Abstract
The epicardium has recently been identified as an active and essential element of cardiac development. Recent reports have unveiled a variety of functions performed by the embryonic epicardium, as well as the cellular and molecular mechanisms regulating them. However, despite its developmental importance, a number of unsolved issues related to embryonic epicardial biology persist. In this review, we will summarize our current knowledge about (i) the ontogeny and evolution of the epicardium, including a discussion on the evolutionary origins of the proepicardium (the epicardial primordium), (ii) the nature of epicardial–myocardial interactions during development, known to be essential for myocardial growth and maturation, and (iii) the contribution of epicardially derived cells to the vascular and connective tissue of the heart. We will finish with a note on the relationships existing between the primordia of the viscera and their coelomic epithelial lining. We would like to suggest that at least a part of the properties of the embryonic epicardium are shared by many other coelomic cell types, such that the role of epicardium in cardiac development is a particular example of a more general mechanism for the contribution of coelomic and coelomic-derived cells to the morphogenesis of organs such as the liver, kidneys, gonads or spleen.
Keywords: epicardium, cardiac development, coronary vessels, myocardium, retinoic acid, coelomic epithelium
Introduction
Cardiac development has traditionally focused on the autonomous genetic and molecular programs controlling the progressive morphogenesis of myocardial chambers (atria and ventricles). The understanding of the complex morphogenetic processes that transform the primary myocardial tube into the fully formed, four-chambered heart of the amniotes has required decades of profound studies. Moreover, the severity of cardiac congenital malformations due to the failure of these processes quite obviously supported the emphasis in the study of the cellular and molecular substrata of myocardial differentiation, growth and maturation. However, the discovery that the valvuloseptal tissue of the heart was an embryonic derivative of the endocardium immediately attracted the attention of the cardiac embryologists in the late 1970s. This finding highlighted, for the first time, the importance of non-muscular tissues during cardiac development, a concept that gained more and more attention with the first description of the participation of neural crest cells in cardiac outflow tract septation and the implication of (pro)epicardial cells in coronary development [1, 2].
The development of the epicardium has been neglected during decades. As a matter of fact, despite the pioneering work of Kurkiewicz on the origin of the epicardium (published in 1909), who suggested an extracardiac origin for this tissue, the scientific literature and the embryology textbooks continued for almost a whole century considering the epicardium as a derivative of a primitive embryonic cell layer called ‘epimyocardium’. This epimyocardium supposedly gave rise to the contractile myocardium and, on its outer surface, to the epicardial epithelium. This old fashioned and out of date concept has surprisingly survived through time in recent papers and even in the Merriam Webster Medical Dictionary online.
Luckily, along the last 30 years we have witnessed an increasing interest for the embryonic epicardium. Today, epicardial studies are a main subject in the cardiovascular developmental field, since investigators realized that the epicardium, instead of being a passive enveloping of the myocardium, is an essential player in cardiac development. The epicardium contributes cells to the vascular (coronary) and connective tissue compartment of the heart, and supplies the myocardium with soluble factors which induce myocardial growth and maturation. We will herein review the three most outstanding aspects that have been recently reported on the embryonic epicardium, namely (i) its peculiar ontogenetic and phylogenetic origin, (ii) the still poorly known nature of epicardial/myocardial molecular interactions and (iii) the substantial contribution of the epicardially derived cells (EPDC) to the vascular and connective tissue of the heart. We will finish discussing the possibility of these new findings on epicardial development representing a more general developmental mechanism, in which coelomic epithelial cells provide other visceral primordia with vascular and connective tissue progenitors as well as with specific paracrine signals.
Origin of the epicardium: ontogeny and phylogeny
As stated above, the epicardium was considered for a long time as a derivative of the outer cell layer of the primitive cardiac tube. However, as first noted by Kurkiewicz in 1909, and later demonstrated by a number of anatomists in the 1980s and 1990s, the epicardium develops from a proliferation of coelomic cells located on the pericardial side of the septum transversum or in the limit between the sinus venosus and the liver, when the septum has not yet developed. This cell cluster, called the proepicardium, can be paired or not, depending on the vertebrate model considered. In amniote vertebrates, the proepicardium is slightly more prominent to the right side of the septum transversum (mouse) and becomes an isolated single structure located at the right side [3, 4]. The separation of the (pro)epicardial lineage from the precardiac mesoderm seems to be mediated by FGF signalling transduced via Mek1/2 (MAPK kinases), while the closely located myocardium requires BMP signals and inhibition of the Mek1/2 pathway for its differentiation [5].
