Abstract
Cystamine, a disulphide metabolite, has been demonstrated to ameliorate various lupus-associated tissue damages by animal models. However, effects of cystamine on apoptosis of cardiac tissue, a main cardiac damage attributing to lupus, are less obvious. Therefore, we aimed to investigate whether or not cystamine possesses anti-apoptotic effects with emphasis on LV tissue of lupus-prone mice NZB/W-F1. Cystamine treatment was performed by daily intraperitoneal administration. Morphology and apoptotic status of ventricular tissues in the treated mice were assessed by microscopy and TUNEL assay, respectively. Levels of apoptotic biomarkers were determined using immunoblot. Our results revealed that cystamine significantly attenuated the apoptosis of LV tissues in NZB/W-F1 mice, whereas the morphology of the tissues was slightly altered. In addition, cystamine reduced level of Fas and inhibited activation of caspase-8. Cystamine also increased level of Bcl-2 and phosphorylation of Bad, and decreased level of Bad and truncated Bid (tBid). Moreover, level of cytosolic cytochrome c and Apaf-1, and activation of caspase-9 and caspase-3 were suppressed in response to cystamine treatment. In Balb/c mice, as normal control mice, changes in cell morphology and levels of the tested apoptotic components were found insignificant in the LV tissues. These findings indicate that cystamine treatment attenuates apoptosis of LV tissues of NZB/W-F1 mice through suppressing both intrinsic and extrinsic apoptotic pathways. Therefore, cystamine is considered beneficial to alleviating lupus-associated cardiac damages.
Keywords: apoptosis, cystamine, Fas, lupus-prone mice, ventricular tissue
Introduction
Systemic lupus erythematosus (SLE), a prototypic autoimmune disease, is characterized by production of autoantibodies of different specificities and the abnormal apoptosis [1]. Systemic lupus erythematosus is associated with a series of disease manifestations, including glomerulonephritis, nephritis, arthritis, pleuritis, pericarditis and vasculitis. In addition, mounting evidence has demonstrated that SLE patients have relatively high mortality and morbidity of cardiovascular diseases (CVD) [2–5]. Notably, incidence rate of SLE is found significant in some particular populations such as women aged 44–50 who have a 50-fold increased risk of myocardial infarction [2, 6]. As a result, CVD is considered as a critical manifestation to patients with SLE.
Autoantibodies reactive with heart tissue associating certain heart diseases were found in autoimmune diseases [7–9]. Previous study indicated that autoantibodies, particularly anti-phospholipid and anti-oxidized low-density lipoprotein antibodies, were highly associated with CVD in both SLE and general population [10]. Of patients with SLE, 30–50% have anti-phospholipid antibodies and develop anti-phospholipid syndrome [11]. The humoural immune response may play a pivotal role through induction of cardiomyocyte apoptosis [12], activation of the complement system and cell-mediated cytotoxicity [13], attributing to alteration of myocardial mechanical and electrophysiological functions. Therefore, suppression of cardiomyocyte apoptosis is suggested to alleviate or ameliorate autoantibody-induced cardiac injuries.
Cystamine, precursor of cysteamine, has been reported to inactivate protein kinase C-epsilon, gamma-glutamylcysteine synthetase and tissue transglutaminase by S-cysteaminylation-triggered mechanisms [14–16]. Our previous study found that cystamine suppressed the production of anti-cardiolipin autoantibody in lupus-prone mice NZB/W-F1 [17]. Moreover, cystamine also shows neuroprotective activities, prolonging effect on cell survival and alleviation of abnormal cell movements in the transgenic model of Huntington disease [18]. However, the effects of cystamine on apoptosis in cardiac tissues in vivo are of interest to be determined.
In this study, we aimed to investigate whether or not cystamine alleviates apoptosis of cardiac tissues in NZB/W-F1 mice with emphasis on the underlying mechanisms. Morphology and cellular apoptosis of LV tissue in NZB/W-F1 mice treated with cystamine was analysed by haematoxylin and eosin staining and terminal deoxynucleotide transferase-mediated dUTP nick end labelling (TUNEL) assay, respectively. Activation of apoptotic cascades was demonstrated using immunoblot. In addition, the effects of cystamine on LV tissues of Balb/c mice were also determined and referred as a normal control.
