Abstract
Mesenchymal stem cells (MSCs) inhibit proliferation of allogeneic T cells and express low levels of major histocompatibility complex class I (MHCI), MHCII and vascular adhesion molecule-1 (VCAM-1). We investigated whether their immunosuppressive properties and low immunophenotype protect allogeneic rat MSCs against cytotoxic lysis in vitro and result in a reduced immune response in vivo. Rat MSCs were partially protected against alloantigen-specific cytotoxic T cells in vitro. However, after treatment with IFN-γ and IL-1β, MSCs upregulated MHCI, MHCII and VCAM-1, and cytotoxic lysis was significantly increased. In vivo, allogeneic T cells but not allogeneic MSCs induced upregulation of the activation markers CD25 and CD71 as well as downregulation of CD62L on CD4+ T cells from recipient rats. However, intravenous injection of allo-MSCs in rats led to the formation of alloantibodies with the capacity to facilitate complement-mediated lysis, although IgM levels were markedly decreased compared with animals that received T cells. The allo-MSC induced immune response was sufficient to lead to significantly reduced survival of subsequently injected allo-MSCs. Interestingly, no increased immunogenicity of IFN-γ stimulated allo-MSCs was observed in vivo. Both the loss of protection against cytotoxic lysis under inflammatory conditions and the induction of complement-activating antibodies will likely impact the utility of allogeneic MSCs for therapeutic applications.
Keywords: mesenchymal stem cells, allo-antibody, complement, immunogenicity
Introduction
Mesenchymal stem cells are non-hematopoietic adult stem cells that can be isolated from bone marrow (BM) and various other sources such as umbilical cord blood or adipose tissue [1]. Mesenchymal stem cells have elicited considerable interest in recent years because of their ability to migrate to sites of tissue injury, their multi-lineage differentiation potential, their immunomodulatory properties, and the ease of isolation and ex vivo expansion [2, 3]. Therefore, MSCs are a promising tool for cell-based therapies and several studies using syngeneic MSCs have demonstrated their therapeutic potential in a variety of applications both in animal models [4–6], and in humans [7–9]. Mesenchymal stem cells are considered to be immunoprivileged because of their low immunogenicity, that is, they express very low levels of MHC class I, no MHC class II and do not induce activation of allogeneic lymphocytes [10]. In vitro, MSCs inhibit proliferation of syngeneic and allogeneic T cells equally effectively, suggesting that their immunosuppressive properties are independent of MHC expression by MSCs and lymphocytes [11, 12]. Hypothetically, their low immunogenicity would enable allogeneic MSCs to evade the allogeneic immune system and allow their usage across MHC barriers.
Allogeneic MSCs have been used in a number of studies with variable results. They have been shown to prolong skin graft survival in baboons [13] and were as effective as syngeneic MSCs in enhancing wound healing in mice [14]. Transplantation of allogeneic MSCs ameliorated systemic lupus erythematosus disease activities in mice and humans [15]. A clinical study that investigated the safety and efficacy of intravenous allogeneic human MSCs in patients with myocardial infarction showed a moderate benefit in the absence of adverse events [16]. On the other hand, application of MSCs in solid organ transplantation produced conflicting results. Semi-allogeneic and allogeneic MSCs improved the outcome after heterotopic heart transplantation in mice [17, 18]. Allogeneic MSCs failed to prolong survival of heterotopic heart transplants in rats, showed accelerated rejection in some studies [19–21] and prolonged graft survival in another [22]. Concomitant low-dose immunosuppression with mycophenolate, but not with cyclosporin A, restored the beneficial effect of allogeneic MSCs in heart transplantation [19, 21]. Equally conflicting results are reported for the use of third party MSCs in graft-versus-host disease [12, 23, 24]. The most likely reason for the failure of allogeneic MSCs to provide beneficial effects is their rejection by the allogeneic immune system [11, 25–27]. It has been shown that allogeneic MSCs are lysed by activated NK cells [28], can induce memory T cells [25, 26] and led to the formation of IgG antibodies after subcutaneous or intracardial injection in pigs [29]. However, the exact nature of the immune response to allogeneic MSCs still remains poorly characterized.
The objective of this study was to further elucidate the nature of the immune response upon intravenous injection of allogeneic BM-derived MSCs in a rat model.
Materials and methods
Animals
All procedures performed were conducted under animal licences no. B100/3859 and B100/4186 and were approved by the Animals Ethics Committee of the National University of Ireland, Galway. In addition, animal care and management followed the Standard Operating Procedures of the Animal Facility at the National Centre for Biomedical Engineering Science. Male Lewis (LEW) and Dark Agouti (DA) rats were obtained from Harlan Laboratories (UK). All animals used were between 8 and 12 weeks of age. Lewis rats were briefly anaesthetized with isofluorane for injection with either 1–5 × 106 syngeneic (LEW) or allogeneic (DA) MSCs or 1–5 × 106 allogeneic (DA) T cells. At several time points animals were killed by CO2-inhalation and organs were harvested for further analysis.
Isolation of rMSCs
Bone marrow cells were extracted from male LEW or DA rats (8–12 weeks old). The animals were killed by inhalation of CO2 and BM cells were flushed out of the tibias and femurs. After washing, centrifuged cells were transferred to T-175 flasks at a density of 9 × 105 cells/cm2 and rat MSC medium (10% FCS in 1 volume F-12 nutrient mixture + 1 volume αMEM; both Lonza, Walkersville, MD, USA) was added to a final volume of 30 ml. The cultures were maintained at 37°C, 5% CO2 and 90% humidity. On day 3, medium and non-adherent cells were removed and fresh rat MSC complete medium was added. The medium was changed every 3–4 days.
