Abstract
Sunitinib is an anti-angiogenic receptor tyrosine kinase inhibitor used to treat advanced metastatic renal cell carcinoma and other types of cancer. Sutent is effective in only approximately 70% of clear cell renal cell carcinoma (CCRCC) patients, has significant adverse side effects and no method is available to predict which patients will not respond. Our purpose was to explore the possibility of introducing an effective prediction method based on a marker of the tumour vasculature, the follicle stimulating hormone receptor (FSHR). Fifty patients diagnosed with advanced metastatic CCRCC have been subjected to surgery for removal of the primary tumour and were subsequently treated with sunitinib. After three months of therapy the patients were categorized as ‘responsive’, ‘stable’ or ‘non-responsive’ based on the RECIST guidelines. The blood vessel density and the percentage of FSHR-positive vessels were determined by immunofluorescence on sections from the primary tumours removed by surgery, prior to the sunitinib treatment. The percentage of FSHR-stained vessels was on average fivefold higher for the patients who responded to the treatment in comparison with the stable group and almost eightfold higher than in the non-responsive group. The percentage allowed the detection of responders with 87–100% sensitivity and specificity. No significant differences were detected in the total density of vessels among the three groups. The data suggest that FSHR expression levels in the blood vessels of CCRCC primary tumours can be used to predict, with high sensitivity and specificity, the patients who will respond to sunitinib therapy.
Keywords: FSHR, sunitinib, kidney cancer, blood vessels, immunohistochemistry
Introduction
Renal cell carcinoma (RCC) represents 3.6% of all new cancer cases in the United States [1]. Clear cell RCC (CCRCC) represents 85% of all renal cancers and by far the most lethal urologic cancer [2].
Both sporadic and inherited CCRCCs are strongly associated with mutations in von Hippel Lindau tumour suppressor gene [3]. Clear cell renal cell carcinomas are known to be highly vascular tumours with high expression of vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptors (PDGFR) [4].
Sunitinib (sunitinib malate, SU11248, Sutent) is used to treat advanced and/or metastatic RCC and gastrointestinal stromal tumours (GISTs) in patients with tumours that were not treated successfully with imatinib (Gleevec) or people who cannot take imatinib [5]. Sunitinib was also recently approved for pancreatic neuroendocrine tumours in patients with locally advanced or metastatic disease. For patients diagnosed with metastatic RCC, sunitinib is usually prescribed after the primary tumour is surgically removed.
Sunitinib is a selective, orally administered receptor tyrosine kinase (RTK) inhibitor that targets platelet-derived growth factor receptors (PDGFRs) alpha and beta, vascular endothelial growth factor receptors (VEGFR) 1, 2 and 3, c-kit, colony stimulating factor-1 receptor (CSF-1R), fms-related tyrosine kinase 3 (FLT3) and rearranged during transfection (RET) [4, 6–8]. The anti-tumoural effects are thought to occur to a large extent due to the prominent role of these kinases in tumour angiogenesis.
In a Phase III trial in previously untreated patients with metastatic RCC, Sutent was associated with median progression-free survival of 11 months, which was more than double that observed with interferon-α[9]. Approximately one third of the RCC patients do not respond to sunitinib treatment [10–12].
Sunitinib has significant adverse side effects. Most common toxicities include hypertension, bleeding, fatigue, diarrhoea, nausea and/or vomiting, hand foot syndrome and myelosuppression [13, 14]. No criteria are currently available to predict, before a full course of treatment is applied, which patients belong to the 30% subset in which sunitinib treatment is not beneficial.
There is a critical need for strategies to increase complete responses (now rare). One strategy is to combine sunitinib with other agents available for RCC therapy [15–18], but trials have revealed difficulties with combination therapy. By combining these agents, the toxicity of one or more can be enhanced [19]. Unexpected toxicity characterized by micro-angiopathic haemolytic anaemia occurred late in treatment with sunitinib and bevacizumab. Toxicity may be more severe in patients with RCC, who frequently have one kidney and poor renal function [19]. Other combinations are intolerable (sunitinib with temsirolimus or sunitinib with bevacizumab). Thus, if the efficacy of sunitinib therapy would be known in advance for each patient, the selection of the most appropriate agent or combination would be simplified and would have a better chance of success.