Materials and methods
Animals and reagents
Female Balb/c mice and NZB/W-F1 mice were obtained from the Animal Center, National Taiwan University, Taiwan and housed under supervision of the Institutional Animal Care and Use Committee at Chung Shan Medical University. To monitor lupus development, proteinuria was determined biweekly by Albustix test strips (Bayer Diagnostics, Hong Kong, China) as previously described [17]. Antibodies against mouse Apaf-1, Bad, Bcl-2, cytochrome c, Fas (also known as CD95), caspase-3, caspase-8, caspase-9 and α-tubulin (α-TN) were purchased from Upstates (Charlottesville, VA, USA). Antibody against phosphorylated extracellular signal-regulated kinase 1/2 (pErk1/2) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Alkaline phosphatase-conjugated secondary antibodies were purchased from BioSource International (Camarillo, CA, USA). Chemicals for immunoblot and phagocytic analysis were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cystamine treatment
The NZB/W F1 mouse is a lupus-prone mouse strain in which the clinical symptoms appear at the age of 8–12 weeks and reach the peak at the age of 24–28 weeks [19, 20]. Accordingly, 6-month-old NZB/W-F1 mice were used for cystamine treatment. The mice were randomly divided into two groups (12 mice for each group). Treatment was performed by daily intraperitoneal injection (100 μl of 10 mM cystamine in normal saline or 100 μl of normal saline as control) for 14 days as previously described [17, 18]. After the 14-day injection, the mice were killed by CO2 asphyxiation and the cardiac samples were dissected. All samples were stored at −70°C until use.
Morphology examination by haematoxylin and eosin staining
The left ventricles of hearts from mice were soaked in formalin and embedded with paraffin. Cardiac tissue sections were prepared by microtome, followed by deparaffinization and dehydration. Slides were stained with haematoxylin. After gently rinsing with distilled water, each slide was soaked with 85% alcohol and 100% alcohol for 15 min., respectively. At the end, the slides were immersed with xylene. Photomicrographs were obtained using Zeiss Axiophot microscopy (Oberkochen, Germany).
TUNEL assay
Left ventricles of hearts from animals were surgically removed, embedded in OCT compound (Tissue-Tek; Miles Inc., Elkhart, IN, USA) and snap frozen in liquid nitrogen. Cryoblocks were sectioned at 5 μm and fixed in 4% phosphate-buffered paraformaldehyde (Sigma-Aldrich) for 20 min. at room temperature. Tissue sections were washed with PBS for 30 min. and incubated with 3% H2O2 in methanol for 10 min. at room temperature. The TUNEL reaction mixture was freshly prepared according to the manufacturer's instructions (Roche Applied Science, Mannheim, Germany). In brief, a total volume of 100 μl of terminal deoxytransferase reaction mixture was incubated with the tissue sections for 1 hr at room temperature in dark. Cardiac myocytes were identified using α-sarcomeric actinin antibodies (Sigma-Aldrich). DAPI staining was used to count the total number of nuclei. The index of apoptosis was the average of the percentages of apoptotic myocyte nuclei per total number of nuclei from five random observation fields at 200× magnitude. All measurements were performed by at least three independent animals in a blinded manner.
Protein extraction
The LV tissue samples were resuspended in ice-cold PBS (200 mg tissue/ml PBS) containing 1% v/v Triton X-100 (Sigma-Aldrich) and protease inhibitors (Complete protease inhibitor cocktail; Roche Applied Science), homogenized by polytron, and incubated for 15 min. on ice. The crude extracts were centrifuged at 12,000 × g for 30 min., and then the supernatant was collected and stored at −70°C for further analyses. Concentration of crude protein was determined using BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA).
Immunoblot
For immunoblot, protein extracts from four mice were pooled with equal amount for the analysis. A quantity of 20 μg of crude protein was electrophoresed on 10% sodium dodecyl sulphate-polyacrylamide gel at 140 V for 3.5 hr. After electrophoresis, the proteins were transferred onto a nitrocellulose membrane (Millipore, Bedford, MA, USA) using Bio-Rad Scientific Instruments Transphor Unit (Hercules, CA, USA). The blots were blocked with 5% w/v skimmed milk in PBS, and then incubated for 1 hr with 1000-fold diluted primary antibodies followed by incubation with 2000-fold diluted peroxidase-conjugated secondary antibodies (BioSource International). Antigen-antibody complexes were revealed using ECL chemiluminescence. The photographic density was quantified by using image analysis system (Alpha Imager 2000; Alpha Innotech, San Leandro, CA, USA). Reacted density of α-TN was used as internal control for relative quantification.
Statistical analysis
Data were presented as means ± S.D. of three independent experiments. Statistical significance analysis was determined by using One-way anova followed by Dunnett for multiple comparisons with the control. The differences were considered significant for P < 0.05.