Proepicardial cells adhere to the bare myocardial surface through two different mechanisms, whose relative importance varies depending on the taxonomic group. Proepicardial cellular outgrowths called ‘proepicardial villi’ can directly attach to the inner curvature of the cardiac tube or can be released as free floating cells/cell clusters into the pericardial cavity. These cell clusters will eventually adhere to the bare myocardial surface. In both cases, primitive epicardial cells migrate and spread over the myocardium, enveloping the atrioventricular canal and the left ventricle first, and then progressing towards the atrium, right ventricle and the trabeculated portion of the outflow tract. The most distal, non-trabeculated outflow tract is covered by coelomic epithelium through a different mechanism, namely incorporation of the conotruncal pericardial epithelium [6]. The molecular mechanism of adhesion and migration of the proepicardial cells over the myocardium seems to be mediated by the interaction of the α4β1 integrin (expressed by the proepicardial and epicardial cells) with their ligands fibronectin and VCAM-1 (expressed by the myocardium) [7], although much more research on this aspect is necessary to understand embryonic epicardial patterns.
Thus, the heart is enveloped by a subtype of coelomic epithelium through a mechanism clearly different to that of other viscera, which are covered by the coelomic epithelium ab initio. This should be interpreted in evolutionary terms. The myocardium itself, unlike other endodermal or mesodermal viscera, is a derivative of the coelomic epithelium, the same tissue regarded as the evolutionary origin of myoepithelial hemal pumps of invertebrates (reviewed in [8]). This coelomic origin of the heart made necessary a specific, new mechanism to externally protect the organ with an epithelial cover. We nevertheless think that the epicardium should not be regarded as a mere ad hoc structure ‘invented’ along the evolution of the chordate heart to supply additional coelomic cells to the myocardium. We have suggested elsewhere that the proepicardium is an evolutionary derivative of the ancestral external glomeruli of the pronephros, a structure that is still originating the embryonic epicardium in the lampreys, a group of phylogenetically primitive vertebrates [9]. External glomeruli filtering the blood towards the coelomic cavity were the ancient excretory structures of the vertebrates, and they are still present in lamprey and amphibian larvae. Along vertebrate evolution, these external glomeruli became associated with the system of collecting tubules draining the filtrate from the coelomic cavity to the outside, giving rise to the characteristic renal corpuscle of the kidneys. According to our hypothesis, the external glomeruli of the pronephros did not completely disappear when the glomeruli became internal. Instead, they gave rise to the proepicardium, which retained the embryonic function of supplying the heart with coelomic (epicardial) cells (Fig. 1A and B).
Fig 1.
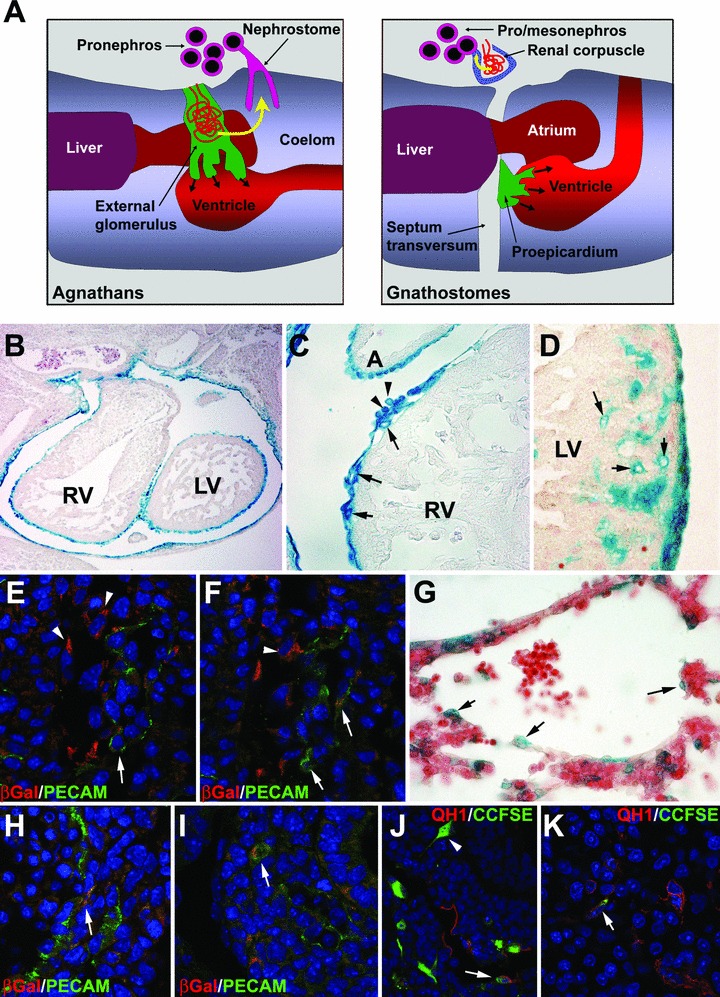