The mean per-patient lifetime cost of treatment with sunitinib was estimated at over $220,000 [20]. If only the patients who respond to the agent would be treated, at the level of the health care system the positive outcome of sunitinib treatment, which as mentioned consists in a median progression-free survival of 11 months [9], would be achieved at substantially lower costs.
Therefore, prediction of the RCC patients who would benefit from sunitinib treatment would allow physicians to make better decisions about which therapy to select for each RCC patient. Moreover, prediction of the responsiveness would increase the average progression-free survival of RCC patients, would avoid the unnecessary exposure to sunitinib toxicity of the patients who do not respond and would reduce the average cost/benefit of sunitinib treatment. The ability to predict in advance of sunitinib treatment the efficacy of the drug based on particular features of the tumours may allow selection for clinical trials of patient cohorts that are enriched in responders, which would facilitate adoption of sunitinib as a therapy for other types of cancers, besides RCC, GIST and progressive neuroendocrine pancreatic tumours.
We discovered recently a new marker of the tumour vasculature, the follicle stimulating hormone receptor (FSHR), which is expressed by the endothelial cells in a wide range of tumour types [21]. No information exists so far about a functional contribution of tumour vascular FSHR to the disease or to the response to therapies. We hypothesized that FSHR localization in the tumour vasculature indicates a role in tumour angiogenesis [21]. A corollary of this hypothesis is that the response of tumours to anti-angiogenic therapies could be correlated with FSHR expression. As an initial test of this hypothesis, we investigated if a correlation exists between FSHR levels in surgically removed primary CCRCC tumours and the response to subsequent treatment with sunitinib. Here we report that such correlation exists and appears to be strong enough to allow clinically relevant predictions of sunitinib efficacy.
Patients and methods
We analysed retrospectively patients diagnosed with advanced metastatic CCRCC, who have been subjected to surgery for removal of the primary tumour at the French hospitals Henri Mondor, Créteil (18 patients), CHU Rennes (9 patients), Rangueil Toulouse (7 patients), CHU Bordeaux (9 patients), Lyon-Sud (5 patients) and CHU Lille (2 patients). Patients’ characteristics are summarized in Table 1. Distant metastases were present in 41% of the patients. Regional lymph nodes metastases were absent in 55% of the patients, 23% had metastasis in a single regional lymph node and 22% had metastases in more than one regional lymph node. According to the Memorial Sloan-Kettering Cancer Center (MSKCC) classification [12], 22% of the patients were in the favourable risk group, 64% in the intermediate and 14% in the poor risk group.
Table 1.
Clinico-pathological characteristics of the patients
| Patient characteristics | Number of cases | Percentage (%) |
|---|---|---|
| Gender | ||
| Male | 31 | 62 |
| Female | 19 | 38 |
| Age (years): 57.6 ± 1.2 | ||
| Fuhrman grade | ||
| 1 | 2 | 4 |
| 2 | 4 | 8 |
| 3 | 30 | 60 |
| 4 | 14 | 28 |
| Tumour size (cm): 9.8 ± 0.5 | ||
| Tumour stage at diagnosis | ||
| T1 | 5 | 10 |
| T2 | 3 | 6 |
| T3 | 42 | 84 |
| MSKCC classification | ||
| Favourable | 11 | 22 |
| Intermediate | 32 | 64 |
| Poor | 7 | 14 |
| Immunotherapy before Sutent | 25 | 50 |
| Treatment response at 3 months | ||
| Partial response | 15 | 30 |
| Stable disease | 18 | 36 |
| Failure | 17 | 34 |
| Progression-free survival (months) | ||
| Partial response 20 ± 3 | ||
| Stable disease 19 ± 2 | ||
| Failure 4.1 ± 0.3 | ||
MSKCC: Memorial Sloan-Kettering Cancer Center.
