Abstract
The Neurofibromatosis 2 (NF2) gene product merlin is a tumour suppressor, which in addition to inhibiting cell proliferation regulates cell morphology. The morphogenic properties of merlin may play a role in tumour suppression, as patient-derived tumour cells demonstrate cytoskeletal abnormalities. However, it is still unclear how these functions are linked. The N-terminal FERM-domain of merlin is highly homologous to the oncogenic protein ezrin, while the C-termini are less conserved, suggesting that the opposite effect of the proteins on proliferation could be mediated by their distinct C-terminal regions. In this study we characterize the role of the most C-terminal residues of merlin in the regulation of proliferation, cytoskeletal organization, phosphorylation and intramolecular associations. In addition to the two full-length merlin isoforms and truncating mutations found in patients, we focused on the evolutionally conserved C-terminal residues 545-547, also harbouring disease-causing mutations. We demonstrate that merlin induces cell extensions, which result from impaired retraction of protrusions rather than from increased formation of filopodia. The residues 538-568 were found particularly important for this morphogenic activity. The results further show that both merlin isoforms are able to equally inhibit proliferation, whereas C-terminal mutants affecting residues 545-547 are less effective in growth suppression. This study demonstrates that the C-terminus contains distinct but overlapping functional domains important for regulation of the morphogenic activity, intramolecular associations and cell proliferation.
Keywords: NF2, merlin, ezrin
Introduction
Neurofibromatosis 2 (NF2) is a dominantly inherited tumour suppressor syndrome manifested by tumours of the nervous system, mainly schwannomas and meningiomas [1]. The disease develops as a result of inactivation of the NF2 gene, which codes for the tumour suppressor merlin. Merlin not only links membrane proteins to the cytoskeleton, but also has functions in the nucleus [2–4]. In spite of several models, the growth inhibitory mechanism of merlin is still not completely elucidated.
Alternative splicing of the NF2 gene exon 16 gives rise to the two major isoforms of merlin. The isoforms 1 and 2 are identical over their first 579 residues and differ only in their very C-terminal sequence [5]. The two isoforms are expressed at roughly equivalent ratio, but isoform 2 is slightly more prevalent [6]. However, isoform 2 has a low expression level in the eighth cranial nerve [5], where the NF2-related schwannomas typically occur. The functional difference of the isoforms is not known, but it has been suggested that only isoform 1 possesses tumour suppressor activity [7].
Merlin is related to the ERM (ezrin–radixin–moesin) protein family. All family members contain an N-terminal FERM (band four point-one, ezrin, radixin, moesin)-domain (residues 19-314 in merlin) followed by an α-helical coiled-coil region (314-492) and a globular C-terminus (492-595). Whereas the N-termini of ERM proteins are highly homologous, the α-helix and C-terminal regions are less conserved; the homology between the C-terminus of merlin and ezrin is only 22% [8]. The ERM family members share some functional properties as well as binding partners with merlin including the heterodimerization capacity [9, 10]. Merlin is, however, the only ERM family member known to contain growth inhibitory properties, whereas ezrin has been linked to oncogenic activities in vitro and in vivo [11–13]. It is still unclear how these two structurally very similar proteins mediate such opposite functions.
Merlin and the ERM proteins are able to form intramolecular interactions by N- to C-terminal binding [7, 10, 14–17]. This intramolecular folding, suggested to control the activity of merlin, is regulated by phosphorylation of serine 518 (S518) catalysed by p21-activated kinase or protein kinase A [18–20]. The unfolded S518 phosphorylated merlin is incapable of inhibiting cell proliferation, whereas the closed dephosphorylated form of the molecule is presumably the active tumour suppressive form [21, 22]. In addition to S518, three other merlin phosphorylation sites; serine 10, threonine 230 and serine 315, have been described so far [23–25].
Although several reports have demonstrated the link between merlin and intracellular signalling pathways, little information is available on the functional domains of merlin, especially of the C-terminal part of the protein. As merlin C-terminus is most divergent from the ERMs, the functional difference between merlin and ezrin could partly be regulated by their distinct C-terminal regions. In this study we have dissected the functional regions of merlin C-terminus in the prevalent isoforms and defined their role in morphogenic activity and growth inhibition.
Materials and methods
Plasmids and samples
For expression of recombinant GST-fusion proteins, merlin fragments 1-314, 314-477, 492-595 and full-length ezrin in pGEX4T1 vector (Amersham Biosciences, Uppsala, Sweden) were used. Human merlin isoform I (WT, amino acids 1-595) and isoform 2 (amino acids 1-590) in pcDNA3 vector (Invitrogen, Carlsbad, CA, USA) were used for transfection experiments of full-length proteins. C-terminally truncated constructs 1-587, 1-569, 1-547, 1-537 and 1-518 were created from merlin isoform 1 by digestion with restriction endonucleases and ligation into the pCDNA3 vector. The point mutations S518A, E547K, E545K + E547K, T581A, S584A and S587A of merlin were made by site-directed mutagenesis performed with the QuikChange Kit (Stratagene, La Jolla, CA, USA). The Δ2 merlin construct containing a deletion of residues 50-70 in exon 2 [26] and GFP-α-actinin used in live cell imaging [27] have been described before. GFP in pCDNA3 vector was used as control in the proliferation assay and the c-Ha-Ras C61L construct (Addgene plasmid 13485) used in soft agar experiments has been previously described [28]. The frozen tumour material was obtained from the Neurofibromatosis Clinic of Massachusetts General Hospital, and contains a 1646 delT mutation in the NF2 gene and LOH compared to the matching blood sample [29]. The tumour was resolved in reducing Laemmli sample buffer, sonicated and analysed by SDS-PAGE and western blotting.
Cells and antibodies
293 human embryonic kidney (HEK) cells were maintained in RPMI 1640 medium supplemented with 10% foetal bovine serum (FBS) (PromoCell, Heidelberg, Germany) and antibiotics. COS-7 cells, mouse embryonic fibroblasts (MEFs), Nf2−/− MEFs (obtained from Dr. Giovannini) and ezrin−/− MEFs (obtained from Dr. McClatchey) were all grown in DMEM (Gibco, Invitrogen, Carlsbad, CA, USA) with 10% FBS. Primary Nf2 knock-out Schwann cells [30] were isolated and cultured as previously described [31] and used before passage 20.
Anti-merlin A-19 sc-331 pAb (epitope 2-21), C-18 sc-332 pAb (both from Santa Cruz Biotechnology, Santa Cruz, CA, USA), HB7 mAb (epitope 192-209) [32], and KF10 mAb (epitope 561-578) [33] were used to detect merlin. S518 phosphorylated merlin was detected with pS518 pAb (Biodesign, Saco, ME, USA). 3C12 mAb [34] was used for ezrin detection, Alexa-568 conjugated phalloidin (Molecular Probes, Invitrogen, Carlsbad, CA, USA) to stain F-actin, α-tubulin mAb (Sigma-Aldrich, St. Louis, MO, USA) to detect tubulin and anti-GST pAb (GE Healthcare, Uppsala, Sweden) to detect GST-proteins. Ki-67 Ab sc-7846 (Santa Cruz Biotechnology) was used as a marker of cell proliferation. The nuclei were stained with TO-PRO 3-iodide probe (Molecular Probes). Alexa-488- and Alexa-594-conjugated goat antimouse and goat anti-rabbit antibodies (Molecular Probes) were used as secondary antibodies in immunofluorescence and HRP-conjugated rabbit antimouse, swine anti-rabbit (Dako A/S, Glostrup, Denmark), and swine anti-goat (Santa Cruz Biotechnology) secondary antibodies in western blot analysis.
Transfection, immunofluorescence and immunoblotting
Cells were transiently transfected using FuGENE6 reagent (Hoffmann-La Roche, Basel, Switzerland) or Lipofectamine 2000 (Invitrogen) for Schwann cells and incubated for 48 hrs before analysis. For immunofluorescence, cells grown on glass coverslips were fixed in 3.5% paraformaldehyde (pH 7.5), and detected with appropriate antibodies using standard protocols. For western blotting, cells were lysed in ELB buffer (50 mM HEPES pH 7.4, 150 mM NaCl, 5 mM EDTA) containing 0.5% Nonidet P-40 (NP-40), HALT phosphatase inhibitors (Pierce, Rockford, IL, USA), and Complete protease inhibitors (Roche). Lysed cells were centrifuged at 13,000 × g for 20 min at +4°C and the pellet and supernatant were resolved in equal amounts of reducing Laemmli buffer. Whole cell lysates were prepared by adding reducing Laemmli sample buffer on the plates. Samples were resolved in SDS-PAGE (8% for electrophoretic mobility assays), transferred to nitrocellulose filters, and analysed by immunoblotting with appropriate antibodies using enhanced chemiluminescence detection (Amersham Biosciences).
Quantification of extensions
Transfected Nf2−/− and ezrin−/− MEFs were stained with merlin A-19 Ab and untransfected cells with ezrin 3C12 Ab for Nf2−/− MEFs or phalloidin for ezrin−/− MEFs. Cells were imaged with immunofluorescence microscopy (Zeiss Axiophot equipped with Axiocam cooled CCD camera, Carl Zeiss, Esselingen, Germany). The extension length was measured from twenty untransfected cells and cells expressing the different constructs. The total length of all extensions from each cell was calculated and the mean length per cell is shown in graph. Student's t-test was used for calculation of P values and in figures mean ± S.D. is given.