(A) Hypothesis about the origin of the proepicardium of the gnathostomes from an ancestral pronephric external glomerulus. In agnathans, the external glomerulus filters the blood of the vascular network and releases the filtrate (yellow arrow) into the coelomic cavity, where it is aspirated by the nephrostome and eliminated through the pronephric tubules. Before its differentiation, the glomerular primordium from lamprey larvae originates the epicardial cells that spread over the heart surface. In gnathostomes, the excretory glomeruli have become internal (within the renal corpuscles), the pro and mesonephros are located caudal to the heart, but the primordium of the ancestral external glomerulus (in green), now located on the pericardial side of the septum transversum, is still providing the heart with epicardial cells as the proepicardium. (B–D) Labelling of the epicardium and the cells of the epicardial lineage in a transgenic mouse model (Wt1cre/Rosa26) that expresses β-Gal in cells that have previously expressed Wt1. We can see in (B) the lining of the heart from an E11.5 mouse embryo. In (C), EPDC appear in the subepicardial space by E11.5 (arrows). It is still possible to see some cells presumably released by the proepicardium and attached to the epicardium (arrowheads). In (D) we can see epicardially derived, intramyocardial coronary vessels by E15.5 (arrows). (E), (F) Early endothelial differentiation of EPDC in the atrioventricular groove of a Wt1cre/Rosa26 mouse embryo. β-Gal co-localizes with the endothelial marker PECAM-1 (arrows). Epicardial cells are shown by arrowheads. (G) Contribution of coelomic-derived cells to the liver sinusoids in a E10.5 mouse embryo. This is a different transgenic model expressing β-Gal under control of a Wt1 promoter (described in reference 37). Some presumptive endothelial cells are positive for this marker (arrows). (H), (I) Wt1cre/Rosa26 embryos of E11.5. Co-localization of β-Gal with the endothelial marker PECAM-1 is observed in organs other than the heart, such as the intestine (H) or the lung (I). (J), (K) The endothelial differentiation of coelomic-derived cells is also shown by this experiment of staining of the visceral coelomic epithelium of quail embryos (stage HH15) with the fluorescent tracer CCFSE. After 24 (J) and 48 hrs (K), fluorescence can be observed in cells of the stomach wall that are positive for the endothelial marker QH1 (arrows). Note the presence of CCFSE-labelled cells that area apparently migrating within the tissue (arrowhead in J).
The origin of the proepicardium from a primitive pronephric glomerulus accounts for two peculiarities of this tissue, i.e. the expression of genes critical to the development of the kidneys (Wt1, Pod1/epicardin, Tbx18, podoplanin) and the high vasculogenic potential of the EPDC. The primary fate of the cells of the glomerular primordium is essentially vasculogenic, a potential that is maintained by proepicardial cells. As a result of this event, the developing heart receives an abundant supply of vascular progenitors that will build a characteristic coronary vascular bed, a prerequisite to develop a thick cardiac wall. From the evolutionary point of view, this allowed a substantial increase in cardiac size and performance.