After surgery the patients have been subjected to sunitinib treatment delivered orally for ≥3 months with a dose of 50 mg/day for 4 weeks followed by 2 weeks off. For 40% of the patients the dose was subsequently reduced to 37.5 or 25 mg/day. Depending on the effects of the treatment, after 3 months of therapy the patients have been designated as ‘responsive’, ‘stable’ or ‘non-responsive’ according to the revised RECIST guidelines [22]. (No patient stopped the treatment before 3 months.) The patients were categorized as ‘responsive’ if there was at least a 30% decrease in the sum of the diameters of the lesions, and ‘non-responsive’ if the sum of the lesion sizes increased by at least 20%. Patients who did not meet the criteria for any the two categories were categorized as ‘stable’.
The primary tumours removed by surgery prior to the sunitinib treatment have been fixed in formaldehyde and embedded in paraffin. Sections have been cut subsequently from the archived paraffin blocks and stained for the FSH receptor using the monoclonal antibody FSHR323 (5 μg/ml) [23], and for the von Willebrand Factor (vWF) using a rabbit polyclonal antibody (Cat. no. F3520, dilution 1:500; Sigma-Aldrich, St. Quentin Fallavier, France) [21]. The sections were subsequently incubated with a goat anti-mouse IgG antibody-Alexa555 (Cat no. A21424, dilution 1:750; Invitrogen, Villebon sur Yvette, France) and a goat anti-rabbit IgG antibody-Alexa488 (Cat no. A11034, dilution 1:750; Invitrogen). Digital images were taken with a fluorescence microscope using a 20χ objective. Twenty microscope fields were photographed for each tumour. The fields were randomly located within the tumour at a distance of 5 mm or less from the border between the normal and the tumour tissue. The numbers of FSHR323-positive vessels and the total number of vessels (vWF-positive) were counted on the images.
Some sections were incubated only with the FSHR323 antibody followed by a goat anti-mouse IgG horseradish peroxidase–coupled antibody (Cat no A9309, dilution 1:500; Sigma-Aldrich), visualized using AEC (Cat. no. A6926; Sigma-Aldrich).
Sections of normal human testis, in which Sertoli cells express FSHR, were used as positive controls. Sections from biopsies of kidney transplants were used as negative controls. Supplementary controls consisted in omissions of the primary antibodies.
The study was performed in accordance with the precepts established by the Helsinki Declaration and approved by the Ethic Committees of the institutions; patients were enrolled after giving written consent. All data were analysed anonymously.
Results
We analysed 50 patients treated with sunitinib: 15 who were ‘responsive’ to the sunitinib treatment, 18 who were ‘stable’ and 17 who were ‘non-responsive’.
In this limited sets of patients (only 10% low grade patients), no statistically significant correlation was found between FSHR expression and the tumour grade.
There are no significant differences in the total density of vessels among the three groups: the densities of vWF-positive vessels are: 49.1 ± 4.9, 42.7 ± 4.4 and 46.7 ± 5.4 (average ± S.E.M.) for the responsive, stable and non-responsive patients, respectively (P= 0.16 for responsive versus stable and P= 0.68 for stable versus non-responsive).
A much higher number of FSHR-positive vessels are visible for the patients who responded to the treatment (Fig. 1A–C).
Fig 1.
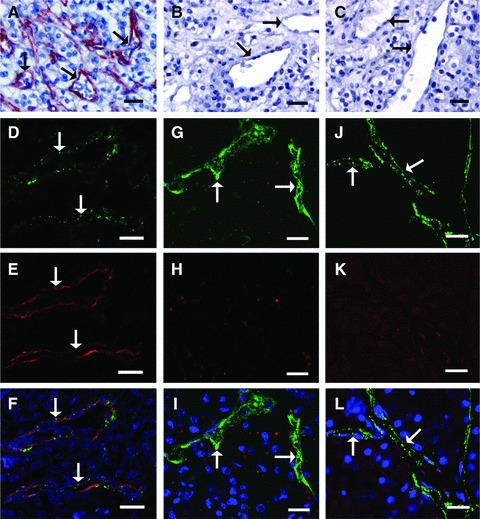
The CCRCC patients who respond to sunitinib treatment have a much higher density of FSHR-expressing vessels in the primary tumours, compared with patients who have stable disease or do not respond to the treatment. (A–C) Immunoperoxidase staining (red) for FSHR of paraffin sections from primary tumours removed by surgery. Representative images of tumour sections from responsive (A), stable (B) or non-responsive (C) patients. (D–L) Representative double immunofluorescence images of tumour sections used to determine the percentage of FSHR-positive vessels. Paraffin sections from primary tumours have been stained with antibodies against vWF and FSHR, followed by fluorescently labelled green and red, respectively, secondary antibodies. Tumour sections from patients responsive to sunitinib treatment (D–F), stable (G–I) or non-responsive (J–L). The arrows point to blood vessels. Bar: 20 μm.