Cytofluorimetric analysis
The following monoclonal antibodies were used for phenotypic characterization of MSCs and T cells: anti-rat MHC class I-FITC (OX18, Cat. No. MCA5IFT; AbD Serotec, Kidlington, UK), anti-rat MHC class II-PE (OX6, Cat. No. MCA46PEB; AbD Serotec), anti-rat CD4-APC (OX35, Cat. No. 17-0040; eBioscience, Hatfield, UK), anti-rat CD25-FITC (OX39, Cat. No. 554865), anti-rat CD29-FITC (Ha2/5, Cat. No. 555005), anti-rat CD44H-FITC (OX-49, Cat. No. 550974), anti-rat CD45-PE (OX-1, Cat. No. 554878), anti-rat CD62L-PE (HRL1, Cat. No. 551398), anti-rat CD71-PE (OX-26, Cat. No. 554891), anti-rat CD73-PE (5F/B9, Cat. No. 551124), anti-rat CD90-FITC (OX-7, Cat. No. 551401), anti-CD106-PE (MR106, Cat. No. 559229), anti-rat CD134-PE (OX-40, Cat. No. 204508) and anti-rat CD172-PE (OX-41, Cat. No. 552298) (all from BD Biosciences, San Jose, CA, USA). For staining, cells were washed with FACS buffer (PBS containing 2% FCS and 0.1% NaN3, all from Sigma-Aldrich, Dublin, Ireland) and incubated for 5 min. on ice with anti-rat CD32 [Fcγ receptor; (D34-485, Cat. No. 550271) BD Biosciences] to reduce unspecific binding. Then, without washing, mAbs were added and the cells were incubated for 30–45 min. on ice. Finally, unbound reagents were removed by washing twice with FACS buffer and the cells were resuspended in FACS buffer for analysis with a FACS Canto (BD Biosciences). In some cases, cells were fixed by adding 2% Paraformaldehyde in FACS buffer. Data were analysed with Diva software (BD Biosciences) or FlowJo (Tree Star Inc., Ashland, OR, USA).
Alloantibody staining
LEW rats were injected intravenously (tail vein) with 1 × 106 syngeneic or allogeneic DA MSCs or 1 × 106 DA T cells. Mesenchymal stem cells were cultured with LEW serum for a minimum of 2–3 days prior to injection to avoid formation of antibodies against FCS. T cells were freshly prepared. After 2 weeks, rats were killed and blood was collected. The serum was obtained by centrifugation for 15 min. at 2000 × g. To detect the presence of alloantibodies in serum, freshly prepared DA splenocytes were washed with FACS buffer and incubated for 5 min. with anti-rat CD32. Then, serum diluted 1:2 with FACS buffer was added and the cells were incubated for 45 min. on ice. After washing twice with FACS buffer, monoclonal antibodies against rat immunoglobulins [anti-rat IgG1-FITC, anti-rat IgG2-FITC or anti-rat IgM-PE (all Antibodies-Online, Aachen, Germany)] were added. In case of anti-IgM-PE staining, anti-CD45RA-FITC (BD Biosciences) was added later to allow exclusion of B cells from analysis. Splenocytes were incubated for another 45 min. on ice, washed twice with FACS buffer to remove unbound reagents and resuspended in FACS buffer for analysis with a FACS Canto (BD Biosciences). Data were analysed with Diva software (BD Biosciences).
T cell proliferation assay
T cells were washed with 0.1% BSA/PBS and stained in prewarmed (37°C) 10 μM Vybrant CFDA SE (CFSE)/PBS staining solution (Invitrogen, Carlsbad, CA, USA) at a concentration of 2 × 107 cells/ml. Cells were incubated for 6 min./37°C protected from light and the reaction was stopped by adding 5 volumes of ice-cold medium containing 10% FCS. T cells were washed three times with final culture medium to remove all traces of unbound CFSE. 2 × 106 CFSE-stained T cells were stimulated in 24-well plates with an equal amount of anti-rCD3/anti-rCD28-labelled beads in MLC medium [2% heat-inactivated rat serum, 10% FCS, 50 μM β-mercaptoethanol (β-ME) in RPMI 1690 (Sigma-Aldrich)]. Varying amounts of MSCs were added. T cells were harvested after 3 days, counterstained with Violet Dead Cell Stain (Invitrogen) and CFSE fluorescence of living cells was analysed with a FACS Aria (BD Biosciences).
Cytotoxicity assay
To test whether MSCs are susceptible to cytotoxic lysis by alloantigen-specific T cells, cytotoxicity assays were performed with allogeneic DA MSCs either untreated or pretreated for 24 hr with 100 U/ml of IFN-γ, IL-1β or IFN-γ + IL-1β, and syngeneic Lewis rMSCs as control. Alloantigen-specific cytotoxic T cells (CTLs) were generated in one-way mixed lymphocyte cultures with LEW lymphocytes and γ-irradiated DA lymphocytes (ratio 2:1) in MLC medium [2% heat-inactivated rat serum, 10% FCS, 50 μM β-ME in RPMI (Sigma-Aldrich)]. After 5 days, T cells were harvested, washed and resuspended in MLC medium. Mesenchymal stem cells were washed twice with serum-free medium and resuspended in serum-free medium containing 10 μM calcein AM (1 × 106 cells/ml). After 30 min. incubation at 37°C, cells were washed three times with MLC medium to remove superfluous calcein and resuspended in MLC medium. In a 96-well round bottom plate in a total volume of 200 μl, 1 × 104 MSCs/well were cocultured with 1 × 106/well (1:100) or 5 × 105/well (1:50) T cells, or treated with 0.9% Triton-X (for maximum lysis), or treated with medium (spontaneous release) in five replicates each. After 4 hr, 100 μl of the supernatant was harvested and calcein fluorescences (F) measured with an ELISA (Perkin-Elmer, Waltham, MA, USA) plate reader. Specific lysis was calculated from mean fluorescence of replicates as follows:
Complement binding assay
After red blood cell lysis, 4.5 × 105 DA splenocytes were washed with PBS and incubated with serum diluted with PBS (1:10) for 30 min. at 4°C. Serum was removed by washing the splenocytes twice with PBS. Ten per cent baby rabbit complement (AbD Serotec) in 30 μl PBS was added and the cells were incubated for 30 min. at 37°C. Complement-mediated lysis was stopped by adding 1 ml of ice-cold PBS. After washing twice with PBS and directly before analysis, the cells were stained with 0.5 μg propidium iodide (PI)/sample. The splenocytes were analysed for percentage of PI-positive cells with a FACS Canto (BD Biosciences).
In vivo survival assay
LEW rats were injected intravenously with 3.5 × 106 LEW or DA MSCs, or with DA T cells (three animals per group). Two weeks later, all animals were injected intravenously with CFSE-labelled LEW MSCs and Far Red DDAO-SE (Invitrogen) labelled DA MSCs in a ratio of 1:1 (2.5 × 106 cells in total). MSCs were stained for 6 min. in 10 μM CFSE or Far Red; for the staining procedure, see T cell proliferation assay as described before. After 24 hr, lungs and spleens were harvested. Lungs were cut into pieces and collagenase D-digested (Roche Applied Science, Burgess Hill, UK) for 1 hr at 37°C. Lungs and spleens were forced through a 100 μm cell strainer. The cells were collected in 5 ml PBS (Ca++ Mg++), and filtered through a 40 μm cell strainer. After centrifugation (5 min. at 400 × g), the cells were resuspended in 4 ml PBS (Ca++ Mg++). Four millilitre cell suspension was layered on top of 3 ml Ficoll Paque PLUS solution (GE Healthcare, Uppsala, Sweden), centrifuged for 40 min. at 400 × g and the ring of mononuclear cells at the interphase harvested. The cells were washed twice, treated with anti-CD32 (Fcγ receptor block) and stained with 1 μl anti-rat CD90-PE (BD Biosciences) or an appropriate isotype control.