Results
Effect of cystamine treatment on morphology of LV tissues
The LV tissues of NZB/W-F1 and Balb/c mice treated with cystamine or PBS were harvested and morphology of LV tissues was evaluated by using microscopy after haematoxylin and eosin stain. As shown in Figure 1, no significant morphology changes were observed in LV tissues of NZB/W-F1 mice treated with cystamine as compared to that treated with PBS. In addition, cystamine treatment also slightly affected morphology of LV tissues from Balb/c mice as compared with PBS treatment.
Fig 1.
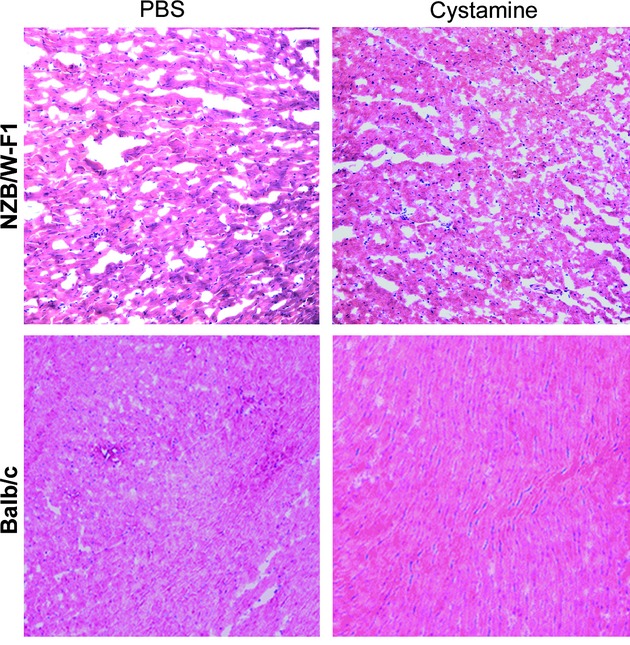
Effects of cystamine treatment on morphology of LV tissues. LV samples were obtained from NZB/W-F1 mice and Balb/c mice, which were treated with PBS or cystamine as described in Materials and methods, stained by haematoxylin and eosin and then observed using microscopy (magnification 200×).
Attenuation of apoptosis in LV tissues by cystamine treatment
Cell apoptosis in many organs is associated with development of lupus syndrome in NZB/W-F1 mice. Therefore, whether or not cystamine treatment attenuates apoptosis of LV tissues was investigated. As shown in Figure 2A, number of apoptotic cells (TUNEL-stained cells) in LV tissues of NZB/W-F1 mice with PBS treatment was higher than that in LV tissues of Balb/c mice. In addition, cystamine treatment reduced the number of apoptotic cells in LV tissues of NZB/W-F1 mice compared with that of animals treated with PBS. Quantitative analysis revealed that percentage of apoptotic cells of LV tissues in PBS-treated NZB/W-F1 mice was 53.6 ± 13.3%, which was lowered to 22.6 ± 7.4% in cystamine-treated mice (P < 0.05) (Fig. 2B). Moreover, apoptotic phenomena were not detected in LV tissues of Balb/c mice treated with cystamine or PBS (Fig. 2B).
Fig 2.
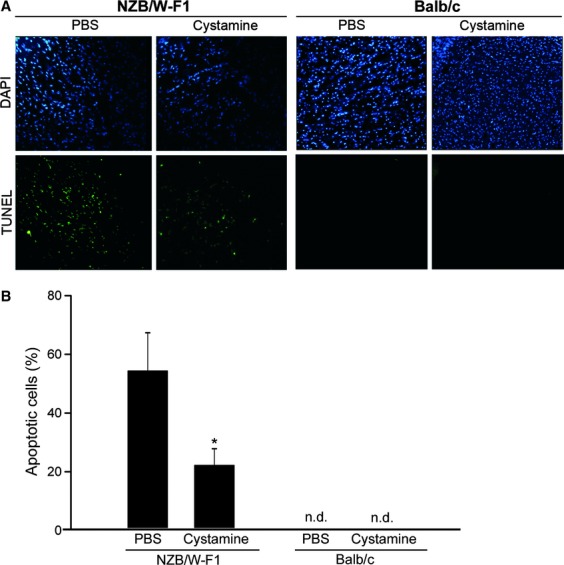
Effects of cystamine treatment on lupus-associated cardiac cell apoptosis in NZB/W-F1 mice. (A) LV samples were obtained from NZB/W-F1 mice and Balb/c mice, which were treated with PBS or cystamine as described in Materials and methods, reacted with TUNEL reagents and then observed using fluorescence microscopy (magnification 200×). (B) Quantitative analysis for cell apoptosis was presented as percentage of TUNEL-stained cells in total DAPI-stained cells. Data were obtained from five random observe fields at magnification 200× (total cells >300) for each sample and three independent experiments were performed for statistic analysis. *P < 0.05 as compared to PBS control. n.d.: not detected.
