JL, et al. Polyamines are present in mast cell secretory granules and are important for granule homeostasis. PLoS One. 2010;5:e15071. doi: 10.1371/journal.pone.0015071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrighach H, Fajardo I, Sanchez-Jimenez F, et al. Exploring polyamine regulation by nascent histamine in a human-transfected cell model. Amino Acids. 2010;38:561–73. doi: 10.1007/s00726-009-0417-6. [DOI] [PubMed] [Google Scholar]
- 6.Ochiai Y, Itoh K, Sakurai E, et al. Substrate selectivity of monoamine oxidase A, monoamine oxidase B, diamine oxidase, and semicarbazide-sensitive amine oxidase in COS-1 expression systems. Biol Pharm Bull. 2006;29:2362–6. doi: 10.1248/bpb.29.2362. [DOI] [PubMed] [Google Scholar]
- 7.Ogasawara M, Yamauchi K, Satoh Y, et al. Recent advances in molecular pharmacology of the histamine systems: organic cation transporters as a histamine transporter and histamine metabolism. J Pharmacol Sci. 2006;101:24–30. doi: 10.1254/jphs.fmj06001x6. [DOI] [PubMed] [Google Scholar]
- 8.Schneider E, Bertron AF, Dy M. Modulation of hematopoiesis through histamine receptor signaling. Front Biosci (Schol Ed) 2011;3:467–73. doi: 10.2741/s165. [DOI] [PubMed] [Google Scholar]
- 9.Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov. 2008;7:41–53. doi: 10.1038/nrd2465. [DOI] [PubMed] [Google Scholar]
- 10.Leurs R, Chazot PL, Shenton FC, et al. Molecular and biochemical pharmacology of the histamine H4 receptor. Br J Pharmacol. 2009;157:14–23. doi: 10.1111/j.1476-5381.2009.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akdis CA, Simons FE. Histamine receptors are hot in immunopharmacology. Eur J Pharmacol. 2006;533:69–76. doi: 10.1016/j.ejphar.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 12.Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 13.Arrang JM, Morisset S, Gbahou F. Constitutive activity of the histamine H3 receptor. Trends Pharmacol Sci. 2007;28:350–7. doi: 10.1016/j.tips.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Connelly WM, Shenton FC, Lethbridge N, et al. The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS. Br J Pharmacol. 2009;157:55–63. doi: 10.1111/j.1476-5381.2009.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musio S, Gallo B, Scabeni S, et al. A key regulatory role for histamine in experimental autoimmune encephalomyelitis: disease exacerbation in histidine decarboxylase-deficient mice. J Immunol. 2006;176:17–26. doi: 10.4049/jimmunol.176.1.17. [DOI] [PubMed] [Google Scholar]
- 16.Mondillo C, Falus A, Pignataro O, et al. Prolonged histamine deficiency in histidine decarboxylase gene knockout mice affects Leydig cell function. J Androl. 2007;28:86–91. doi: 10.2164/jandrol.106.000257. [DOI] [PubMed] [Google Scholar]
- 17.Burgess CR. Histamine and orexin in the control of arousal, locomotion, and motivation. J Neurosci. 2010;30:2810–1. doi: 10.1523/JNEUROSCI.0045-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakurai E, Kuramasu A, Watanabe T, et al. Multiple histamine receptor gene knockout mice and their phenotypes. Inflamm Res. 2009;58(Suppl 1):41–2. doi: 10.1007/s00011-009-0659-5. [DOI] [PubMed] [Google Scholar]
- 19.Moya-Garcia AA, Pino-Angeles A, Gil-Redondo R, et al. Structural features of mammalian histidine decarboxylase reveal the basis for specific inhibition. Br J Pharmacol. 2009;157:4–13. doi: 10.1111/j.1476-5381.2009.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wijtmans M, de Graaf C, de Kloe G, et al. Triazole ligands reveal distinct molecular features that induce histamine H4 receptor affinity and subtly govern H4/H3 subtype selectivity. J Med Chem. 2011;54:1693–703. doi: 10.1021/jm1013488. [DOI] [PubMed] [Google Scholar]
- 21.Yildirim MA, Goh KI, Cusick ME, et al. Drug-target network. Nat Biotechnol. 2007;25:1119–26. doi: 10.1038/nbt1338. [DOI] [PubMed] [Google Scholar]
- 22.Tanabe M, Sakura N. Hyperhistaminemia in patients with histidinemia due to increased decarboxylation of histidine. Clin Chim Acta. 1989;186:11–7. doi: 10.1016/0009-8981(89)90197-6. [DOI] [PubMed] [Google Scholar]