Statistical analysis
Significance was assessed by student's t-test or non-parametric Mann–Whitney test. Differences were considered significant if P ≤ 0.05.
Results
Characterization of rat MSCs
Rat MSCs (rMSCs) were isolated from the BM of Lewis (LEW) and DA rats and subsequently characterized for the expression of relevant cell surface markers, their capacity to differentiate into various lineages and their immunomodulatory properties. Rat MSCs are shown to be CD29+, CD73+, CD90+ and MHC class I (MHCI), MHC class II (MHCII), CD44H, CD45, CD71 and CD172 low or negative (Fig. 1A). They can differentiate along the adipogeneic, osteogeneic and chondrogeneic lineages (data not shown) and, under coculture conditions, rMSCs significantly inhibit the proliferation of polyclonally activated T cells stimulated by anti-CD3/anti-CD28 labelled beads (Fig. 1B).
Fig 1.
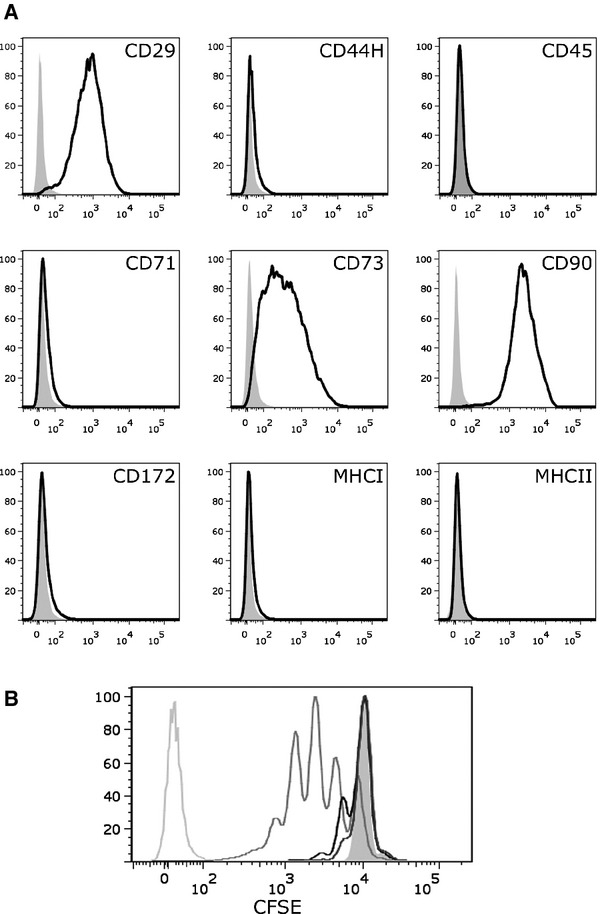
Characterization of rat mesenchymal stem cells (MSCs). (A) rMSCs are CD29+, CD73+, CD90+, and major histocompatibility complex class I (MHCI), MHCII, CD44H, CD45, CD71, CD172 low or negative. Shown are FACS histograms of Dark Agouti (DA) rMSCs (passage 5) stained with antibodies against surface markers as indicated (black) or with appropriate isotype controls (grey). (B) CFSE-labelled T cells were polyclonally stimulated with anti-CD3/anti-CD28-labelled beads in the absence (medium grey line) or presence of MSCs in different ratios (black line: 1:10; dark grey line: 1:5). CFSE fluorescence was analysed on day 3. Unstimulated T cells (filled grey line) and unstained T cells (light grey line) served as controls. Shown is a representative experiment of >3.
Allogeneic MSCs lose protection against CTLs after stimulation with pro-inflammatory cytokines IFN-γ and IL-1β
Rat MSCs do not express MHCII and only low levels of MHCI molecules on their cell surface. It is therefore conceivable that rMSCs can escape recognition by alloantigen-specific T cells. However, MSCs up-regulate MHCI and to a lesser extent MHCII as well as the adhesion molecule VCAM-1 in the presence of pro-inflammatory cytokines (Fig. 2A), which might increase the visibility of MSCs for CTLs. It is also known that VCAM-1 is essential for specific and efficient immune responses [30].
Fig 2.
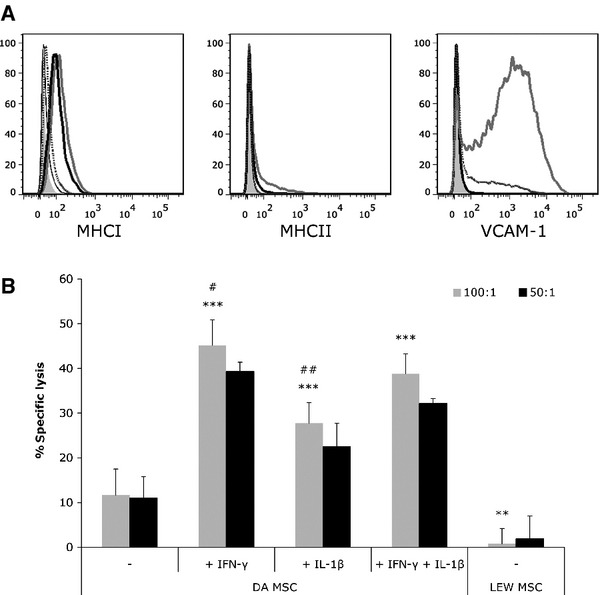
Pretreatment with inflammatory cytokines leads to upregulation of major histocompatibility complex class I (MHCI), MHCII and vascular adhesion molecule-1 (VCAM-1), and renders allogeneic rat mesenchymal stem cells (rMSCs) susceptible to cytotoxic lysis by alloantigen-specific T cells. (A) MSCs were treated with 100 U/ml IFN-γ, IL-1β or IFN-γ + IL-1β for 24 hr and analysed for MHCI, MHCII and VCAM-1 expression by FACS. IFN-γ (thick black line) and IFN-γ + IL-1β (grey line) induced upregulation of MHCI and MHCII, while MSCs stimulated with IL-1β alone (dotted line) did not change MHCII expression and only slightly increased MHCI expression compared to untreated MSCs (thin black line). VCAM-1 expression was induced by IL-1β and IFN-γ + IL-1β, but not by IFN-γ alone. Isotype controls are shown in filled grey (representative experiment from >3). (B) Alloantigen-specific cytotoxic T cells (CTLs) were generated in a mixed lymphocyte culture of LEW and γ-irradiated DA T cells. Syngeneic LEW or allogeneic DA rMSCs, either untreated or pretreated with 100 U/ml IFN-γ, IL-1β or IFN-γ + IL-1β for 24 hr, were stained with the fluorescent dye calcein and cocultured with alloantigen-specific CTLs in an effector to target ratio of 100:1 or 50:1 for 4 hr. MSCs that are lysed by CTLs release calcein into the cell culture supernatant and fluorescence of the supernatant is proportional to the amount of cells lysed. Percentage of specific lysis is calculated in relation to spontaneous release of calcein of MSCs in medium alone and maximum release of calcein by Triton-X treated MSCs. Shown is a representative experiment with means of five replicates ± S.D. (**P < 0.01; ***P < 0.001 compared to untreated DA MSC; #P < 0.05; ##P < 0.01 compared to DA MSC pretreated with IFN-γ + IL-1β; student's t-test).