Decrease of death receptor Fas and inhibition of caspase-8 activation in LV tissues by cystamine treatment
To investigate the anti-apoptotic mechanisms of cystamine, the level of death receptor Fas in LV tissues was examined. As shown in Figure 3, cystamine treatment diminished the level of Fas and resulted in a significant reduction of Fas/α-TN ratio from 0.39 ± 0.06 to 0.31 ± 0.02 (P < 0.005) in LV tissues from NZB/W-F1 mice compared to that of PBS group. Interestingly, reduction of the Fas/α-TN ratio in LV tissues of Balb/c mice compared with that of PBS-treated animals was found in response to cystamine treatment (P = 0.342).
Fig 3.
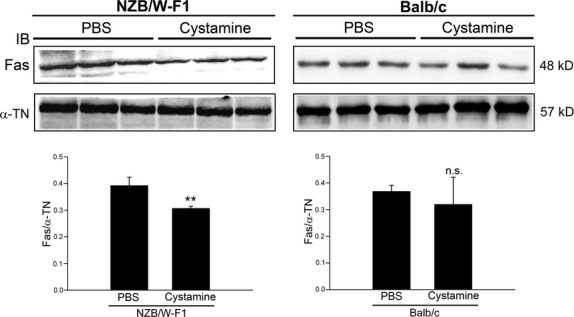
Effects of cystamine treatment on expression level of Fas in LV tissues of NZB/W-F1 mice. LV samples were obtained from NZB/W-F1 mice and Balb/c mice, which were treated with PBS or cystamine as described in Materials and methods. Crude proteins were extracted from the LV samples for immunodetection of Fas using specific antibody. Quantitative data were obtained by densitometric analysis and presented as ratio of Fas/α-tubulin (α-TN). **P < 0.005 as compared to PBS control. n.s.: not significant.
Given decreased Fas level in LV tissues by cystamine treatment, we investigated the activation of caspase-8 that is its downstream apoptotic effecter. As shown in Figure 4A, levels of active forms of caspase-8 (40 and 23 kD) were higher in LV tissues of NZB/W-F1 mice than those of Balb/c mice (PBS treatment). Cystamine treatment resulted in significant reduction of 40 kD-active form/α-TN ratio from 0.36 ± 0.02 to 0.28 ± 0.03 in LV tissues of NZB/W-F1 (P < 0.05) compared with that of PBS-treated ones, whereas change in level of 55 kD-precursor form and 23 kD-active form was not significant (P = 0.325 and P = 0.127) (Fig. 4B). On the contrary, cystamine treatment slightly affected the levels of three forms of caspase-8 in LV tissues of Balb/c mice (Fig. 4A).
Fig 4.
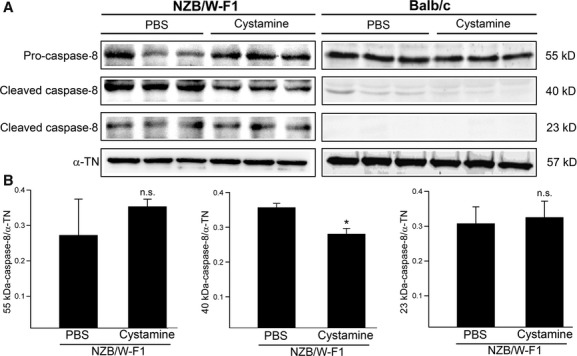
Effects of cystamine treatment on activation of caspase-8 in LV tissues of NZB/W-F1 mice. LV samples were obtained from NZB/W-F1 mice and Balb/c mice, which were treated with PBS or cystamine as described in Materials and methods. (A) Immunoblot for level of caspase-8 (55 kD-precursor form and 40 and 23 kD-cleaved form as active form). (B) Quantitative data for caspase-8 (55, 40 or 23 kD) from NZB/W-F1 mice were obtained by densitometric analysis and presented as ratio of the tested protein/α-tubulin (α-TN). *P < 0.05 as compared to PBS control. n.s.: not significant.