The correlation between the density of FSHR-stained vessels and the progression-free survival of RCC patients is 0.50 (n= 43; P= 0.0005, double-sided).
Subsequent measurement revealed that the three groups of patients are better differentiated by the ratio between the FSHR-positive vessel density and the total vessel density, detected as vWF-positive vessels, than by the density of FSHR-positive vessels alone. The ratios have been determined on double immunofluorescence images like those shown in Figure 1D–L.
The ratios for the three groups of patients are shown in Figure 2. In the group of patients who responded to treatment the proportion of FSHR-stained vessels was on average fivefold higher than in the stable group [56.8 ± 5.4 (S.E.M.) versus 11.4 ± 2.0, respectively], and almost eightfold higher than in the non-responsive group (7.3 ± 0.7) (P= 3 χ 10−9 for the difference between responsive and stable patients, and P= 0.5 χ 10−16 for the difference between responsive and non-responsive patients (t-test, two tails, equal variance). The difference between the stable and non-responsive groups was significant at P= 0.02.
Fig 2.
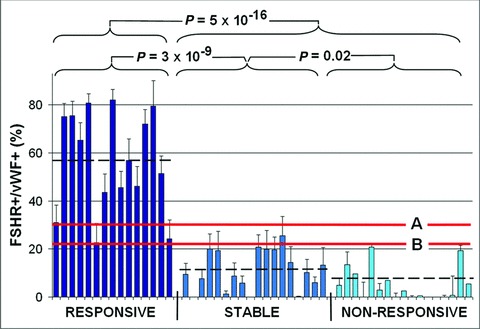
The ratio between the density of the vessels that show a FSHR signal and vessels positive for von Willebrand factor (vWF) is correlated with the response of the patients to subsequent treatment with sunitinib. The bars correspond to 15 patients who responded to the treatment, 18 patients who were stable and 17 patients who did not respond. Errors bars: standard errors of the means computed for 20 microscopic fields for each patient. The three thick horizontal lines correspond to the averages for the three groups of patients (56.8 ± 5.4%, 11.4 ± 2% and 7.3 ± 0.7% for the responsive, stable and non-respective patients, respectively. The lines marked A and B correspond to the two thresholds (31% and 23%, respectively) used for the points A and B in Figure 3 to determine the sensitivities and the specificities of discriminating between the patients who are responsive versus the combined stable or non-responsive set.
The correlation between the density of FSHR-stained vessels and the response to sunitinib brings to attention the possibility of predicting the outcome of sunitinib treatment based on the density of FSHR vessels in the primary tumour. The potential of such method can be assessed based on the graph shown in Figure 3. If the threshold between the two categories of response is placed at 23% FSHR-positive vessels (level B from Fig. 2) the patients who responded to treatment can be distinguished with 97% specificity and 100% sensitivity (Fig. 3). If the threshold is placed anywhere between 31% and 23% (levels A and B, respectively from Fig. 2, respectively), the patients who responded to treatment can be distinguished with 87–100% sensitivity and specificity (Fig. 3). The area under the curve is 0.996.
Fig 3.