Going further in this evolutionary approach, we have also suggested that the primitive association of the vertebrate embryonic heart with a pronephric glomerulus can be related with the heart–kidney complex described in hemichordates [8, 9]. In these animals, an excretory glomerulus is closely associated to a ‘heart’ constituted of a myoepithelial cell layer. The contractility of this ‘heart’ increases the blood pressure and allows filtration by the podocytes of the glomerulus. The dorsal localization of this heart–kidney complex traditionally ruled out any relationship of this structure with the vertebrate heart. However, this argument has turned out to be not valid, because the dorsoventral axis of hemichordates is probably reversed respect to that of chordates [10]. Thus, the epicardial–myocardial association present in vertebrates might be regarded as an evolutionary remnant of an original excretory/circulatory axis from deuterostomians.
Epicardial/myocardial cellular and molecular interactions
Soon after the epicardial lining of the heart is completed, a space constituted of amorphous extracellular matrix is produced between the embryonic epicardial cell layer and the myocardial surface. This space, called the subepicardium, is rapidly populated by mesenchymal cells originated from the epicardium through an epithelial–mesenchymal transition (EMT) event, and their subsequent migration into the subepicardial matrix. These mesenchymal cells are known as EPDC [2, 11, 12]. The factors regulating the transformation of epicardial epithelial cells into mesenchymal EPDC are still subject to study and many different candidates have been proposed. The main ones are FGFs [13], in contrast to the reported role of transforming growth factor-β signalling in the other major cardiac EMT (the endocardial transformation giving rise to cardiac valve primordia). The transcription factors Snail in mammals and Slug in avians, two main regulators of embryonic EMTs, are strongly expressed in the embryonic epicardium, and they probably are the most relevant transcriptional agents of the epicardial EMT. These factors repress the expression of the epithelial marker E-cadherin and other intercellular adhesion proteins and promote the expression of mesenchymal markers such as vimentin and N-cadherin. In the mouse epicardium, Snail is under transcriptional control of another zinc-finger transcription factor, the product of the Wilms’ tumour suppressor gene Wt1, which is an E-cadherin repressor. Wt1 is strongly expressed in the embryonic epicardium (Fig. 1B–D), where it probably controls epicardial EMT, as we will discuss below [14].
A most relevant issue is that of the molecular cross-talk that exists between epicardial/EPDC and the developing myocardium. This exchange of signals is pivotal to the promotion of the proliferation and maturation of the myocardium. In accordance with this finding, a number of mutations affecting genes exclusively expressed in the epicardium, or the mere delay of epicardial development, leads to a severe hypoplasia of the neighbouring compact myocardium [15]. This ‘thin myocardium’ frequently causes a lethal cardiac failure by midgestation.
The identities of the factor(s) secreted by the epicardium/EPDC, which are essential for the development of the compact layer, are still elusive (reviewed in [15]). In the trabecular layer proliferation is mediated by neuregulin through a heterodimer receptor constituted by ErbB2 and ErbB4, but the epicardium does not express neuregulin. The most promising epicardial candidates to induce compact layer proliferation are FGFs [16]. Conditional mutation of FGFR1 and 2 in the myocardium lead to myocardial hypoplasia, particularly in the ventricular apex [17], but this does not demonstrate that epicardially derived FGFs must be the mitogenic signal, because FGFs are also secreted by the endocardium. Moreover, FGF9 and FGF16, two candidates proposed to be the main signal for myocardial proliferation, are also secreted by the endocardium [17, 18; discussed in 15]. Finally, FGFR inhibition does not decrease the levels of phosphorylated ERKs in the E10.5 heart [19], suggesting that FGF-independent transduction pathways converge in this intermediate of the MAPK proliferation pathway.
A more elaborated possibility is that the epicardial cells secrete Wnt9b which would stabilize β-catenin in the myocardial cells through the canonical Wnt pathway, leading to myocardial FGF secretion and mitogenic autocrine signalling via FGFRs [20]. Related with this observation is the fact that epicardial β-catenin is required for coronary artery formation [21]. Other recently reported possibilities to explain this epicardial–myocardial signalling would be PDGFs [22] and sonic hedgehog [23].
Anyhow, what seems to be well established is that the mitogenic signal(s) produced by the epicardium are dependent on erythropoietin (Epo) and retinoic acid (RA) receptor signalling [24, 25]. Epo and its receptor EpoR are expressed in the epicardium, and their deficiency leads to myocardial thinning [26]. Epo signalling is thus required for the production of the epicardial mitogenic signal [25]. On the other hand, in the absence of RA signalling, the myocardium differentiates prematurely and stops proliferation. RALDH2, the main RA producer enzyme in the mesodermal tissue, is highly expressed in the epicardium and EPDC. However, RA is not directly involved in an epicardial-to myocardial signalling mechanism, because it is probably acting on the epicardium itself in an autocrine fashion [17, 20, 27]. Deletion of the RXRα receptor in the myocardium or endocardium has no effect, whereas the loss of function of this receptor in the epicardium leads to thin myocardium. RA production by the epicardium could be related with FGF signalling because RA induces epicardial expression of FGF9 [17] and FGF2 [20].
