Abstract
In the past decade, researchers have gained important insights on the role of bone marrow (BM)-derived cells in adult neovascularization. A subset of BM-derived cells, called endothelial progenitor cells (EPCs), has been of particular interest, as these cells were suggested to home to sites of neovascularization and neoendothelialization and differentiate into endothelial cells (ECs) in situ, a process referred to as postnatal vasculogenesis. Therefore, EPCs were proposed as a potential regenerative tool for treating human vascular disease and a possible target to restrict vessel growth in tumour pathology. However, conflicting results have been reported in the field, and the identification, characterization, and exact role of EPCs in vascular biology is still a subject of much discussion. The focus of this review is on the controversial issues in the field of EPCs which are related to the lack of a unique EPC marker, identification challenges related to the paucity of EPCs in the circulation, and the important phenotypical and functional overlap between EPCs, haematopoietic cells and mature ECs. We also discuss our recent findings on the origin of endothelial outgrowth cells (EOCs), showing that this in vitro defined EC population does not originate from circulating CD133+ cells or CD45+ haematopoietic cells.
Keywords: endothelial progenitor cells, haematopoietic cells, CD34, CD45, CD133
Introduction
The development of the vascular system is an essential event during embryonic development of many animal species. During this process, local mesodermal precursors differentiate into vascular and endothelial cells (ECs) to form a primary vascular plexus, a process referred to as vasculogenesis [1]. Until the description of circulating endothelial progenitors (CEPCs), this process of vasculogenesis was thought only to occur during embryonic development, but not in postnatal life. Indeed, a decade ago, two groups reported that human CD34+ cells isolated from circulating peripheral blood (PB), umbilical cord blood (UCB) and bone marrow (BM), could differentiate into ECs in vitro and in vivo in mouse models, thereby contributing to neoendothelialization and neovascularization in the adult organism [2, 3]. Additional studies in mice suggested incorporation of BM-derived cells into blood vessels at tumour sites [4, 5], healing wounds [6, 7], areas of endothelial denudation [8, 9], induced hindlimb ischaemia [10] and following experimental myocardial infarction [11].
These landmark studies on endothelial progenitor cells (EPCs) thus challenged the traditional concept that endothelial regeneration and angiogenesis occurs exclusively via the proliferation of pre-existing resident vessel wall ECs, and thus it appeared that vasculogenesis also occurs in postnatal life. Moreover, this novel concept that EPCs enter the blood stream via the BM and provide a pool of CEPCs in postnatal life was even more striking, because there is no clear evidence that circulating vascular precursors contribute to vasculogenesis during murine or human embryonic development.
In patients that had undergone BM or PB stem cell transplantation, evidence was also presented that some ECs lining the blood vessels in the recipient were chimeric in origin, (i.e. of both recipient and donor origin), pointing to the existence of circulating cells in blood and BM that contribute to endothelial turnover of blood vessels in humans [12, 13]. Indirect evidence for the concept that EPCs act as a back-up system for maintaining vascular homeostasis in humans was suggested by showing an inverse correlation between the number of PB CEPCs and presence of atherosclerosis, an adverse cardiovascular risk score, level of cardiovascular dysfunction, or cardiovascular morbidity and mortality risk in human subjects [14–18]. Also, the functional properties of EPCs such as cell adherence, migration, invasion and vessel formation appear to be attenuated in patients with increased cardiovascular risk factors and established cardiovascular disease [15, 19–21]. Therefore, EPCs could also serve as a biomarker to predict cardiovascular outcomes and might help in monitoring the effects of primary and secondary cardiovascular prevention strategies.
In spite of the growing number of reports on EPCs as a potential tool in regenerative medicine or a therapeutic target in oncology, contradicting results have been reported at the pre-clinical and clinical level, and several issues such as the definition, identification, characterization and the role of EPCs in vascular homeostasis and disease remain controversial [22–36]. In this review, we will discuss these issues based on published results conducted at the pre-clinical phase.