To test whether MSCs are protected against alloantigen-specific CTLs, and what impact cytokine-induced upregulation of MHCI, MHCII and VCAM-1 might have on the susceptibility of MSCs to cytotoxic lysis, we performed cytotoxicity assays with cytokine-stimulated and unstimulated MSCs. Untreated MSCs were indeed almost fully protected against CTL-mediated lysis, whereas IFN-γ-primed MSCs (100 U/ml; 24 hr) upregulated MHCI and MHCII and were effectively lysed by CTLs added in a ratio of 100:1 (45.1%) (Fig. 2A and B). Stimulation with IL-1β (100 U/ml; 24 hr) led to an enhanced expression of VCAM-1 and, to a lesser extent, of MHCI. In combination with IFN-γ, VCAM-1 expression was increased even more and both MHCI and MHCII were upregulated (Fig. 2A). IL-1β stimulation resulted in at least a doubling of the specific lysis of MSCs compared to no stimulation (27.8% and 11.7%, respectively), while 38.8% of MSCs primed with IFN-γ + IL-1β were lysed (Fig. 2B).
Allogeneic MSCs do not induce markers of T cell activation in vivo
Next, we analysed whether injection of allogeneic MSCs leads to the activation of T cells in vivo. First, we tested for systemic early activation of CD4+ T cells in response to allogeneic MSCs. Spleens were harvested from animals 24 or 48 hr after injection of 5 × 106 MSCs, T cells or PBS and expression of the early activation markers CD25 and CD71 as well as expression of CD62L on CD4+ T cells was analysed by flow cytometry (Fig. 3). We found that in allo-T cell-injected animals, more CD4+CD25+CD71+ and CD4+CD62L− cells were detectable at 24 and 48 hr compared to PBS and allo-MSC-injected rats. Levels of T cell activation were similar in allo-MSC-treated and control-treated rats at both time points. In addition to the characterization of T cell activation in the spleen, T cells from mesenteric lymph nodes were also analysed for the expression of pro-inflammatory cytokines upon injection of allo-MSCs. The relative quantities of IL-1β, IL-2, IL-6 and IFN-γ mRNAs compared with β-actin mRNA were analysed with TaqMan semi-quantitative real time PCR (Invitrogen). No significant changes in the mRNA expression profile of any of these immune markers could be detected in lymphocytes of allo-MSC-injected animals after 24 and 48 hr when compared to PBS controls (Fig. S1). In contrast, injection of allogeneic T cells led to a transient upregulation of mRNAs of all inflammatory cytokines investigated after 24 hr.
Fig 3.
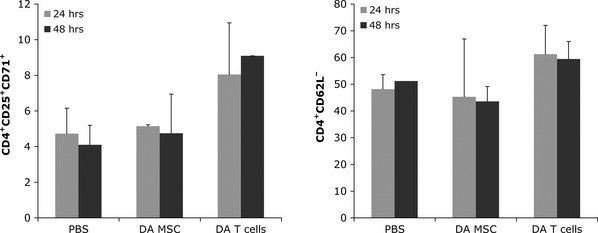
Allogeneic mesenchymal stem cells (MSCs) do not induce markers of T cell activation. LEW rats were injected with 5 × 106 DA MSCs, DA T cells or PBS. Spleens were harvested 24 or 48 hr after injection and expression of early activation markers was analysed. Cells were stained with anti-CD4, anti-CD25, and anti-CD71 (left) or anti-CD4 and anti-CD62L (right), or appropriate isotype controls. Shown are mean percentages of positive cells ± S.D. (n = 2–3).
Injection of allogeneic MSCs elicits an alloantibody response in vivo
To investigate whether allogeneic MSCs elicit an antibody response in vivo which could contribute to accelerated rejection of the cells, we intravenously injected 1 × 106 syngeneic or allogeneic MSCs or allogeneic T cells as control and collected the serum of treated animals after 14 days. The presence of alloantibodies in serum was detected by the binding of these antibodies to indicator-splenocytes followed by flow cytometric analysis. We could show that injection of allogeneic MSCs induced the formation of both IgG1 and IgG2 antibodies but, interestingly, only low levels of IgM antibodies (mean RFI: 574; naive control: 393) could be found after allo-MSC injection (Fig. 4). In contrast, injection of allogeneic T cells induced a strong IgM response (mean RFI: 1805) in treated animals. The level of IgG1 and IgG2 antibodies generated after MSC application was slightly lower or comparable with those generated by injection of 1 × 106 allogeneic T cells (Fig. 4).
Fig 4.
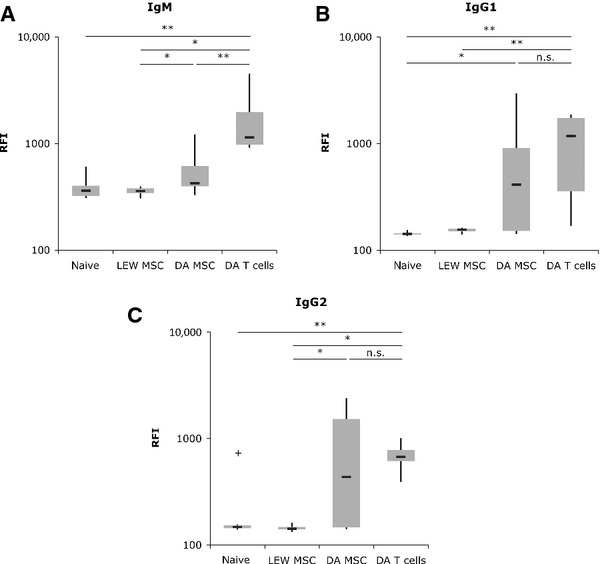
Allogeneic mesenchymal stem cells (MSCs) elicit an antibody response. LEW rats were injected with 1 × 106 syngeneic or allogeneic (DA) MSCs or allogeneic T cells. Serum was harvested 2–3 weeks after injection. Splenocytes of allogeneic DA rats were incubated with serum aliquots and, consequently, bound alloantigen-specific antibodies were stained with anti-rat IgM (A), anti-rat IgG1 (B) or anti-rat IgG2 (C) antibodies. B cells were excluded from analysis of IgM by counterstaining with CD45RA (n = 6 animals/group; *P < 0.05; **P < 0.01; n.s.: not significant; non-parametric Mann–Whitney test).
