Abstract
Previous studies have shown that the transforming growth factor (TGF)β/Alk1/Smad1 signaling pathway is constitutively activated in a subset of systemic sclerosis (SSc) fibroblasts and this pathway is a critical regulator of CCN2 gene expression. Caveolin-1 (cav-1), an integral membrane protein and the main component of caveolae, has also been implicated in SSc pathogenesis. This study was undertaken to evaluate the role of caveolin-1 in Smad1 signaling and CCN2 expression in healthy and SSc dermal fibroblasts. We show that a significant subset of SSc dermal fibroblasts has up-regulated cav-1 expression in vitro, and that cav-1 up-regulation correlates with constitutive Smad1 phosphorylation. In addition, basal levels of phospho-Smad1 were down-regulated after inhibition of cav-1 in SSc dermal fibroblasts. Caveolin-1 formed a protein complex with Alk1 in dermal fibroblasts, and this association was enhanced by TGFβ. By using siRNA against cav-1 and adenoviral cav-1 overexpression we demonstrate that activation of Smad1 in response to TGFβ requires cav-1 and that cav-1 is sufficient for Smad-1 phosphorylation. We also show that cav-1 is a positive regulator of CCN2 gene expression, and that it is required for the basal and TGFβ-induced CCN2 levels. In conclusion, this study has revealed an important role of cav-1 in mediating TGFβ/Smad1 signaling and CCN2 gene expression in healthy and SSc dermal fibroblasts.
Keywords: scleroderma, caveolin-1, Smad1, CCN2
Background
Systemic sclerosis is an autoimmune connective tissue disease characterized by vasculopathy and tissue fibrosis. Although the exact pathogenetic mechanism leading to SSc manifestations is currently unknown, increasing evidence implicates activation of the TGFβ pathway in the disease process.
The TGFβ is a pleiotropic growth factor with important roles in tissue homeostasis that has been implicated in cell proliferation, differentiation, apoptosis, extracellular matrix deposition and cell motility. Deregulated TGFβ signaling has been linked to various pathological processes, including tissue fibrosis, cancer and metastasis. Canonical TGFβ signaling is accomplished through ligand binding to its cognate receptor TGFβRI/Alk5, which in turn binds and activates TGFβRII, leading to recruitment of Smad2/3, phosphorylation and nuclear translocation. Several recent reports have described additional, non-canonical TGFβ pathways, including the endoglin/Alk1/Smad1, PI3K/Akt, Erk1/2, PKCδ/c-abl/Fli1 pathways, and there is evidence that these pathways are important contributors to SSc pathogenesis [1–5]. In endothelial cells and dermal fibroblasts, activation of Smad1 in response to TGFβ involves the Alk1 and Alk5 types of TGFβRI, as well as the type III TGFβ receptor endoglin [1, 6–8]. Constitutive Smad1 phosphorylation was reported in a subset of SSc dermal fibroblasts and increased endoglin expression was also shown in these cells in vitro. Furthermore, the increases in endoglin and phospho-Smad1 correlated with higher expression of the pro-fibrotic marker CCN2 (connective tissue growth factor) [1, 3].
CCN2 is a member of the CNN family of matrix-associated proteins encoded by immediate early genes that play important roles in embryonic development, differentiation, angiogenesis, chondrogenesis and wound healing. CCN2 is an important mediator of TGFβ in the induction of type I collagen and is overexpressed in various types of fibrotic diseases, including SSc, thus being considered a major player in fibrosis [9].
Caveolin-1 is a ubiquitously expressed scaffolding membrane protein and a major structural component of caveolae. Caveolae are membrane invaginations that serve as organizing centres, participating in signal transduction. Through its scaffolding sequence, cav-1 can either sequester signaling molecules, down-regulating receptor activities, or can contribute to assembling of signaling molecules, facilitating their function. Cav-1 has inhibitory effects on the majority of interacting proteins, resulting in down-regulation of various signaling pathways, including ERK1/2, eNOS and EGFR [10]. Several exceptions have been reported: cav-1 up-regulates insulin receptor signaling and activates the Pi3K/Akt pathway [11, 12]. Caveolae have also been implicated in cholesterol and vesicular trafficking. Internalization of cell surface receptors is mediated via two major endocytic pathways: clathrin-mediated endocytosis and lipid-raft/caveolae-mediated endocytosis. Internalization of TGFβ receptors via clathrin-coated pits into the early endosome promotes TGFβ/Smad3 signaling while lipid-raft/caveolar internalization promotes Smad7-Smurf dependent receptor degradation, thus inhibiting the canonical TGFβ signaling [13].
The Cav-1 down-regulation has been previously implicated in the pathogenesis of lung fibrosis, both for idiopathic pulmonary fibrosis and SSc-associated interstitial lung disease [14, 15]. Thus, in SSc lung fibroblasts, low levels of cav-1 were linked to constitutive activation of JNK, ERK, and Akt signaling, leading to overexpression of the profibrotic markers collagen and alpha smooth muscle actin [16]. Previous studies by Del Galdo and collaborators in dermal fibroblasts have shown that cav-1 is down-regulated in SSc skin, contributing to increased collagen deposition via activation of the canonical TGFβ pathway [17]. Although in fibroblasts and epithelial cells association of cav-1 with the Alk5 TGFβ type I receptor inhibits signaling through Smad2/3 [18], the effects of cav-1/Alk1 association in dermal fibroblasts are currently unknown. This study was undertaken to determine the role of cav-1 in TGFβ/Alk1/Smad1 signaling in normal and SSc dermal fibroblasts and to evaluate its functional significance. We found that in normal and SSc dermal fibroblasts cav-1 promotes TGFβ/Smad1 signaling and that cav-1 is a positive regulator of CCN2 gene expression.