Live cell imaging
The Nf2−/− MEFs, grown on LabTek glass chamber slides (Nunc, Naperville, IL, USA), were co-transfected with GFP-α-actinin and merlin isoform 2 24–48 hrs prior to imaging. Live cells expressing merlin were chosen by their GFP expression and imaged with an inverted Olympus IX81 microscope and CellR programme at +37°C, 5% CO2. Images were taken once every 30 sec. for 5 hrs. GFP-expression images were taken before and after the experiment, and the cells were stained for merlin to verify expression.
Affinity pull-down
Transfected COS-7 cells were lysed in cold binding buffer (50 mM HEPES pH 7.4, 150 mM NaCl, protease inhibitors) containing 0.5% Triton X-100 and Complete protease inhibitors, and the homogenates were cleared by centrifugation at +4°C for 20 min at 13,000 × g. GST-merlin and ezrin fusion proteins were bacterially expressed and purified. Glutathione Sepharose beads carrying 4 μg of proteins were washed in binding buffer and incubated with the lysates under rotation over night at +4°C. Beads were washed with binding buffer, reducing Laemmli buffer was added, and bound proteins were separated by SDS-PAGE. Proteins were detected with merlin, ezrin and GST antibodies.
Proliferation assay
The Nf2−/− MEFs transfected with merlin or GFP as a control were first serum starved followed by serum treatment for 12 hrs. Cells were stained for merlin and the proliferation marker Ki-67. Ki-67 positivity from 400 cells of each construct from four experiments was quantified. Student's t-test was used for calculation of P values and in graph mean ± S.D. is given.
Soft agar colony formation assay
The Nf2−/− MEFs were transfected with c-Ha-Ras C61L CMV together with merlin constructs or empty pcDNA3 vector. Forty-eight hours after transfection 5 × 104 cells were plated in triplicates in 0.3% agar containing DMEM and 10% FBS on a layer of precast 0.6% agar. After 2–3 weeks of growing in soft agar cells were stained by 0.005% Crystal Violet for 2–3 hrs. Plates were scanned and the number of the colonies on each plate was determined by counting. The experiment was repeated three times.
Results
Characterization of C-terminally truncated merlin proteins
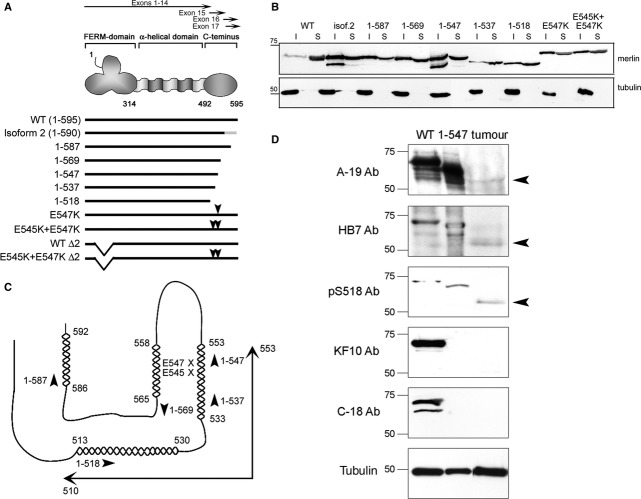
To characterize functionally important regions in the C-terminal part of merlin, several merlin constructs were created (Fig. 1A). Merlin wild-type isoform 1 (WT), produced from exons 1-15 and 17, is a 595 amino acid protein. In isoform 2 exon 16 codes for 11 unique residues followed by a termination codon, which results in a 590 amino acid protein (Fig. 1A) [5, 35, 36]. Shorter truncation constructs of merlin isoform 1, producing proteins that terminate between residues 518-587 (representing exons 14, 15 and 17), were also created to elucidate the importance of the C-terminus. Several of the used truncating mutants have been detected in NF2 patient-derived tumours [37, 38]. The motif of residues E545-I546-E547 in merlin is evolutionally conserved in many species [39] and contains one of the most C-terminal missense mutations reported so far, E547K [40], highlighting the importance of this region for merlin function. Therefore, the point mutation E547K, and a double mutant of both glutamic acids 545 and 547 (E545K + E547K), disrupting the whole conserved domain, were generated. In addition, deletion constructs disrupting exon 2 and lacking the cytoplasmic retention signal [2], were used to analyse the effect of merlin C-terminus on nuclear shuttling.
Fig 1.
Characterization of the merlin C-terminal constructs. (A) Schematic overview of merlin's exon composition and structural domains (upper figure) and the constructs in this study (lower figure). Merlin wild-type (WT) isoform 1, isoform 2, several C-terminally truncated molecules of isoform 1, constructs with C-terminal point mutations (arrowheads) and deletion of residues 50-70 in exon 2 (Δ2) were used. The unique C-terminus of isoform 2 is indicated in grey, and numbers represent amino acids. (B) Lysates from COS-7 cells transfected with merlin constructs were separated into insoluble (I) and soluble (S) fractions and detected with the N-terminal A-19 merlin antibody. Merlin WT is mainly soluble whereas isoform 2 and the truncated constructs are distributed to both the insoluble and soluble fraction. All merlin constructs migrate at expected molecular size. (C) Schematic picture of merlin C-terminus based on moesin structure and merlin predictions (combined from [39, 41]). Alpha-helices are indicated with helical structures, and the predicted conserved α-helical region is shown by arrows. Arrowheads point out the termination of the truncations and the missense mutations are marked with X. (D) Expression of a truncated merlin protein corresponding to residues 1-548 in a patient-derived tumour with a mutation in the NF2 gene. The tumour material was run in SDS-PAGE together with COS-7 cell lysates expressing WT and 1-547 merlin as controls, and detected with various merlin antibodies. The N-terminal antibodies A-19 and HB7 and the phospho-serine 518 antibody (p-S518 Ab) detect merlin in the tumour (arrowheads), whereas C-terminal merlin antibodies KF10 and C-18 do not. Tubulin is used as a loading control.
To confirm the expression of these merlin proteins and to analyse their solubility, COS-7 cells transfected with the various constructs were lysed in a buffer containing neutral detergent and separated into soluble and insoluble fractions. All merlin constructs migrated at expected molecular sizes and distributed to both fractions of the lysates (Fig. 1B). However, although merlin WT (isoform 1) was detected mainly in the soluble fraction, isoform 2 was clearly more insoluble, suggesting a stronger cytoskeletal association. A smaller N-terminal immunoreactive band was detected in the insoluble fraction of both isoform 2 and merlin 1-547, indicating that these constructs could be more susceptible to degradation.
Even though the C-terminal domain of merlin is unique compared to the ERMs, the critical residues involved in the binding interface between the FERM-domain and the C-terminus are conserved [41]. Therefore, moesin was used as a predictive model for the conformation of merlin C-terminus, which has not been determined. The moesin structure [41] and the predicted organization of merlin [39] were combined for a schematic model of the merlin C-terminal domain and the constructs used in this study, and is shown in Figure 1C. The C-terminal tail of moesin consists of four major helices which form hydrophobic contacts with the F2- and F3-subdomains covering an extensive area of the FERM-domain surface [41]. The C-terminus of merlin is predicted to contain also an α-helical region between amino acids 513-553, which is highly conserved among merlin species [39]. The missense mutations E547K and E545K + E547K and the truncated constructs 1-537 and 1-547 most likely disrupt this α-helix (Fig. 1C).
Previous work has shown that most mutant merlin variants are undetectable in cells, as the transcripts are unstable and the translated polypeptides susceptible to proteolytic degradation [42, 43]. However, C-terminal truncations may be an exception [44]. We therefore tested, whether truncated forms of merlin relevant for this study can be detected in a tumour from an NF2 patient with a germline deletion of nucleotide T1646 (in residue L549), predicted to result in a premature stop codon and translation of residues 1-548. The presence of merlin was analysed by dissolving the sample, separating proteins in SDS-PAGE, and detection of merlin with several N- and C-terminal merlin antibodies. As shown in Figure 1D, a truncated merlin product of the predicted ∼60 kD size is present in the tumour as detected by the N-terminal antibodies A-19 and HB7 and the antibody against phosphorylated S518. The product is not recognized by the C-terminal antibodies KF10 and C-18. Thus, C-terminally truncated proteins can be expressed in human tumours.