Effects of cystamine treatment on proapoptotic proteins in LV tissues
Proapoptotic proteins involved in intrinsic apoptosis pathway including Bcl-2, Bad and tBid were investigated. As shown in Figure 5A, cystamine treatment increased levels of Bcl-2 and phosphorylated Bad (p-Bad) parallel to decreases in levels of Bad and tBid. Quantitative analysis revealed that ratios of Bcl-2/α-TN and p-Bad/α-TN were increased from 0.17 ± 0.02 to 0.39 ± 0.05 (P < 0.05) and from 0.42 ± 0.02 to 0.52 ± 0.54 (P < 0.05), respectively, in LV tissues of NZB/W-F1 treated with cystamine compared with those of PBS-treated ones (Fig. 5B). Significantly decreased ratios of Bad/α-TN (0.42 ± 0.05 to 0.23 ± 0.04; P < 0.005) and tBid/α-TN (0.46 ± 0.06 to 0.23 ± 0.11; P < 0.05) were discovered in LV tissues of NZB/W-F1 mice in comparison with PBS–treated group (Fig. 5B). In addition, slight effect of cystamine on levels of tested proapoptotic proteins in LV tissues of Balb/c mice was discovered (Fig. 5A).
Fig 5.
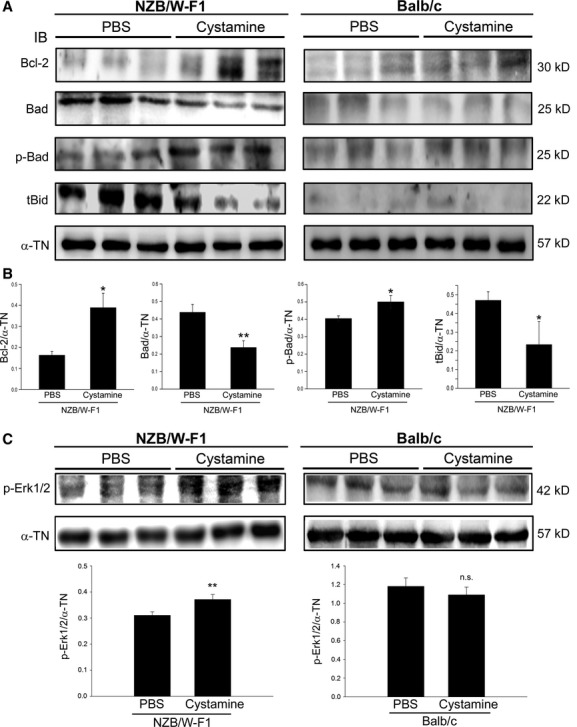
Effects of cystamine treatment on expression level of pro-apoptotic proteins and survival signal. LV samples were obtained from NZB/W-F1 mice and Balb/c mice, which were treated with PBS or cystamine as described in Materials and methods. (A) Immunoblot for expression level of Bcl-2, Bad, phosphorylated Bad (p-Bad) and truncated Bid (tBid). (B) Quantitative data for tested targets from NZB/W-F1 mice were obtained by densitometric analysis and presented as ratio of the tested protein/α-tubulin (α-TN). (C) Immunoblot for level of phosphorylation of Erk1/2 (p-Erk1/2), quantitative data were obtained by densitometric analysis and presented as ratio of p-Erk1/2/α-TN. *P < 0.05 as compared to PBS control. n.s.: not significant.
Effect of cystamine on survival signal such as extracellular signal-regulated kinase 1/2 (Erk1/2) was also investigated. As shown in Figure 5C, cystamine treatment enhanced phosphorylation of Erk1/2 (p-Erk1/2) in LV tissues of NZB/W-F1 mice as compared to that in PBS treatment. Quantitative analysis revealed that cystamine treatment increased ratio of p-Erk1/2/α-TN from 0.31 ± 0.03 to 0.35 ± 0.0.02 (P < 0.05) in LV tissues of NZB/W-F1 mice as compared to that in PBS treatment. Moreover, phosphorylation of Erk1/2 in LV tissues of Balb/c mice was slightly affected by cystamine treatment (Fig. 5C).