Materials and methods
Reagents
The following antibodies were used: monoclonal β actin (Sigma-Aldrich, St. Louis, MO, USA), anti-cav-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-ALK1 (Santa Cruz Biotechnology), anti-CTGF (Santa Cruz Biotechnology), goat anti-type-1 collagen (Southern Biotech, Birmingham, AL, USA), anti-SMAD1/5/8 (Cell Signaling, Beverly, MA, USA), anti-phospho-SMAD1/5(S463/465)/SMAD8(S426/428) (Cell Signaling), DMEM and 100× Antibiotic-Antimycotic solution (penicillin streptomycin and amphotericin B) were obtained from Gibco BRL (Grand Island, NY, USA). Foetal bovine serum was purchased from HyClone (Logan, UT, USA). Enhanced chemiluminescence reagent and bovine serum albumin (BSA) protein assay reagent were obtained from Pierce (Rockford, IL, USA).
Cell culture
Human dermal fibroblast cultures were established from biopsy specimens obtained from the dorsal forearms of SSc patients with diffuse cutaneous disease and from age, race and gender-matched healthy donors, upon informed consent and in compliance with the Institutional Review Board. Dermal fibroblasts were cultured from the biopsy specimens as described previously [19]. Normal and SSc skin fibroblasts were cultured in DMEM supplemented with 10% FBS and 1% antibiotic-antimycotic solution.
Adenovirus transfection
The Ad-Caveolin-1 human adenovirus and the Ad-Alk1 human adenovirus utilizing CMV promoters and the Ad-luciferase control vector were purchased from Vector Biolabs (Philadelphia, PA, USA). Dermal fibroblasts were grown to 80–90% confluence, changed to serum free media, and treated with adenovirus for 48 hrs before mRNA was collected.
RNA interference
SMARTpool siRNA against Caveolin-1 was purchased from Dharmacon RNA Technologies (Lafayette, CO, USA). Negative-control siRNA and Hiperfect siRNA transfection reagent were purchased from Qiagen (Germantown, MD, USA). Dermal fibroblasts were grown to 70–80% confluence and transiently transfected using 50 nM of gene-specific siRNA, or scrambled non-silencing siRNA. Transfection was performed in serum containing media according the manufacturer's protocol, and 5 hrs later cultures were changed to serum free DMEM containing 0.1% BSA, and left for 72 hrs. A second transfection was performed in the same manner, and cell lysates were collected 72 hrs later.
Western blot analysis
Cells were collected and washed with PBS. Cell pellets were suspended in lysis buffer containing 20 mM Tris-HCl, pH 7.5, 15 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, and 1 mM glycerophosphate with freshly added phosphatase inhibitors (5 mM sodium fluoride and 1 mM Na3VO4) and a protease inhibitor mixture (Sigma-Aldrich). Protein concentration was quantified using the BCA Protein Assay kit (Pierce). Equal amounts of total protein for each sample were separated via SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). Membranes were blocked in 2% milk in TBST for 1 hr and incubated with primary Ab overnight at 4°C. After TBST washes, membranes were probed with HRP-conjugated secondary Ab against the appropriate species for 1 hr at room temperature. Protein was visualized using ECL reagent (Amersham Biosciences, Piscataway, NJ, USA).
Immunoprecipitation
Whole cell lysates prepared in lysis buffer as described above were pre-cleared with antimouse IgG-coated beads (protein G-Sepharose beads; GE Healthcare, Uppsala, Sweden) for 2 hrs, and incubated on a rotator overnight in monoclonal mouse anti-Alk1 primary antibody at 4°C. Twenty-five microlitres of new beads were added and lysates were again rotated for 24 hrs at 4°C. Beads were spun down and washed four times with lysis buffer, boiled for 3 min. in loading buffer, and loaded on a 10% SDS-PAGE gel for Western blot analysis.