C-terminally deleted merlin variants induce prominent cell extensions
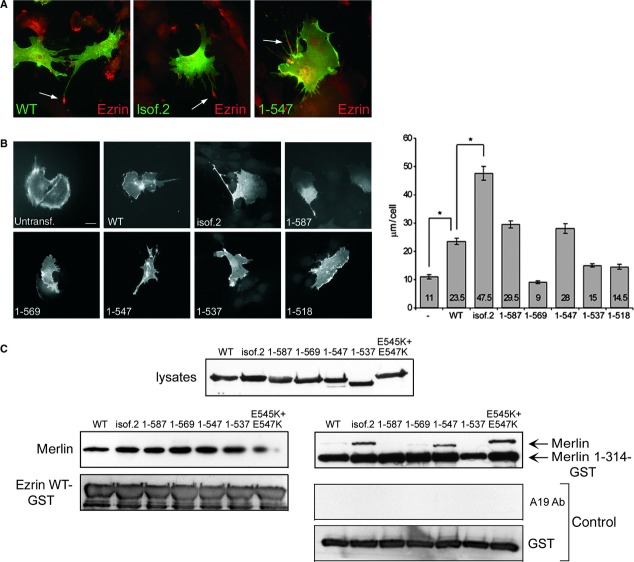
Overexpression of merlin is known to induce formation of cell extensions, membrane ruffles and filopodia [45, 46]. To analyse the effect of merlin C-terminus on cell morphology, full-length isoforms and truncated variants were transfected into COS-7 cells and mouse embryonic fibroblasts lacking merlin (Nf2−/− MEFs). As previously shown for endogenous merlin [45, 47], both full-length merlin isoforms localized underneath the plasma membrane. All truncated variants showed an identical subcellular distribution and were also present in protrusions in both cell types (Fig. 2A and B). The amount and length of the extensions and the total extension length per cell were quantified from Nf2−/− MEFs (Fig. 2B, right panel). Expression of all merlin constructs affected cell morphology by increasing the amount of membrane protrusions compared to untransfected cells. Both full-length isoforms induced the formation of cell extensions, but the extensions were more abundant and longer in isoform 2-expressing cells. Interestingly, the effect of isoform 2 on cell extensions was phenocopied by some of the truncation variants (Table 1). The removal of the last eight C-terminal amino acids from merlin isoform 1, construct 1-587, generated long cell protrusions similarly to isoform 2. An additional deletion of 18 residues changed this phenotype; cells expressing merlin 1-569 had fewer extensions and only rarely induced longer cellular projections. A distinct difference was observed between merlin 1-547 and the other truncated constructs, as expression of this truncation induced the most dramatic phenotype with long, thin cellular processes. Expression of constructs 1-537 and 1-518 resulted in phenotypes with short extensions that differed markedly from merlin 1-547, but resembled that of merlin 1-569. Interestingly, only merlin isoform 2, 1-587 and 1-547 induced the formation of extremely long cell protrusions over 50 μm in length, which were not observed in other merlin expressing cells. The results were similar in both COS-7 cells and Nf2−/− MEFs, as well as in 239 HEK cells (data not shown), and were consistent for multiple independent transfection experiments.
Fig 2.
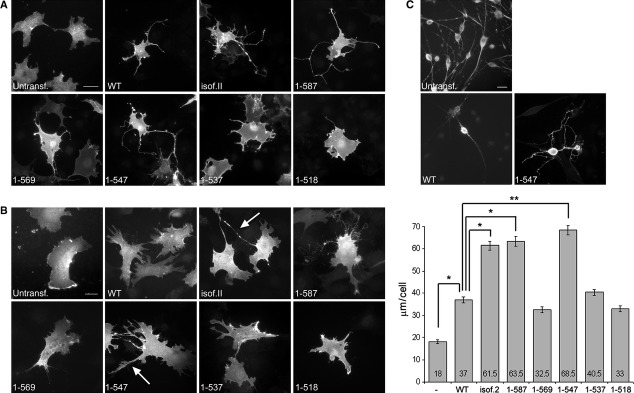
The morphogenic activity of different merlin constructs. COS-7 cells (A), mouse embryonic fibroblasts lacking merlin (Nf2−/− MEFs) (B), and Schwann cells (C) were transiently transfected with merlin constructs and stained for merlin. Untransfected cells were stained for ezrin (COS-7 cells and Nf2−/− MEFs) or phalloidin (Schwann cells). The total extension length per cell was quantified from Nf2−/− MEFs, and the values indicate the average length of extensions per cell (B, right panel). Merlin induces the formation of cell extensions and a significant increase in the total extension length is observed in isoform 2, 1-587 and 1-547 expressing cells compared to WT. Extensions over 50 μm in length in Nf2−/− MEFs are marked with arrows. Scale bars 20 μm. *P < 0.05, **P < 0.01.
Table 1.
Summary of the effects of the C-terminal merlin variants
| Merlin construct | Morphogenic effect | Intramolecular association | Growth suppression |
|---|---|---|---|
| WT (isoform 1) | + | + | + |
| Isoform 2 | ++ | − | + |
| 1-587 | ++ | ++ | Nd |
| 1-569 | + | ++ | Nd |
| 1-547 | ++ | − | − |
| 1-537 | + | ++ | Nd |
| 1-518 | + | ++ | Nd |
| E547K | ++ | Nd | Nd |
| E545K+E547K | ++ | − | − |
Nd: not determined.
To study whether or not morphogenic activities of merlin also affect the cells most relevant to the NF2 disease, merlin WT and 1-547 were transiently transfected into mouse Schwann cells lacking merlin. Merlin expression in these cells has been shown to induce the formation of short membrane protrusions [48]. As in fibroblasts, the morphology of the Schwann cells changed as a result of merlin 1-547 expression (Fig. 2C). Whereas WT merlin expressing cells usually showed a bipolar morphology with two long cell extensions, cells expressing merlin 1-547 induced the formation of multiple branched protrusions in various directions untypical for Schwann cells, but more resembling the phenotype of merlin-deficient schwannoma cells [49–51].
Merlin-induced cell extensions are formed as a result of impaired tail retraction
To study the composition of the merlin-induced long protrusions, isoform 2 and 1-547 transfected Nf2−/− MEFs were stained for actin and tubulin. The merlin-induced long processes contained both actin and microtubuli (Fig. 3A), indicating that the extensions are not only actin-containing long filopodia, but represent other membrane structures.
Fig 3.
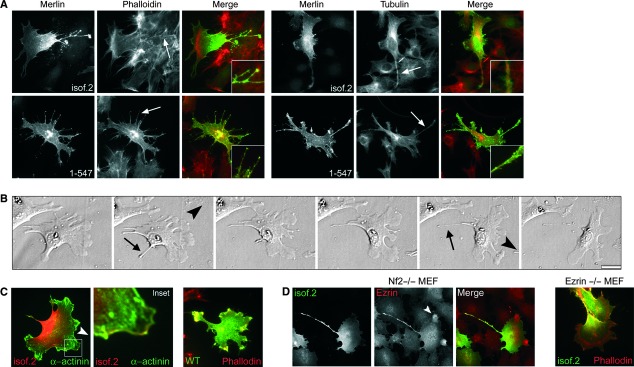
Cytoskeletal components and time-lapse analysis of merlin-induced extensions. (A) Nf2−/− MEFs expressing merlin isoform 2 and 1-547 were stained for merlin and either phalloidin or α-tubulin. Both actin and tubulin are present in longer merlin-induced extensions (arrows). (B) Still pictures from live cell imaging of Nf2−/− MEFs transfected with merlin isoform 2. Arrowheads show the direction of movement and arrows the forming extension. The long extensions are formed when protrusions cannot detach as the cell moves in the opposite direction. (C) Migrating cells used in live cell imaging expressing isoform 2 (left picture) and WT expressing cells (right picture) were stained for merlin. GFP-α-actinin was used to identify isoform 2 expressing cells used in live cell imaging. A gradient of merlin isoform 2 is observed in moving cells in the trailing edge, in addition merlin is present at the leading edge (inset). (D) Nf2−/− MEFs expressing merlin isoform 2 were stained for merlin and endogenous ezrin (left panel). Ezrin does not form a gradient but is present in membrane structures which are devoid of merlin (arrowhead). Mouse embryonic fibroblasts lacking ezrin (ezrin−/− MEFs) expressing merlin isoform 2 were stained for merlin and phalloidin (right picture). A gradient of merlin is detected also in ezrin−/− MEFs.
To further investigate the formation of merlin-induced cell extensions, isoform 2 expressing Nf2−/− MEFs were examined by live cell imaging. The live supplemental video (Video S1) and still images (Fig. 3B) indicate the mechanism by which the extensions form. The long protrusions are formed as a result of impaired release of adhesion sites upon movement, consequently forming a tail behind the cell. Interestingly, analysis of the imaged isoform 2 expressing cells revealed a gradient of merlin (Fig. 3C). In these migrating cells merlin was localized diffusely in the cytoplasm and extensions mainly in the trailing edge of the cell, but was also present as a thin band at the outline of lamellipodia at the leading edge. Such gradient was not detected in WT merlin expressing cells. Merlin isoform 2 and ezrin did not colocalize, as ezrin was present in the protrusions of the leading edge, which were devoid of merlin (Fig. 3D, left image). As the gradient of merlin staining was also observed in ezrin−/− MEFs expressing isoform 2 (Fig. 3D, right image), this subcellular distribution of merlin in migrating MEFs is not ezrin dependent.
Disruption of the E545-E547 domain leads to a cell-extension phenotype but does not affect the nuclear export signal
Residues conserved in merlin of various species, but not in the ERMs, can be important for elucidating the functional difference between merlin and ERM proteins. The sequence EIE (residues 545-547 in human merlin) is conserved among merlins, while I546 is replaced by a leucine residue in ERM proteins (Fig. 4A) [39]. The importance of the region was studied by analysing missense mutations E547K and E545K + E547K. Expression of these molecules resulted in the formation of long cell extensions in both COS-7 cells and Nf2−/− MEFs (Fig. 4B), similarly to merlin 1-547 (Table 1). This phenotypic change indicates that these residues are essential in regulating the formation of merlin-induced cell protrusions.
Fig 4.