- 23.Krauth MT, Agis H, Aichberger KJ, et al. Immunohistochemical detection of histidine decarboxylase in neoplastic mast cells in patients with systemic mastocytosis. Hum Pathol. 2006;37:439–47. doi: 10.1016/j.humpath.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Wardlaw R, Smith JW. Gastric carcinoid tumors. Ochsner J. 2008;8:191–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Raithel M, Nagel A, Zopf Y, et al. Plasma histamine levels (H) during adjunctive H1-receptor antagonist treatment with loratadine in patients with active inflammatory bowel disease (IBD) Inflamm Res. 2010;59:S257–8. doi: 10.1007/s00011-009-0120-9. [DOI] [PubMed] [Google Scholar]
- 26.Roubertoux PL, de Vries PJ. From molecules to behavior: lessons from the study of rare genetic disorders. Behav Genet. 2011;41:341–8. doi: 10.1007/s10519-011-9469-y. [DOI] [PubMed] [Google Scholar]
- 27.Goh KI, Cusick ME, Valle D, et al. The human disease network. Proc Natl Acad Sci USA. 2007;104:8685–90. doi: 10.1073/pnas.0701361104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang M, Zhu C, Jacomy A, et al. The orphan disease networks. Am J Hum Genet. 2011;88:755–66. doi: 10.1016/j.ajhg.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medina MA, Correa-Fiz F, Rodriguez-Caso C, et al. A comprehensive view of polyamine and histamine metabolism to the light of new technologies. J Cell Mol Med. 2005;9:854–64. doi: 10.1111/j.1582-4934.2005.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 30.Ananiadou S, Kell DB, Tsujii J. Text mining and its potential applications in systems biology. Trends Biotechnol. 2006;24:571–9. doi: 10.1016/j.tibtech.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Jimenez F, Montanez R, Correa-Fiz F, et al. The usefulness of post-genomics tools for characterization of the amine cross-talk in mammalian cells. Biochem Soc Trans. 2007;35:381–5. doi: 10.1042/BST0350381. [DOI] [PubMed] [Google Scholar]
- 32.Hur J, Schuyler AD, States DJ, et al. SciMiner: web-based literature mining tool for target identification and functional enrichment analysis. Bioinformatics. 2009;25:838–40. doi: 10.1093/bioinformatics/btp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachrach CA, Charen T. Selection of MEDLINE contents, the development of its thesaurus, and the indexing process. Med Inform. 1978;3:237–54. doi: 10.3109/14639237809014183. [DOI] [PubMed] [Google Scholar]
- 34.Neveol A, Shooshan SE, Claveau V. Automatic inference of indexing rules for MEDLINE. BMC Bioinformatics. 2008;9(Suppl 11):S11. doi: 10.1186/1471-2105-9-S11-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neveol A, Shooshan SE, Humphrey SM, et al. A recent advance in the automatic indexing of the biomedical literature. J Biomed Inform. 2009;42:814–23. doi: 10.1016/j.jbi.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Mahony L, Akdis M, Akdis CA. Regulation of the immune response and inflammation by histamine and histamine receptors. J Allergy Clin Immunol. 2011;128:1153–62. doi: 10.1016/j.jaci.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 37.Chazot PL. Advances in histamine pharmacology reveal new drug targets. Br J Pharmacol. 2009;157:1–3. doi: 10.1111/j.1476-5381.2009.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suwa E, Yamaura K, Oda M, et al. Histamine H(4) receptor antagonist reduces dermal inflammation and pruritus in a hapten-induced experimental model. Eur J Pharmacol. 2011;667:383–8. doi: 10.1016/j.ejphar.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura Y, Kambe N, Saito M, et al. Mast cells mediate neutrophil recruitment and vascular leakage through the NLRP3 inflammasome in histamine-independent urticaria. J Exp Med. 2009;206:1037–46. doi: 10.1084/jem.20082179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lasiglie D, Traggiai E, Federici S, et al. Role of IL-1 beta in the development of human T(H)17 cells: lesson from NLPR3 mutated patients. PLoS One. 2011;6:e20014. doi: 10.1371/journal.pone.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon T, Laszlo V, Lang O, et al. Histamine regulates relevant murine dendritic cell functions via H4 receptor. Front Biosci. 2011;3:1414–24. doi: 10.2741/e343. [DOI] [PubMed] [Google Scholar]