Fewer complement-fixing alloantibodies are generated after rMSCs injection
Not all antibodies bind and activate complement equally well. IgM antibodies, because of their pentameric nature, are very efficient at fixing complement because of multimeric interactions between the secreted antibody and the antigen. Monomeric IgG antibodies are generally less efficient at fixing complement and the affinity for C1q, which is the first component of the complement pathway, differs between the IgG subclasses (IgG3 > IgG1 > IgG2; IgG4 fails to bind complement) [31]. Therefore, we wanted to know whether the alloantibodies produced in response to injection of allogeneic MSCs were equally effective in fixing and activating complement as allogeneic T cell-induced antibodies. A complement-mediated lysis assay using serum from either allogeneic MSC or T cell-injected LEW rats showed that MSC-induced alloantibodies were significantly less effective at fixing complement than alloantibodies induced by T cells (Fig. 5). On average, 55.1% (S.D.: ± 13.3) of splenocytes treated with allogeneic T cell serum were lysed compared to only 16.6% (S.D.: ± 7.3) of splenocytes incubated with allogeneic MSC serum. Nevertheless, complement-mediated lysis of MSC serum-treated splenocytes was still significantly higher than ‘background’ lysis of splenocytes incubated with serum from naive or syngeneic MSC-injected LEW rats (11.6 ± 1.3 and 11.8 ± 1.2, respectively).
Fig 5.
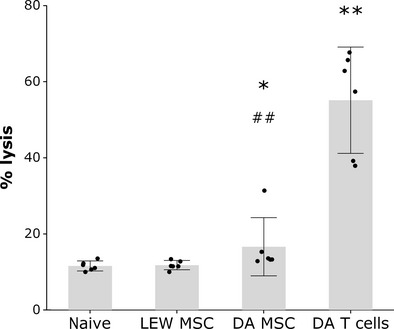
Allogeneic mesenchymal stem cell (MSC)-induced antibodies mediate significant albeit reduced complement activation. LEW rats were injected with 1 × 106 syngeneic or allogeneic MSCs or allogeneic T cells. Serum was harvested 2–3 weeks after injection. Splenocytes of allogeneic DA rats were incubated with serum (diluted 1:10) and treated with baby rabbit complement (diluted 1:10). Splenocytes were stained with PI to detect complement-mediated lysis. The grey bars show the means and 95% confidence intervals of serums from six animals per group tested and the black dots show means of triplicates from serum of individual animals. *P < 0.05, **P < 0.01 compared to LEW MSC and naïve; ##P < 0.01 compared to DATc (non-parametric Mann–Whitney test).
Survival of allogeneic MSCs is reduced in vivo
Next, we wanted to know if the presence of alloantigen-specific antibodies is likely to have an influence on survival of allogeneic MSCs in vivo. Therefore, we injected LEW rats (three animals per group) intravenously with 3.5 × 106 allogeneic or syngeneic MSCs or allogeneic T cells. Two weeks later, all animals received a second i.v. injection with a 1:1 mixture of Far Red-labelled allogeneic and CFSE-labelled syngeneic MSCs (2.5 × 106 cells in total). Lungs and spleens were harvested 24 hr later. Mononuclear cells were enriched using a Ficoll gradient and the cells were stained with CD90-PE. While CD90 is not a unique MSC marker, all MSCs are CD90+ (Fig. 1). This allowed us to further verify that recovered CFSE+ and Far Red+ cells were indeed MSCs. Gates were set by using data from preliminary experiments (data not shown), isotype controls, and by analysing the pre-injection mixture of labelled MSCs (data not shown). CD90+ CFSE+ cells in the MSC gate were counted as LEW MSCs and CD90+ Far Red+ cells as DA MSCs (Fig. 6A). The majority of MSCs was recovered from the lungs of injected animals, although a few MSCs were also found in the spleen (Table 1).
Fig 6.
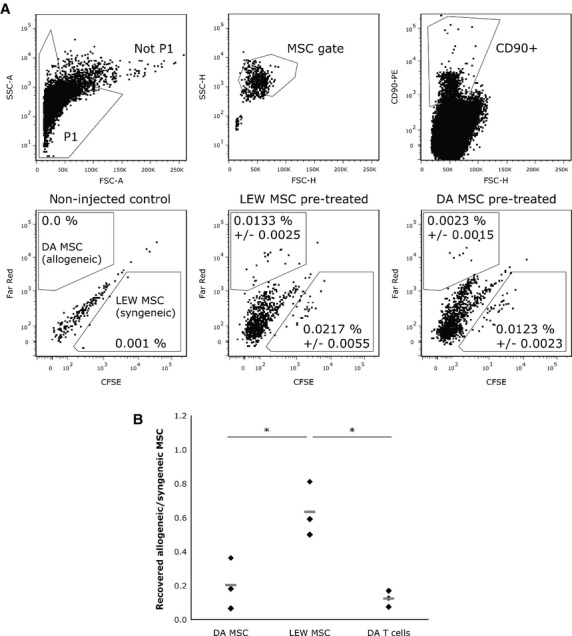
Reduced survival of allogeneic mesenchymal stem cells (MSCs) in vivo. LEW rats were injected with 3.5 × 106 syngeneic or allogeneic MSCs or allogeneic T cells (three animals per group). After 2 weeks, all groups received 2.5 × 106 CFSE-stained LEW MSCs and Far Red-stained DA MSCs (ratio 1:1). Lungs were harvested 24 hr after injection. Mononuclear cells were enriched using a Ficoll gradient and stained with anti-rat CD90-PE. (A) Gating strategy: Not P1 → MSC gate → CD90+ (top row). Bottom row: Shown are representative dot plots of CD90+ cells recovered from lungs of non-injected controls, LEW MSC pre-treated and DA MSC pre-treated rats (left to right). The percentages given are percentage of events ± S.D. in the MSC gate that are CD90+ CFSE+ (LEW MSCs) or CD90+ Far Red+ double positive (DA MSCs), respectively. (B) Ratio of allogeneic MSCs (CD90+ Far Red+) to syngeneic MSCs (CD90+ CFSE+) in LEW rats pre-treated with DA MSCs or LEW MSCs. Rats pre-treated with DA T cells were used as positive controls. Each black diamond represents an individual animal (n = 3/group), the grey lines show the mean (*P < 0.05; non-parametric Mann–Whitney test).