Immunohistochemistry
The study group consisted of seven patients with diffuse cutaneous scleroderma (SSc) and eight healthy volunteers (NS) (Table 1). Upon obtaining informed consent and in compliance with the Institutional Review Board for Human Studies, skin biopsy samples were obtained from the affected areas (dorsal forearm) of the SSc patients. Skin biopsy samples were embedded in paraffin and used for immunohistochemistry. Five-micrometre sections were cut and mounted on Fisherbrand Superfrost Plus Microscope Slides (Fisher Scientific, Pittsburg, PA, USA). Slides were deparaffinized using Histoclear, and passed through a graded alcohol series of decreasing percentage of ethyl alcohol in water. Antigen unmasking was performed for 1 min. in a pressure cooker containing boiling water and antigen unmasking solution (Vector, Burlingame, CA, USA). Slides were washed in PBS and treated with 0.3% hydrogen peroxide for 30 min. to block endogenous peroxide activity, and then washed again in PBS. Blocking was performed with normal horse serum in PBS for 1 hr. Tissue was then incubated in primary antibody overnight at 4°C in a humidified chamber using mouse anti-caveolin-1 (sc-53564; Santa Cruz Biotechnology) at a concentration of 1:2000 in blocking solution. Primary antibody binding sites were detected by incubation with a biotinilated antimouse IgG antibody at a concentration of 1:200 in blocking solution. Biotin labelled antibody was detected using the Vectastain ABC kit (Vector), and the sites of peroxidase activity were visualized by using diaminobenzidine (DAB). The sections were then counterstained with haematoxylin. Immunostaining was detected by using light microscopy. Normal mouse IgG was used as a negative control (data not shown). Quantification was performed by two independent observers, and the intensity of staining was recorded for at least 200 individual fibroblasts using a scale of 0–3 (0 = no visible DAB staining, 1 = faint staining observable, 2 = clear brown staining, 3 = dark brown staining). The average intensity of fibroblast DAB staining was calculated based on images from random fields throughout the dermis of the whole tissue section. All the blood vessels observable in the section were quantified for caveolin-1 staining using the same scale, but counting the entire vessel as one unit.
Table 1.
Demographic characteristics of scleroderma and control skin biopsy donors matched with caveolin-1 protein levels
| Demographics | ||||||
|---|---|---|---|---|---|---|
| Sample | Avg fbl | Avg bv | Race | Gender | Age | Skin score |
| Controls | ||||||
| NS346 | 0.35 | 1.85 | AA | F | 38 | 0 |
| NS350 | 0.28 | 0.5 | AA | M | 57 | 0 |
| NS384 | 0.63 | 2.22 | C | M | 53 | 0 |
| NS380 | 0.44 | 2.64 | C | F | 56 | 0 |
| NS348 | 0.14 | 1.6 | C | F | 52 | 0 |
| NS362 | 0.73 | 2.66 | AA | F | 44 | 0 |
| NS364 | 0.51 | 1.71 | C | M | 54 | 0 |
| NS366 | 0.72 | 3 | C | M | 48 | 0 |
| Avg controls | 0.47 | 2.02 | ||||
| SSc | ||||||
| SSc377 | 0.73 | 1.5 | C | F | 50 | 20 |
| SSc361 | 0.51 | 2.6 | AA | F | 49 | 24 |
| SSc383 | 0.47 | 2.3 | C | M | 59 | 47 |
| SSc345 | 0.14 | 0.77 | AA | F | 41 | 10 |
| SSc365 | 0.69 | 2.77 | C | M | 42 | 24 |
| SSc347 | 0.09 | 1 | C | F | 60 | 41 |
| SSc379 | 0.60 | 1.25 | C | F | 56 | 15 |
| Avg SSc | 0.46 | 1.74 | ||||
The average caveolin-1 staining intensity in fibroblasts (avg fbl) and blood vessels (avg bv) are displayed for each control and scleroderma biopsy section used in this study, along with the demographic characteristics and skin score of each patient. NS: healthy control; SSc: scleroderma; avg fbl: average fibroblasts score/sample; avg bv: average blood vessels score/sample; AA: African-American; C: caucasian.
Results
SSc fibroblasts with constitutive Smad1 phosphorylation have elevated cav-1 expression
We evaluated the expression levels of cav-1, phospho-Smad1 and total Smad1 in SSc dermal fibroblasts. Age, race, and gender-matched normal and SSc cells were grown to confluence then serum starved overnight, followed by Western blot analysis of the protein levels of total and phospho-Smad1, and of cav-1. Representative results are presented in Figure 1, showing that the majority (approximately 80%) of SSc cell lines tested had higher levels of cav-1 when compared with their matched controls, and this was also confirmed at the mRNA level (data not shown). Confirming our previously published data, we found up-regulation of phosphorylated Smad1 in most of the SSc cell lines. Interestingly, cav-1 levels were up-regulated in cells with constitutive Smad1 activation, whereas cells with low phospho-Smad1 levels had decreased cav-1 gene expression. The positive correlation suggested a direct causal relationship between the expression levels of cav-1 and Smad1 activation in SSc dermal fibroblasts.
Fig 1.
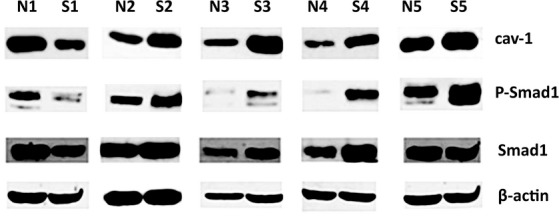
Scleroderma fibroblasts with constitutive Smad1 phosphorylation have high levels of cav-1 Primary cultures of human dermal fibroblasts from matched pairs of normal (N1-5) and diffuse scleroderma patients (S1-5) were grown to subconfluence and lysates were analysed by Western blot for caveolin-1 (cav-1), Smad1, phospho-Smad1, and β-actin.