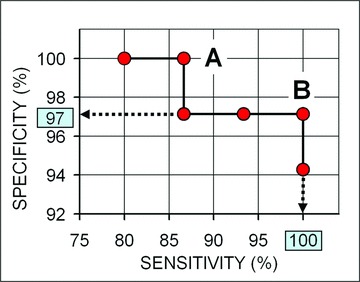
The ratio of the density of FSHR-stained vessels divided by the density of vWF-stained vessels predicts with high sensitivity and selectivity the patients who will be responsive to sunitinib. Horizontal axis: sensitivity (%); vertical axis: specificity (%). For point A (for a threshold of the FSHR+/vWF+ stained vessels = 31%), the sensitivity is 87% and the specificity is 100% (i.e. 87% of the patients who will respond are correctly predicted, and none of the patients who will be stable or non-responsive are incorrectly predicted to be responsive); for point B (FSHR+/vWF+= 23%) the sensitivity is 100% and the specificity is 97% (dashed arrows) (i.e. all patients who will respond are correctly predicted, and only 3% of the stable or non-responsive patients are incorrectly predicted to be responsive).
Discussion
The data show an association between the densities of the FSHR-stained vessels in the primary tumours and the efficacy of subsequent sunitinib treatment.
For the set of 50 patients analysed by us the association allows discrimination with 87–100% sensitivity and specificity of the patients who responded to treatment. Such discrimination is achieved for our dataset if the threshold between the two categories of response is placed between 23% and 31% FSHR-positive vessels. If the set analysed by us is viewed as a training set, we would place the threshold for future prospective tests at 27% FSHR-positive vessels.
The data indicate that high discrimination is possible not only for a precise value of the threshold, but also for a range that is not very narrow. This observation suggests that independent replications of this study would lead to equally good discriminations, although, as expected, the thresholds determined by such studies would be somewhat different.
In principle, better prediction could be achieved if FSHR expression is combined with other predictors of response to sunitinib, especially if their predictive ability reflects different processes/mechanisms. Previous studies pointed to the potential predictive role of the cumulative baseline of VEGF titer [24] and the ratio of VEGF soluble isoforms (VEGF121/VEGF165) [25]. The statistical significance of the differences for these factors is, however, much weaker than for FSHR, which raises doubt that addition of these predictors can improve significantly the outcome based on FSHR alone.
A recent study showed that the fibroblast growth factor 2 (FGF2) supports pro-angiogenic signalling in cell cultures in the presence of sunitinib [26]. The authors suggested that therapeutic strategies designed to simultaneously target both VEGF and FGF2 signalling may prove more efficacious than sunitinib in renal cancer patients whose tumours express FGF2.
The predictions can guide physicians in the process of selecting if sunitinib should be tried first, or rather other treatments have better chance of success. Such guided decisions would have two types of benefits for the very large number of patients who are treated unsuccessfully with sunitinib. The first benefit is that they would be saved from the burden of the serious side effects. The second benefit is that they could be treated from the beginning with some other drug. Conversely, our finding would be useful for the patients who would benefit from sunitinib treatment, but because this is not known they would be treated first with other medication.
For clinical use the density of FSHR-expressing vessels determined by peroxidase staining could be used instead of double immunofluorescence.
As mentioned in the Introduction, predicting the success of sunitinib treatment could be also useful for clinical trials aimed at extending the use of sunitinib in other types of cancer. We showed recently that FSHR is expressed by the tumour ECs not only in RCC, but also in a wide range of other tumour types [21]. It remains to be determined if FSHR expression level is correlated with the response to therapy in these other tumour types.
The NIH website http://clinicaltrials.gov/ lists 373 clinical trials involving sunitinib, of which 212 are closed and 161 are active. The majority of the studies refer to kidney cancer, but many other tumour types have been or are under investigation. It remains possible that sunitinib is effective in subsets of patients who have these types of tumours, but the subsets are substantially smaller than the 30% valid for RCC. For instance, in the pancreatic neuroendocrine cancer, for which sunitinib was recently approved, only 9.3% of the patients had an objective response to sunitinib treatments [27]. Smaller percentages of patients responsive to sunitinib could lead to failure to detect statistically significant effects unless prohibitively large numbers of patients are enrolled. The ability to select in advance of the treatment cohorts of patients who are likely to respond may ultimately lead to the adoption of sunitinib for other types of malignancies. Although the percentage of responsive patients for cancers other than those already approved may be relatively small, their treatment would be a highly desirable goal.