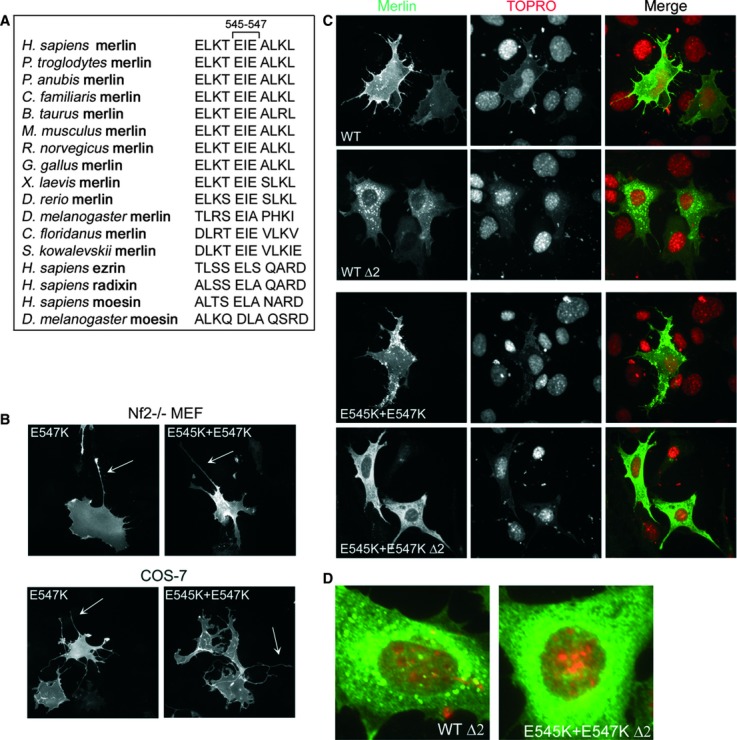
The E545 + E547 motif and its impact on morphogenic properties. (A) Sequence alignment of residues 541-551 of merlin proteins from different species and the ERM proteins of human and Drosophila. (B) Nf2−/− MEFs (upper panel) and COS-7 cells (lower panel) transfected with merlin E547K and E545K + E547K were stained for merlin. Mutation of E547K or E545K + E547K leads to the formation of cell extensions in both cell types (arrows). (C) Nf2−/− MEFs expressing WT or E545K + E547K with or without a deletion in exon 2, containing a cytoplasmic retention factor, were stained for merlin (green) and the nuclei (red). Mutation of E545K + E547K does not disrupt the nuclear export sequence, as the protein shows a cytoplasmic distribution. (D) Enlargement of Nf2−/− MEFs expressing merlin WT Δ2 and E545K + E547K Δ2. WT Δ2 cells display a punctate distribution of merlin which is absent from E545K + E547K Δ2 cells.
A nuclear export sequence (NES) is present in exon 15 between residues 535-551, while exon 2 contains a cytoplasmic retention factor [2]. Truncation of merlin at 547 or point mutations of E545 and E547 could result in aberrant nuclear activity resulting from disruption of the C-terminal NES. We therefore analysed the subcellular localization of merlin E545K + E547K with and without the cytoplasmic retention factor sequence. When exon 2 is disturbed, the constructs should be free to relocate from the membrane and if the mutants disrupt the NES, the protein should be trapped in the nucleus. However, merlin E545K + E547K Δ2 localized similarly to WT Δ2 in the cytoplasm of Nf2−/− MEFs and did not show increased nuclear accumulation indicating that mutation of E545 + E547 does not disrupt the NES (Fig. 4C). Thus, the morphogenic activity of the C-terminally mutated constructs is not likely linked to changes in nuclear localization of merlin.
It has been shown that when the FERM-domain is misfolded merlin forms aggresomes that require the C-terminal sequence of amino acids 535-595 [52]. Interestingly, although WT Δ2 displayed a punctate distribution, as expected, this was absent from E545K + E547K Δ2 (Fig. 4D). These results suggest that the region of residues E545-E547 is likely to function as an aggresome determinant.
Ezrin is not required for merlin-induced extensions but influences their formation
Merlin and ezrin are able to heterodimerize and the proteins colocalize in cells at areas of membrane remodelling [10, 45]. Similarly to merlin, overexpression of ezrin constructs promotes formation of cell extensions [53]. To investigate whether or not the C-terminally deleted merlin variants affect ezrin distribution, Nf2−/− MEFs expressing endogenous ezrin were transfected with merlin constructs and analysed. Double staining of the cells revealed only partial colocalization of merlin and ezrin at the membrane. Ezrin was often localized to the distal end of the long cell protrusions and to specific membrane areas lacking merlin (Fig. 5A). However, no difference in the subcellular distribution or the amount and solubility of endogenous ezrin was observed in cells expressing the different merlin variants (data not shown). Therefore, expression of the merlin truncations does not appear to affect ezrin localization or cytoskeletal association.
Fig 5.
The role of merlin-ezrin association in extension formation. (A) Nf2−/− MEFs expressing endogenous ezrin were transfected with merlin constructs and stained for merlin (green) and ezrin (red). Merlin and ezrin co-localize only partially at the cell membrane. Ezrin is present at the tip of extensions and membrane regions which lack merlin (arrows). (B) Ezrin−/− MEFs transfected with merlin constructs were stained for merlin and untransfected cells with phalloidin. The total extension length per cell was quantified, and values indicate the average length of extensions (right panel). Merlin induces cell-extension formation in ezrin−/− MEFs, but the extensions are shorter than in Nf2−/− MEFs. An increase in the total extension length is observed in isoform 2 expressing cells, whereas the truncated constructs did not induce a significant increase in extensions compared to WT merlin. Scale bar 20 μm. *P < 0.05. (C) Pull-down analysis of COS-7 cell lysates expressing merlin constructs (upper panel) incubated with GST-ezrin and merlin fusion proteins. Bound proteins were separated on SDS–PAGE and immunoblotted with merlin A-19 Ab or GST to ensure equal loading of the GST-fusion proteins. All C-terminally deleted merlin constructs bind full-length ezrin (left panels), but not the GST-control (right lower panels). Merlin isoform 2, 1-547, and E545K + E547K bind to merlin 1-314 FERM-domain whereas only weak binding was observed with WT merlin (right upper panel).
Even though merlin is able to bind actin directly [54, 55], the effect of merlin on cell morphology and cytoskeletal organization could also be mediated through ezrin, which interacts with F-actin through a conserved C-terminal region, not present in merlin [56]. To study whether ezrin is required for merlin's morphogenic activity, the phenotypic effect of merlin constructs was analysed in mouse embryonic fibroblasts lacking ezrin (ezrin−/− MEFs) but expressing endogenous merlin. The total extension length per cell was quantified from the cells as previously described for MEFs lacking merlin. Expression of merlin in ezrin−/− MEFs induced generally shorter protrusions than in Nf2−/− MEFs (Fig. 5B). We did, however, observe a significant increase in the total extension length of cells expressing WT merlin versus no merlin and isoform 2 compared to WT, indicating that ezrin is not required for the morphogenic activity of full-length merlin. Interestingly, the extension formation of C-terminally deleted merlin constructs did not markedly differ from the protrusions of WT merlin expressing cells, in contrast to results seen in Nf2−/− MEFs.
To analyse whether the reduced morphogenic activity of merlin truncations detected in ezrin−/− MEFs could be explained by their increased association with ezrin compared to the full-length isoforms, binding assays were conducted. Lysates from COS-7 cells transfected with the different merlin constructs were incubated with GST-ezrin immobilized on glutathione Sepharose beads. The constructs were expressed in mammalian cells to ensure that the proper three-dimensional conformation of the proteins is restored. All merlin constructs bound WT ezrin in the pull-down analysis, and no differences were observed in the binding affinities (Fig. 5C, left panel). The reduced ability of the C-terminal truncated constructs to form protrusions in absence of ezrin does therefore not result from lack of direct association.
Merlin isoform 2 and truncated molecules lacking the most C-terminal residues critical for intramolecular association are thought to represent more open forms of merlin [7, 16, 17]. To analyse the intramolecular interactions of the C-terminally deleted molecules and to determine if the truncating mutations disrupt merlin self-association, a pull-down assay was conducted. If a construct possesses a more open conformation, it should show increased association with N-terminal merlin. Interestingly, a significant difference in the binding of the constructs to the FERM-domain was observed (Fig. 5C, right panel). Merlin isoform 2, 1-547 and E545K + E547K displayed strong association with merlin 1-314, although the binding of WT, 1-587, 1-569 and 1-537 merlin to 1-314 was weak. Thus, the dimerization ability of merlin variants is not directly associated with their morphogenic properties (Table 1).
The C-terminus regulates merlin's electrophoretic mobility independently of serine 518 phosphorylation
We next wanted to test whether or not the functional properties of merlin variants are dependent on phosphorylation. WT merlin expressed in 293 cells migrates as a triplet and the bands are considered to represent differentially phosphorylated forms of the protein. The slowest migrating band corresponding to the hyperphosphorylated form, has been shown to be S518 phosphorylation dependent, where as the middle band represents the phosphorylated and the fastest migrating band the hypophosphorylated form of merlin [20, 57]. When S518 is mutated to alanine the electrophoretic mobility changes, leading to a protein that migrates as a single band in the gel [20].
To analyse the electrophoretic mobilities of the C-terminally truncated merlin constructs, lysates from transfected 293 cells were divided into insoluble and soluble fractions and run in SDS-PAGE. Consistent with earlier studies, WT merlin migrated as a triplet in the gel (Fig. 6A), whereas the thicker, faster migrating band is a doublet (Fig. 6A, lower panel). Isoform 2 did not have the same triplet migration pattern as WT, whereas the longest truncation 1-587 did. The shorter variants 1-569 and 1-547 displayed distinct mobilities by migrating as single bands.
Fig 6.