Table 1.
Total number of CFSE+/FarRed+ CD90+ cells in 2.5 × 106 ungated lung and spleen cells of injected animals
| Lung | Spleen | |||||
|---|---|---|---|---|---|---|
| Syngeneic | Allogeneic | Allo/syn ratio | Syngeneic | Allogeneic | Allo/syn ratio | |
| LEW MSC | 35 ± 10 | 18 ± 1 | 0.62 | 8 ± 6 | 11 ± 10 | 1.65 |
| DA MSC | 25 ± 5 | 5 ± 5 | 0.19 | 10 ± 9 | 3 ± 4 | 0.19 |
| DA T cells | 259 ± 121 | 34 ± 24 | 0.14 | 9 ± 2 | 5 ± 4 | 0.42 |
We compared the ratios of recovered allogeneic to syngeneic MSCs in the lungs of animals pre-treated either with syngeneic MSCs, allogeneic MSCs or allogeneic T cells. If the allo-MSC-induced immune response was too weak to facilitate rejection of allo-MSCs we would not see a difference between the allo/syn MSC ratios of LEW and DA MSC pre-treated groups. In addition, if survival of allogeneic MSCs in vivo was not influenced by prior contact with the allo-antigen we would expect equal allo/syn MSC ratios in all groups. However, we measured a significantly higher ratio of allogeneic cells in the lungs of animals pre-treated with syngeneic MSCs (0.64 ± 0.16) than in animals pre-treated with allogeneic MSCs (0.20 ± 0.15) and T cells (0.12 ± 0.05) (Fig. 6B). A comparably reduced survival of allogeneic MSCs in the allo-MSC and T cell treated groups was found in the spleen (Table 1). These data indicate that allo-MSCs are indeed subject of rejection in pre-treated animals. In a separate experiment, we compared the survival of allo-MSCs in animals (n = 4/group) that were pre-treated with either allo-MSCs or IFN-γ stimulated allo-MSC (24 hr stimulation with 100 U/ml IFN-γ, then cultured for additional 12 hr). We wanted to see whether the immunogenicity-enhancing effect of IFN-γ would lead to even more pronounced rejection of subsequently injected allo-MSCs in vivo. However, we did not find a difference in the allo/syn MSC ratio in these two groups (Fig. S2).
Discussion
In preclinical studies, therapeutic treatment with MSCs has provided beneficial effects in several acute and subacute conditions. Mesenchymal stem cells are considered to be immunoprivileged because of their low immunogenicity in vitro and in some preclincial studies [13–15], which would support the possible use of ‘universal donor’ MSCs in the clinic. In most acute clinical conditions allogeneic MSCs would be the only option for a timely treatment [32], for example, after myocardial infarction or in organ transplantation from non-living donors. However, there is increasing evidence to suggest that allogeneic MSCs elicit an immune response and can be rejected by an allogeneic recipient which might reduce their therapeutic potential [12, 21, 26]. The elucidation of the exact nature of the immune response against allogeneic MSCs might help in devising strategies to optimize the modalities of therapeutic treatments with allogeneic MSCs that minimize possible negative effects and/or rejection. In this study, we analysed cytotoxic activity against allogeneic MSCs in vitro and early and late cellular and humoural immune responses after i.v. injection in vivo.
First, we tested whether allogeneic MSCs are protected against lysis by alloantigen-specific activated T cells in vitro. Mesenchymal stem cells express only very low levels of MHCI and MHCII (Fig. 1 and Hoare et al. [33]) and Potian et al. [34] demonstrated that third party MSCs can reduce lysis of allogeneic target cells in a cytotoxicity assay with alloantigen-specific activated CTLs. It is therefore conceivable that allogeneic MSCs can evade lysis by alloantigen-specific CTLs because of their low immunophenotype and/or an intrinsic anti-cytotoxic activity. Indeed, we found that the majority of allogeneic MSCs remained intact and were not lysed by CTLs in vitro (Fig. 2B), as has been described previously [35]. However, as we (Fig. 2A) and others [36] have shown, MSCs upregulate MHCI and to a lesser extent MHCII, as well as the adhesion molecule VCAM-1 in the presence of inflammatory cytokines. Recently, it has been demonstrated in a mouse model that cytokine-induced upregulation of VCAM-1 and ICAM-1 plays an important role in the immunosuppressive function of MSCs because it enables adhesion of T cells [36]. This likely enhances the impact of immunosuppressive mechanisms of MSCs which rely on close proximity between MSCs and T cells to function, for example, nitric oxide secretion [37, 38]. Ren et al. [38] found that IFN-γ in combination with any of the inflammatory cytokines TNF-α, IL-1α or IL-1β induced expression of inducible nitric oxide synthase and several chemokines by MSCs and increased their ability to inhibit T cell proliferation. On the other hand, it is also known that VCAM-1 and ICAM-1 are essential for specific and efficient immune responses [30]. In an allogeneic setting, upregulation of MHCI, MHCII and VCAM-1 together might override the immunosuppressive capabilities of MSCs. Indeed, MSCs that were preincubated with IFN-γ, IL-1β or IFN-γ and IL-1β in combination for 24 hr before coculture with alloantigen-specific CTLs were effectively lysed (Fig. 2B). Notably, pretreatment with IFN-γ alone increased lysis of MSCs more than combined IFN-γ and IL-1β stimulation despite the even more pronounced upregulation of MHCI, MHCII and VCAM-1 induced by the latter treatment. It is possible that the loss of their low immunophenotype by upregulation of MHCI, MHCII and VCAM-1 is counteracted by a gain in immunosuppressive activity through combined IFN-γ + IL-1β stimulation. Cytotoxic T cell-mediated lysis might play a role in the rejection of allogeneic MSCs in vivo as well. Rafei et al. [39] reported recently that allogeneic MSCs at least transiently improved the outcome of experimental autoimmune encephalomyelitis in mice but IFN-γ-primed allogeneic MSCs completely lost their suppressive effects suggesting an accelerated immune clearance. However, injection of IFN-γ pretreated allo-MSCs did not lead to significantly reduced survival of subsequently injected allo-MSCs (Fig.S2). Therefore, it is possible that the presence of other cytokines in vivo negate the immunogenicity-enhancing effect of IFN-γ while still reducing their immunosuppressive properties. In addition, we showed that in vivo, MSCs failed to induce T cell activation. Both levels of CD25+CD71+ and CD62L on CD4+ T cells were comparable in MSC- and PBS-injected animals while rats that received allogeneic T cells showed elevated numbers of CD4+CD25+CD71+ and downregulation of CD62L after 24 and 48 hr (Fig. 3). This suggests that allo-MSCs fail to induce the generation of CTLs in vivo.