The proof-of-concept in vivo: the cell, the read-out and the animal model
After 10 years of vigorous research in EPC biology, we have not yet reached a consensus on the definitive appearance and function of an EPC. What the field is currently suffering from is the use of a single term (EPC) to refer to BM-derived or circulating cells of diverse lineages. It is apparent that progress in the use of EPCs to treat human disorders may not reach its ultimate potential unless we can specifically identify all of the component cell types that contribute to neovasculogenesis, define the role that each cell lineage plays in the process, and identify strategies to assess the in vivo function of these diverse cell types in patients with vascular disorders, thus defining which cell type may need to be replaced to reinvigorate the vascular repair process. Therefore, if an EPC is defined as an immature precursor cell that individually displays postnatal vasculogenic activity, then any cell called an EPC should be capable of forming new ECs and blood vessels in vivo. Unfortunately, attempts to illustrate this defining property of a (candidate) EPC have often yielded conflicting results, which probably relates to the highly variable approaches undertaken in different research venues with regard to cell source, cell purification, the animal model or assay used, method of detection and analysis, and data interpretation.
The CEPC: still a putative cell
In many studies addressing the identity of EPCs in vascular biology unselected PB mononuclear cells (MNCs) were used, or the purity of the (starting) CEPCs population was low, making it difficult to identify the CEPCs within the MNC population that gave rise to the ECs (i.e. the exact precursor (CEPC)–product (EC) relationship in these studies was not addressed). Many investigators have identified or designated putative CEPCs with flow cytometry using a single surface marker such as CD34 or CD133 in humans, or various combinations of surface markers were used, which has actually resulted in a complicated list of putative CEPC immunophenotypes in man and mice (Table 1). As shown in Table 1, most of the surface marker combinations used in flow cytometry studies included the marker CD34 and vascular endothelial growth factor receptor-2 (VEGFR-2), because initial studies in the field have reported that CD34+ and VEGFR-2+ cells purified from various sources (UCB, PB and BM) are able to generate ECs in vitro, suggesting that CD34+ cells contains CEPCs [2, 3, 37]. One specific subset of CD34+ cells, designated as CD34+VEGFR-2+CD133+ cells are widely accepted to identify ‘true CEPCs’ in humans but yet these cells were never directly tested for generating new ECs in vitro or in vivo, which is essential to validate CD34+VEGFR-2+CD133+ cells as true CEPCs [38–40]. Recently, however, Case et al. reported for the first time that isolated human UCB or mobilized adult PB CD34+VEGFR-2+CD133+ cells in fact represent an enriched population of CD45+ haematopoietic precursors using in vitro haematopoiesis assays, but CD34+VEGFR-2+CD133+ did not contribute to the formation of ECs in vitro[41]. Similarly, CD34+CD45+CD146+ cells previously reported to represent CEPCs, were not directly assayed in vitro, nor in vivo for contribution to newly formed endothelium, and thus, it is difficult to know whether this cell type acts as a true CEPC [42] Therefore, the scientific foundation for using the variable surface marker combinations and CD34+ subsets depicted in Table 1 remains elusive. Moreover, the use of these diverse combinations to define a singular entity (the CEPC), makes the significance of flow cytometric studies difficult to interpret, creates obstacles to the direct comparison of data between laboratories, and may result in discrepancies in the interpretations of study results among different laboratories. Therefore, investigators should strongly consider that any ‘putative’ CEPC, whatever its phenotype, be carefully assessed by validating its postnatal endothelial differentiation capacity in vitro and in vivo.
1.
