Abstract
The clinical relevance of human leucocyte antigen-G (HLA-G) has been postulated in malignancies. Hepatocellular carcinoma (HCC) is a major contributor to cancer incidence and mortality worldwide; however, potential roles of HLA-G in HCC remain unknown. In the current study, HLA-G expression in 219 primary HCC lesions and their adjacent non-tumourous samples was analysed with immunohistochemistry. Correlations among HLA-G expression and various clinical parameters were evaluated. Meanwhile, functional analysis of transfected cell surface HLA-G expression on NK cell cytolysis was performed in vitro. HLA-G expression was observed in 50.2% (110/219) of primary HCC lesions, and undetectable in corresponding adjacent normal liver tissues. HLA-G expression was found in 37.8%, 41.9% and 71.4% of stage I, II and III HCC lesions, respectively. Data revealed that HLA-G expression in HCC was strongly correlated to advanced disease stage (I versus II, P= 0.882; I versus III, P= 0.020; II versus III, P= 0.037). HLA-G expression was also more frequently observed in elder patients (≥median 52 years, 57.5%versus 43.4%, P= 0.004). Meanwhile, plasma soluble HLA-G in HCC patients was significantly higher than that in normal controls (median, 92.49U/ml versus 9.29U/ml, P= 0.000). Functional assay showed that HLA-G expression in transfected cells could dramatically decrease the NK cell cytolysis (P= 0.036), which could be markedly restored by the blockade of HLA-G (P= 0.004) and its receptor ILT2 (P= 0.019). Our finding indicated that HLA-G expression was strongly correlated to advanced disease stage, and more frequently observed in elder patients. Its relevance to HCC progression might be result from the inhibition of NK cell cytolysis.
Keywords: HLA-G, hepatocellular carcinoma, NK cell, cytotoxicity
Introduction
A variety of strategies are developed by tumour cells to avoid recognition by different immune effector mechanisms [1]. Alteration of human leucocyte antigen (HLA) class I cell surface expression is one of the mechanisms most widely used by tumour cells, which seems to provide tumour cells with a mechanism to escape cytotoxic T lymphocyte recognition and destruction. HLA deficient tumour clones can escape T-cell immune responses, but are in theory more susceptible to elimination by NK cells [2]. Unfortunately, tumours appear and develop by uncontrolled growth, metastatic progression and poor clinical outcome. It seems that, specific HLA loss could provide the escape from cellular immunity, while retaining at the same time the inhibitory effects on NK cells [3]. In this scenario, tumour tissue specific up-regulated HLA-G plays a vital role. Ample evidence indicated that up-regulation of the HLA-G in tumour cells is involved in every phase of cancer immunoediting including elimination, equilibrium and escape [4]. During the procession of tumorigenesis, various microenvironmental factors such as gene demethylation and histone acetylation, hypoxia, inflammatory stimuli (via NF-κB) and the cytokines including interleukin-10 and interferon-α or γ, could up-regulate HLA-G transcriptional activation and protein expression [5–9]. However, the exact time-point of HLA-G expression in tumour cells remains elusive.
Unlike classical HLA class I molecules, seven HLA-G isoforms including four membrane-bound (HLA-G1–G4) and three soluble HLA-G isoforms (HLA-G5–G7) are generated by alternative splicing its primary transcripts [10, 11]. Additionally, another soluble form of HLA-G molecule could be generated by shedding of the proteolytically cleaved surface HLA-G1 (sHLA-G1) [12]. Both the membrane-bound and sHLA-G isoforms were postulated as important immunotolerants. HLA-G could suppress the functions of various immune cells such as NK cells, CD4+ and CD8+ T lymphocytes and dendritic cells (DC) by binding to the cell surface expressed receptors including ILT2 (CD85j, LIR-1), ILT4 (CD85d, LIR-2) and KIR2DL4 (CD158d) [13]. Furthermore, HLA-G involved suppressor cells such as HLA-G induced regulatory T cells, DCs and NK cells, or even the HLA-G1 tumour cells, have a long-term immune modulatory function to block the immune effectors [14].
Apart from initially addressed in development of foetal maternal tolerance during pregnancy, clinical implication of HLA-G has been involved in a broad spectrum of physio-pathological situations [15]. In normal conditions, HLA-G is expressed in foetal trophoblast cells, and in other tissues such as adult thymic medulla, cornea, nail matrix, pancreatic islets, erythroid and endothelial precursors, and mesenchymal stem cells [16–22]. Importantly, an increasing number of studies have highlighted the clinical relevance of HLA-G expression in cancer. Since Paul et al.[23] described the expression of HLA-G in melanoma for the first time, augmented HLA-G expression in situ was observed in nearly 20 types of tumours. HLA-G was preferentially detected in the tumour tissue and only rarely in the adjacent normal tissue, suggesting its specific association with tumour growth and progression [24, 25].
To date, little information was available for the clinical relevance of HLA-G expression in hepatocellular carcinoma (HCC). In this study, HLA-G expression in primary HCC lesions was analysed, and its correlation to clinical parameters was evaluated. Furthermore, functions of transfected HLA-G expression in HCC cell line Hep-G2 on NK cell cytolysis were also analysed.
Materials and methods
Patients and specimens
Primary HCC lesions and their adjacent non-tumourous tissues were obtained from 219 consecutive patients who were diagnosed and treated between November 2000 and January 2008 at Taizhou Hospital of Zhejiang Province. None of the patients received preoperative anticancer treatment. HCC diagnosis was based on World Health Organization criteria [26]. Tumour differentiation was defined according to the Edmondson grading system [27]. Tumour staging was determined according to the sixth edition of the tumour-node metastasis (TNM) classification of the International Union Against Cancer [28]. Patient data collected included age, gender, date of initial diagnosis, histological diagnosis, tumour grade and clinical stage. All tissue specimens underwent a microscopic confirmation for pathological features prior to their inclusion in the study. This study was performed following an Institutional Ethics Review Board approved protocol and informed consent was obtained from all patients.