A potentially interesting issue is the possibility that the signalling function of the embryonic epicardium on the myocardium can extend beyond foetal life. This is supported by the observation that myocardial cells cocultured with epicardial cells preserve better the differentiated phenotype, suggesting some kind of physiological epicardial–myocardial interaction in the adult heart [28].
EPDC contribute to coronary vessels and cardiac connective tissue
As stated above, EPDC are mesenchymal cells that delaminate from the surface epicardium and populate the subepicardial matrix, first, and then invade the myocardial wall. These cells contribute to the vascular tissue of the heart, including the coronary endothelium, the smooth muscle media and the fibrous adventitia of at least a part of coronary blood vessels [2, 11, 12, 29, 30]. The contribution of EPDC to the coronary endothelium has been largely debated. The use of murine lineage tracing models has shown a relatively little number of epicardially derived endothelial cells in the adult coronary vessels. However, early coronary vessels of mouse and avian embryos seem to bear a significant EPDC-derived endothelial contribution [1, 2, 29, 31; see also Fig. 1E and F]. It is conceivable that other sources of endothelial cells, e.g. circulating progenitors, can progressively replace the embryonic endothelium of the coronary vessels in postnatal stages, thus accounting for this difference between the embryonic and the adult coronary endothelium. It is widely accepted that EPDC contribute significantly to the media of coronary vessels. However, epicardial lineage tracing in mouse has revealed that not all the smooth muscle cells of coronary walls are epicardial derivatives [20], opening the possibility for the contribution of other cell progenitors. Even more intriguing is the case of EPDC contribution to coronary adventitial and intramyocardial fibroblasts, because it raises a question on the developmental origin of myocardial connective tissue and suggests a potential role for EPDC in the establishment of this non-muscular interstitial cardiac compartment. Another unsolved question is that of the multipotent properties of EPDC. Are endothelial cells, smooth muscle cells and fibroblasts all derivatives of single multipotent epicardial progenitor cell type? Is there any molecular heterogeneity in epicardial cells that could explain the different developmental fates of their derivatives?
On a final note, EPDC have also claimed to be potential progenitors of myocardial cells in vivo, as suggested by lineage tracing studies [32, 33]. However, most of these results lie on the assumption that the lineage tracing markers used (Wt1 and Tbx18, respectively) are not expressed in myocardial progenitors, a conclusion challenged by alternative findings and interpretations (see [34] for a discussion of this issue).
Wt1 is a crucial gene in the regulation of the developmental fate of the EPDC. Wt1 probably plays a role, not only for EMT promotion, but also for regulation of the origin of cardiovascular lineages, in part through the regulation of the cadherin-repressor Snail. In a model of epicardial-specific deletion of Wt1, coronary arteries fail to form [14]. In this paper, the authors report on the in vitro construction of embryoid bodies from Wt1-null embryonic stem cells. As expected, Snail and E-cadherin were down- and up-regulated, respectively, in these embryoid bodies. Quite surprisingly, Wt1-deficient embryoid bodies lacked important markers of endothelial, haemopoietic and myocardial cells, such as VEGFR-2, VE-cadherin, Hbb-b1, Nkx2.5, Hand1 or Isl1. However, when Snail expression was forced in Wt1-deficient embryoid bodies, the expression of the cardiovascular markers was rescued. These findings suggest that a process of EMT, dependent on Wt1 and Snail, is required in this system to give rise to cardiovascular progenitors.
Finally, a question that will deserve attention in the near future is the possibility of using epicardial ‘rejuvenation’ to repair and neovascularize the damaged heart, a regenerative approach that would allow these cells for the reacquisition of their embryonic multipotent potential. In this context, treatment of cultured adult epicardial cells with thymosin-β4 or prokineticin apparently results in their recovery of pluripotentiality and their ability to transdifferentiate into endothelial or smooth muscle cells [35, 36].