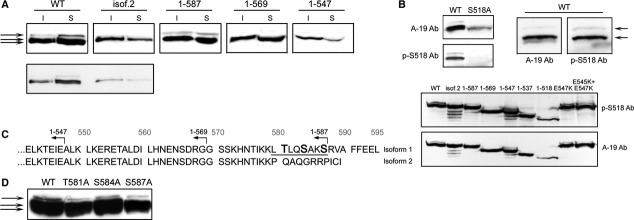
Electrophoretic mobility and phosphorylation status of merlin constructs. (A) 293 lysates transfected with merlin constructs were separated into insoluble (I) and soluble fractions (S), run in SDS–PAGE and detected with merlin A-19 Ab. The migration pattern is shown for the constructs in the long exposure (upper panel), and in the short exposure (lower panel) for WT and isoform 2. Merlin isoform 2 migrates as a single band whereas the longest truncated construct 1-587 migrates as a triplet like WT. (B) S518 phosphorylation of merlin constructs. WT and S518A merlin expressed in 293 cells were detected with A-19 and phospho-serine 518 Ab (p-S518 Ab, upper panel). S518 phosphorylated merlin is detected both in the hyperphosphorylated (upper) and phosphorylated (middle) band (arrows), but not in the hypophosphorylated (lower) band. All merlin truncations and missense mutants expressed in COS-7 cells are S518 phosphorylated (lower panel). (C) The C-terminal sequence of merlin isoforms 1 and 2. Isoform 2 and merlin 1-587 differ only in their last eight residues (580-587, underlined). Potential phosphorylation sites in this region are bolded. (D) Immunoblot analysis of lysates from 293 cells expressing WT merlin or missense mutants T581A, S584A and S587A detected with A-19 Ab. The hyperphosphorylated band (arrow) is detected in all constructs and the alanine mutations of the indicated residues do not change the electrophoretic mobility of merlin.
To study whether or not the merlin bands contain phosphorylated S518 residue, lysates from WT and S518A expressing 293 cells were probed with phospho-S518 antibody (pS518 Ab). S518 phosphorylated merlin is detected in both the phosphorylated (middle) and hyperphosphorylated (upper) bands, but not in the hypophosphorylated (lower) form (Fig. 6B, upper panel, right image). As expected, the pS518 antibody did not detect S518A merlin (Fig. 6B, upper panel, left image). To investigate whether or not merlin variants display abnormal S518 phosphorylation, which would explain their distinct electrophoretic mobilities and lack of hyperphosphorylated bands, the phosphorylation status was assessed by pS518 Ab (Fig. 6B, lower panel). All merlin constructs were phosphorylated on S518, demonstrating that the S518 phosphorylation status does not explain the electrophoretic mobilities and morphogenic effects of the variants.
The difference in electrophoretic mobility of isoform 2 and 1-587 suggested that residues other than S518 influence the migration pattern of merlin, we further analysed the region distinguishing the two constructs. Merlin isoforms are identical up to amino acid 579. Therefore, isoform 2 and merlin 1-587 differ only in eight residues corresponding to 580-587 (Fig. 6C). As phosphorylation affects merlin's electrophoretic mobility, all potential phosphorylation sites between residues 580 and 587 in merlin WT were substituted with alanines and lysates from transfected 293 cells were analysed for their migration pattern in SDS-PAGE. Mutation of threonine 581, serine 584, or serine 587 did not affect the mobility of merlin, as all mutated constructs migrated as triplets in the gel (Fig. 6D). Hence, the potential phosphorylation sites between residues 580 and 587 were ruled out as additional regulators of merlin's electrophoretic mobility pattern.
C-terminally mutated merlin possess reduced ability to inhibit proliferation and growth
Truncating NF2 gene mutations are found in patients and their protein products expressed in tumour (Fig. 1D) [44], suggesting that the C-terminally deleted merlin proteins possess no or reduced tumour suppressor function. To study the effect of merlin C-terminus in growth inhibition, a colony formation assay was performed. Previous studies have shown that overexpression of merlin can reverse the Ha-Ras-induced anchorage-independent growth of fibroblasts [58, 59].
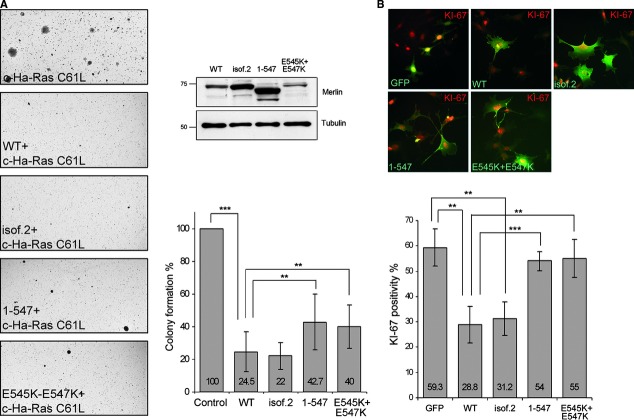
The MEFs were transfected with either c-Ha-Ras C61L, the oncogenic mutant form of Ras, alone or cotransfected together with WT merlin. Transfection of WT merlin did not reduce the amount of c-Ha-Ras-induced colonies compared to cells expressing only c-Ha-Ras in MEFs (data not shown), suggesting that the endogenous merlin in MEFs is able to block colony formation of Ras transfectants. Therefore, merlin WT, isoform 2, 1-547, E545K + E547K and empty plasmid were co-transfected with c-Ha-Ras C61L into Nf2−/− MEFs without endogenous merlin. Cells were plated on soft agar, incubated for 2-3 weeks before staining, and their colony amount was quantified. Expression of the constructs was verified by immunoblotting (Fig. 7A, upper right panel).
Fig 7.
The effect of C-terminal mutations on growth inhibition and proliferation. (A) Soft agar colony formation of Nf2−/− MEFs. Cells transfected with c-Ha-Ras C61L and merlin were grown in soft agar (left panel). Merlin expression levels of transfectants were verified (upper right panel). The number of colonies was quantified from triplicate plates of each construct from three independent experiments (lower right panel). The chart shows the number of colonies in comparison to control (cells transfected with c-Ha-Ras C61L and empty vector), and average number of colonies in control (206) is regarded as 100%. Merlin WT and isoform 2 suppress Ras-induced growth, whereas 1-547 and E545K + E547K have reduced growth inhibitory effect. (B) Nf2−/− MEFs transfected with GFP or merlin were serum starved and then serum induced for ∼12 hrs. Cells were stained for merlin and the proliferation marker Ki-67, and Ki-67 positivity was quantified. Both merlin WT and isoform 2 decrease proliferation compared to GFP whereas merlin 1-547 and E545K + E547K do not suppress proliferation. ***P < 0.001; **P < 0.01.
The Nf2−/− MEFs formed colonies when expressing c-Ha-Ras C61L, whereas cells transfected with merlin alone did not induce colony formation (data not shown). As shown in the quantification in Figure 7A (lower right panel), expression of merlin WT or isoform 2 in c-Ha-Ras C61L expressing cells reduced the amount of colonies by approximately 65% compared to the control without merlin. Interestingly, even though the colony formation of merlin 1-547 and E545K + E547K transfectants was lower than that for the control, expression of these constructs could not block colony formation to the same extent as WT and isoform 2.
To verify the effect of merlin C-terminus on proliferation, Nf2−/− MEFs transfected with GFP or merlin constructs were stained for the proliferation marker Ki-67 and merlin (Fig. 7B, upper panel) and the positivity of the cells was quantified (Fig. 7B, lower panel). Both merlin WT and isoform 2 significantly decreased the number of Ki-67 positive cells, whereas the effect of merlin 1-547 and E545K + E547K was insignificant. Hence, the region around residue 547 in merlin is essential for inhibition of proliferation (Table 1).
Discussion
Several patient mutations have been identified in the C-terminal part of merlin, suggesting that proper merlin function and growth inhibition requires an intact C-terminus. The functions of merlin C-terminus are of special interest also because the distinct regions of merlin and ezrin could explain their opposite biological effects on proliferation. By analysing a NF2 patient-derived tumour we demonstrate that C-terminally truncated forms of merlin can be expressed in vivo. Therefore, by defining functional defects of these proteins we can gain insight into merlin's role in disease progression. In this study we show that the C-terminal domains important for merlin's morphogenic properties and growth inhibiting function are distinct.
Merlin-deficient schwannoma cells display cytoskeletal defects such as cell extensions [49, 50], suggesting that the morphogenic activity previously identified for merlin could be linked to its tumour suppression. Even though merlin is known to promote the formation of membrane structures including extensions [45, 47, 60], the mechanism of their formation and cytoskeletal organization is unknown. Our live cell imaging shows that the formation of merlin-induced cell extensions is a dynamic process. The long extensions are not actively formed by extending out from the cell body, but instead, the retraction of protrusions during movement is impaired leading to long extensions forming at the lagging edge. Merlin interacts with several proteins that regulate cell adhesion formation and detachment. It binds focal adhesion proteins β1 integrin [61] and paxillin [26]. The paxillin interaction is involved in regulating actin-based morphological changes in Schwann cells [26] and is required for neurite outgrowth in neuroblastoma cells [62]. The tail retraction defect could also be a consequence of merlin influencing the Rho signalling pathway, as tail retraction requires active RhoA [63–65], or regulating microtubule dynamics through its association with tubulin [54, 66]. Supporting this hypothesis, unlike WT merlin isoform 1, isoform 2 and 1-547 with morphogenic activity have been described to associate more efficiently with polymerized microtubules [54]. However, although merlin isoform 2 is clearly more insoluble than WT, no significant differences in the solubility of the truncation mutants were detected. Therefore, the phenotypic changes observed in merlin transfected cells are not a direct consequence of stronger association to the cytoskeleton. It remains to be investigated which of the molecular interactions are essential for the merlin-induced morphogenic activity.