Tissue microarray and immunohistochemistry
After screening haematoxylin and eosin stained slides for optimal tumour content, we constructed tissue microarray (TMA) slides (Shanghai Biochip Company, Ltd., Shanghai, China). Two cores were taken from each formalin-fixed, paraffin-embedded HCC samples by using punch cores that measured 1.0 mm in greatest dimension from the centre of tumour foci. Immunohistochemistry was performed by a two-step method using primary antibody including heat-induced antigen-retrieval procedures. TMA slides were dewaxed in xylene and rehydrated through a graded series of ethanol. After de-paraffinization, antigen retrieval treatment was performed at 120°C for 5 min. in a 10 μM sodium citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked by using a 3% hydrogen peroxide solution at room temperature for 15 min. Then, anti-HLA-G mAb 4H84 (1:300, Exbio, Prague, Czech Republic) was applied and incubated at 4°C overnight. After that, a thorough washing in a 0.01 M phosphate-buffered saline (PBS) solution was performed. Subsequently, binding sites of the primary antibody were visualized using a Dako EnVison kit (Dako, Glostrup, Denmark). Finally, sections were counterstained with haematoxylin and mounted with glycerol gelatin. Cytotrophoblast from first trimester human placenta served as a HLA-G1 control and negative controls were achieved by including isotype matched IgG in immunostaining. HLA-G expression in partial samples (n 5 20) was tested by Western blot using the mAb 4H84 in case-matched fresh HCC lesions.
Evaluation of staining
HLA-G staining in HCC tissues was determined by three pathologists. The pathologists were blinded to any clinical details related to the patients. Membrane or/and cytoplasmic expression of HLA-G were interpreted as positive. Percentage of HLA-G1 tumour cells was determined by each observer, and the average of three scores was calculated. Percentage of HLA-G expression was graded as negative for the absence of HLA-G staining, and lesions with positive HLA-G staining was grouped as 1–25%, 26–50%, 51–75% and >75%, respectively. The percentage of positive cells was assigned a value based on the presence or absence of HLA-G staining, irrespective of staining intensity. For the HLA-G staining control, first trimester cytotrophoblast sections were used. The HLA-G intensity was scored from 0 to 3, with a score of 3 for intensity comparable to the staining of the cytotrophoblast, 0 comparable to the staining of the corresponding negative control using an isotype matched IgG1, and 1 and 2 as gradations between the two.
Tissue protein extraction and Western blot analysis
For preparation of protein extracts, 18 case-matched fresh primary HCC lesions and two normal liver tissues were crushed with a mortar under liquid nitrogen. Harvested cells were washed three times with cold PBS. Cell pellets were collected and lysed with lysis buffer (pH7.4, 50 μM Tris-base, 150 μM NaCl, 1mM ethylenediaminetetraacetic acid, 1% Triton X-100, PMSF 1 mM) with the final concentration of 1 × 107 cells/ml. After centrifugation at 15,000 ×g at 4°C for 30 min., cell lysate aliquots were separated in 10% SDS-PAGE gel. All samples were heated for 5 min. at 100°C before loading. Proteins were then electroblotted onto PVDF membranes (Millipore, Bedford, MA, USA) and blocked by incubation with PBS containing 5% non-fat dry milk for at least 4 hrs. After blocking, membranes were washed in PBS containing 0.2% Tween-20, three times and then probed with the HLA-G specific mAb 4H°4 (Exbio) overnight at 4°C and washed with 0.2% Tween-PBS three times. The membranes were subsequently incubated for 30 min. at room temperature with Peroxidase/DAB + Rabbit /Mouse (Dako), washed thoroughly with 0.1% Tween-PBS. Finally, membranes were developed with Dako REAL™ EnVision™ Detection System (Dako) for 1–3 min. Samples from JEG-3 and JAR cells (ATCC, Rockville, MD, USA) were used as positive and negative controls, respectively. A mouse IgG1 isotype antibody (1:1000, Exbio) and anti-Calnexin (a house keeping protein, molecular weight: 90 kD) mAb (1:1000, Stressgen, Victoria, BC, Canada) were used as internal controls, respectively (data not shown).
sHLA-G ELISA
sHLA-G concentrations were determined with the sHLA-G specific ELISA kit (sHLA-G kit; Exbio), which measures sHLA-G1 and HLA-G5. Each sample (50 μl) was measured in triplicates. The optical densities were measured at 450 nm (Spectra Max 250, Molecular Devices, Sunnyvale, CA, USA). The final concentration was determined by optical density according to the standard curves (range: 0–125 U/ml). When the concentration exceeds 125 U/ml, diluted samples were used and dilution factors were considered to calculate the sHLA-G concentration. The detection limits were 1 U/ml. Details of the performance were according to the manufacturer’s instruction.
HLA-G transfection of the HCC cell line Hep-G2
The Hep-G2 cells were transfected with the recombinant pVITRO2-mcs vector (Invivogen, San Diego, CA, USA) containing HLA-G1 using Lipofectamine®2000 reagent (Invitrogen, Grand Island, NY, USA) according to the manual instructions. Details of transfection were described previously [29]. Transfectants were screened with hygromycin B (Amresco, Solon, OH, USA) and HLA-G expression was monitored by flow cytometry (BD FACSCalibur, San Jose, CA, USA) with MEM-G/09 and Western blot with mAb 4H84. Meanwhile, HLA I antigen expression was also analysed by flow cytometry with mAb W6/32 (Exbio) and by Western blot with mAb anti-HLA-A, B, C (MBL, Woburn, MA, USA). Both cell surface HLA-G and HLA I expression status were checked with flow cytometry for each experiment.
Lactic dehydrogenase releasing cytotoxicity and antibody blocking assay
Cytotoxicity was performed with CytoTox96® Non-Radioactive Cytotoxicity Assay Kit (Promega, Madison, WI, USA). Details of the performance were as described previously [29]. HLA-G was stably expressed in transfected cells. In this study, the NK cell sensitive target K562 cells were used as a positive control. The effector/target ratio was optimized (data not shown). For antibody blocking assay, the amount of anti-HLA-G mAb 87G, anti-HLA class I mAb W6/32 and HLA-G receptor anti-ILT2 mAb GHI/75 (Biolegend, San Diego, CA, USA) were optimized, where 10 μg/ml was applied for each antibody. Experiments were performed in quadruplicate with an effector/target ratio of 20:1, and the results were expressed as percentage of specific lysis ± S.D.