Alk1 interacts with cav-1 in human dermal fibroblasts and TGFβ enhances this association
Our previous studies showed that, similar to endothelial cells, TGFβ-mediated activation of Smad1 in dermal fibroblasts is dependent of the Alk1-TGFβ type I receptor. To further explore the potential role of cav-1 in Smad1 activation in dermal fibroblasts we used co-immunoprecipitation to investigate the interaction between cav-1 and Alk1. Figure 2A shows that immunoprecipitation of Alk1 resulted in co-immunoprecipitation of cav-1, thus suggesting complex formation between these two proteins. To assess the role of TGFβ on the association of Alk1 with cav-1, we treated dermal fibroblasts with Alk1-overexpressing adenovirus in the presence or absence of TGFβ. Thus, quiescent cells were transduced with the virus for 48 hrs then treated for 15 min. with 2.5 ng/ml of recombinant human TGFβ. Treatment of cells with TGFβ enhanced the association between cav-1 and Alk1 (Fig. 2B), further supporting a role for cav-1 in TGFβ/Alk1/Smad1 signaling.
Fig 2.
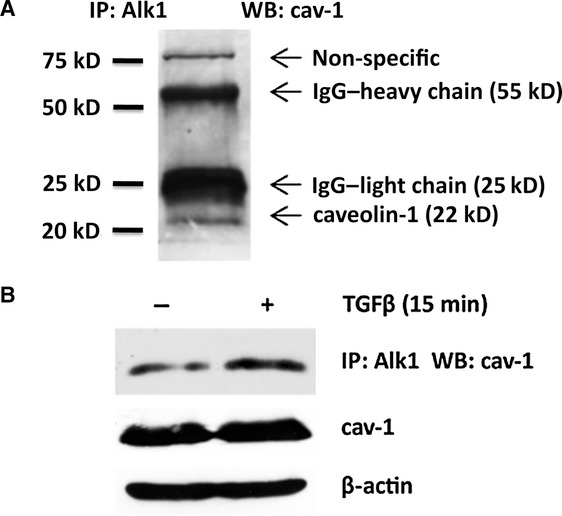
Association between cav-1 and TGFβ-RI/Alk1 in dermal fibroblasts. (A) Primary cultures of human dermal fibroblasts were grown to subconfluence and serum starved for 24 hrs. Protein from whole cell lysate was subjected to immunoprecipitation using mouse anti-Alk1 antibody (Alk1), and cav-1 was detected by immunoblotting using rabbit anti-caveolin-1 antibody (caveolin 1). The specific cav-1 protein appears in a band at 22 kD. (B) Alk1 was up-regulated in human dermal fibroblasts using adenovirus mediated overexpression, and cells were serum starved for 24 hrs and treated with TGFβ for 15 min prior to collection of whole cell lysates. Immunoprecipitation was again performed using mouse anti-Alk1 antibody, and caveolin-1 levels were detected by western blot of the Alk1 bound fraction (top panel), and input whole cell lysate (bottom panels). Western blot of whole cell lysate was stripped and reprobed for β-actin as a loading control.
Cav-1 is required and sufficient for Smad1 signaling in human dermal fibroblasts
We next sought to evaluate the role of cav-1 in activation of the TGFβ/Alk1/Smad1 pathway by loss and gain of function assays. First we determined the effects of cav-1 depletion on basal levels of phospho-Smad1 in SSc and healthy dermal fibroblasts. To do this, we decreased endogenous levels of cav-1 using specific cav-1 siRNA oligos, and examined by using Western Blot the levels of phospho-Smad1. As presented in Figure 3A, a 50% depletion of cav-1 resulted in significant down-regulation of phospho-Smad1 levels, both in SSc and normal dermal fibroblasts. To determine if cav-1 is required for TGFβ-induced Smad1 phosphorylation, we suppressed endogenous cav-1 expression in the presence and absence of TGFβ. Depletion of cav-1 in human dermal fibroblasts completely abrogated phosphorylation of Smad1 by TGFβ, suggesting that cav-1 is required for activation of the TGFβ/Smad1 pathway (Fig. 3B).
Fig 3.
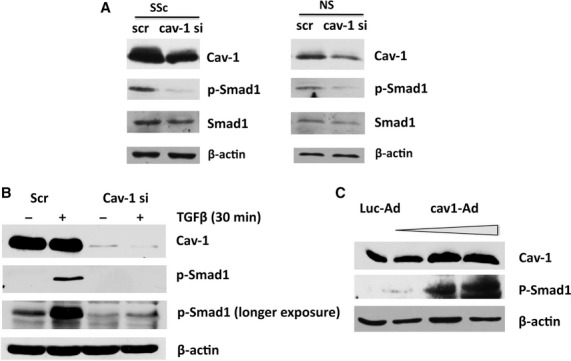
Cav-1 promotes Alk1/Smad1 signaling and is required for the TGFβ induction of Smad1 phosphorylation in dermal fibroblasts. (A) Human dermal fibroblast cultures isolated from normal (NS) and scleroderma (SSc) patients were treated with scrambled siRNA (scr) or siRNA targeted against cav-1 (cav-1 si) as described, and protein levels of cav-1 (cav-1), Smad1, phospho-Smad1, and β–actin were determined by Western blot. (B) Normal human dermal fibroblast cultures were treated with scrambled siRNA or siRNA targeted against cav-1, serum starved for 24 hrs, and treated with or without TGFβ for 30 min prior to collection of cell lysates. Levels of cav-1, phospho-Smad1, and β-actin protein were detected by Western blot. Basal P-Smad1 levels were detectable only after longer exposure compared to levels after TGFβ treatment. (C) Human dermal fibroblasts were grown to confluence and transduced with either cav1-Ad or control adenovirus (LucAd) for 24 hrs, followed by serum starvation for 24 hrs. A dose response of cav1-Ad was performed and a dose-dependent increase in phosphorylated Smad1 was detected by Western blot.