Suppression of mitochondrial apoptotic signals by cystamine treatment
Since proapoptotic Bad and tBid involved in mitochondrial apoptotic signals were found decreased in LV tissues of NZB/W-F1 mice, effects of cystamine on mitochondrial apoptotic signals were further investigated. Cytosolic cytochrome c and Apaf-1 in LV tissues of NZB/W-F1 mice were higher than those in Balb/c mice (Fig. 6A). Cystamine treatment significantly diminished cytosolic cytochrome c and Apaf-1 in LV tissues of NZB/W-F1 mice compared with those of PBS-treated ones. Moreover, cystamine treatment also resulted in decrease in activated form of caspase-9 (35 kD) and caspase-3 (12 kD) in LV tissues of NZB/W-F1 mice. Ratios of cytochrome c/α-TN, Apaf-1/α-TN, active caspase-9/α-TN and active caspase-3/α-TN were decreased from 0.35 ± 0.02 to 0.25 ± 0.02 (P < 0.005), from 0.32 ± 0.03 to 0.11 ± 0.01 (P < 0.005), from 0.23 ± 0.04 to 0.10 ± 0.01 (P < 0.005) and from 0.55 ± 0.09 to 0.21 ± 0.03 (P < 0.05), respectively, in LV tissues of NZB/W-F1 mice compared with PBS-treated animals (Fig. 6B). In addition, cystamine treatment slightly affected the levels of these mitochondrial apoptotic proteins in LV tissues of Balb/c mice (Fig. 6A).
Fig 6.
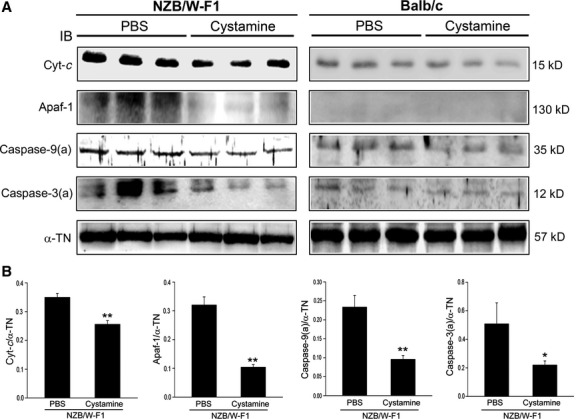
Effects of cystamine treatment on mitochondrial apoptotic signals. LV samples were obtained from NZB/W-F1 mice and Balb/c mice, which were treated with PBS or cystamine as described in Materials and methods. (A) Immunoblot for level of cytochrome c (Cyt-c), Apaf-1, active caspase-9 and active caspase-3 using specific antibodies. (B) Quantitative data for tested targets from NZB/W-F1 mice were obtained by densitometric analysis and presented as a ratio of tested target/α-tubulin (α-TN). *P < 0.05 and **P < 0.005 as compared to PBS controls.
Discussion
Autoantibody-induced apoptosis has been considered to be involved in tissue damage in patients with autoimmune disorders. Consistent with previous studies, our results showed that apoptosis of LV tissues was greatly evoked in lupus-prone NZB/W-F1 mice compared with normal Balb/c mice. It suggests that attenuation of apoptosis should ameliorate cardiac injury induced by autoantibodies in autoimmune diseases such as SLE.
Two well-known pathways, extrinsic and intrinsic pathways, are responsible for triggering apoptosis [21]. In the case of the intrinsic pathway, a release of cytochrome c from mitochondria results in binding to Apaf-1 and subsequently leading to activation of procaspase-9 and following caspase-3 [22]. Activated caspase-3 exerts as the key executioner of apoptosis, which induces the cleavage and inactivation of key cellular protein [22, 23]. In the present study, we demonstrated that cystamine treatment decreased the level of cytosolic Cyt-c and Apaf-1 and inhibited activation of caspase-9 and caspase-3. Extrinsic pathway-induced activation of caspase-3 through caspase-8 is also known as a major cause to trigger apoptosis [24]. In addition, Fas/Fas ligand system triggering extrinsic apoptosis has been demonstrated for its important role in pathogenesis of autoimmune myocarditis in rats [25]. Our previous study demonstrated that high-cholesterol diet elevated both Fas ligand and Fas expressions in the LV tissues of NZB/W-F1 mice, and the increased levels of Fas ligand and Fas was diminished upon cystamine treatment [26]. Supportively, the findings of the present study further showed that cystamine treatment simultaneously suppressed Fas expression and activation of caspase-8 in LV tissue of NZB/W-F1 mice. Taken together, these findings indicate that both inhibited intrinsic and extrinsic pathways are responses to exposure to cystamine in LV tissues as a result of apoptosis.