Statistical analysis
Statistical analysis was performed with SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). Correlations between the HLA-G expression and clinical parameters were calculated with Pearson chi-square test. Comparison of cytotoxicity differences and age distribution among groups were analysed for significance by the two-tailed Student’s t-test. Difference of sHLA-G between groups was analysed with Mann-Whitney U-test. P< 0.05 was considered to be significant.
Results
HLA-G expression in primary HCC lesions
Overall, 50.2% (110/219) of HCC lesions was classified as HLA-G+ (Table 1). In the malignant tumour sections, the intensity of staining varied from tumour to tumour and from one area to another within the same tumour. Heterogeneous staining was observed in HCC lesions. Some tumours showed focal patchy positive staining, and others displayed uniform staining pattern in tumour nests. Positive staining was observed in both cell membrane and cytoplasm region. Cytotrophoblasts were used as internal positive control for HLA-G expression (data not shown). No staining was detected in adjacent normal liver tissues and tissue sections incubated with irrelevant mouse IgG1 (Fig. 1A).
Table 1.
Association of HLA-G expression in HCC lesions with clinicopathological parameters
| Variables | No. of cases | HLA-G expression | P* | ||||
|---|---|---|---|---|---|---|---|
| Negative (%) | 1–25% | 26–50% | 51–75% | >75% | |||
| Gender | |||||||
| Male | 186 | 88 (47.3) | 16 | 13 | 22 | 47 | 0.264 |
| Female | 33 | 21 (63.6) | 0 | 2 | 2 | 8 | |
| Age | |||||||
| ≤Median (52 years) | 113 | 64 (56.6) | 11 | 10 | 6 | 22 | 0.004 |
| ≥Median | 106 | 45 (42.5) | 5 | 5 | 18 | 33 | |
| Tumour differentiation | |||||||
| I | 56 | 29 (51.8) | 5 | 4 | 3 | 15 | 0.587† |
| II | 145 | 73 (50.3) | 9 | 8 | 19 | 36 | |
| III | 18 | 7 (38.9) | 2 | 3 | 2 | 4 | |
| TNM stage | |||||||
| I | 37 | 23 (62.2) | 4 | 3 | 5 | 2 | 0.029‡ |
| II | 31 | 18 (58.1) | 3 | 5 | 4 | 1 | |
| III | 21 | 6 (28.6) | 1 | 4 | 3 | 7 | |
Comparison of HLA-G expression status between or among each variable using the Pearson chi-square test.
The overall P-value among tumour differentiation stage I, II and III is 0.587; P-values for the comparison between I and II, between I and III, between II and III are 0.575, 0.624 and 0.400, respectively.
The overall P-value among TNM stage I, II and III is 0.029; P-values for the comparison between I and II, between I and III, between II and III are 0.882, 0.020 and 0.037, respectively.
Fig 1.
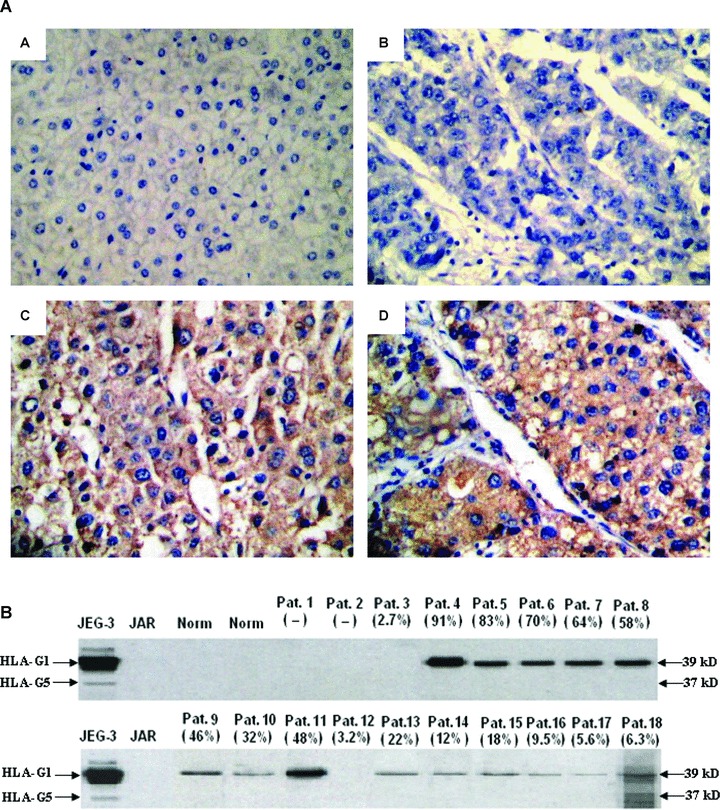
(A) Immunohistochemical detection of HLA-G expression in normal liver tissue and in primary HCC lesions. (A) Normal liver tissue is HLA-G–; (B) A representative of HCC lesion with HLA-G–; (C, D) A representative of HLA-G+ HCC lesions. HLA-G mAb 4H84 (1:300) was used to detect the HLA-G expression. Original magnification: 200×. (B) Western blot analysis of HLA-G expression in HCC lesions. Two normal liver tissues and eighteen primary HCC lesions were analysed with Western blot. The degree of HLA-G expression was shown in brackets according to the case-matched immunohistochemistry data. JEG-3 and JAR lysates were used as HLA-G+ and HLA-G– controls, respectively. The analysis was performed with the HLA-G mAb 4H84 (1:1000).
Specificity for detecting HLA-G in tissues raises concerns as Apps et al. mentioned [30]. Here, HLA-G expression in partial samples (n 5 20) was tested by Western blot. Only two HLA-G+ samples (2.7% and 3.2%, respectively) were undetectable in Western blot, data of other samples were highly consistent with that of immunohistochemistry (Pearson correlation r= 0.764, P= 0.000, Fig. 1B).