Interestingly, WT merlin isoform 1 but not isoform 2 is able to reverse the cytoskeletal abnormalities of schwannoma cells [29]. According to our results, the morphogenic activity is lower in the full-length isoform 1 which predominantly exists as a monomer in a stable largely closed conformation [67], while the C-terminus of isoform 2 is more accessible which may promote cell extension formation. The region important for the morphogenic activity was mapped to the C-terminal α-helix and specifically to residues 538-568. This was further supported by the fact that disruption of E545-E547 in the full-length isoform 1 is sufficient to release the full cell extension phenotype. Similarly to merlin, expression of full-length ezrin causes few extensions but deletion of residues at either end of the protein releases the extension phenotype [53]. The truncated merlin proteins had a reduced effect on the protrusion formation in ezrin−/− MEFs in contrast to the full-length isoforms, suggesting that C-terminally-induced extension formation is partly regulated through the interplay with ezrin, although colocalization of the proteins was not detected in extensions.
Merlin undergoes conformational regulation and its intramolecular associations play an important functional role, as many of merlin's interactions depend on its conformation. Similarly to the ERMs, merlin's self-association is mediated by the binding of its C-terminal domain to the N-terminus [7, 10, 16, 17] and an internal folding of the N-terminal domain [17]. Our study shows, that the conformational regulation is even more complex, as C-terminal truncations affect the intramolecular associations in varying degrees. Merlin isoform 2, 1-547 and E545K + E547K appear to contain a C-terminal conformation where the binding sites for the N-terminus are more accessible. Truncations other than 1-547 do not increase binding affinities, suggesting that the other variants fold in a different manner. This is similar to moesin, where the interactions between the different C-terminal helices with the FERM-domain are largely independent of each other [41]. Merlin E545 and I546 correspond to moesin residues E528 and L529 which contact subdomain F2 of the FERM-domain [41]. According to our results, mutations of E545 and E547 disrupt N-terminal binding suggesting that this region is involved in the FERM-domain C-terminal interface. Although isoform 2 contains an intact region around 547, it lacks the last α-helix which in moesin forms the interaction to the F3-domain [41]. The absence of the most C-terminal helix in isoform 2 is apparently enough to reduce its C-terminal folding and intramolecular association. These results demonstrate that not only the extreme C-terminus, but also the conserved C-terminal α-helix, are essential for the conformational regulation of merlin. The complexity of merlin's conformational regulation is further confirmed by the recent crystal structure of the merlin isoform 1 head-to-tail complex, where the FERM F2-domain is unfurled. Thus, the N- to C-terminus bound molecule represents a more open conformation than previously thought [68].
Merlin is regulated by phosphorylation, which causes it to migrate as a triplet in SDS-PAGE [20, 57]. Interestingly, in addition to merlin WT, only 1-587 displayed all three migrating forms, whereas isoform 2, which contains unique C-terminal residues, and the shorter truncation constructs, migrated as single bands. Despite the different electrophoretic mobilities of the constructs, they all are phosphorylated on S518, showing that the extreme C-terminus is not directly required for S518 phosphorylation in vivo. The results also demonstrate that S518 phosphorylation alone is not sufficient to induce the slowly migrating merlin form (hyperphosphorylated), but the region of residues 580-587 are involved in regulating the electrophoretic mobility of merlin. As mutation of the potential phosphorylation sites in this region did not change the electrophoretic mobility of merlin, it is unlikely that the presence of a hyperphosphorylated band is a direct consequence of a second C-terminal phosphorylation additional to S518, but rather represents conformational differences resulting from intramolecular associations or other post-translational modifications.
Inactivating mutations in the NF2-gene occur in all of the first 15 coding exons, but have not been reported in the last exons 16 and 17 that distinguish the two isoforms from each other [69–71]. This observation has led to the notion that inactivation of the C-terminus would not be sufficient to eliminate merlin's tumour suppressor function [69]. It appeared, however, that the presence of the extreme C-terminus of merlin isoform 1 is required for tumour suppression, as expression of both merlin isoform 2 and 1-547 were shown not to affect the growth rate of rat schwannoma cells and cells expressing isoform 2 did not reduce tumour growth when injected into nude mice [7]. Therefore, isoform 2 has been considered to possess reduced ability to inhibit proliferation [7], thus differing from the wild-type protein in its tumour suppressor ability. Our results, however, show that both merlin isoform 1 and 2 are able to suppress Ras-induced cell growth in soft agar and decrease cell proliferation. Schwannoma cells show an accumulation of growth factor receptors and a high basal activity of mitogenic signalling components, e.g. Ras, MEK1/2 and ERK1/2 [72, 73], thus indicating that these signalling pathways are regulated by merlin. It was previously reported that full-length merlin was able to reduce the growth of Ras transformed cells by inhibiting the activation of both Ras and Rac by counteracting the ERM dependent activation of Ras [74]. Importantly, our results indicate that the closed conformation of isoform 1 is not required to negatively regulate cell proliferation. This is in agreement with a recent report where growth inhibition of both isoforms requiring the N-terminal F2-subdomain and C-terminal residues 532-579 was observed in Schwann cells [48]. Our results demonstrate that the growth inhibitory function of both 1-547 and E545K + E547K merlin is significantly reduced. In accordance, mutations resulting in merlin truncations around residue 547 have been described in both NF2 patients and in sporadic schwannomas [37, 38, 69]. Our data further define the importance of the conserved residues around 545-547 and confirm that an intact C-terminus is required for full growth inhibitory effect of merlin. These results differ from experiments done in Drosophila, where the essential functions of Dmerlin reside within the FERM-domain [75]. However, it should be noted that the motif of 545-547 in Dmerlin slightly differs from that of other species (Fig. 4A).
Even though many of the patient mutations described localize to the N-terminal part of merlin [70, 71], also several interesting C-terminal mutations have been characterized. According to our results, C-terminally truncated merlin may not always be degraded but could perform unique functions in tumour cells, which may be a cause for tumourigenesis. However, as it appears that truncated merlin 1-548 is expressed at a low level in the tumour, and may partially be degraded, it might not perform all the functions detected in our experiments with overexpressed merlin truncations. Missense mutations occur at a low frequency in the NF2 gene representing only ∼5% of all NF2 mutations. Interestingly, of these almost one-third are located in exon 15 (representing residues 525-579) [2], highlighting the importance of this region. Four C-terminal point mutations in exon 15 resulting in K533T [76], L535P [77], Q538P [78] and E547K [40] amino acid substitutions have been characterized, and of these L535P and Q538P have been shown to impair merlin's growth inhibitory function [42, 79], indicating that missense alterations within the C-terminus can inactivate the tumour suppressor activity of merlin. The E547K NF2 mutation identified in a patient with atypical hybrid neurofibroma-perineurioma [40], in turn, appears to change the C-terminal domain folding based on our assays, which could result in reduced growth inhibition. When considering the genotype-phenotype correlation, caution is needed, as also additional alternative mechanisms for reduced tumour suppressor activity can exist. Recently, the loss of function of NF2 missense mutations was suggested to result from reduction in mutant protein half-life and increased degradation rather than abnormal function [80].
In conclusion, this study demonstrates that the C-terminus is important for proper merlin function, both for cytoskeletal regulation and growth inhibition. Our results suggest that merlin can form complex C-terminal intramolecular associations that require several C-terminal regions. Both merlin isoform 1 and 2 are able to suppress Ras-induced cell growth and decrease proliferation indicating that full association between the N- and C-terminal domains is not required to negatively regulate proliferation. The region around the conserved domain of E545-E547 is particularly important for merlin function, as mutations at these residues impair both morphogenic and growth inhibitory activities of merlin. Taken together, this study demonstrates that overlapping but specific C-terminal regions of merlin mediate functions related to growth suppression, cellular morphology and control of molecular interactions.