Surface immunophenotype of human and murine CEPCs
| Immunophenotype in humans | Reference | Immunophenotype in mice | Reference |
|---|---|---|---|
| CD34+CD31+ | [135] | Sca-1+ | [10] |
| CD34+CD62L+ | [135] | Sca-1− Lin− cKit− | [145] |
| CD34+CD133+ | [136] | ||
| CD34+CD11b+ | [137] | Lin− cKit+ Flk1+ CD13+ CD133+VE-Cadherin+ | [50] |
| CD34+CD45+ | [110] | ||
| CD34+CD133+CD45+ | [138] | ||
| CD34+FGFR1+ | [111] | CD45dimCD34+ Flk-1 + | [9] |
| CD34+VEGFR-2+ | [112] | CD31 +Flk-1 +CXCR4+ | [146] |
| CD34+CD133+VEGFR-2+ | [38] | Sca-1+cKit+Lin− | [56] |
| CD34−CD133+VEGFR-2+ | [55] | CD45−CD34+Flk-1 + | [98] |
| CD34+VE-Cadherin+CD3− | [139] | cKit+CD31+ | [147] |
| CD34+CD133+VEGFR-2+CD45+ | [40] | cKit+CD34+Flk-1 + | [148] |
| CD34+CD45+CD146+ | [42] | CD45− cKit+CD13+ | [149] |
| CD34+CD45− | [140] | ||
| CD133+ | [17] | ||
| CD133+CD45− | [141] | ||
| CD133+VEGFR-2+ | [142] | ||
| CD14+VEGFR-2+ | [71] | ||
| CD14+CD34+ | [70] | ||
| ALDHbright | [143] | ||
| CD31+ | [144] |
A critical set of variables to consider when performing endothelial differentiation studies is that putative CEPCs might only give rise to ECs depending on the exact combination of growth factors to which the cells have been exposed in vitro, and/or depending on the animal model used to assess postnatal vasculogenic activity, and/or the nature, extent, or method of delivery of the angiogenic stimulus applied in vivo. These important caveats, in what should be the most important criterion to validate EPCness, makes the field of EPCs intriguing, but at the same time complicated. Therefore, further efforts should be focused on the development of a straightforward standard assay, or set of assays, that are accessible to all investigators, to specifically define and validate the function of (candidate) EPCs, so that investigators would have a benchmark for comparison, and a rationale for the examination and clinical translation of selected cell subsets in targeted clinical disorders.
To validate the EC differentiation capacity of a candidate CEPC, single cell studies offer a rigorous approach to address the precursor (CEPC)–product (EC) relationship However, numerous technical and biological limitations must be overcome in performing a clonal assay. For instance, one of the potential limits of single cell experiments is that the isolated single cell may behave differently in the absence of other cells. Therefore, transplanting or culturing a single cell may not be an appropriate EPC assay, but may require the presence of (supportive) cells with a different phenotype or function. However, one of the greatest obstacles to date in the field of EPC biology is the lack of a unique marker, or combination of markers, that solely identifies the rare CEPCs in humans, making a prospective isolation and thus single cells studies impossible. In the murine BM transplantation system, two single cell transplantation studies have been reported, showing that a single mouse haematopoietic stem cell (HSC) could give rise to endothelial progeny after retinal injury [43, 44]. However, these studies have been challenged by other groups that failed to detect endothelial differentiation from transplanted HSCs in vivo using different donor cell marking strategies [23, 45].
Considering the remarks discussed in this section, two important questions remain with regard to the phenotype of CEPCs: (i) If CD34+VEGFR-2+CD133+ appear not to be true CEPCs, what then is the exact surface phenotype of the CEPCs? and (ii) How can we explain the statistical (inverse) correlation between the several cell subsets depicted in Table 1 and cardiovascular indices and outcome, even if these cell types would not represent true CEPC? As we will discuss later in this review, the phenotypes shown in Table 1 mostly represent haematopoietic-derived cells, rather than true CEPCs, similar to the CD34+VEGFR-2+CD133+ cells discussed above. Nevertheless, haematopoietic-derived cells may contribute to vascular repair and homeostasis in an indirect manner, as suggested by several investigators [25, 31, 46–48]. Indeed, haematopoietic lineage cells might be recruited to injured or angiogenic sites and secrete regulatory cytokines that promote vessel homeostasis and repair by local cells, including local vessel wall ECs. It is possible that cardiovascular risk factors and established cardiovascular disease decrease the circulating number and properties of the haematopoietic-derived cells. Hence, low levels of these circulating cells might correlate with adverse cardiovascular outcome. However, this concept is entirely different from the EPC concept proposed 10 years ago where EPCs were suggested to function as a structural backup from the BM [2].
The question as to what the exact phenotype is of true CEPCs will be discussed at the end of the review after we have outlined several crucial issues related to the identification, definition and differentiated progenies of putative CEPCs.
Do CEPCs play an essential role in vascular (patho)physiology?