HCC HLA-G expression relative to clinicopathological parameters
HLA-G expression was observed in 37.84% (14/37) in disease stage I, 41.94% (13/31) in stage II and 71.43% (15/21) in stage III, respectively. HLA-G expression in primary HCC lesions was strongly associated with disease stage with an overall P-value of 0.029. When sub-grouped, significant difference was found for stage I versus III (P= 0.020), stage II versus III (P= 0.037), whereas no significance was observed between stage I and II (P= 0.882) (Table 1). Furthermore, HLA-G expression was found in 43.4% (49/113) of patients with age equal or less than median age (52 years), and in 57.5% (61/106) of patients with age above the median, respectively (P= 0.004) (Table 1). Age distribution between HLA-G– and positive HCC patients by normal curve analysis showed that the mean age for HLA-G1 patients was dramatically elder than that of the HLA-G– patients (50.0 years versus 56.2 years, P= 0.000) (Fig. 2). However, HLA-G expression in HCC lesions was not significantly associated with patient gender and tumour grade (Table 1).
Fig 2.
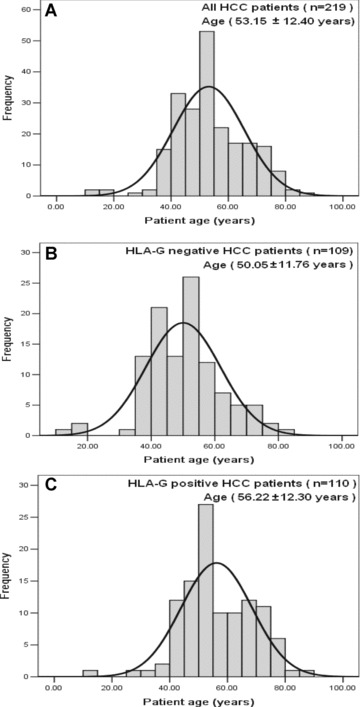
Frequency of age distribution of HCC patients in the study. (A) Age distribution of all HCC patients (53.15 ± 12.40 years, n 5 219). (B) Age distribution of HLA-G– HCC patients (50.05 ± 11.78 years, n= 109). (C) Age distribution of HLA-G+ HCC patients (56.22 ± 12.30 years, n= 110). Significance of age distribution between HLA-G– (B) and HLA-G+ (C) HCC patients was observed (50.0 years versus 56.2 years, P= 0.000).
Plasma sHLA-G expression in HCC patients
Plasma sHLA-G levels in 19 HCC patients and 86 normal healthy individuals were determined by ELISA. Concentration of the plasma sHLA-G was with the median of 92.49 U/ml (range: 14.69–501.38) for HCC patients, 9.29 U/ml (range: 4.38–50.81) for normal controls, respectively. Data revealed that sHLA-G expression in HCC patients was significantly higher than that in normal controls (P= 0.000) (Fig. 3). Furthermore, no correlation was observed between sHLA-G levels and lesion HLA-G expression evaluated by immunohistochemistry in 19 case-matched HCC samples (Pearson correlation, r 5 0.123, P= 0.735).
Fig 3.
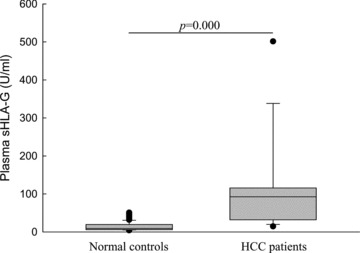
Comparison of plasma sHLA-G between HCC patients and normal controls. Plasma sHLA-G concentrations from 19 HCC patients (median: 92.49 U/ml, range: 14.69–501.38 U/ml) were significantly higher (P= 0.000) than those detected in 86 normal controls (median: 9.29 U/ml, range: 4.38–50.81 U/ml).
Inhibition of NK-92 cytolysis against Hep-G2 cells by cell surface HLA-G in vitro
We then analysed the effects of HLA-G expression in Hep-G2 on the NK-mediated cytolysis. Here, the IL-2 activated NK-92 cells were introduced as the effector. The expression of HLA-G in transfected cells (Hep-G2-G) was confirmed by flow cytometry (Fig. 4A) and Western blot (Fig. 4B). Meanwhile, HLA I antigen expression was also analysed. Effectors were challenged with the target Hep-G2 or Hep-G2-G cells. K562 cells were used as the NK cell cytolysis sensitive control (specific lysis 83.41 6 7.48%). HLA-G expression in Hep-G2-G cells could significantly inhibit the cytolysis of the NK-92 cells (specific lysis 38.98 ± 4.04%versus 28.98 ± 3.90%, P= 0.036) (Fig. 5A), which could be markedly restored either by the functional HLA-G specific antibody 87G (specific lysis 50.53 ± 4.77%versus 28.98 ± 3.89%, P= 0.004) or the HLA-G receptor ILT2 mAb GHI/75 (27.65 ± 5.70%versus 47.91 ± 6.67%, P= 0.019), whereas no significant lysis restoration was found for the HLA-G– Hep-G2 cells (Fig. 5B and C).
Fig 4.
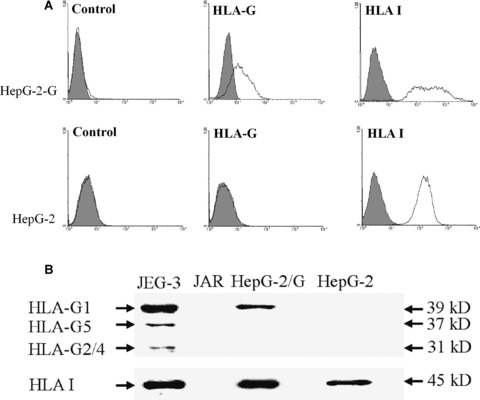
Expression of transfected HLA-G on HCC cell line Hep-G2. (A) Flow cytometry analysis of cell surface HLA-G expression on Hep-G2 and Hep-G2-G cells with mAb MEM-G/9; HLA I expression was detected with mAb W6/32, and an appropriate isotope IgG1 was used as a control. (B) Western blot analysis of HLA-G expression in Hep-G2 and Hep-G2-G cells with mAb 4H84 (1:1000, Exbio). Heavy chain of HLA-I antigens was detected with mAb D226–3 (anti-HLA-ABC, 1:1000, MBL). Choriocarcinoma cell line JAR and JEG-3 were used as HLA– and HLA+ controls, respectively.