Acknowledgments
We would like to thank Dr. Giovannini for the Nf2−/− MEFs and Schwann cells, Dr. McClatchey for the Ezrin−/− MEFs, and Dr. Fernandez-Valle for the exon 2 deletion construct. We also thank H. Ahola for her skilful technical assistance. This work was supported by the grants of the Finnish Cancer Organizations, the Academy of Finland, the Medical Research Fund of Turku University Central Hospital District, Sigrid Juselius foundation, Magnus Ehrnrooths stiftelse, Medicinska Understödsföreningen Liv och Hälsa, Svenska kulturfonden, Stiftelsen Leo, Mary och Mary-Ann Hackman, Maire Taponen säätiö, Maud Kuistila Memorial Foundation, K. Albin Johanssons stiftelse and Waldemar von Frenckells stiftelse.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Video S1. Time-lapse analysis of extension formation. The live cell imaging of isoform 2 transfected Nf2−/− MEFs indicates the mechanism by which the merlin-induced extensions form. The long protrusions are formed as a result of impaired release of adhesion sites upon movement, consequently forming a tail behind the cell.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Evans DG, Huson SM, Donnai D, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84:603–18. [PubMed] [Google Scholar]
- 2.Kressel M, Schmucker B. Nucleocytoplasmic transfer of the NF2 tumour suppressor protein merlin is regulated by exon 2 and a CRM1-dependent nuclear export signal in exon 15. Hum Mol Genet. 2002;11:2269–78. doi: 10.1093/hmg/11.19.2269. [DOI] [PubMed] [Google Scholar]
- 3.Muranen T, Grönholm M, Renkema GH, et al. Cell cycle-dependent nucleocytoplasmic shuttling of the neurofibromatosis 2 tumour suppressor merlin. Oncogene. 2005;24:1150–8. doi: 10.1038/sj.onc.1208283. [DOI] [PubMed] [Google Scholar]
- 4.Li W, You L, Cooper J, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140:477–90. doi: 10.1016/j.cell.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi AB, Hara T, Ramesh V, et al. Mutations in transcript isoforms of the neurofibromatosis 2 gene in multiple human tumour types. Nat Genet. 1994;6:185–92. doi: 10.1038/ng0294-185. [DOI] [PubMed] [Google Scholar]
- 6.Chang LS, Akhmametyeva EM, Wu Y, et al. Multiple transcription initiation sites, alternative splicing, and differential polyadenylation contribute to the complexity of human neurofibromatosis 2 transcripts. Genomics. 2002;79:63–76. doi: 10.1006/geno.2001.6672. [DOI] [PubMed] [Google Scholar]
- 7.Sherman L, Xu HM, Geist RT, et al. Interdomain binding mediates tumour growth suppression by the NF2 gene product. Oncogene. 1997;15:2505–9. doi: 10.1038/sj.onc.1201418. [DOI] [PubMed] [Google Scholar]
- 8.Turunen O, Sainio M, Jääskeläinen J, et al. Structure-function relationships in the ezrin family and the effect of tumour-associated point mutations in neurofibromatosis 2 protein. Biochim Biophys Acta. 1998;1387:1–16. doi: 10.1016/s0167-4838(98)00103-4. [DOI] [PubMed] [Google Scholar]
- 9.Gary R, Bretscher A. Heterotypic and homotypic associations between ezrin and moesin, two putative membrane-cytoskeletal linking proteins. Proc Natl Acad Sci USA. 1993;90:10846–50. doi: 10.1073/pnas.90.22.10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grönholm M, Sainio M, Zhao F, et al. Homotypic and heterotypic interaction of the neurofibromatosis 2 tumour suppressor protein merlin and the ERM protein ezrin. J Cell Sci. 1999;112:895–904. doi: 10.1242/jcs.112.6.895. [DOI] [PubMed] [Google Scholar]
- 11.Khanna C, Wan X, Bose S, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–6. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- 12.Yu Y, Khan J, Khanna C, et al. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med. 2004;10:175–81. doi: 10.1038/nm966. [DOI] [PubMed] [Google Scholar]
- 13.Heiska L, Melikova M, Zhao F, et al. Ezrin is key regulator of Src-induced malignant phenotype in three-dimensional environment. Oncogene. 2011;30:4953–62. doi: 10.1038/onc.2011.207. [DOI] [PubMed] [Google Scholar]
- 14.Gary R, Bretscher A. Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol Biol Cell. 1995;6:1061–75. doi: 10.1091/mbc.6.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang L, Ichimaru E, Pestonjamasp K, et al. Merlin differs from moesin in binding to F-actin and in its intra- and intermolecular interactions. Biochem Biophys Res Commun. 1998;248:548–53. doi: 10.1006/bbrc.1998.9009. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Agosti C, Wiederhold T, Herndon ME, et al. Interdomain interaction of merlin isoforms and its influence on intermolecular binding to NHE-RF. J Biol Chem. 1999;274:34438–42. doi: 10.1074/jbc.274.48.34438. [DOI] [PubMed] [Google Scholar]
- 17.Gutmann DH, Haipek CA, Hoang Lu K. Neurofibromatosis 2 tumour suppressor protein, merlin, forms two functionally important intramolecular associations. J Neurosci Res. 1999;58:706–16. [PubMed] [Google Scholar]
- 18.Kissil JL, Johnson KC, Eckman MS, et al. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem. 2002;277:10394–9. doi: 10.1074/jbc.M200083200. [DOI] [PubMed] [Google Scholar]
- 19.Xiao GH, Beeser A, Chernoff J, et al. p21-activated kinase links Rac/Cdc42 signaling to merlin. J Biol Chem. 2002;277:883–6. doi: 10.1074/jbc.C100553200. [DOI] [PubMed] [Google Scholar]
- 20.Alfthan K, Heiska L, Grönholm M, et al. Cyclic AMP-dependent protein kinase phosphorylates merlin at serine 518 independently of p21-activated kinase and promotes merlin-ezrin heterodimerization. J Biol Chem. 2004;279:18559–66. doi: 10.1074/jbc.M313916200. [DOI] [PubMed] [Google Scholar]
- 21.Morrison H, Sherman LS, Legg J, et al. The NF2 tumour suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001;15:968–80. doi: 10.1101/gad.189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw RJ, Paez JG, Curto M, et al. The Nf2 tumour suppressor, merlin, functions in Rac-dependent signaling. Dev Cell. 2001;1:63–72. doi: 10.1016/s1534-5807(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 23.Tang X, Jang SW, Wang X, et al. Akt phosphorylation regulates the tumour-suppressor merlin through ubiquitination and degradation. Nat Cell Biol. 2007;9:1199–207. doi: 10.1038/ncb1641. [DOI] [PubMed] [Google Scholar]
- 24.Laulajainen M, Muranen T, Carpén O, et al. Protein kinase A-mediated phosphorylation of the NF2 tumour suppressor protein merlin at serine 10 affects the actin cytoskeleton. Oncogene. 2008;27:3233–43. doi: 10.1038/sj.onc.1210988. [DOI] [PubMed] [Google Scholar]
- 25.Laulajainen M, Muranen T, Nyman TA, et al. Multistep phosphorylation by oncogenic kinases enhances the degradation of the NF2 tumour suppressor merlin. Neoplasia. 2011;13:643–52. doi: 10.1593/neo.11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez-Valle C, Tang Y, Ricard J, et al. Paxillin binds schwannomin and regulates its density-dependent localization and effect on cell morphology. Nat Genet. 2002;31:354–62. doi: 10.1038/ng930. [DOI] [PubMed] [Google Scholar]
- 27.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383–94. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khosravi-Far R, White MA, Westwick JK, et al. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumourigenic transformation. Mol Cell Biol. 1996;16:3923–33. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bashour AM, Meng JJ, Ip W, et al. The neurofibromatosis type 2 gene product, merlin, reverses the F-actin cytoskeletal defects in primary human Schwannoma cells. Mol Cell Biol. 2002;22:1150–7. doi: 10.1128/MCB.22.4.1150-1157.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giovannini M, Robanus-Maandag E, van der Valk M, et al. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 2000;14:1617–30. [PMC free article] [PubMed] [Google Scholar]
- 31.Manent J, Oguievetskaia K, Bayer J, et al. Magnetic cell sorting for enriching Schwann cells from adult mouse peripheral nerves. J Neurosci Methods. 2003;123:167–73. doi: 10.1016/s0165-0270(02)00349-7. [DOI] [PubMed] [Google Scholar]
- 32.den Bakker MA, Riegman PH, Hekman RA, et al. The product of the NF2 tumour suppressor gene localizes near the plasma membrane and is highly expressed in muscle cells. Oncogene. 1995;10:757–63. [PubMed] [Google Scholar]
- 33.den Bakker MA, Tascilar M, Riegman PH, et al. Neurofibromatosis type 2 protein co-localizes with elements of the cytoskeleton. Am J Pathol. 1995;147:1339–49. [PMC free article] [PubMed] [Google Scholar]
- 34.Böhling T, Turunen O, Jääskeläinen J, et al. Ezrin expression in stromal cells of capillary hemangioblastoma. An immunohistochemical survey of brain tumours. Am J Pathol. 1996;148:367–73. [PMC free article] [PubMed] [Google Scholar]
- 35.Rouleau GA, Merel P, Lutchman M, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–21. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- 36.Trofatter JA, MacCollin MM, Rutter JL, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumour suppressor. Cell. 1993;75:826. doi: 10.1016/0092-8674(93)90501-g. [DOI] [PubMed] [Google Scholar]
- 37.Jacoby LB, MacCollin M, Louis DN, et al. Exon scanning for mutation of the NF2 gene in schwannomas. Hum Mol Genet. 1994;3:413–9. doi: 10.1093/hmg/3.3.413. [DOI] [PubMed] [Google Scholar]
- 38.Wallace AJ, Watson CJ, Oward E, et al. Mutation scanning of the NF2 gene: an improved service based on meta-PCR/sequencing, dosage analysis, and loss of heterozygosity analysis. Genet Test. 2004;8:368–80. doi: 10.1089/gte.2004.8.368. [DOI] [PubMed] [Google Scholar]