Although many papers have been reported on EPCs in man and mice, the direct proof-of-concept or true relevance of these cells in vascular homeostasis, vascular growth and disease (atherosclerosis) is still uncertain. Perhaps one of the most solid approaches to address the relevance of CEPCs in vascular homeostasis is to look at the natural history of vascular aging or the extent of vascular disease in animal models lacking these CEPCs. This could be achieved by selectively eliminating the CEPCs in mice with pharmacological agents, or by means of genetic knockdown. The vascular outcome in mice with knockedout EPCs could then be compared with control mice in diverse pathological settings. However, (genetic) knockdown studies to assess the mechanistic underpinning between CEPCs and vascular (patho)biology, requires that one possess the ability to prospectively identify and thus specifically target the CEPCs, without targeting the mature vessel wall ECs. Indeed, the current approaches used to abolish or enhance the function and/or number of endogenous CEPCs might not have been selective enough, and are likely to have targeted mature vessel wall ECs, and other (BM-derived) cells involved in vascular repair as well, thus complicating the interpretation of the study results of CEPCs in modulating the process of vascular repair [49]. Recently, some studies have focused on the role of CEPCs in tumour angiogenesis in murine models using several knockdown tools [5, 50, 51]. In these studies, murine BM-derived CEPCs were immunophenotyped as cKit+Flk-1+CD13+CD133+ cells. Moreover, it was suggested that these candidate CEPCs express Id-1 proteins and a monomeric form of the surface markers VE-Cadherin (i.e. CD144) in a unique fashion, whereas the resident mature ECs did not. This allowed the investigators to selectively inhibit the CEPCs without targeting the resident mature vessel wall ECs, and thus address the relevance of CEPCs in tumour angiogenesis. However, studies have shown that local, mature vessel wall ECs also express the monomeric VE-Cadherin epitope upon vessel disassembly and tumour angiogenesis [52]. Therefore, it is possible that the neutralizing antibody used to target the CEPCs might also have tackled the proliferating mature vessel wall ECs in this study [52].
In another study, it was suggested that murine CEPCs selectively express Id-1, a protein previously shown to be involved in angiogenesis [4, 51]. Using genetically manipulated BM cells, the expression of this Id-1 gene could be turned off ‘à la demande’, resulting in a profound reduction of tumour angiogenesis and tumour growth. However, because Id-1 is also expressed in haematopoietic lineage cells, it remains uncertain whether the inhibition of tumour angiogenesis is merely because of reduced tumour-recruitment and/or paracrine angiogenic function of haematopoietic lineage cells, instead of reduced CEPC function, differentiation or incorporation [50, 53]. Therefore, although these studies illustrate the role of BM cells in tumour angiogenesis, because of a lack of specificity, they do not formally prove the unique or dominant position of putative CEPCs in the modulation of tumour angiogenesis. Therefore, at the end, the identification of a unique marker gene that specifically identifies CEPCs will allow us to unambiguously address the ‘proof-of-concept’ or causal role of CEPCs in cardviovascular (patho)-physiology and tumour pathology.
In Figure 1, we propose a genetic knock-down model based on the expression of a unique cell marker in CEPCs (being either a surface or intracellular marker). As illustrated, in this conditional knock-down model, only CEPCs that express a unique marker gene will be targeted and die after challenge with the specific agent, whereas mature vessel wall ECs and haematopoietic cells that do not express this unique cell marker, will not be targeted. Comparative studies between knockedout mice and control mice will then accurately answer the question whether CEPCs are truly causal or act as an essential backup for vascular disease and physiology, respectively.
1.

Conditional EPC knockout mouse model based on a unique CEPC marker. A modified estrogen receptor (mER; in red) is co-expressed on CEPCs that expresses a unique marker (indicated in grey). This unique marker may be either a membrane (as shown) or a cytoplasmatic marker. Upon challenge with a receptor specific agent, the mER activates the Cre enzyme (indicated by scissors) in CEPCs. The Cre-enzyme recognizes the specific LoxP sequences (white) that flank a survival gene (black) resulting in its deletion and eventually CEPC death.