Fig 5.
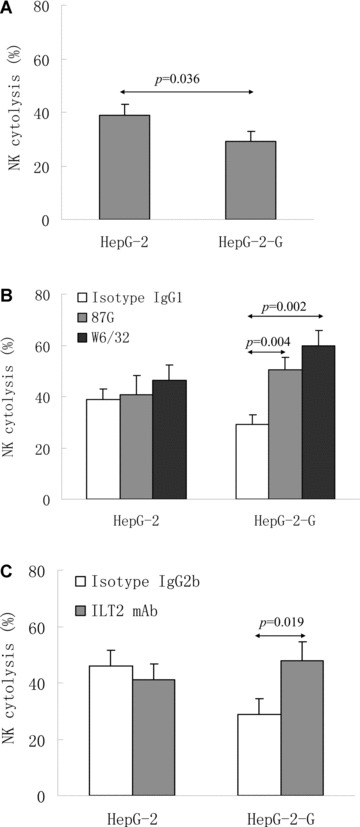
NK-92 cytotoxicity inhibition induced by HLA-G expression. (A) Comparison of the mean cytolytic percentage of NK-92 to different target cells (Hep-G2 and Hep-G2-G). (B) Restoration of cytotoxicity by anti-HLA-G mAb 87G and anti-HLA class I mAb W6/32 blockade. (C) Restoration of NK-92 cytotoxicity by anti-ILT2 mAb GHI/75 blocking. Target cell Hep-G2 and Hep-G2-G cells were pre-incubated with 10 μg/ml mAb 87G, W6/32 and GHI/75, respectively. Isotopes IgG1 (for mAb 87G and mAb W6/32) and IgG2b (for mAb GHI/75) were used as internal controls. Experiments were performed in quadruplicate with an effector/target ratio of 20:1, and the results were expressed as percentage of specific lysis ± S.D.
Blocking experiments with the pan anti-HLA class I mAb W6/32, which reacts with HLA class I antigens including HLA-G were also performed. As shown in Fig. 5, mAb W6/32 could enhance NK-specific killing of HLA-G1 Hep-G2-G cells (specific lysis 59.90 ± 6.03%versus 28.98 ± 3.90%, P= 0.002), where no significant lysis restoration was observed for the blockade between 87G and W6/32 in Hep-G2-G cells (specific lysis 50.53 ± 4.77%versus 59.90 ± 6.03%, P= 0.102). Similarly, no significant restoration was found with either 87G (40.70 ± 7.46%versus 38.98 ± 4.04%, P= 0.743), W6/32 (46.45 ± 6.00%versus 38.98 ± 4.04%, P= 0.148) and ILT2 (45.08 ± 5.48%versus 41.03 ± 5.92%, P= 0.349) blockade for the Hep-G2 cells (Fig. 5B and C).
Discussion
Loss or down-regulation of HLA class I molecules has been thought to be one of critical tumour escape mechanisms by circumventing antigen-specific T-cell response [2]. Lacking HLA class I molecules should render the tumour cells be susceptible to elimination by NK cells. However, tumour cells continue to grow and are not being efficiently destroyed by NK cells [3]. It is common that, during tumour development, tumour-specific and micro-environment cues induced antigen expression could be modulated on tumour cells. Among them, HLA-G is one of important antigens whose expression could be induced and provides immunoinhibitory effects on immune competent cells, and its aberrant expression in malignant cells may represent one of the various mechanisms used by the tumour cells to escape [31].
Since the first address of HLA-G expression by tumour cells in 1998, numerous studies have been performed and HLA-G in situ expression was observed in various malignancies, such as oesophageal squamous cell carcinoma, colorectal cancer, retinoblastoma [32–34], ovarian carcinoma [29, 35], acute myeloid leukaemia [36]. Furthermore, the clinical relevance of HLA-G expression in certain tumours was also addressed. A recent study by Yie et al. revealed that HLA-G expression was detectable in 90.9% (110/121) of oesophageal squamous cell carcinoma lesions, and that could be considered as an independent prognostic factor [32]. In non-small cell lung carcinoma (NSCLC), aberrant HLA-G expression was detected 75% (79/106) of the primary sites by the same group, and HLA-G+ NSCLC patients had a significantly shorter survival time [37]. An early study on B-chronic lymphocytic leukaemia (B-CLL) showed that positive HLA-G surface expression on B-CLL cells has been associated with a poor prognosis [38]. However, discrepant results were observed in B-CLL in a larger cohort, where HLA-G expression in B-CLL cells was found to be irrelevant to the prognosis [39]. These findings suggested that HLA-G expression in malignant cells might be relevant to unfavourable clinical outcome; however, further investigation is absolutely necessary.
Though HLA-G expression was reported in a wide variety of tumours as above mentioned, a considerable controversy remains. In B-CLL, much lower HLA-G expression (ranges from 0% to 14.5%) was observed in a study by Perez-Chacon et al. than that by Nückel group (ranges from 1% to 54%) [38, 39]. Similar discrepancy remains in the studies on NSCLC. A recent report showed that HLA-G expression was observed in 75.0% (79/106) of the lesions analysed, whereas only 24.2% (8/33) was detectable in another study [37, 40]. For the controversial, in immunohistochemistry or in flow cytometry, FcR mediated binding of anti-HLA-G antibodies by macrophages might be a major problem, and all HLA I molecules share 80% amino acid sequence identity in their extracellular domain, cross-reactivity to other HLA I molecules by mAbs to HLA-G is possible [41, 42]. The only unambiguous means of detecting HLA-G expression is to identify the 39-kD heavy chain of HLA-G, which is distinct from the 45-kD classical HLA I molecules [43]. In our study, HLA-G expression in partial
HCC lesions was tested with Western blot, where distinct bands with 39-kD were observed in most of the HLA-G+ lesions (14/16). The two HCC lesions with low expression of HLA-G (3.2% and 2.7%, respectively) were of negative signal in Western blot. High consistence between immunohistochemistry and Western blot suggests that, using HLA-G mAb 4H84, immunohistochemistry is a reliable method to detect HLA-G expression in HCC lesions. However, low expression of HLA-G (such as less than 3%) in HCC lesion may be beyond the detection limit for Western blot.