To investigate the role of cav-1 in various cellular processes, most published studies have used a cell permeable cav-1 scaffolding domain peptide (C1-SD82-101). Although this peptide was shown to mimic the effects of cav-1 protein on several signaling pathways, there is evidence suggesting that the cav-1 scaffolding domain is not sufficient to fully reproduce cav-1 functions, and it may even have inhibitory effects on cav-1 signaling [20, 21]. To further confirm the role of cav-1 in Smad1 activation we next examined the effects of full-length cav-1 overexpression in human dermal fibroblasts. For this, we transduced cells with an adenoviral vector overexpressing cav-1 (cav1-Ad) or with a control virus expressing luciferase (Luc-Ad). Cav1-Ad induced cav-1 expression in a dose dependent manner (Fig. 3C). Consistent with the siRNA results, cells with overexpression of cav-1 showed a dose-dependent increase in Smad1 activation, as seen by measuring the levels of phosphorylated Smad1, even in the absence of TGFβ stimulation. Taken together, these results demonstrate for the first time that cav-1 is a positive regulator of the TGFβ/Alk1/Smad1 signaling pathway in human dermal fibroblasts.
Cav-1 is a positive regulator of CCN2 gene expression in healthy dermal fibroblasts
Previous reports indicate that CCN2 expression is increased in SSc dermal fibroblasts at the mRNA and protein levels [22]. In our recent studies CCN2 expression correlated with activation of Smad1 in SSc dermal fibroblasts and depletion of Smad1 using siRNA in these cells resulted in normalization of CCN2 levels [3]. We next wanted to investigate whether or not cav-1 has a role in CCN2 gene expression in human dermal fibroblasts. To do this, we overexpressed full-length cav-1 in healthy dermal fibroblasts and evaluated effects on activation of Smad1 and expression levels of CCN2. Up-regulation of cav-1 to physiologically expressed levels (2- to 4-fold) induced a prominent Smad1 activation, accompanied by up-regulation of the CCN2 protein levels (Fig. 4A), thus suggesting that cav-1 is a positive regulator of CCN2 gene expression in human dermal fibroblasts. CCN2 is potently and rapidly induced by TGFβ at the transcriptional level, and published studies show that CCN2 is required for TGFβ-mediated up-regulation of collagen. Thus, we next tested whether or not cav-1 is required for TGFβ-induced CCN2 gene expression. Healthy human dermal fibroblasts were transiently transfected with siRNA oligos against cav-1 or scrambled siRNA oligos. After 72 hrs, cells were further treated with 2.5 ng/ml of recombinant TGFβ1 and then cell pellets were collected after an additional 48-hr incubation. Cav-1 siRNA treatment efficiently depleted cav-1 protein levels by more than 80%. Confirming previously published reports showing that TGFβ negatively regulates cav-1, TGFβ treatment further decreased cav-1 levels (Fig. 4B). Cells depleted of cav-1 had low basal and TGFβ-induced CCN2 protein levels, thus further confirming the positive role of cav-1 in CCN2 gene expression. Although cav-1 siRNA treatment reduced CCN2 expression, depletion of cav-1 did not completely prevent CCN2 induction by TGFβ, suggesting that other non-cav-1 dependent mechanisms may contribute to TGFβ-stimulated CCN2 gene expression.
Fig 4.
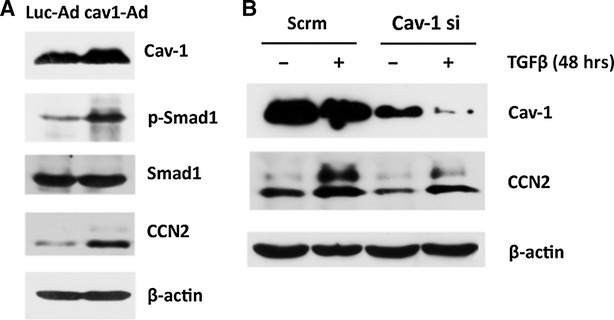
Cav-1 overexpression increases CCN2 protein levels, while cav-1 depletion decreases both endogenous and TGFβ induced CCN2 protein in normal dermal fibroblasts. (A) Human dermal fibroblasts were grown to confluence and transduced with either cav1-Ad or control adenovirus (LucAd) for 24 hrs, followed by serum starvation for 24 hrs. A dose of cav1-Ad corresponding to a 2- to 4-fold increase in cav-1 protein was used, and a representative Western blot shows levels of cav-1, Smad1, phospho-Smad1, CCN2 and β-actin (left panel). (B) Human dermal fibroblast cultures were treated with scrambled siRNA (scr) or siRNA targeted against cav-1, serum starved and treated with or without TGFβ for 48 hrs prior to collection of cell lysates. Protein levels of cav-1, CCN2, and β-actin in the lysate were determined by Western blot.