The in vivo read-out and animal model
Perhaps the most important but often disregarded issue in the assessment of the precursor–product relationship are the criteria used to dentify the EPC progeny as ‘true ECs’ in vivo. In fact, identifying a cell as a bona fide EC is crucial for classifying its precursor as a true EPC. To identify ECs generated from CEPCs in vivo, most reports have relied on the anatomical position of the donor cell population within the context of a perfused vascular structure in combination with a single marker or combination of cell surface markers that typically are expressed on normal vascular ECs such as CD31 or CD34, or the uptake of acetylated low density lipoprotein (LDL), as well as the binding of certain plant lectins (Ulex Europaeus Agglutinin-1 or UEA-1) to identify ECs [54–56]. However, most of the characteristics, or cell markers used in previous studies such as CD31, CD34, lectin binding or LDL ingestion, are not specific for the endothelial lineage, but also characterize haematopoietic lineage cells (Table 2) [57, 58]. This notion is important because vascular homeostasis and repair are multi-cellular processes, recruiting several cellular participants such as local vascular wall cells (vessel wall progenitors, smooth muscle cells, pericytes, ECs and macrophages) and circulating BM-derived cells, including putative CEPCs, but also haematopoietic (derived) cells [22, 25, 31, 32, 59, 60–63].
2.
Immunophenotype of haematopoietic cells and endothelial cells
| Embryonic HPC | Postnatal HPC | ECs | References | |
|---|---|---|---|---|
| CD14 | − | − | − | [58, 85] |
| CD31 (PECAM-1) | + | + | + | [58, 85, 106, 150] |
| CD34 | + | + | + | [41, 106, 109, 150, 151, 152, 153] |
| CD38 | −/+ | −/+ | − | [152, 153] |
| CD43 | + | + | − | [109, 151] |
| CD44 | ? | + | ? | [153] |
| CD45 | −/+ | + | − | [41, 58, 106, 108, 109, 153] |
| CD54 (ICAM-1) | ? | + | + | [154] |
| CD90 | + | + | (+)a | [109, 153] |
| CD105 (Endoglin) | ? | + | + | [58, 155] |
| CD106 (VCAM-1) | ? | ? | (+)b | [133] |
| CD117 (C-kit) | + | + | (+)a | [58, 79, 151, 153] |
| CD133 | (?)c | + | − | [58, 153] |
| CD143 | + | + | ? | [156] |
| CD146 (MUC-18) | ? | ? | + | [58] |
| CD164 | + | + | − | [157] |
| VEGFR-2 | + | (+)d | (+)e | [58, 106, 132, 151] |
| Tie-2 | + | + | + | [106, 158] |
| VE-Cadherin (CD144) | + | − | + | [58, 106, 109] |
Note: The surface marker profile of human embryonic haematopoietic precursors (HPC), postnatal HPCs and ECs is depicted. The surface marker profile of the EC markers shown is based on the analysis of both in vivo ECs and in vitro cultured vessel wall ECs.
(+)a indicates that we identified a small CD90+ or CD117+ population on non-passaged vessel wall ECs.
(+)b indicates that CD106 is upregulated following endothelial activation [133].
(+)c indicates that the haematopoietic potential (T cells) of embryonic stem cell-derived CD133+ HPCs has been demonstrated only in vivo [134].
(+)d refers to a report on the expression of VEGFR-2 on a small HSC population [132].
(+)e indicates that the precursor–product relation between circulating VEGFR-2+ cells and EOCs generated in vitro has not yet been proven.
Mature haematopoietic cells include red blood cells, platelets, myeloid cells such as monocytes/macrophages and granulocytes, dendritic cells and lymphoid cells including B cells, T cells, NK cells and NKT cells. Haematopoietic cells derive from HSCs and haematopoietic progenitor cells (HPC respectively) that reside within the BM. Importantly, haematopoietic-derived cells such as monocytes, granulocytes, platelets and even HSCs/HPCs have been shown to be involved in vascular repair [48, 64]. However because both endothelial lineage cells and haematopoietic cells are present at sites of neovascularization and co-express a host of similar surface markers, it might be difficult to discriminate them from each other at sites of vascular repair, and appreciate their individual contribution to the healing or regenerative process. Therefore, the diverse recruited cell types now known to populate sites of neovascularization are highly likely to have previously been lumped into the single term ‘EPC in many early studies of postnatal vasculogenesis, explaining some of the apparent controversy in the field.
One of the strategies that have been used to directly assay donor cell differentiation into ECs in vivo has been the use of transgenic mice that express a fluorescent marker (e.g. green fluorescent protein (GFP)) only in cells expressing an endothelial specific gene, such as Tie-2 [65]. Therefore, transplantation of BM cells from transgenic mice into wild-type mice allows the tracking of the cells of interest, and their fate during mobilization from the BM into sites of vascular injury, and discriminates them from other cell types and host cells involved in vascular repair and regeneration. However, even these sophisticated approaches have often yielded contradictory results, probably because expression of Tie-2 is not entirely restricted to the endothelial lineage, and is also expressed by pericytes and haematopoietic (derived) cells such as monocytes, that also migrate to sites of vascular repair [22].