In our study, HLA-G was expressed in 50.2% (110/219) of primary HCC lesions. HLA-G expression status was significantly associated with patient age and tumour stage, which is preferentially observed in patients with more advanced tumour stage and in older patients; however, neither tumour grade nor patient gender was found to be associated with HLA-G expression. Similarly, HLA-G expression was found to be strongly correlated with the disease stage in oesophageal squamous cell carcinoma, colorectal cancer, lung cancer and gastric carcinoma, indicating that HLA-G status might be involved in tumour progression and yields unfavourable prognosis for cancer [32, 33, 37, 44]. The mechanisms for the association between age and HLA-G expression remain uncertain. Previous studies indicated that factors such as IFN-γ, IL-10 and TGF-β1, or the hormone progesterone could up-regulate HLA-G expression [45–47]. Whether these microenvironmental cues varied in HCC patients with different age needs further investigated.
In addition to HLA-G expression in situ, sHLA-G molecules have been found circulating at high concentrations in certain cancer patients including glioblastoma multiforme, breast and ovarian cancers, lymphoblastic and monocytic acute leukaemia, malignant melanoma and neuroblastoma [13, 45, 48, 49]. Our study showed that plasma sHLA-G from HCC patients was dramatically increased compared to that in normal controls. However, no correlation was observed between the lesion HLA-G expression and plasma sHLA-G level in the case-matched patients. Previous studies indicated that sHLA-G was preferentially released by peripheral blood rather than melanoma cells, and this was supported by the fact that peripheral blood monocytes are the predominant cells secreting HLA-G5 [12, 45]. High serum sHLA-G level in neuroblastoma patients was found to be strongly correlated with relapse, and glioblastoma multiforme patients with high plasma sHLA-G levels had a significantly shorter survival than those with low sHLA-G levels [48, 50]. Given its immune inhibitory functions, high levels of systemically sHLA-G in HCC patients may synergistically help malignant cells to overcome the host immune system. However, for the limited patient cohort included in the current study, the clinical relevance of sHLA-G in HCC needs further investigated.
It is now widely acknowledged that HLA-G expression could protect tumour cells from anti-tumour immune responses, such as NK cell cytolysis. Moreover, HLA-G could induce the generation of suppressive/regulatory cells and block the immune effectors [14]. Thereby, the immune clearance of tumour cell might be enhanced by blocking HLA-G. We then analysed the roles of HLA-G generated artificially on Hep-G2 cells on NK cell-mediated cytotoxicity. Data showed that HLA-G expression could inhibit the cytolysis activities of activated NK cells in vitro. Cytolysis inhibition induced by HLA-G was confirmed by using the HLA-G conformational blocking mAb 87G, which could be dramatically recovered when HLA-G was blocked. Of note is that other HLA antigens such as HLA-E may also participate in the inhibitory effect on cytolysis via their specific inhibitory receptors present on NK cells [51]. Our study showed that HLA I antigens were also expressed on Hep-G2 cells. To test whether the NK cell cytolysis inhibition is mainly induced by HLA-G, blockade experiment was performed with the pan anti-HLA I mAb W6/32 which recognizes all HLA class I antigens such as HLA-A, -B, -C, -G and HLA-E [52]. Similarly, W6/32 could dramatically restore the NK cell lysis activity against Hep-G2-G cells, whereas no significance for the restoration was observed between the 87G and W6/32. Furthermore, the blockade experiments dose not markedly change NK cell cytolysis against the HLA-G– Hep-G2 cells. We finally analysed whether the inhibition of NK-92 cytotoxicity was induced by the HLA-G inhibitory receptor ILT2, which could preferentially recognize HLA-G [53]. ILT2 was moderately expressed on NK-92 cells with the mean percentage of 39.2 ± 4.3% in the current study. Our data demonstrated that blocking HLA-G receptor ILT2 could markedly restore the NK-92 cytotoxicity capacity. These findings suggested that the NK cell inhibition was predominately induced by HLA-G. In a similar scenario, a study by Bukur et al. indicated that cytotoxicity of lymphokine activated killer against the HLA-G+ renal carcinoma cell line MZ2733RC could be restored with the mAb W6/32 [54]. The restoration of NK cell cytolysis was also observed in our previous studies on ovarian cancer and acute myeloid leukaemia when HLA-G was blocked by HLA-G specific mAb 87G [29, 36].
Taken together, we confirmed that HCC lesion could express HLA-G, and that HLA-G expression in HCC is significantly related to the disease stage and patient age. Moreover, HLA-G expression in HCC cells could exert immunosuppressive to the NK cell cytolysis. Our findings indicated that HLA-G expression is involved in tumour progression and may exert unfavourable clinical relevance in HCC.
Acknowledgments
This work was supported by Zhejiang Provincial program for the cultivation of high-level innovative health talents, Natural Science Foundation of Zhejiang Province, China (Y205531, Y205575), the Science and Technology Bureau of Zhejiang Province (2008C33013, 2009C23SA800001), Health Bureau of Zhejiang Province (2007A195) and by grants from Ministry of Personnel and Education, China.