Expression of cav-1 in SSc skin in vivo
As the majority of SSc cell lines express higher levels of cav-1 than matched, healthy dermal fibroblasts (Fig. 1), we next examined cav-1 in the skin of seven SSc patients and eight healthy controls, using immunohistochemistry. The demographic characteristics of the patients are summarized in Table 1. Representative staining from the skin of SSc and healthy controls is shown in Figure 5. An average of 200 fibroblasts were counted in each specimen and scored as described in materials and methods, and the summary of the results is included in Table 1. Staining revealed heterogeneity of cav-1 expression among SSc and control skin sections. Although cav-1 was expressed at high levels in blood vessels, the majority of SSc and healthy skin fibroblasts showed low levels of cav-1 in vivo. In addition, although SSc fibroblasts had higher expression of cav-1 in culture, when matched for gender, race and age, this pattern was only observed in some of the healthy skin biopsies (Fig. 5 and Table 1). The average intensity of staining in blood vessels from SSc skin was slightly decreased compared to control, however, this result did not reach statistical significance. Our present results show that cav-1 is neither consistently down-regulated nor up-regulated in SSc skin in vivo. This result is in contradiction with the conclusion by Del Galdo et al., showing that fibroblasts from SSc biopsies express cav-1 at lower levels [17]. One potential explanation is that for that study the investigators used a small sample size of only three SSc and three control biopsies, compared to the sample size used in our study, which was larger (seven SSc and eight healthy biopsies).
Fig 5.
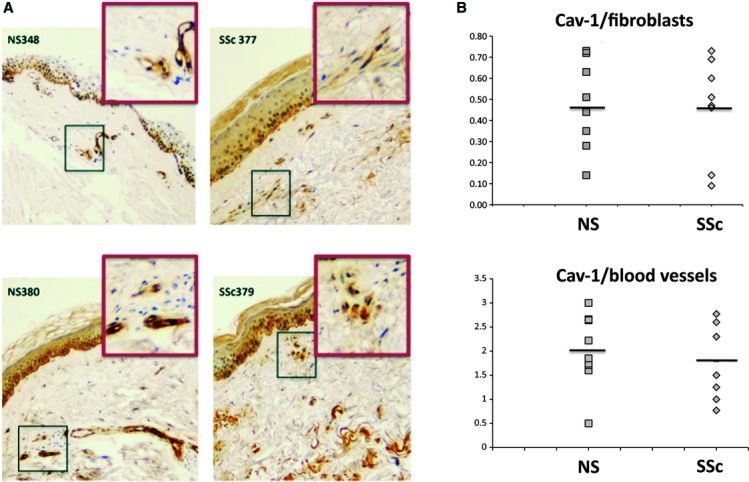
Heterogeneous expression of caveolin-1 among scleroderma and control skin sections. (A) Representative images of two healthy (NS348, NS380) and two scleroderma (SSc377, SSc379) skin biopsy sections stained with caveolin-1 antibody as described in materials and methods. Regions containing typical fibroblasts were outlined and enlarged. (B) The intensity of staining for each individual fibroblast was quantified on a scale of 0–3 for an average of 200 fibroblasts per section. Average fibroblast caveolin-1 levels were plotted for comparison of normal (NS) and scleroderma (SSc) biopsies. The intensity of caveolin-1 staining in blood vessels in each section was also quantified and plotted in the same manner.
Discussion
Activation of non-canonical TGFβ signaling pathways is a well described feature of SSc dermal fibroblasts and includes elevated expression of Smad1 and constitutive Smad1 phosphorylation. This study was undertaken to characterize the role of cav-1 overexpression in the constitutive activation of the TGFβ/Alk1/Smad1 pathway in SSc dermal fibroblasts. In contrast to the published observation showing down-regulation of cav-1 in cultured skin fibroblasts from two SSc patients [17], our data show that a significant subset of SSc dermal fibroblasts have up-regulated cav-1 expression in vitro. In addition, we found that constitutive Smad1 phosphorylation correlates with cav-1 overexpression in these cells, that cav-1 is found in a complex with the Smad1 specific TGFβ type I receptor Alk1, and that this association is further enhanced by TGFβ treatment. Furthermore, using siRNA against cav-1 and adenovirus mediated cav-1 overexpression, we demonstrated that cav-1 is a positive regulator of TGFβ/Smad1 signaling, thus suggesting that cav-1 overexpression in cultured SSc dermal fibroblasts is responsible for enhanced Smad1 signaling.
Our previously published data demonstrated that similar to the signaling complex described in endothelial cells, Smad1 activation in dermal fibroblasts involves cooperation between the TGF-β ALK5 and ALK1 receptors and the co-receptor endoglin [1, 8]. In this study we show that cav-1 is also required for the activation of the Smad1 pathway in human dermal fibroblasts. Although previous reports from other laboratories have shown that the direct interaction of the Alk5/TGFβ type I receptor with cav-1 in caveolae results in inhibition of canonical signaling [17], herein we demonstrate that cav-1 also associates with Alk1 in dermal fibroblasts and promotes non-canonical Smad1 signaling. Our results showing that cav-1 is a positive regulator of TGFβ/Smad1 signaling in dermal fibroblasts are in agreement with previously published studies in vascular smooth muscle cells, endothelial cells and hepatic stellate cells, where cav-1 was shown to enhance Smad1 signaling [18, 21, 23]. However, in a mouse myoblast cell line Smad1 signaling was not affected by cav-1, and in LPS-treated bronchial epithelial cells cav-1 down-regulation was accompanied by increased Smad2/3 and unchanged Smad1 activation [24, 25], thus confirming that cav-1 influence on signaling pathways is cell-type specific. Although our study clearly shows that in human dermal fibroblasts cav-1 is both necessary and sufficient for Smad1 phosphorylation, elucidating the exact mechanism and the complex interaction between cav-1 and components of the TGFβ signaling pathway requires further study.