In contrast, allogeneic MSCs clearly induced a humoural immune response. In vitro, mouse and human MSCs have been demonstrated to inhibit B cell differentiation and proliferation, minimize Ig production, and reduce the number of Ig-producing cells [40, 41], although it is possible that the influence on Ig production is simply a consequence of MSCs’ anti-proliferative effect on B cells [42]. In addition, the best results were obtained with 1:1 or 1:4 ratios of responder B cell to MSC ratios. At lower MSC to responder cell ratios (1:10), MSCs failed to inhibit B cell proliferation [43] and IgG production [41, 44]. Furthermore, none of the studies analysed antibody production directly in response to allogeneic MSCs.
Very little is known about induction of alloantibodies by MSCs in vivo. In one study, immunocompetent non-human primates (baboons) injected with two doses of allogeneic MSCs (5 × 106 cells/kg bodyweight) developed alloantibodies [45]. In a clinical study with 12 patients which were treated with MSCs (0.8–2.0 × 106 cells/kg bodyweight) after hematopoietic stem cell transplantation, none of the patients developed anti-MSC antibodies. However, in this study only five patients received fully HLA-mismatched MSCs instead of haploidentical or sibling-derived MSCs and all patients were immunosuppressed [46].
Our results indicate that a single injection of allogeneic MSCs can induce a substantial alloantibody response in an immunocompetent host. We choose a MSC cell number for injection, 1 × 106 cells/animal (= approximately 3 × 106 cells/kg bodyweight), that is comparable to cell concentrations most commonly used in humans, between 1 and 5 × 106 cells/kg bodyweight [47]. Two weeks after injection, substantial amounts of both IgG1 and IgG2 antibodies were detectable in the serum of rats that received 1 × 106 allogeneic MSCs but not in the serum of rats injected with syngeneic MSCs. The serum IgG1 and IgG2 antibody levels were comparable with those of rats injected with allogeneic T cells. The mean increase in IgG1 and IgG2 antibodies compared with syngeneic MSC-injected animals was 5.5 ± 7.0 and 6.2 ± 6.7 in allogeneic MSC-injected animals and 6.9 ± 5.0 and 4.8 ± 1.4 in allogeneic T cell-injected animals, respectively. Interestingly, allogeneic MSCs induced only very low levels of IgM antibodies (mean increase: 1.6 ± 0.9), whereas allogeneic T cells led to a pronounced IgM response (mean increase: 5.0 ± 3.9) (Fig. 4).
Several studies found that FCS contained in the culture medium used to expand MSCs or other cell types can lead to anti-FCS antibody formation in recipients after i.v. injection [46, 48]. We minimized the possibility that the presence of FCS interfered with our results in multiple ways. First, we cultured MSCs with 10% rat serum instead of FCS for at least 48 hr prior to injection, which has been shown to drastically reduce the amount of FCS associated per cell [49]. Second, we used binding of serum antibodies to allogeneic cells as the detection method, ensuring that we measure alloantigen-specific antibodies rather than total antibodies. Finally, we used serum from rats injected with syngeneic MSCs cultured in parallel as additional negative controls, which showed no difference in antibody levels compared to serum from naive rats. However, we cannot completely exclude that anti-FCS antibodies may have been generated.
Antibodies that are specific for graft antigens (typically MHC molecules) can mediate or contribute to rejection by complement activation. In addition, complement activation leads to the recruitment of effector cells such as monocytes, macrophages and neutrophils [50]. To further evaluate the differences in the antibody responses against allogeneic MSCs and T cells, we analysed the complement-fixing and activating properties of alloantibody sera from injected animals. Complement-mediated lysis of DA splenocytes incubated with allo-MSC sera was only marginally, albeit significantly, elevated compared to control sera-treated cells, while treatment with allo-T cell-induced sera led to substantial lysis of the target cells (1.4 and 4.7 times higher than lysis of control sera-treated cells, respectively) (Fig. 5). Most likely, this difference in the extent of complement-mediated lysis is explained both by differing amounts and isotype compositions of the induced alloantibodies. Mesenchymal stem cell-induced allosera contain slightly less IgG1 and much less IgM-type antibodies than T cell-induced allosera but slightly more IgG2 antibodies. Of the analysed isotype classes and subclasses, IgM possesses the highest complement affinity, followed by IgG1, while IgG2 binds and activates complement with relatively low efficiency [31]. It is not obvious how MSCs can influence the isotype composition of the antibody response, whether by direct contact to B lymphocytes or indirectly, for example, by interfering with the CD4 T cell response.
Finally, we showed that the immune reaction induced by allogeneic MSCs was sufficient to lead to rejection of subsequently injected allogeneic MSCs in vivo. Compared to animals injected with syngeneic MSCs, survival of allogeneic MSCs was considerably reduced in allo-MSC pre-treated rats, both in lung and spleen (Fig. 6; Table 1). While other mechanisms of rejection cannot be excluded, it is likely that the allo-MSC induced formation of complement-fixing allo-antibodies is partly responsible for this diminished survival of allogeneic MSCs in vivo.
Our results add to the growing evidence that MSCs are not fully immunoprivileged in an immunocompetent allogeneic host. Both the activation of CD4+ T cells and the humoural immune response were substantially reduced or altered in MSC-injected animals compared to the immune responses to allogeneic T cells. Nevertheless, even a single injection of allo-MSCs was sufficient to induce production of complement-fixing alloantibodies and to facilitate rapid rejection of subsequently injected allo-MSCs. The success of therapeutic treatments with allogeneic MSCs in conditions where pro-inflammatory cytokines are present or that involve multiple injections might be limited by rapid clearance of the allogeneic MSCs.
Acknowledgments
The material presented in this manuscript is based upon works supported by the Health Research Board (http://www.hrb.ie) under Grant No. (RP/2007/60). Contribution: S.S., L.O'F., M.N., O.T. and G.S. performed experiments; S.S. analysed results and made the figures; S.S., M.M., F.B., T.O'B. and T.R. designed the research and wrote the paper.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1 Allogeneic MSCs do not induce cytokine production or T cell activation.