References
- 1.Reiman JM, Kmieciak M, Manjili MH, et al. Tumor immunoediting and immunosculpting pathways to cancer progression. Semin Cancer Biol. 2007;17:275–87. doi: 10.1016/j.semcancer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrone S, Whiteside TL. Tumor microenvironment and immune escape. Surg Oncol Clin N Am. 2007;16:755–74. doi: 10.1016/j.soc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–43. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 4.Urosevic M, Dummer R. Human leukocyte antigen-G and cancer immunoediting. Cancer Res. 2008;68:627–30. doi: 10.1158/0008-5472.CAN-07-2704. [DOI] [PubMed] [Google Scholar]
- 5.Polakova K, Bandzuchova E, Tirpakova J, et al. Modulation of HLA-G expression. Neoplasma. 2007;54:455–62. [PubMed] [Google Scholar]
- 6.Mouillot G, Marcou C, Zidi I, et al. Hypoxia modulates HLA-G gene expression in tumor cells. Hum Immunol. 2007;68:277–85. doi: 10.1016/j.humimm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Zidi I, Guillard C, Marcou C, et al. Increase in HLA-G1 proteolytic shedding by tumor cells: a regulatory pathway controlled by NF-kappaB inducers. Cell Mol Life Sci. 2006;63:2669–81. doi: 10.1007/s00018-006-6341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urosevic M, Dummer R. HLA-G and IL-10 expression in human cancer–different stories with the same message. Semin Cancer Biol. 2003;13:337–42. doi: 10.1016/s1044-579x(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 9.Wagner SN, Rebmann V, Willers CP, et al. Expression analysis of classic and non-classic HLA molecules before interferon alfa-2b treatment of melanoma. Lancet. 2000;356:220–1. doi: 10.1016/S0140-6736(00)02486-7. [DOI] [PubMed] [Google Scholar]
- 10.Ishitani A, Geraghty DE. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc Natl Acad Sci USA. 1992;89:3947–51. doi: 10.1073/pnas.89.9.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul P, Cabestre FA, Ibrahim EC, et al. Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5, -G6, and -G7 transcripts in human transfected cells. Hum Immunol. 2000;61:1138–49. doi: 10.1016/s0198-8859(00)00197-x. [DOI] [PubMed] [Google Scholar]
- 12.Rebmann V, Busemann A, Lindemann M, et al. Detection of HLA-G5 secreting cells. Hum Immunol. 2003;64:1017–24. doi: 10.1016/j.humimm.2003.08.354. [DOI] [PubMed] [Google Scholar]
- 13.Pistoia V, Morandi F, Wang X, et al. Soluble HLA-G: are they clinically relevant. Semin Cancer Biol. 2007;17:469–79. doi: 10.1016/j.semcancer.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carosella ED, Howangyin KY, Favier B, et al. HLA-G-dependent suppressor cells: diverse by nature, function, and significance. Hum Immunol. 2008;69:700–7. doi: 10.1016/j.humimm.2008.08.280. [DOI] [PubMed] [Google Scholar]
- 15.Carosella ED, Moreau P, Lemaoult J, et al. HLA-G: from biology to clinical benefits. Trends Immunol. 2008;29:125–32. doi: 10.1016/j.it.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Kovats S, Main EK, Librach C, et al. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220–3. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 17.Crisa L, McMaster MT, Ishii JK, et al. Identification of a thymic epithelial cell subset sharing expression of the class Ib HLA-G molecule with fetal trophoblasts. J Exp Med. 1997;186:289–98. doi: 10.1084/jem.186.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Discorde M, Moreau P, Sabatier P, et al. Expression of HLA-G in human cornea, an immune-privileged tissue. Hum Immunol. 2003;64:1039–44. doi: 10.1016/j.humimm.2003.08.346. [DOI] [PubMed] [Google Scholar]
- 19.Ito T, Ito N, Saathoff M, et al. Immunology of the human nail apparatus: the nail matrix is a site of relative immune privilege. J Invest Dermatol. 2005;125:1139–48. doi: 10.1111/j.0022-202X.2005.23927.x. [DOI] [PubMed] [Google Scholar]
- 20.Cirulli V, Zalatan J, McMaster M, et al. The class I HLA repertoire of pancreatic islets comprises the nonclassical class Ib antigen HLA-G. Diabetes. 2006;55:1214–22. doi: 10.2337/db05-0731. [DOI] [PubMed] [Google Scholar]
- 21.Menier C, Rabreau M, Challier JC, et al. Erythroblasts secrete the nonclassical HLA-G molecule from primitive to definitive hematopoiesis. Blood. 2004;104:3153–60. doi: 10.1182/blood-2004-03-0809. [DOI] [PubMed] [Google Scholar]
- 22.Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–22. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 23.Paul P, Rouas-Freiss N, Khalil-Daher I, et al. HLA-G expression in melanoma: a way for tumor cells to escape from immunosurveillance. Proc Natl Acad Sci USA. 1998;95:4510–5. doi: 10.1073/pnas.95.8.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tripathi P, Agrawal S. Non-classical HLA-G antigen and its role in the cancer progression. Cancer Invest. 2006;24:178–86. doi: 10.1080/07357900500524579. [DOI] [PubMed] [Google Scholar]
- 25.Rouas-Freiss N, Moreau P, et al. HLA-G proteins in cancer: do they provide tumor cells with an escape mechanism. Cancer Res. 2005;65:10139–44. doi: 10.1158/0008-5472.CAN-05-0097. [DOI] [PubMed] [Google Scholar]
- 26.Ishak KG, Anthony PP, Sobin LH. Nonepithelial tumors. In: Ishak KG, editor. Histological typing of tumors of the liver. Berlin: Springer; 1994. pp. 22–7. [Google Scholar]
- 27.Wittekind C. Pitfalls in the classification of liver tumors [in German] Pathologe. 2006;27:289–93. doi: 10.1007/s00292-006-0834-1. [DOI] [PubMed] [Google Scholar]
- 28.Sobin LH, Wittekind C. TNM classification of malignant tumors. 6th ed. New York, NY: Wiley-Liss; 2002. pp. 81–3. [Google Scholar]
- 29.Lin A, Yan WH, Xu HH, et al. HLA-G expression in human ovarian carcinoma counteracts NK cell function. Ann Oncol. 2007;18:1804–9. doi: 10.1093/annonc/mdm356. [DOI] [PubMed] [Google Scholar]