We have recently shown that CCN2 is indispensable for TGF-β-induced phosphorylation of Smad1, and that in the absence of CCN2, TGF-β is not able to activate Smad1 signaling [26]. Furthermore, data from our laboratory show that Smad1 is a direct activator of CCN2 gene expression, and that Smad1 is required for TGFβ-induced CCN2 gene expression. This study provides new insight into the mechanism of CCN2 gene regulation in dermal fibroblasts. We show herein that cav-1 is a positive regulator of CCN2 protein levels, and that it contributes to basal and TGFβ-induced CCN2 gene expression. Our results are in agreement with previously published studies in hepatocytes demonstrating that cav-1 can induce CCN2 gene expression via activation of the non-canonical TGFβ/Akt signaling pathway [23]. Although Akt signaling is also enhanced by cav-1 in dermal fibroblasts, leading to collagen up-regulation, it is unlikely that this would contribute to increased CCN2 gene expression, because we have previously shown that Akt negatively regulates CCN2 in dermal fibroblasts [27]. Although cav-1 most probably induces CCN2 via activation of Smad1 signaling, we cannot exclude the possibility that other signaling pathways affected by cav-1 could also contribute to this effect. Further supporting this hypothesis, cav-1 down-regulation only partially prevented CCN2 induction by TGFβ. Additional studies are required to completely understand the mechanism of cav-1-induced CCN2 gene expression.
Decreased levels of cav-1 have been previously reported in lung fibrosis for both idiopathic pulmonary fibrosis and scleroderma interstitial lung disease, and cav-1 down-regulation has been shown to contribute to the pathogenesis of fibrotic lung disease. Investigation of cav-1 levels in scleroderma dermal fibroblasts has generated contradictory results, one report showing increased levels in SSc [14] and another report showing down-regulation [17]. Both studies used a relatively small sample size of only three SSc and normal control pairs, which could explain the discrepancies. In the present study we have examined a larger number of cell lines and found that although cav-1 can be either under-expressed or overexpressed in SSc, the majority of cultured SSc fibroblasts had higher levels of cav-1 than those in their healthy matched fibroblasts. However, when we examined the levels of cav-1 in SSc skin biopsies, we found that both SSc and healthy skin samples show heterogeneity in cav-1 staining, with no trend towards up-regulation or down-regulation in SSc. Although fibroblast phenotype in vivo can be influenced by communication with other skin cell types, these influences are lost when fibroblasts are cultured, thus potentially explaining the differences observed between our in vivo and in vitro data. Furthermore, a change in fibroblast phenotype might also be due to a selection of a subpopulation of cells with higher cav-1 expression in culture.
In conclusion, this study shows for the first time that cav-1 is a positive regulator of Smad1 signaling and CCN2 gene expression in human dermal fibroblasts. Furthermore, we found increased cav-1 levels in a subset of SSc fibroblasts with constitutive Smad1 activation, and down-regulation of cav-1 normalized Smad1 phosphorylation levels. Given the importance of CCN2 and Smad1 in the pathogenesis of SSc fibrosis, a better understanding of the mechanisms that are responsible for deregulated Smad1 signaling and CCN2 expression may contribute to development of more effective therapeutic strategies to treat this disease.
Acknowledgments
Supported in part by the grants: Scleroderma Foundation grant (AB) and NIAMS AR42334 (MT).