- 42.de Benedetti F, Martini A. Targeting the interleukin-6 receptor: a new treatment for systemic juvenile idiopathic arthritis? Arthritis Rheum. 2005;52:687–93. doi: 10.1002/art.20946. [DOI] [PubMed] [Google Scholar]
- 43.Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–76. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conti P, Kempuraj D, Di Gioacchino M, et al. Interleukin-6 and mast cells. Allergy Asthma Proc. 2002;23:331–5. [PubMed] [Google Scholar]
- 45.Ales K, Wraber B, Lipnik-Stangelj M. The synergistic effect of histamine and IL-6 on NGF secretion from cultured astrocytes is evoked by histamine stimulation of IL-6 secretion via H1-receptor-PKC-MAPK signalling pathway. Inflamm Res. 2008;57:S33–4. doi: 10.1007/s00011-007-0617-z. [DOI] [PubMed] [Google Scholar]
- 46.Lindsley CB, Miner PB., Jr Seronegative juvenile rheumatoid arthritis and mast cell-associated gastritis. Arthritis Rheum. 1991;34:106–9. doi: 10.1002/art.1780340117. [DOI] [PubMed] [Google Scholar]
- 47.Desai P, Thurmond RL. Histamine H(4) receptor activation enhances LPS-induced IL-6 production in mast cells via ERK and PI3K activation. Eur J Immunol. 2011;41:1764–73. doi: 10.1002/eji.201040932. [DOI] [PubMed] [Google Scholar]
- 48.Dunford PJ, O'Donnell N, Riley JP, et al. The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cells. J Immunol. 2006;176:7062–70. doi: 10.4049/jimmunol.176.11.7062. [DOI] [PubMed] [Google Scholar]
- 49.Verma A, Deb DK, Sassano A, et al. Cutting edge: activation of the p38 mitogen-activated protein kinase signaling pathway mediates cytokine-induced hemopoietic suppression in aplastic anemia. J Immunol. 2002;168:5984–8. doi: 10.4049/jimmunol.168.12.5984. [DOI] [PubMed] [Google Scholar]
- 50.Xia T, Wang QR, Xu YH. Cimetidine inhibits production of interferon gamma and tumor necrosis factor alpha by splenocytes in aplastic anemic mice. Acta Pharmacol Sin. 2001;22:239–42. [PubMed] [Google Scholar]
- 51.Gantner F, Sakai K, Tusche MW, et al. Histamine h(4) and h(2) receptors control histamine-induced interleukin-16 release from human CD8(+) T cells. J Pharmacol Exp Ther. 2002;303:300–7. doi: 10.1124/jpet.102.036939. [DOI] [PubMed] [Google Scholar]
- 52.Leite-de-Moraes MC, Diem S, Michel ML, et al. Cutting edge: histamine receptor H4 activation positively regulates in vivo IL-4 and IFN-gamma production by invariant NKT cells. J Immunol. 2009;182:1233–6. doi: 10.4049/jimmunol.182.3.1233. [DOI] [PubMed] [Google Scholar]
- 53.Winterkamp S, Weidenhiller M, Otte P, et al. Urinary excretion of N-methylhistamine as a marker of disease activity in inflammatory bowel disease. Am J Gastroenterol. 2002;97:3071–7. doi: 10.1111/j.1572-0241.2002.07028.x. [DOI] [PubMed] [Google Scholar]
- 54.Fogel WA, Lewinski A, Jochem J. Histamine in idiopathic inflammatory bowel diseases – not a standby player. Folia Med Cracov. 2005;46:107–18. [PubMed] [Google Scholar]
- 55.Passani MB, Blandina P. Histamine receptors in the CNS as targets for therapeutic intervention. Trends Pharmacol Sci. 2011;32:242–9. doi: 10.1016/j.tips.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Yanai K, Tashiro M. The physiological and pathophysiological roles of neuronal histamine: an insight from human positron emission tomography studies. Pharmacol Ther. 2007;113:1–15. doi: 10.1016/j.pharmthera.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Celanire S, Wijtmans M, Talaga P, et al. Keynote review: histamine H3 receptor antagonists reach out for the clinic. Drug Discov Today. 2005;10:1613–27. doi: 10.1016/S1359-6446(05)03625-1. [DOI] [PubMed] [Google Scholar]