Fig. S2 IFNγ stimulation does not alter the rejection rate of allogeneic MSCs in sensitized animals.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Sensebe L, Bourin P. Mesenchymal stem cells for therapeutic purposes. Transplantation. 2009;87:S49–53. doi: 10.1097/TP.0b013e3181a28635. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 3.Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009;1176:101–17. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- 4.Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 5.Togel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Chen J, Wang L, et al. Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Neurosci Lett. 2001;316:67–70. doi: 10.1016/s0304-3940(01)02384-9. [DOI] [PubMed] [Google Scholar]
- 7.Dryden GW. Overview of stem cell therapy for Crohn's disease. Exp Opin Biol Ther. 2009;9:841–7. doi: 10.1517/14712590902956615. [DOI] [PubMed] [Google Scholar]
- 8.Bang OY, Lee JS, Lee PH, et al. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–82. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 9.Lipinski MJ, Biondi-Zoccai GGL, Abbate A, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–7. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 10.Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–6. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 11.Nauta AJ, Westerhuis G, Kruisselbrink AB, et al. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–20. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudres M, Norol F, Trenado A, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006;176:7761–7. doi: 10.4049/jimmunol.176.12.7761. [DOI] [PubMed] [Google Scholar]
- 13.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–8. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Tredget EE, Liu C, et al. Analysis of allogenicity of mesenchymal stem cells in engraftment and wound healing in mice. PLoS ONE. 2009;4:e7119. doi: 10.1371/journal.pone.0007119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun L, Akiyama K, Zhang H, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–32. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (Prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casiraghi F, Azzollini N, Cassis P, et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol. 2008;181:3933–46. doi: 10.4049/jimmunol.181.6.3933. [DOI] [PubMed] [Google Scholar]
- 18.Ge W, Jiang J, Baroja ML, et al. Infusion of mesenchymal stem cells and rapamycin synergize to attenuate alloimmune responses and promote cardiac allograft tolerance. Am J Transplant. 2009;9:1760–72. doi: 10.1111/j.1600-6143.2009.02721.x. [DOI] [PubMed] [Google Scholar]
- 19.Popp FC, Eggenhofer E, Renner P, et al. Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl Immunol. 2008;20:55–60. doi: 10.1016/j.trim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Renner P, Eggenhofer E, Rosenauer A, et al. Mesenchymal stem cells require a sufficient, ongoing immune response to exert their immunosuppressive function. Transplant Proc. 2009;41:2607–11. doi: 10.1016/j.transproceed.2009.06.119. [DOI] [PubMed] [Google Scholar]
- 21.Inoue S, Popp FC, Koehl GE, et al. Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation. 2006;81:1589–95. doi: 10.1097/01.tp.0000209919.90630.7b. [DOI] [PubMed] [Google Scholar]
- 22.Zhou HP, Yi DH, Yu SQ, et al. Administration of donor-derived mesenchymal stem cells can prolong the survival of rat cardiac allograft. Transplant Proc. 2006;38:3046–51. doi: 10.1016/j.transproceed.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 23.von Bonin M, Stolzel F, Goedecke A, et al. Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transplant. 2008;43:245–51. doi: 10.1038/bmt.2008.316. [DOI] [PubMed] [Google Scholar]
- 24.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–86. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 25.Zangi L, Margalit R, Reich-Zeliger S, et al. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells. 2009;27:2865–74. doi: 10.1002/stem.217. [DOI] [PubMed] [Google Scholar]
- 26.Eliopoulos N, Stagg J, Lejeune L, et al. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057–65. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 27.Prigozhina TB, Khitrin S, Elkin G, et al. Mesenchymal stromal cells lose their immunosuppressive potential after allotransplantation. Exp Hematol. 2008;36:1370–6. doi: 10.1016/j.exphem.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Spaggiari GM, Capobianco A, Becchetti S, et al. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–90. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 29.Poncelet AJ, Vercruysse J, Saliez A, et al. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation. 2007;83:783–90. doi: 10.1097/01.tp.0000258649.23081.a3. [DOI] [PubMed] [Google Scholar]
- 30.Lebedeva T, Dustin ML, Sykulev Y. ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr Opin Immunol. 2005;17:251–8. doi: 10.1016/j.coi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Schroeder HW, Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125:S41–52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–36. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 33.Hoare M, Greiser U, Schu S, et al. Enhanced lipoplex-mediated gene expression in mesenchymal stem cells using reiterated nuclear localization sequence peptides. J Gene Med. 2010;12:207–18. doi: 10.1002/jgm.1426. [DOI] [PubMed] [Google Scholar]
- 34.Potian JA, Aviv H, Ponzio NM, et al. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–34. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 35.Rasmusson I, Ringden O, Sundberg B, et al. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–13. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 36.Ren G, Zhao X, Zhang L, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–8. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–34. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 38.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–50. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 39.Rafei M, Birman E, Forner K, et al. Allogeneic mesenchymal stem cells for treatment of experimental autoimmune encephalomyelitis. Mol Ther. 2009;17:1799–803. doi: 10.1038/mt.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–72. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 41.Asari S, Itakura S, Ferreri K, et al. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol. 2009;37:604–15. doi: 10.1016/j.exphem.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siegel G, Schafer R, Dazzi F. The immunosuppressive properties of mesenchymal stem cells. Transplantation. 2009;87:S45–9. doi: 10.1097/TP.0b013e3181a285b0. [DOI] [PubMed] [Google Scholar]
- 43.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–98. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 44.Rasmusson I, Blanc KL, Sundberg B, et al. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol. 2007;65:336–43. doi: 10.1111/j.1365-3083.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 45.Beggs KJ, Lyubimov A, Borneman JN, et al. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006;15:711–21. doi: 10.3727/000000006783981503. [DOI] [PubMed] [Google Scholar]
- 46.Sundin M, Ringden O, Sundberg B, et al. No alloantibodies against mesenchymal stromal cells, but presence of anti-foetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007;92:1208–15. doi: 10.3324/haematol.11446. [DOI] [PubMed] [Google Scholar]
- 47.Wagner J, Kean T, Young R, et al. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol. 2009;20:531–6. doi: 10.1016/j.copbio.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Tuschong L, Soenen SL, Blaese RM, et al. Immune response to foetal calf serum by two adenosine deaminase-deficient patients after T cell gene therapy. Hum Gene Ther. 2002;13:1605–10. doi: 10.1089/10430340260201699. [DOI] [PubMed] [Google Scholar]
- 49.Spees JL, Gregory CA, Singh H, et al. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9:747–56. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 50.Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5:807–17. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