The Bcl-2 family proteins act as double-edged sword in apoptosis regulation. Interaction of Bcl-2 with other molecules constituting BH3-domain has been demonstrated as an important process in the regulation of apoptosis [27]. Bad and Bid, belonging to BH3-only protein family, are proapoptotic molecules and their proapoptotic activity is believed to be mediated via interaction with Bcl-2 family proteins. BH3-only proteins are categorized into two groups by their biological functions. Proteins in the first group including Bad and Bim selectively inactivate anti-apoptotic molecules through binding activity. Bad is phosphorylated by survival signals, bound to 14-3-3 scaffold proteins and localized in the cytoplasm in an inactive form [28]. Once survival signals are abrogated, Bad is dephosphorylated, dissociated from 14-3-3 and localized to mitochondria. Free, mitochondrial Bad molecules then interact with either Bcl-2 or Bcl-XL and neutralize their anti-apoptotic functions. Bid, belonging to the second group of BH3-only proteins, can bind to both anti-apoptotic and pro-apoptotic Bcl-2 family members. Bid is localized in the cytoplasm in non-apoptotic cells. Activated caspase-8 cleaves 22-kD Bid to generate a 15-kD-active tBid fragment which is then targeted to the mitochondria where it binds to the pro-apoptotic Bcl-2 family members Bax or Bak as well as to the anti-apoptotic Bcl-2 [29, 30]. Formation of tBid-Bax or tBid-Bak oligomers evokes apoptotic signals. Importantly, neutralization of anti-apoptotic Bcl-2 family members is believed to play only a marginal role in induction of apoptosis by these proteins. Our findings showed that cystamine treatment enhanced survival Erk1/2 signal, reduced Fas expression, elevated anti-apoptotic Bcl-2 and phosphorylation of Bad and diminished levels of BAD and tBid, leading to inhibited activation of caspase-8, caspase-9 and caspase-3 activation and the consequent apoptosis (Fig. 7).
Fig 7.
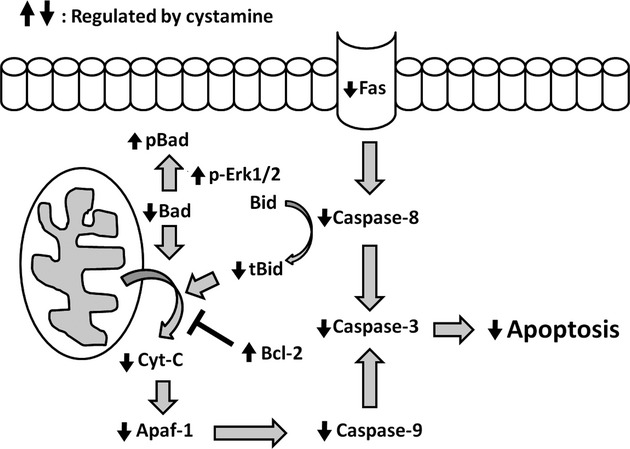
Schematic representation of mechanism underlying the anti-apoptotic effects of cystamine on the lupus-associated apoptosis of LV tissues. Based on present data, cystamine attenuates the lupus-associated apoptosis of LV myocardiac cells, which may be due to the down-regulation of Fas, reduction of tBid, cytochrome c (Cyt-c) and Apaf-1, and the subsequent inhibition of caspase cascades, as well as the enhancement of Erk1/2 survival signalling and the increase of anti-apoptotic Bcl-2.
In conclusion, the present study provides evidence that cystamine treatment significantly suppresses lupus-associated apoptosis of LV tissues, which may attribute to augment of survival Erk1/2 signal as well as inhibition of both extrinsic and intrinsic apoptosis signals. These findings indicate that cystamine may protect LV tissues from lupus-induced apoptosis and represents a potential therapeutic for CVD in SLE patients.