Epicardium as the tip of the iceberg: coelomic-derived cells can be essential for vascularization, growth and maturation of the visceral compartment
We have previously discussed EPDC involvement in early cardiac vascularization and growth, and have also suggested that the vascular fate of EPDC is probably related to the evolutionary origin of the epicardium from an ancestral external pronephric glomerulus. The transcription factor Wt1 could be a master regulator of this process by promoting EMT and modulating EPDC differentiation into different vascular cell lineages. However, we think that these observations might just be part of a more general mechanism of interaction between cells derived from the embryonic coelomic epithelium and other cell types constituting the developing viscera such as the liver, kidneys, gonads or spleen. Interestingly, all these viscera bear a Wt1-expressing coelomic epithelium and Wt1-null mice display dramatic anomalies in all these tissues [37]. We think that coelomic-derived cells can be playing a dual role in visceral development, first providing signals for growth and maturation and then supplying vascular progenitors. This relationship between signalling and vascular functions in visceral development has been already described for the liver, where vascular progenitors stimulate liver growth before differentiating in vessels [38]. These vascular progenitors have been found to derive from the coelomic epithelium [39; see also Fig. 1G]. Preliminary lineage studies of the Wt1-expressing cells and direct fluorescent coelomic epithelium labelling seem to confirm this generalized coelomic contribution to the visceral vascularization (unpublished results from our laboratory, see Fig. 1H–K).
The origin of endothelial progenitors (angioblasts) could thus be a process much more extended in time than previously supposed. The origin of a wave of angioblasts from the primitive mesoderm (somites and splanchnopleura) shortly after gastrulation is a well-established embryonic phenomenon [40]. This event also involves an EMT, and it is probably Snail- but not Wt1 dependent (Wt1 expression is initiated relatively late during development, around E9.5 in the mouse). The main somatic vessels (aorta, cardinal veins) and the endocardium would be derived from this first vasculogenic wave. According to our hypothesis, during the development of the viscera from endodermal outgrowths (e.g. liver, pancreas) or from mesenchymal condensations (e.g. spleen, kidneys and gonads), the coelomic epithelium covering these viscera would supply them with mesenchymal cells with a dual function, to sustain their growth and to contribute to their vascularization. This second wave of angioblast differentiation would be both, Snail- and Wt1 dependent, with Wt1 acting as a local activator of Snail, and inducing in this way the EMT of the coelomic epithelium. This model is consistent with the phenotype of Wt1-null embryos, as well as with the published data on the epicardial function of Wt1 and the reported signalling activities of endothelial progenitors. Future studies using Wt1 lineage tracing methods will allow further research on this topic.
Concluding remarks
Epithelial–mesenchymal interactions constitute a leitmotiv of vertebrate development, being involved in the morphogenesis of organs so diverse as limbs, teeth or lungs. On the other hand, the transformation of epithelial cells into invasive mesenchymal cells is an essential developmental process that accounts for the formation of multiple cell populations, such as the neural crest or the limb myoblasts and sclerotomic cells migrating from the epithelial somite. It is surprising that both processes have not been recognized in the epicardium until relatively recent times. The epicardium plays a very active role in cardiac development, as probably does the coelomic epithelium lining other developing viscera. To obtain the whole picture of these interactions will be a fascinating task for the near future, as it will be also exciting to appraise the clinical applications that can be derived from this knowledge.