- 39.Golovnina K, Blinov A, Akhmametyeva EM, et al. Evolution and origin of merlin, the product of the Neurofibromatosis type 2 (NF2) tumour-suppressor gene. BMC Evol Biol. 2005;5:69. doi: 10.1186/1471-2148-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazakov DV, Pitha J, Sima R, et al. Hybrid peripheral nerve sheath tumours: Schwannoma-perineurioma and neurofibroma-perineurioma. A report of three cases in extradigital locations. Ann Diagn Pathol. 2005;9:16–23. doi: 10.1016/j.anndiagpath.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Pearson MA, Reczek D, Bretscher A, et al. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell. 2000;101:259–70. doi: 10.1016/s0092-8674(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 42.Gutmann DH, Geist RT, Xu H, et al. Defects in neurofibromatosis 2 protein function can arise at multiple levels. Hum Mol Genet. 1998;7:335–45. doi: 10.1093/hmg/7.3.335. [DOI] [PubMed] [Google Scholar]
- 43.Gautreau A, Manent J, Fievet B, et al. Mutant products of the NF2 tumour suppressor gene are degraded by the ubiquitin-proteasome pathway. J Biol Chem. 2002;277:31279–82. doi: 10.1074/jbc.C200125200. [DOI] [PubMed] [Google Scholar]
- 44.Sainio M, Jääskeläinen J, Pihlaja H, et al. Mild familial neurofibromatosis 2 associates with expression of merlin with altered COOH-terminus. Neurology. 2000;54:1132–8. doi: 10.1212/wnl.54.5.1132. [DOI] [PubMed] [Google Scholar]
- 45.Sainio M, Zhao F, Heiska L, et al. Neurofibromatosis 2 tumour suppressor protein colocalizes with ezrin and CD44 and associates with actin-containing cytoskeleton. J Cell Sci. 1997;110:2249–60. doi: 10.1242/jcs.110.18.2249. [DOI] [PubMed] [Google Scholar]
- 46.Shaw RJ, McClatchey AI, Jacks T. Localization and functional domains of the neurofibromatosis type II tumour suppressor, merlin. Cell Growth Differ. 1998;9:287–96. [PubMed] [Google Scholar]
- 47.Gonzalez-Agosti C, Xu L, Pinney D, et al. The merlin tumour suppressor localizes preferentially in membrane ruffles. Oncogene. 1996;13:1239–47. [PubMed] [Google Scholar]
- 48.Lallemand D, Saint-Amaux AL, Giovannini M. Tumour-suppression functions of merlin are independent of its role as an organizer of the actin cytoskeleton in Schwann cells. J Cell Sci. 2009;122:4141–9. doi: 10.1242/jcs.045914. [DOI] [PubMed] [Google Scholar]
- 49.Pelton PD, Sherman LS, Rizvi TA, et al. Ruffling membrane, stress fiber, cell spreading and proliferation abnormalities in human Schwannoma cells. Oncogene. 1998;17:2195–209. doi: 10.1038/sj.onc.1202141. [DOI] [PubMed] [Google Scholar]
- 50.Rosenbaum C, Kluwe L, Mautner VF, et al. Isolation and characterization of Schwann cells from neurofibromatosis type 2 patients. Neurobiol Dis. 1998;5:55–64. doi: 10.1006/nbdi.1998.0179. [DOI] [PubMed] [Google Scholar]
- 51.Flaiz C, Kaempchen K, Matthies C, et al. Actin-rich protrusions and nonlocalized GTPase activation in Merlin-deficient schwannomas. J Neuropathol Exp Neurol. 2007;66:608–16. doi: 10.1097/nen.0b013e318093e555. [DOI] [PubMed] [Google Scholar]
- 52.Gautreau A, Fievet BT, Brault E, et al. Isolation and characterization of an aggresome determinant in the NF2 tumour suppressor. J Biol Chem. 2003;278:6235–42. doi: 10.1074/jbc.M210639200. [DOI] [PubMed] [Google Scholar]
- 53.Martin M, Roy C, Montcourrier P, et al. Three determinants in ezrin are responsible for cell extension activity. Mol Biol Cell. 1997;8:1543–57. doi: 10.1091/mbc.8.8.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu HM, Gutmann DH. Merlin differentially associates with the microtubule and actin cytoskeleton. J Neurosci Res. 1998;51:403–15. doi: 10.1002/(SICI)1097-4547(19980201)51:3<403::AID-JNR13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 55.James MF, Manchanda N, Gonzalez-Agosti C, et al. The neurofibromatosis 2 protein product merlin selectively binds F-actin but not G-actin, and stabilizes the filaments through a lateral association. Biochem J. 2001;356:377–86. doi: 10.1042/0264-6021:3560377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turunen O, Wahlström T, Vaheri A. Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J Cell Biol. 1994;126:1445–53. doi: 10.1083/jcb.126.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shaw RJ, McClatchey AI, Jacks T. Regulation of the neurofibromatosis type 2 tumour suppressor protein, merlin, by adhesion and growth arrest stimuli. J Biol Chem. 1998;273:7757–64. doi: 10.1074/jbc.273.13.7757. [DOI] [PubMed] [Google Scholar]
- 58.Tikoo A, Varga M, Ramesh V, et al. An anti-Ras function of neurofibromatosis type 2 gene product (NF2/Merlin) J Biol Chem. 1994;269:23387–90. [PubMed] [Google Scholar]
- 59.Jung JR, Kim H, Jeun SS, et al. The Phosphorylation status of merlin is important for regulating the Ras-ERK pathway. Mol Cells. 2005;20:196–200. [PubMed] [Google Scholar]
- 60.Stokowski RP, Cox DR. Functional analysis of the neurofibromatosis type 2 protein by means of disease-causing point mutations. Am J Hum Genet. 2000;66:873–91. doi: 10.1086/302812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Obremski VJ, Hall AM, Fernandez-Valle C. Merlin, the neurofibromatosis type 2 gene product, and beta1 integrin associate in isolated and differentiating Schwann cells. J Neurobiol. 1998;37:487–501. [PubMed] [Google Scholar]
- 62.Yamauchi J, Miyamoto Y, Kusakawa S, et al. Neurofibromatosis 2 tumour suppressor, the gene induced by valproic acid, mediates neurite outgrowth through interaction with paxillin. Exp Cell Res. 2008;314:2279–88. doi: 10.1016/j.yexcr.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 63.Somlyo AV, Bradshaw D, Ramos S, et al. Rho-kinase inhibitor retards migration and in vivo dissemination of human prostate cancer cells. Biochem Biophys Res Commun. 2000;269:652–9. doi: 10.1006/bbrc.2000.2343. [DOI] [PubMed] [Google Scholar]
- 64.Alblas J, Ulfman L, Hordijk P, et al. Activation of Rhoa and ROCK are essential for detachment of migrating leukocytes. Mol Biol Cell. 2001;12:2137–45. doi: 10.1091/mbc.12.7.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Worthylake RA, Lemoine S, Watson JM, et al. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol. 2001;154:147–60. doi: 10.1083/jcb.200103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muranen T, Grönholm M, Lampin A, et al. The tumour suppressor merlin interacts with microtubules and modulates Schwann cell microtubule cytoskeleton. Hum Mol Genet. 2007;16:1742–51. doi: 10.1093/hmg/ddm122. [DOI] [PubMed] [Google Scholar]
- 67.Hennigan RF, Foster LA, Chaiken MF, et al. Fluorescence resonance energy transfer analysis of merlin conformational changes. Mol Cell Biol. 2010;30:54–67. doi: 10.1128/MCB.00248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yogesha SD, Sharff AJ, Giovannini M, et al. Unfurling of the band 4.1, ezrin, radixin, moesin (FERM) domain of the merlin tumour suppressor. Protein Sci. 2011;20:2113–20. doi: 10.1002/pro.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacoby LB, MacCollin M, Barone R, et al. Frequency and distribution of NF2 mutations in schwannomas. Genes Chromosomes Cancer. 1996;17:45–55. doi: 10.1002/(SICI)1098-2264(199609)17:1<45::AID-GCC7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 70.Baser ME Contributors to the International NF2 Mutation Database. The distribution of constitutional and somatic mutations in the neurofibromatosis 2 gene. Hum Mutat. 2006;27:297–306. doi: 10.1002/humu.20317. [DOI] [PubMed] [Google Scholar]
- 71.Ahronowitz I, Xin W, Kiely R, et al. Mutational spectrum of the NF2 gene: a meta-analysis of 12 years of research and diagnostic laboratory findings. Hum Mutat. 2007;28:1–12. doi: 10.1002/humu.20393. [DOI] [PubMed] [Google Scholar]
- 72.Ammoun S, Hanemann CO. Emerging therapeutic targets in schwannomas and other merlin-deficient tumours. Nat Rev Neurol. 2011;7:392–9. doi: 10.1038/nrneurol.2011.82. [DOI] [PubMed] [Google Scholar]
- 73.Lallemand D, Manent J, Couvelard A, et al. Merlin regulates transmembrane receptor accumulation and signaling at the plasma membrane in primary mouse Schwann cells and in human schwannomas. Oncogene. 2009;28:854–65. doi: 10.1038/onc.2008.427. [DOI] [PubMed] [Google Scholar]
- 74.Morrison H, Sperka T, Manent J, et al. Merlin/neurofibromatosis type 2 suppresses growth by inhibiting the activation of Ras and Rac. Cancer Res. 2007;67:520–7. doi: 10.1158/0008-5472.CAN-06-1608. [DOI] [PubMed] [Google Scholar]
- 75.LaJeunesse DR, McCartney BM, Fehon RG. Structural analysis of Drosophila merlin reveals functional domains important for growth control and subcellular localization. J Cell Biol. 1998;141:1589–99. doi: 10.1083/jcb.141.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hung G, Faudoa R, Baser ME, et al. Neurofibromatosis 2 phenotypes and germ-line NF2 mutations determined by an RNA mismatch method and loss of heterozygosity analysis in NF2 schwannomas. Cancer Genet Cytogenet. 2000;118:167–8. doi: 10.1016/s0165-4608(99)00201-0. [DOI] [PubMed] [Google Scholar]
- 77.Bourn D, Evans G, Mason S, et al. Eleven novel mutations in the NF2 tumour suppressor gene. Hum Genet. 1995;95:572–4. doi: 10.1007/BF00223872. [DOI] [PubMed] [Google Scholar]
- 78.Kluwe L, Mautner VF. A missense mutation in the NF2 gene results in moderate and mild clinical phenotypes of neurofibromatosis type 2. Hum Genet. 1996;97:224–7. doi: 10.1007/BF02265270. [DOI] [PubMed] [Google Scholar]
- 79.Gutmann DH, Hirbe AC, Haipek CA. Functional analysis of neurofibromatosis 2 (NF2) missense mutations. Hum Mol Genet. 2001;10:1519–29. doi: 10.1093/hmg/10.14.1519. [DOI] [PubMed] [Google Scholar]
- 80.Yang C, Asthagiri AR, Iyer RR, et al. Missense mutations in the NF2 gene result in the quantitative loss of merlin protein and minimally affect protein intrinsic function. Proc Natl Acad Sci USA. 2011;108:4980–5. doi: 10.1073/pnas.1102198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.