- 58.Moya-Garcia AA, Rodriguez CE, Morilla I, et al. The function of histamine receptor H4R in the brain revealed by interaction partners. Front Biosci. 2011;3:1058–66. doi: 10.2741/210. [DOI] [PubMed] [Google Scholar]
- 59.Thakkar MM. Histamine in the regulation of wakefulness. Sleep Med Rev. 2011;15:65–74. doi: 10.1016/j.smrv.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yasuko S, Atanda AM, Masato M, et al. Alpha-fluoromethylhistidine, a histamine synthesis inhibitor, inhibits orexin-induced wakefulness in rats. Behav Brain Res. 2010;207:151–4. doi: 10.1016/j.bbr.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 61.Sundvik M, Kudo H, Toivonen P, et al. The histaminergic system regulates wakefulness and orexin/hypocretin neuron development via histamine receptor H1 in zebrafish. FASEB J. 2011;25:4338–47. doi: 10.1096/fj.11-188268. [DOI] [PubMed] [Google Scholar]
- 62.Anaclet C, Parmentier R, Ouk K, et al. Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J Neurosci. 2009;29:14423–38. doi: 10.1523/JNEUROSCI.2604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grimaldi D, Agati P, Pierangeli G, et al. Hypocretin deficiency in narcolepsy with cataplexy is associated with a normal body core temperature modulation. Chronobiol Int. 2010;27:1596–608. doi: 10.3109/07420528.2010.504907. [DOI] [PubMed] [Google Scholar]
- 64.Wallace TL, Ballard TM, Pouzet B, et al. Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacol Biochem Behav. 2011;99:130–45. doi: 10.1016/j.pbb.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 65.Ercan-Sencicek AG, Stillman AA, Ghosh AK, et al. L-histidine decarboxylase and Tourette's syndrome. N Engl J Med. 2010;362:1901–8. doi: 10.1056/NEJMoa0907006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ligneau X, Landais L, Perrin D, et al. Brain histamine and schizophrenia: potential therapeutic applications of H3-receptor inverse agonists studied with BF2.649. Biochem Pharmacol. 2007;73:1215–24. doi: 10.1016/j.bcp.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 67.Yanovsky Y, Li S, Klyuch BP, et al. L-Dopa activates histaminergic neurons. J Physiol. 2011;589:1349–66. doi: 10.1113/jphysiol.2010.203257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–77. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- 69.Ito C, Onodera K, Sakurai E, et al. The effect of methamphetamine on histamine level and histidine decarboxylase activity in the rat brain. Brain Res. 1996;734:98–102. [PubMed] [Google Scholar]
- 70.Kubota Y, Ito C, Sakurai E, et al. Increased methamphetamine-induced locomotor activity and behavioral sensitization in histamine-deficient mice. J Neurochem. 2002;83:837–45. doi: 10.1046/j.1471-4159.2002.01189.x. [DOI] [PubMed] [Google Scholar]
- 71.Morisset S, Pilon C, Tardivel-Lacombe J, et al. Acute and chronic effects of methamphetamine on tele-methylhistamine levels in mouse brain: selective involvement of the D(2) and not D(3) receptor. J Pharmacol Exp Ther. 2002;300:621–8. doi: 10.1124/jpet.300.2.621. [DOI] [PubMed] [Google Scholar]
- 72.Morisset S, Rouleau A, Ligneau X, et al. High constitutive activity of native H3 receptors regulates histamine neurons in brain. Nature. 2000;408:860–4. doi: 10.1038/35048583. [DOI] [PubMed] [Google Scholar]
- 73.Acevedo SF, Pfankuch T, van Meer P, et al. Role of histamine in short- and long-term effects of methamphetamine on the developing mouse brain. J Neurochem. 2008;107:976–86. doi: 10.1111/j.1471-4159.2008.05673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang L, Smith LM, LoPresti C, et al. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 75.Lucotte G, Lagarde JP, Funalot B, et al. Linkage with the Ser9Gly DRD3 polymorphism in essential tremor families. Clin Genet. 2006;69:437–40. doi: 10.1111/j.1399-0004.2006.00600.x. [DOI] [PubMed] [Google Scholar]
- 76.Klein C, Gurvich N, Sena-Esteves M, et al. Evaluation of the role of the D2 dopamine receptor in myoclonus dystonia. Ann Neurol. 2000;47:369–73. [PubMed] [Google Scholar]