Acknowledgments
This work was supported by grants BFU2008–02384, BFU2009–07929 and SAF2008–1883, (Ministerio de Ciencia e Innovación), RD06/0010/0015 (TerCel network, ISCIII), RD96/0014/1009 (RECAVA network, ISCIII), P08-CTS-03618 (Junta de AndalucÌa) and LSHM-CT-2005–018630 (VI framework, UE). We thank Andrea Mattioti, Daniel Castellanos and Agustina Torres for their technical support and also D. Navas for his assistance with confocal microscopy.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci USA. 1992;89:9504–8. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pérez-Pomares JM, Macías D, García-Garrido L, et al. The origin of the subepicardial mesenchyme in the avian embryo, an immunohistochemical and quail-chick chimera study. Dev Biol. 1998;200:57–68. doi: 10.1006/dbio.1998.8949. [DOI] [PubMed] [Google Scholar]
- 3.Männer J, Pérez-Pomares JM, Macías D, et al. The origin, formation and developmental significance of the epicardium, a review. Cells Tiss Org. 2001;169:89–103. doi: 10.1159/000047867. [DOI] [PubMed] [Google Scholar]
- 4.Schulte I, Schlueter J, Abu-Issa R, et al. Morphological and molecular left-right asymmetries in the development of the proepicardium: a comparative analysis on mouse and chick embryos. Dev Dyn. 2007;236:684–95. doi: 10.1002/dvdy.21065. [DOI] [PubMed] [Google Scholar]
- 5.Van Wijk B, Van Den Berg G, Abu-Issa R, et al. Epicardium and myocardium separate from a common precursor pool by crosstalk between bone morphogenetic protein- and fibroblast growth factor-signaling pathways. Circ Res. 2009;105:431–41. doi: 10.1161/CIRCRESAHA.109.203083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pérez-Pomares JM, Phelps A, Sedmerova M, et al. Epicardial-like cells on the distal arterial end of the cardiac outflow tract do not derive from the proepicardium but are derivatives of the cephalic pericardium. Dev Dyn. 2003;227:56–68. doi: 10.1002/dvdy.10284. [DOI] [PubMed] [Google Scholar]
- 7.Sengbusch JK, He W, Pinco KA, et al. Dual functions of [alpha]4[beta]1 integrin in epicardial development: initial migration and long-term attachment. J Cell Biol. 2002;157:873–82. doi: 10.1083/jcb.200203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez-Pomares JM, González-Rosa JM, et al. Building the vertebrate heart – an evolutionary approach to cardiac development. Int J Dev Biol. 2009;53:1427–43. doi: 10.1387/ijdb.072409jp. [DOI] [PubMed] [Google Scholar]
- 9.Pombal MA, Carmona R, Megías M, et al. Epicardial development in lamprey supports an evolutionary origin of the vertebrate epicardium from an ancestral pronephric external glomerulus. Evol Dev. 2008;10:210–6. doi: 10.1111/j.1525-142X.2008.00228.x. [DOI] [PubMed] [Google Scholar]
- 10.Lowe CJ, Terasaki M, Wu M, et al. Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biol. 2006;4:e291. doi: 10.1371/journal.pbio.0040291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, et al. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res. 1998;82:1043–52. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 12.Dettman RW, Denetclaw W, Ordahl CP, et al. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intramyocardial fibroblasts in the avian heart. Dev Biol. 1998;193:169–81. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- 13.Morabito CJ, Dettman RW, Kattan J, et al. Positive and negative reguation of epicardial-mesenchymal transition during avian heart development. Dev Biol. 2001;234:204–15. doi: 10.1006/dbio.2001.0254. [DOI] [PubMed] [Google Scholar]
- 14.Martínez-Estrada OM, Lettice LA, Essafi A, et al. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat Genet. 2010;42:89–93. doi: 10.1038/ng.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sucov HM, Gu Y, Thomas S, et al. Epicardial control of myocardial proliferation and morphogenesis. Pediatr Cardiol. 2009;30:617–25. doi: 10.1007/s00246-009-9391-8. [DOI] [PubMed] [Google Scholar]
- 16.Pennisi DJ, Ballard VL, Mikawa T. Epicardium is required for the full rate of myocyte proliferation and levels of expression of myocyte mitogenic factors FGF2 and its receptor, FGFR-1, but not for transmural myocardial patterning in the embryonic chick heart. Dev Dyn. 2003;228:161–72. doi: 10.1002/dvdy.10360. [DOI] [PubMed] [Google Scholar]
- 17.Lavine KJ, Yu K, White AC, et al. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Lu SY, Sheikh F, Sheppard PC, et al. FGF-16 is required for embryonic heart development. Biochem Biophys Res Commun. 2008;373:270–4. doi: 10.1016/j.bbrc.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corson LB, Yamanaka Y, Lai KM, et al. Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development. 2003;130:4527–37. doi: 10.1242/dev.00669. [DOI] [PubMed] [Google Scholar]