Author contribution
Paul Haines performed the research; analysed the data. Faye Hant contributed essential reagents and tools; analysed the data. Robert Lafyatis contributed essential reagents or tools; analysed the data. Maria Trojanowska contributed essential reagents or tools; analysed the data; designed the research study. Andreea M. Bujor designed the research study; performed the research; analysed the data; wrote the article.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Morris E, Chrobak I, Bujor A, et al. Endoglin promotes TGF-beta/Smad1 signaling in scleroderma fibroblasts. J Cell Physiol. 2011;226:3340–8. doi: 10.1002/jcp.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bujor AM, Asano Y, Haines P, et al. The c-Abl tyrosine kinase controls protein kinase Cdelta-induced Fli-1 phosphorylation in human dermal fibroblasts. Arthritis Rheum. 2011;63:1729–37. doi: 10.1002/art.30284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pannu J, Asano Y, Nakerakanti S, et al. Smad1 pathway is activated in systemic sclerosis fibroblasts and is targeted by imatinib mesylate. Arthritis Rheum. 2008;58:2528–37. doi: 10.1002/art.23698. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Leask A, Abraham DJ, et al. Heparan sulfate-dependent ERK activation contributes to the overexpression of fibrotic proteins and enhanced contraction by scleroderma fibroblasts. Arthritis Rheum. 2008;58:577–85. doi: 10.1002/art.23146. [DOI] [PubMed] [Google Scholar]
- 5.Jun JB, Kuechle M, Min J, et al. Scleroderma fibroblasts demonstrate enhanced activation of Akt (protein kinase B) in situ. J Invest Dermatol. 2005;124:298–303. doi: 10.1111/j.0022-202X.2004.23559.x. [DOI] [PubMed] [Google Scholar]
- 6.Goumans MJ, Valdimarsdottir G, Itoh S, et al. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell. 2003;12:817–28. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 7.Lebrin F, Goumans MJ, Jonker L, et al. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 2004;23:4018–28. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pannu J, Nakerakanti S, Smith E, et al. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J Biol Chem. 2007;282:10405–13. doi: 10.1074/jbc.M611742200. [DOI] [PubMed] [Google Scholar]
- 9.Abraham D. Connective tissue growth factor: growth factor, matricellular organizer, fibrotic biomarker or molecular target for anti-fibrotic therapy in SSc? Rheumatology (Oxford) 2008;47(Suppl. 5):v8–9. doi: 10.1093/rheumatology/ken278. [DOI] [PubMed] [Google Scholar]
- 10.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–91. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto M, Toya Y, Schwencke C, et al. Caveolin is an activator of insulin receptor signaling. J Biol Chem. 1998;273:26962–8. doi: 10.1074/jbc.273.41.26962. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Lee Y, Seo JE, et al. Caveolin-1 increases basal and TGF-beta1-induced expression of type I procollagen through PI-3 kinase/Akt/mTOR pathway in human dermal fibroblasts. Cell Signal. 2008;20:1313–9. doi: 10.1016/j.cellsig.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Di Guglielmo GM, Le Roy C, Goodfellow AF, et al. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–21. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 14.Tourkina E, Gooz P, Pannu J, et al. Opposing effects of protein kinase Calpha and protein kinase Cepsilon on collagen expression by human lung fibroblasts are mediated via MEK/ERK and caveolin-1 signaling. J Biol Chem. 2005;280:13879–87. doi: 10.1074/jbc.M412551200. [DOI] [PubMed] [Google Scholar]
- 15.Wang XM, Zhang Y, Kim HP, et al. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med. 2006;203:2895–906. doi: 10.1084/jem.20061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tourkina E, Richard M, Gooz P, et al. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2008;294:L843–61. doi: 10.1152/ajplung.00295.2007. [DOI] [PubMed] [Google Scholar]
- 17.Del Galdo F, Sotgia F, de Almeida CJ, et al. Decreased expression of caveolin 1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum. 2008;58:2854–65. doi: 10.1002/art.23791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santibanez JF, Blanco FJ, Garrido-Martin EM, et al. Caveolin-1 interacts and cooperates with the transforming growth factor-beta type I receptor ALK1 in endothelial caveolae. Cardiovasc Res. 2008;77:791–9. doi: 10.1093/cvr/cvm097. [DOI] [PubMed] [Google Scholar]
- 19.Bujor AM, Pannu J, Bu S, et al. Akt blockade downregulates collagen and upregulates MMP1 in human dermal fibroblasts. J Invest Dermatol. 2008;128:1906–14. doi: 10.1038/jid.2008.39. [DOI] [PubMed] [Google Scholar]
- 20.Horton MR, Radler J, Gast AP. Phase behavior and the partitioning of caveolin-1 scaffolding domain peptides in model lipid bilayers. J Colloid Interface Sci. 2006;304:67–76. doi: 10.1016/j.jcis.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 21.Wertz JW, Bauer PM. Caveolin-1 regulates BMPRII localization and signaling in vascular smooth muscle cells. Biochem Biophys Res Commun. 2008;375:557–61. doi: 10.1016/j.bbrc.2008.08.066. [DOI] [PubMed] [Google Scholar]
- 22.Shi-wen X, Pennington D, Holmes A, et al. Autocrine overexpression of CTGF maintains fibrosis: RDA analysis of fibrosis genes in systemic sclerosis. Exp Cell Res. 2000;259:213–24. doi: 10.1006/excr.2000.4972. [DOI] [PubMed] [Google Scholar]
- 23.Meyer C, Godoy P, Bachmann A, et al. Distinct role of endocytosis for Smad and non-Smad TGF-beta signaling regulation in hepatocytes. J Hepatol. 2011;55:369–78. doi: 10.1016/j.jhep.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 24.Kunzmann S, Collins JJ, Yang Y, et al. Antenatal inflammation reduces Cav-1 expression and influences multiple signaling pathways in preterm foetal lungs. Am J Respir Cell Mol Biol. 2011;45:969–76. doi: 10.1165/rcmb.2010-0519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartung A, Bitton-Worms K, Rechtman MM, et al. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol Cell Biol. 2006;26:7791–805. doi: 10.1128/MCB.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakerakanti SS, Bujor AM, Trojanowska M. CCN2 is required for the TGF-beta induced activation of Smad1-Erk1/2 signaling network. PLoS One. 2011;6:e21911. doi: 10.1371/journal.pone.0021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bujor AM, Nakerakanti S, Morris E, et al. Akt inhibition up-regulates MMP1 through a CCN2-dependent pathway in human dermal fibroblasts. Exp Dermatol. 2010;19:347–54. doi: 10.1111/j.1600-0625.2010.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