Another question to be addressed is whether FSHR expression is correlated with the response to other receptor tyrosine kinase inhibitors used for cancer therapy. The results could have clinical relevance and could also help elucidate the mechanism based on which FSHR levels are correlated with the response to sunitinib.
The mechanism that underlies the association between FSHR expression and the response to sunitinib therapy is so far unknown. As FSHR is expressed by the tumour ECs, at least some key components of the mechanism should involve the tumour endothelium. A recent study showed in fact that sunitinib acts primarily on tumour ECs rather than on tumour cells to inhibit RCC growth [28].
Hypotheses that can be advanced to explain the reported data are discussed below.
One possibility is that FSHR stimulation by FSH leads to VEGF secretion by the ECs, which in turn stimulates VEGFR2 on ECs as an autocrine mechanism. This process induces angiogenesis, and this could be the key mechanism of sunitinib action in RCC. In fact it is known that the binding of FSH to FSH receptor in ovarian granulosa cells induces an increase in hypoxia-inducible factor 1α protein levels, which in turn leads to up-regulation of VEGF production [29].
Another possible explanation for the correlation between FSHR expression and sunitinib efficacy is that FSHR activates one or more of the other kinases known to be inhibited by sunitinib (c-kit, PDGFR, CSF-1R, FLT3 or RET) [4, 6–8]. Inhibition of these kinases could represent in fact the mechanism that underlies sunitinib efficacy in CCRCC, and not as assumed its action on VEGFR2. No information is however available about the expression of these kinases in ECs of RCC or about the ability of FSH/FSHR to activate these kinases.
Our data indicate that the level of FSHR expression in the primary tumours is correlated with the subsequent effects of sunitinib in the metastatic tumours. This observation suggests that in each patient common characteristics, which determine the response to sunitinib, exist between the primary and the metastatic tumours, and these characteristics are highly correlated with the FSHR levels. The precise nature of these characteristics, most likely related to angiogenesis, remains to be established by further studies.
In conclusion, plausible mechanisms that could explain the presented correlation could be envisioned and will be investigated. Independently of mechanistic studies, the reported observation appears to be very promising for the clinical management of CCRCC patients.
Acknowledgments
We thank Y. Allory, N. Rioux-Leclerc, C. Deminiére, C. Mazerolles, M. Decossin-Petrucci and X. Leroy for the anatomopathological assessment of the tumour specimens, L. Bastien for the clinico-pathological data, A. de la Taille for contributing to specimen procurement and P. Soyeux for immunohistochemistry technical assistance.
The study was supported by the ‘Institut National de la Santé et de la Recherche Médicale’ (C.P. and N.G.) and a Fellowship from the Higher Education Commission, Pakistan (A.S.).
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Kontovinis LF, Papazisis KT, Touplikioti P, et al. Sunitinib treatment for patients with clear-cell metastatic renal cell carcinoma: clinical outcomes and plasma angiogenesis markers. BMC Cancer. 2009;9:82. doi: 10.1186/1471-2407-9-82. doi: 10.1186/1471-2407-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet. 2011;19:617–23. doi: 10.1038/ejhg.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mena AC, Pulido EG, Guillén-Ponce C. Understanding the molecular-based mechanism of action of the tyrosine kinase inhibitor: sunitinib. Anticancer Drugs. 2010;21:S3–11. doi: 10.1097/01.cad.0000361534.44052.c5. [DOI] [PubMed] [Google Scholar]
- 5.Adams VR, Leggas M. Sunitinib malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumours. Clin Ther. 2007;29:1338–53. doi: 10.1016/j.clinthera.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Faivre S, Demetri G, Sargent W, et al. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734–45. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 7.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–37. [PubMed] [Google Scholar]