- 77.Durr A, Tassin J, Vidailhet M, et al. D2 dopamine receptor gene in myoclonic dystonia and essential myoclonus. Ann Neurol. 2000;48:127–8. doi: 10.1002/1531-8249(200007)48:1<127::aid-ana24>3.3.co;2-4. [DOI] [PubMed] [Google Scholar]
- 78.Ferrada C, Ferre S, Casado V, et al. Interactions between histamine H3 and dopamine D2 receptors and the implications for striatal function. Neuropharmacology. 2008;55:190–7. doi: 10.1016/j.neuropharm.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moreno E, Hoffmann H, Gonzalez-Sepulveda M, et al. Dopamine D1-histamine H3 receptor heteromers provide a selective link to MAPK signaling in GABAergic neurons of the direct striatal pathway. J Biol Chem. 2011;286:5846–54. doi: 10.1074/jbc.M110.161489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ledesma MC, Garcia-Martin E, Alonso-Navarro H, et al. The nonsynonymous Thr105Ile polymorphism of the histamine N-methyltransferase is associated to the risk of developing essential tremor. Neuromolecular Med. 2008;10:356–61. doi: 10.1007/s12017-008-8040-3. [DOI] [PubMed] [Google Scholar]
- 81.Keeling BH, Vilarino-Guell C, Soto-Ortolaza AI, et al. Histamine N-methyltransferase Thr105Ile is not associated with Parkinson's disease or essential tremor. Parkinsonism Relat Disord. 2010;16:112–4. doi: 10.1016/j.parkreldis.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marasovic-Susnjara I, Palada V, Marinovic-Terzic I, et al. No association between histamine N-methyltransferase functional polymorphism Thr105Ile and Alzheimer's disease. Neurosci Lett. 2011;489:119–21. doi: 10.1016/j.neulet.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 83.Lipnik-Stangelj M. Multiple role of histamine H1-receptor-PKC-MAPK signalling pathway in histamine-stimulated nerve growth factor synthesis and secretion. Biochem Pharmacol. 2006;72:1375–81. doi: 10.1016/j.bcp.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 84.Capsoni S, Covaceuszach S, Marinelli S, et al. Taking pain out of NGF: a “painless” NGF mutant, linked to hereditary sensory autonomic neuropathy type V, with full neurotrophic activity. PLoS One. 2011;6:e17321. doi: 10.1371/journal.pone.0017321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carvalho OP, Thornton GK, Hertecant J, et al. A novel NGF mutation clarifies the molecular mechanism and extends the phenotypic spectrum of the HSAN5 neuropathy. J Med Genet. 2011;48:131–5. doi: 10.1136/jmg.2010.081455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stampachiacchiere B, Marinova T, Velikova K, et al. Altered levels of nerve growth factor in the thymus of subjects with myasthenia gravis. J Neuroimmunol. 2004;146:199–202. doi: 10.1016/j.jneuroim.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 87.Einarsdottir E, Carlsson A, Minde J, et al. A mutation in the nerve growth factor beta gene (NGFB) causes loss of pain perception. Hum Mol Genet. 2004;13:799–805. doi: 10.1093/hmg/ddh096. [DOI] [PubMed] [Google Scholar]
- 88.Wang ZY, Ding Y, Miki T, et al. Nerve growth factor and receptors are significantly affected by histamine stimulus through H1 receptor in pancreatic carcinoma cells. Mol Med Report. 2010;3:103–9. doi: 10.3892/mmr_00000225. [DOI] [PubMed] [Google Scholar]
- 89.Theoharides TC, Kempuraj D, Kourelis T, et al. Human mast cells stimulate activated T cells: implications for multiple sclerosis. Ann N Y Acad Sci. 2008;1144:74–82. doi: 10.1196/annals.1418.029. [DOI] [PubMed] [Google Scholar]
- 90.Jadidi-Niaragh F, Mirshafiey A. Histamine and histamine receptors in pathogenesis and treatment of multiple sclerosis. Neuropharmacology. 2010;59:180–9. doi: 10.1016/j.neuropharm.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 91.Pedotti R, DeVoss JJ, Youssef S, et al. Multiple elements of the allergic arm of the immune response modulate autoimmune demyelination. Proc Natl Acad Sci USA. 2003;100:1867–72. doi: 10.1073/pnas.252777399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Medina VA, Rivera ES. Histamine receptors and cancer pharmacology. Br J Pharmacol. 2010;161:755–67. doi: 10.1111/j.1476-5381.2010.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ruffell B, Coussens LM. Histamine restricts cancer: nothing to sneeze at. Nat Med. 2011;17:43–4. doi: 10.1038/nm0111-43. [DOI] [PubMed] [Google Scholar]