- 30.Apps R, Gardner L, Moffett A. A critical look at HLA-G. Trends Immunol. 2008;29:313–21. doi: 10.1016/j.it.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Rouas-Freiss N, Moreau P, Menier C, et al. Expression of tolerogenic HLA-G molecules in cancer prevents antitumor responses. Semin Cancer Biol. 2007;17:413–21. doi: 10.1016/j.semcancer.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Yie SM, Yang H, Ye SR, et al. Expression of HLA-G is associated with prognosis in esophageal squamous cell carcinoma. Am J Clin Pathol. 2007;128:1002–9. doi: 10.1309/JNCW1QLDFB6AM9WE. [DOI] [PubMed] [Google Scholar]
- 33.Ye SR, Yang H, Li K, et al. Human leukocyte antigen G expression: as a significant prognostic indicator for patients with colorectal cancer. Mod Pathol. 2007;20:375–83. doi: 10.1038/modpathol.3800751. [DOI] [PubMed] [Google Scholar]
- 34.Adithi M, Kandalam M, Ramkumar HL, et al. Retinoblastoma: expression of HLA-G. Ocul Immunol Inflamm. 2006;14:207–13. doi: 10.1080/09273940600826497. [DOI] [PubMed] [Google Scholar]
- 35.Singer G, Rebmann V, Chen YC, et al. HLA-G is a potential tumor marker in malignant ascites. Clin Cancer Res. 2003;9:4460–4. [PubMed] [Google Scholar]
- 36.Yan WH, Lin A, Chen BG, et al. Unfavourable clinical implications for HLA-G expression in acute myeloid leukaemia. J Cell Mol Med. 2008;12:889–98. doi: 10.1111/j.1582-4934.2008.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yie SM, Yang H, Ye SR, et al. Expression of human leucocyte antigen G (HLA-G) is associated with prognosis in non-small cell lung cancer. Lung Cancer. 2007;58:267–74. doi: 10.1016/j.lungcan.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Nückel H, Rebmann V, Dürig J, et al. HLA-G expression is associated with an unfavorable outcome and immunodeficiency in chronic lymphocytic leukemia. Blood. 2005;105:1694–8. doi: 10.1182/blood-2004-08-3335. [DOI] [PubMed] [Google Scholar]
- 39.Perez-Chacon G, Rosado S, Rebolleda N, et al. Prognostic irrelevance of HLA-G in B-cell chronic lymphocytic leukemia. Int J Lab Hematol. 2009;31:327–37. doi: 10.1111/j.1751-553X.2008.01030.x. [DOI] [PubMed] [Google Scholar]
- 40.Urosevic M, Kurrer MO, Kamarashev J, et al. Human leukocyte antigen G up-regulation in lung cancer associates with high-grade histology, human leukocyte antigen class I loss and interleukin-10 production. Am J Pathol. 2001;159:817–24. doi: 10.1016/S0002-9440(10)61756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honig A, Rieger L, Kapp M, et al. Immunohistochemistry in human placental tissue–pitfalls of antigen detection. J Histochem Cytochem. 2005;53:1413–20. doi: 10.1369/jhc.5A6664.2005. [DOI] [PubMed] [Google Scholar]
- 42.Sernee MF, Ploegh HL, Schust DJ. Why certain antibodies cross-react with HLA-A and HLA-G: epitope mapping of two common MHC class I reagents. Mol Immunol. 1998;35:177–88. doi: 10.1016/s0161-5890(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 43.Park B, Lee S, Kim E, et al. The truncated cytoplasmic tail of HLA-G serves a quality-control function in post-ER compartments. Immunity. 2001;15:213–24. doi: 10.1016/s1074-7613(01)00179-0. [DOI] [PubMed] [Google Scholar]
- 44.Yie SM, Yang H, Ye SR, et al. Expression of human leukocyte antigen G (HLA-G) correlates with poor prognosis in gastric carcinoma. Ann Surg Oncol. 2007;14:2721–9. doi: 10.1245/s10434-007-9464-y. [DOI] [PubMed] [Google Scholar]
- 45.Ugurel S, Rebmann V, Ferrone S, et al. Soluble human leukocyte antigen–G serum level is elevated in melanoma patients and is further increased by interferon-alpha immunotherapy. Cancer. 2001;92:369–76. doi: 10.1002/1097-0142(20010715)92:2<369::aid-cncr1332>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 46.Nasef A, Chapel A, Mazurier C, et al. Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr. 2007;13:217–26. doi: 10.3727/000000006780666957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yie SM, Xiao R, Librach CL. Progesterone regulates HLA-G gene expression through a novel progesterone response element. Hum Reprod. 2006;21:2538–44. doi: 10.1093/humrep/del126. [DOI] [PubMed] [Google Scholar]
- 48.Rebmann V, Regel J, Stolke D, et al. Secretion of sHLA-G molecules in malignancies. Semin Cancer Biol. 2003;13:371–7. doi: 10.1016/s1044-579x(03)00028-2. [DOI] [PubMed] [Google Scholar]
- 49.Amiot L, Le Friec G, Sebti Y, et al. HLA-G and lymphoproliferative disorders. Semin Cancer Biol. 2003;13:379–85. doi: 10.1016/s1044-579x(03)00029-4. [DOI] [PubMed] [Google Scholar]
- 50.Morandi F, Levreri I, Bocca P, et al. Human neuroblastoma cells trigger an immunosuppressive program in monocytes by stimulating soluble HLA-G release. Cancer Res. 2007;67:6433–41. doi: 10.1158/0008-5472.CAN-06-4588. [DOI] [PubMed] [Google Scholar]
- 51.Middleton D, Curran M, Maxwell L. Natural killer cells and their receptors. Transpl Immunol. 2002;10:147–64. doi: 10.1016/s0966-3274(02)00062-x. [DOI] [PubMed] [Google Scholar]
- 52.Paul P, Rouas-Freiss N, Moreau P, et al. HLA-G, -E, -F preworkshop: tools and protocols for analysis of non-classical class I genes transcription and protein expression. Hum Immunol. 2000;61:1177–95. doi: 10.1016/s0198-8859(00)00154-3. [DOI] [PubMed] [Google Scholar]
- 53.Shiroishi M, Tsumoto K, Amano K, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci USA. 2003;100:8856–61. doi: 10.1073/pnas.1431057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bukur J, Rebmann V, Grosse-Wilde H, et al. Functional role of human leukocyte antigen-G up-regulation in renal cell carcinoma. Cancer Res. 2003;63:4107–11. [PubMed] [Google Scholar]