Acknowledgments
This work was partially supported by the National Science Council grants NSC 99-2320-B-040-003-MY3, NSC 99-2632-B-040-001-MY3(5) and NSC99-2320-B-040-007-MY3 and by the National Health Research Institutes grant DOH98-TD-I-111-TM010.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Ruiz-Irastorza G, Khamashta MA, Castellino G, et al. Systemic lupus erythematosus. Lancet. 2001;357:1027–32. doi: 10.1016/S0140-6736(00)04239-2. [DOI] [PubMed] [Google Scholar]
- 2.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–15. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 3.Manzi S, Wasko MC. Inflammation-mediated rheumatic diseases and atherosclerosis. Ann Rheum Dis. 2000;59:321–5. doi: 10.1136/ard.59.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petri M, Perez-Gutthann S, Spence D, et al. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med. 1992;93:513–9. doi: 10.1016/0002-9343(92)90578-y. [DOI] [PubMed] [Google Scholar]
- 5.Bjornadal L, Yin L, Granath F, et al. Cardiovascular disease a hazard despite improved prognosis in patients with systemic lupus erythematosus: results from a Swedish population based study 1964-95. J Rheumatol. 2004;31:713–9. [PubMed] [Google Scholar]
- 6.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–7. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Che J, Li G, Wang W, et al. Serum autoantibodies against human oxidized low-density lipoproteins are inversely associated with severity of coronary stenotic lesions calculated by Gensini score. Cardiol J. 2011;18:364–70. [PubMed] [Google Scholar]
- 8.Lee HC, Huang KT, Wang XL, et al. Autoantibodies and cardiac arrhythmias. Heart Rhythm. 2011;8:1788–95. doi: 10.1016/j.hrthm.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laczik R, Szodoray P, Veres K, et al. Assessment of IgG antibodies to oxidized LDL in patients with acute coronary syndrome. Lupus. 2011;20:730–5. doi: 10.1177/0961203311398884. [DOI] [PubMed] [Google Scholar]
- 10.Wilson WA, Gharavi AE, Koike T, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999;42:1309–11. doi: 10.1002/1529-0131(199907)42:7<1309::AID-ANR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 11.Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun. 2000;15:145–51. doi: 10.1006/jaut.2000.0409. [DOI] [PubMed] [Google Scholar]
- 12.Jahns R, Boivin V, Schwarzbach V, et al. Pathological autoantibodies in cardiomyopathy. Autoimmunity. 2008;41:454–61. doi: 10.1080/08916930802031603. [DOI] [PubMed] [Google Scholar]
- 13.Zhao P, Sharma AC, Ren J. Pathogenesis and therapy of autoimmunity-induced dilated cardiomyopathy. Front Biosci. 2009;14:1708–15. doi: 10.2741/3334. [DOI] [PubMed] [Google Scholar]
- 14.Chu F, Koomen JM, Kobayashi R, et al. Identification of an inactivating cysteine switch in protein kinase Cepsilon, a rational target for the design of protein kinase Cepsilon-inhibitory cancer therapeutics. Cancer Res. 2005;65:10478–85. doi: 10.1158/0008-5472.CAN-05-1989. [DOI] [PubMed] [Google Scholar]
- 15.Seelig GF, Meister A. Gamma-glutamylcysteine synthetase. Interactions of an essential sulfhydryl group. J Biol Chem. 1984;259:3534–8. [PubMed] [Google Scholar]
- 16.O'Brian CA, Chu F. Post-translational disulfide modifications in cell signaling – role of inter-protein, intra-protein, S-glutathionyl, and S-cysteaminyl disulfide modifications in signal transmission. Free Radic Res. 2005;39:471–80. doi: 10.1080/10715760500073931. [DOI] [PubMed] [Google Scholar]
- 17.Hsu TC, Chiang SY, Huang CY, et al. Beneficial effects of treatment with transglutaminase inhibitor cystamine on macrophage response in NZB/W F1 mice. Exp Biol Med. 2007;232:195–203. [PubMed] [Google Scholar]
- 18.Karpuj MV, Becher MW, Springer JE, et al. Prolonged survival and decreased abnormal movements in transgenic model of Huntington disease, with administration of the transglutaminase inhibitor cystamine. Nat Med. 2002;8:143–9. doi: 10.1038/nm0202-143. [DOI] [PubMed] [Google Scholar]
- 19.Denman AM, Denman EJ, Holborow EJ. Suppression of Coombs-positive haemolytic anaemia in NZB mice by antilymphocyte globulin. Lancet. 1967;1:1084–6. doi: 10.1016/s0140-6736(67)92653-0. [DOI] [PubMed] [Google Scholar]
- 20.Holborow EJ, Denman AM. Autoimmune disease in inbred mice. Clin Exp Immunol. 1967;(2 Suppl):761–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Broker LE, Kruyt FA, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res. 2005;11:3155–62. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- 22.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–6. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 23.Wolf BB, Green DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999;274:20049–52. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- 24.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishiyama S, Hiroe M, Nishikawa T, et al. The Fas/Fas ligand system is involved in the pathogenesis of autoimmune myocarditis in rats. J Immunol. 1998;161:4695–701. [PubMed] [Google Scholar]
- 26.Huang CY, Hsu TC, Kuo WW, et al. Beneficial effects of taurine on cardiac abnormality in NZB/W F1 mice fed with a high-cholesterol diet. J Agric Food Chem. 2009;57:8635–42. doi: 10.1021/jf9020625. [DOI] [PubMed] [Google Scholar]
- 27.Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8:324–30. doi: 10.1016/s0962-8924(98)01321-x. [DOI] [PubMed] [Google Scholar]
- 28.Yang E, Zha J, Jockel J, et al. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–91. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Zhu H, Xu CJ, et al. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 30.Wei MC, Lindsten T, Mootha VK, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–71. [PMC free article] [PubMed] [Google Scholar]