- 94.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang XD, Ai W, Asfaha S, et al. Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b+Ly6G+ immature myeloid cells. Nat Med. 2011;17:87–95. doi: 10.1038/nm.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thoren FB, Aurelius J, Martner A. Antitumor properties of histamine in vivo. Nat Med. 2011;17:537. doi: 10.1038/nm0511-537a. author reply -8. [DOI] [PubMed] [Google Scholar]
- 97.Brune M, Castaigne S, Catalano J, et al. Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood. 2006;108:88–96. doi: 10.1182/blood-2005-10-4073. [DOI] [PubMed] [Google Scholar]
- 98.Donskov F, Hokland M, Marcussen N, et al. Monocytes and neutrophils as ‘bad guys’ for the outcome of interleukin-2 with and without histamine in metastatic renal cell carcinoma–results from a randomised phase II trial. Br J Cancer. 2006;94:218–26. doi: 10.1038/sj.bjc.6602937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Osefo N, Ito T, Jensen RT. Gastric acid hypersecretory states: recent insights and advances. Curr Gastroenterol Rep. 2009;11:433–41. doi: 10.1007/s11894-009-0067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497–516. doi: 10.1586/ehm.10.42. [DOI] [PubMed] [Google Scholar]
- 101.Molderings GJ, Brettner S, Homann J, et al. Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options. J Hematol Oncol. 2011;4:10. doi: 10.1186/1756-8722-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Akin C, Metcalfe DD. Systemic mastocytosis. Annu Rev Med. 2004;55:419–32. doi: 10.1146/annurev.med.55.091902.103822. [DOI] [PubMed] [Google Scholar]
- 103.Hadzijusufovic E, Peter B, Gleixner KV, et al. H1-receptor antagonists terfenadine and loratadine inhibit spontaneous growth of neoplastic mast cells. Exp Hematol. 2010;38:896–907. doi: 10.1016/j.exphem.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garcia-Montero AC, Jara-Acevedo M, Teodosio C, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108:2366–72. doi: 10.1182/blood-2006-04-015545. [DOI] [PubMed] [Google Scholar]
- 105.Laine E, Chauvot de Beauchene I, Perahia D, et al. Mutation D816V alters the internal structure and dynamics of c-KIT receptor cytoplasmic region: implications for dimerization and activation mechanisms. PLoS Comput Biol. 2011;7:e1002068. doi: 10.1371/journal.pcbi.1002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dobigny C, Saffar JL. H1 and H2 histamine receptors modulate osteoclastic resorption by different pathways: evidence obtained by using receptor antagonists in a rat synchronized resorption model. J Cell Physiol. 1997;173:10–8. doi: 10.1002/(SICI)1097-4652(199710)173:1<10::AID-JCP2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 107.Fitzpatrick LA, Buzas E, Gagne TJ, et al. Targeted deletion of histidine decarboxylase gene in mice increases bone formation and protects against ovariectomy-induced bone loss. Proc Natl Acad Sci USA. 2003;100:6027–32. doi: 10.1073/pnas.0934373100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Delsignore JL, Dvoretsky PM, Hicks DG, et al. Mastocytosis presenting as a skeletal disorder. Iowa Orthop J. 1996;16:126–34. [PMC free article] [PubMed] [Google Scholar]
- 109.Lesclous P, Guez D, Saffar JL. Short-term prevention of osteoclastic resorption and osteopenia in ovariectomized rats treated with the H(2) receptor antagonist cimetidine. Bone. 2002;30:131–6. doi: 10.1016/s8756-3282(01)00629-9. [DOI] [PubMed] [Google Scholar]
- 110.Pochampally RR, Ylostalo J, Penfornis P, et al. Histamine receptor H1 and dermatopontin: new downstream targets of the vitamin D receptor. J Bone Miner Res. 2007;22:1338–49. doi: 10.1359/jbmr.070605. [DOI] [PubMed] [Google Scholar]