- 20.Merki E, Zamora M, Raya A, et al. Epicardial retinoid X receptor is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci USA. 2005;102:18455–60. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zamora M, Männer J, Ruiz-Lozano P. Epicardium-derived progenitor cells require beta-catenin for coronary artery formation. Proc Natl Acad Sci USA. 2007;104:18109–14. doi: 10.1073/pnas.0702415104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang J, Gu Y, Li P, et al. PDGF-A as an epicardial mitogen during heart development. Dev Dyn. 2008;237:692–701. doi: 10.1002/dvdy.21469. [DOI] [PubMed] [Google Scholar]
- 23.Lavine KJ, Long F, Choi K, et al. Hedgehog signaling to distinct cell types differentially regulates coronary artery and vein development. Development. 2008;135:3161–71. doi: 10.1242/dev.019919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen TH, Chang TC, Kang JO, et al. Epicardial induction of fetal cardiomyocyte proliferation via a retinoic acid-inducible trophic factor. Dev Biol. 2002;250:198–207. doi: 10.1006/dbio.2002.0796. [DOI] [PubMed] [Google Scholar]
- 25.Stuckmann I, Evans S, Lassar AB. Erythropoietin and retinoic acid, secreted from the epicardium, are required for cardiac myocyte proliferation. Dev Biol. 2003;255:334–49. doi: 10.1016/s0012-1606(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 26.Wu H, Lee SH, Gao J, et al. Inactivation of erythropoietin leads to defects in cardiac morphogenesis. Development. 1999;126:3597–605. doi: 10.1242/dev.126.16.3597. [DOI] [PubMed] [Google Scholar]
- 27.Kang JO, Sucov HM. Convergent proliferative response and divergent morphogenic pathways induced by epicardial and endocardial signaling in fetal heart development. Mech Dev. 2005;122:57–65. doi: 10.1016/j.mod.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Eid H, Larson DM, Springhorn JP, et al. Role of epicardial mesothelial cells in the modification of phenotype and function of adult rat ventricular myocytes in primary coculture. Circ Res. 1992;71:40–50. doi: 10.1161/01.res.71.1.40. [DOI] [PubMed] [Google Scholar]
- 29.Pérez-Pomares JM, Carmona R, González-Iriarte M, et al. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int J Dev Biol. 2002;46:1005–13. [PubMed] [Google Scholar]
- 30.Mellgren AM, Smith CL, Olsen GS, et al. Platelet-derived growth factor receptor beta signaling is required for efficient epicardial cell migration and development of two distinct coronary vascular smooth muscle cell populations. Circ Res. 2008;103:1393–401. doi: 10.1161/CIRCRESAHA.108.176768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guadix JA, Carmona R, Muñoz-Chápuli R, et al. In vivo and in vitro analysis of the vasculogenic potential of avian proepicardial and epicardial cells. Dev Dyn. 2006;235:1014–26. doi: 10.1002/dvdy.20685. [DOI] [PubMed] [Google Scholar]
- 32.Cai CL, Martin JC, Sun Y, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–8. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou B, Ma Q, Rajagopal S, Wu SM, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–13. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christoffels VM, Grieskamp T, Norden J, et al. Tbx18 and the fate of epicardial progenitors. Nature. 2009;458:E8–9. doi: 10.1038/nature07916. [DOI] [PubMed] [Google Scholar]
- 35.Smart N, Risebro CA, Melville AA, et al. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–82. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 36.Urayama K, Guilini C, Turkeri G, et al. Prokineticin receptor-1 induces neovascularization and epicardial-derived progenitor cell differentiation. Arterioscler Thromb Vasc Biol. 2008;28:841–9. doi: 10.1161/ATVBAHA.108.162404. [DOI] [PubMed] [Google Scholar]
- 37.Moore AW, McInnes L, Kreidberg J, et al. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development. 1999;126:1845–57. doi: 10.1242/dev.126.9.1845. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto K, Yoshitomi H, Rossant J, et al. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–63. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 39.IJpenberg A, Pérez-Pomares JM, Guadix JA, et al. Wt1 and retinoic acid signaling are essential for stellate cell development and liver morphogenesis. Dev Biol. 2007;312:157–70. doi: 10.1016/j.ydbio.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Dieterlen-Lièvre F, Pardanaud L, Bollerot K, et al. Hemangioblasts and hemopoietic stem cells during ontogeny. C R Biol. 2002;325:1013–20. doi: 10.1016/s1631-0691(02)01515-9. [DOI] [PubMed] [Google Scholar]