- 8.O'Farrell AM, Abrams TJ, Yuen HA, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597–605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 9.Escudier B. Sunitinib for the management of advanced renal cell carcinoma. Expert Rev Anticancer Ther. 2010;10:305–17. doi: 10.1586/era.10.26. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–24. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alpha in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 13.Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur J Cancer. 2006;42:3127–39. doi: 10.1016/j.ejca.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Bhojani N, Jeldres C, Patard JJ, et al. Toxicities associated with the administration of sorafenib, sunitinib, and temsirolimus and their management in patients with metastatic renal cell carcinoma. Eur Urol. 2008;53:917–30. doi: 10.1016/j.eururo.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 15.Tanji N, Yokoyama M. Treatment of metastatic renal cell carcinoma and renal pelvic cancer. Clin Exp Nephrol. 2011;15:331–8. doi: 10.1007/s10157-011-0438-9. [DOI] [PubMed] [Google Scholar]
- 16.Pirrotta MT, Bernardeschi P, Fiorentini G. Targeted-therapy in advanced renal cell carcinoma. Curr Med Chem. 2011;18:1651–7. doi: 10.2174/092986711795471293. [DOI] [PubMed] [Google Scholar]
- 17.Hutson TE. Targeted therapies for the treatment of metastatic renal cell carcinoma: clinical evidence. Oncologist. 2011;16:14–22. doi: 10.1634/theoncologist.2011-S2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer EA, Gupta GN, Srinivasan R. Update on targeted therapies for clear cell renal cell carcinoma. Curr Opin Oncol. 2011;23:283–9. doi: 10.1097/CCO.0b013e32834479c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sosman J, Puzanov I. Combination targeted therapy in advanced renal cell carcinoma. Cancer. 2009;115:2368–75. doi: 10.1002/cncr.24234. [DOI] [PubMed] [Google Scholar]
- 20.Remák E, Charbonneau C, Négrier S, et al. Economic evaluation of sunitinib malate for the first-line treatment of metastatic renal cell carcinoma. J Clin Oncol. 2008;26:3995–4000. doi: 10.1200/JCO.2007.13.2662. [DOI] [PubMed] [Google Scholar]
- 21.Radu A, Pichon C, Camparo P, et al. Expression of follicle-stimulating hormone receptor in tumour blood vessels. N Engl J Med. 2010;363:1621–30. doi: 10.1056/NEJMoa1001283. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Vannier B, Loosfelt H, Meduri G, et al. Anti-human FSH receptor monoclonal antibodies: immunochemical and immunocytochemical characterization of the receptor. Biochemistry. 1996;35:1358–66. doi: 10.1021/bi952290f. [DOI] [PubMed] [Google Scholar]
- 24.Porta C, Paglino C, De Amici M, et al. Predictive value of baseline serum vascular endothelial growth factor and neutrophil gelatinase-associated lipocalin in advanced kidney cancer patients receiving sunitinib. Kidney Int. 2010;77:809–15. doi: 10.1038/ki.2009.552. [DOI] [PubMed] [Google Scholar]
- 25.Paule B, Bastien L, Deslandes E, et al. Soluble isoforms of vascular endothelial growth factor are predictors of response to sunitinib in metastatic renal cell carcinomas. PLoS One. 2010;5:e10715. doi: 10.1371/journal.pone.0010715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welti JC, Gourlaouen M, Powles T, et al. Fibroblast growth factor 2 regulates endothelial cell sensitivity to sunitinib. Oncogene. 2011;30:1183–93. doi: 10.1038/onc.2010.503. [DOI] [PubMed] [Google Scholar]
- 27.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumours. N Engl J Med. 2011;364:501–13. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 28.Huang D, Ding Y, Li Y, et al. Sunitinib acts primarily on tumour endothelium rather than tumour cells to inhibit the growth of renal cell carcinoma. Cancer Res. 2010;70:1053–62. doi: 10.1158/0008-5472.CAN-09-3722. [DOI] [PubMed] [Google Scholar]
- 29.Alam H, Weck J, Maizels E, et al. Role of the phosphatidylinositol-3-kinase and extracellular regulated kinase pathways in the induction of hypoxia-inducible factor (HIF)-1 activity and the HIF-1 target vascular endothelial growth factor in ovarian granulosa cells in response to follicle-stimulating hormone. Endocrinology. 2009;150:915–28. doi: 10.1210/en.2008-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]