- 111.Bristow MR, Ginsburg R, Harrison DC. Histamine and the human heart: the other receptor system. Am J Cardiol. 1982;49:249–51. doi: 10.1016/0002-9149(82)90298-3. [DOI] [PubMed] [Google Scholar]
- 112.Leurs R, Church MK, Taglialatela M. H1-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects. Clin Exp Allergy. 2002;32:489–98. doi: 10.1046/j.0954-7894.2002.01314.x. [DOI] [PubMed] [Google Scholar]
- 113.Matsuki M, Sato N, Matsuda K, et al. Brugada syndrome whose ST-segment changes were enhanced by antihistamines and antiallergenic drugs. Intern Med. 2009;48:1009–13. doi: 10.2169/internalmedicine.48.2067. [DOI] [PubMed] [Google Scholar]
- 114.Vischer UM, de Moerloose P. von Willebrand factor: from cell biology to the clinical management of von Willebrand's disease. Crit Rev Oncol Hematol. 1999;30:93–109. doi: 10.1016/s1040-8428(98)00045-6. [DOI] [PubMed] [Google Scholar]
- 115.Hamilton KK, Sims PJ. Changes in cytosolic Ca2+ associated with von Willebrand factor release in human endothelial cells exposed to histamine. Study of microcarrier cell monolayers using the fluorescent probe indo-1. J Clin Invest. 1987;79:600–8. doi: 10.1172/JCI112853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jilma B, Pernerstorfer T, Dirnberger E, et al. Effects of histamine and nitric oxide synthase inhibition on plasma levels of von Willebrand factor antigen. J Lab Clin Med. 1998;131:151–6. doi: 10.1016/s0022-2143(98)90157-3. [DOI] [PubMed] [Google Scholar]
- 117.Zarei S, Frieden M, Rubi B, et al. Dopamine modulates von Willebrand factor secretion in endothelial cells via D2-D4 receptors. J Thromb Haemost. 2006;4:1588–95. doi: 10.1111/j.1538-7836.2006.01998.x. [DOI] [PubMed] [Google Scholar]
- 118.Wang KY, Tanimoto A, Yamada S, et al. Histamine regulation in glucose and lipid metabolism via histamine receptors: model for nonalcoholic steatohepatitis in mice. Am J Pathol. 2010;177:713–23. doi: 10.2353/ajpath.2010.091198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nimkarn S, Lin-Su K, New MI. Steroid 21 hydroxylase deficiency congenital adrenal hyperplasia. Pediatr Clin North Am. 2011;58:1281–300. doi: 10.1016/j.pcl.2011.07.012. xii. [DOI] [PubMed] [Google Scholar]
- 120.LaBella FS, Brandes LJ. Interaction of histamine and other bioamines with cytochromes P450: implications for cell growth modulation and chemopotentiation by drugs. Semin Cancer Biol. 2000;10:47–53. doi: 10.1006/scbi.2000.0307. [DOI] [PubMed] [Google Scholar]
- 121.Yang R, Hey JA, Aslanian R, et al. Coordination of histamine H3 receptor antagonists with human adrenal cytochrome P450 enzymes. Pharmacology. 2002;66:128–35. doi: 10.1159/000063794. [DOI] [PubMed] [Google Scholar]
- 122.Imamura I, Watanabe T, Hase Y, et al. Histamine metabolism in patients with histidinemia: determination of urinary levels of histamine, N tau-methylhistamine, imidazole acetic acid, and its conjugate(s) J Biochem. 1984;96:1925–9. doi: 10.1093/oxfordjournals.jbchem.a135027. [DOI] [PubMed] [Google Scholar]
- 123.Correa-Fiz F, Reyes-Palomares A, Fajardo I, et al. Regulatory cross-talk of mouse liver polyamine and methionine metabolic pathways: a systemic approach to its physiopathological consequences. Amino Acids. 2012;42:577–95. doi: 10.1007/s00726-011-1044-6. [DOI] [PubMed] [Google Scholar]
- 124.Smits RA, Adami M, Istyastono EP, et al. Synthesis and QSAR of quinazoline sulfonamides as highly potent human histamine H4 receptor inverse agonists. J Med Chem. 2010;53:2390–400. doi: 10.1021/jm901379s. [DOI] [PubMed] [Google Scholar]
- 125.Hussein G, Sankawa U, Goto H, et al. Astaxanthin, a carotenoid with potential in human health and nutrition. J Nat Prod. 2006;69:443–9. doi: 10.1021/np050354+. [DOI] [PubMed] [Google Scholar]
- 126.Mozsik G, Past T, Abdel Salam OM, et al. Interdisciplinary review for correlation between the plant origin capsaicinoids, non-steroidal antiinflammatory drugs, gastrointestinal mucosal damage and prevention in animals and human beings. Inflammopharmacology. 2009;17:113–50. doi: 10.1007/s10787-009-0002-3. [DOI] [PubMed] [Google Scholar]
- 127.Melgarejo E, Medina MA, Sanchez-Jimenez F, et al. Targeting of histamine producing cells by EGCG: a green dart against inflammation? J Physiol Biochem. 2010;66:265–70. doi: 10.1007/s13105-010-0033-7. [DOI] [PubMed] [Google Scholar]
- 128.Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133:3275S–84S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 129.Zhang G, Miura Y, Yagasaki K. Suppression of adhesion and invasion of hepatoma cells in culture by tea compounds through antioxidative activity. Cancer Lett. 2000;159:169–73. doi: 10.1016/s0304-3835(00)00545-0. [DOI] [PubMed] [Google Scholar]
- 130.Tosetti F, Ferrari N, De Flora S, et al. Angioprevention’: angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J. 2002;16:2–14. doi: 10.1096/fj.01-0300rev. [DOI] [PubMed] [Google Scholar]
- 131.Shimizu M, Deguchi A, Lim JT, et al. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005;11:2735–46. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 132.Hung PF, Wu BT, Chen HC, et al. Antimitogenic effect of green tea (−)-epigallocatechin gallate on 3T3-L1 preadipocytes depends on the ERK and Cdk2 pathways. Am J Physiol Cell Physiol. 2005;288:C1094–108. doi: 10.1152/ajpcell.00569.2004. [DOI] [PubMed] [Google Scholar]
- 133.Liang YC, Chen YC, Lin YL, et al. Suppression of extracellular signals and cell proliferation by the black tea polyphenol, theaflavin-3,3′-digallate. Carcinogenesis. 1999;20:733–6. doi: 10.1093/carcin/20.4.733. [DOI] [PubMed] [Google Scholar]
- 134.Song XZ, Bi ZG, Xu AE. Green tea polyphenol epigallocatechin-3-gallate inhibits the expression of nitric oxide synthase and generation of nitric oxide induced by ultraviolet B in HaCaT cells. Chin Med J (Engl) 2006;119:282–7. [PubMed] [Google Scholar]
- 135.Noh SU, Cho EA, Kim HO, et al. Epigallocatechin-3-gallate improves Dermatophagoides pteronissinus extract-induced atopic dermatitis-like skin lesions in NC/Nga mice by suppressing macrophage migration inhibitory factor. Int Immunopharmacol. 2008;8:1172–82. doi: 10.1016/j.intimp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 136.Jatoi A, Ellison N, Burch PA, et al. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer. 2003;97:1442–6. doi: 10.1002/cncr.11200. [DOI] [PubMed] [Google Scholar]
- 137.Ahn WS, Yoo J, Huh SW, et al. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur J Cancer Prev. 2003;12:383–90. doi: 10.1097/00008469-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 138.Suganuma M, Saha A, Fujiki H. New cancer treatment strategy using combination of green tea catechins and anticancer drugs. Cancer Sci. 2011;102:317–23. doi: 10.1111/j.1349-7006.2010.01805.x. [DOI] [PubMed] [Google Scholar]
- 139.Gundimeda U, McNeill TH, Schiffman JE, et al. Green tea polyphenols potentiate the action of nerve growth factor to induce neuritogenesis: possible role of reactive oxygen species. J Neurosci Res. 2010;88:3644–55. doi: 10.1002/jnr.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ranjith-Kumar CT, Lai Y, Sarisky RT, et al. Green tea catechin, epigallocatechin gallate, suppresses signaling by the dsRNA innate immune receptor RIG-I. PLoS One. 2010;5:e12878. doi: 10.1371/journal.pone.0012878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Katiyar SK, Matsui MS, Elmets CA, et al. Polyphenolic antioxidant (−)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem Photobiol. 1999;69:148–53. [PubMed] [Google Scholar]
- 142.Hsu S, Yamamoto T, Borke J, et al. Green tea polyphenol-induced epidermal keratinocyte differentiation is associated with coordinated expression of p57/KIP2 and caspase 14. J Pharmacol Exp Ther. 2005;312:884–90. doi: 10.1124/jpet.104.076075. [DOI] [PubMed] [Google Scholar]
- 143.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