On the other hand, it has been argued that the failure to retrieve genetically labelled BM-derived Tie-2+ ECs in the paper of De Palma et al. might be because of the fact that the exogenous Tie-2 promoter used, may not mark all the mature ECs, or that during the random genetic manipulation of the cells, the CEPC population might not have been targeted with the viral Tie-2 vector [5]. Also, it is possible that the failure to detect few, if any BM-derived ECs in the neovasculature of experimental models may be related to poor engraftment of the EPC compartment following their transplantation [22, 66]. Although the latter pitfall can be circumvented by the use of a parabiosis model (where two mice are surgically connected and share a common circulatory system allowing exchange of circulating cells), conflicting results have also been reported in the parabiosis model [36].
To unequivocally illustrate in vivo that the EPC-derived progeny in the newly formed vasculature is truly endothelial in nature, a more direct or convincing approach would be the extraction of putative EPC progeny from the tissues (by means of a genetic tracer such as GFP) and FACS analyse (with and without previous culture) these cells using a wide panel (CD11, CD45, VE-Cadherin, CD146, CD31, CD13, CD105, etc.) of antigens and combinations of these markers to illustrate their true endothelial lineage and discriminate them from other cell lineages, especially the haematopoietic lineage. In addition, these extracted cells could also be tested functionally by means of proliferative capacity or tube forming capacity. So far, only one group has used such a FACS strategy to identify EPC progeny in vivo[5], but the marker combination used (CD31+Lectin+GFP+) does not allow a clear discrimination from haematopoietic (derived) cells that also display these properties [5, 57].
Other methodological issues, such as the number of tissue sites sampled, or the time frame of the study intervals, and the microscopic technique employed in the tissue analysis, may explain the variable results of EPCs to contribute to sites of neovascularization in different animal model systems. For instance, while the use of confocal microscopy may permit a complete volumetric three-dimensional rendering of the donor cell contribution to new blood vessels in a damaged tissue, the use of light microscopy might impose difficulty in discriminating whether cells are incorporated at the luminal layer and are integrated within the endothelial layer, or are located at peri-luminal sites, just beneath the endothelial layer [22, 28]. Therefore, differences in the microscopic technique, and/or method of analysis of the imaging data, may also contribute to the highly variable reported rate of incorporation of EPCs in repairing vessels (0–90%). Furthermore, it remains uncertain how many of the ‘luminal integrated cells’ (e.g. monocytes, that phenotypically overlap with ECs) are in fact passenger cells participating in an inflammatory reaction to the vascular injury, or cells in the process of transmigrating deeper into the vessel wall and interstitial tissues. Thus, whenever possible, the use of three-dimensional imaging may provide the most sensitive and specific information on the location and contribution of the donor cells to tissues under repair (given the use of appropriately tested antibodies that are specific and validated for detection of cells, cell nuclei or organelles, basement membrane and extracellular matrix proteins).
Other variables that must be considered when using animal models include the genetic background of the mouse, the type of vascular injury to be incurred and the organ system to be challenged. In the case of a tumour model system where one wishes to examine donor cell contributions to tumour endothelium, the tumour type, stage and therapy may be important [5, 49]. Also, the nature of the angiogenic stimulus may be critical, because the cellular and molecular mechanisms of (neo)vascularization differ depending on whether a tumour, a denuded artery, a myocardial infarction, hindlimb ischaemia or a traumatic injury is used to assay a candidate EPC [67, 68]. Therefore, specific cell subsets may be recruited preferentially, and conflicting results may ensue, depending on the animal or tissue model.
Also, the degree of EPC contribution may also depend on the type of vascular bed that has been studied. Indeed, the degree of CEPC incorporation and contribution to neovessels may be influenced by competition between CEPCs and the resident ECs during vascular repair and regeneration.
Summarizing this section, it is imperative that improved approaches are developed to reconcile the previous conflicting reports on the role of EPCs in vascular repair, to further define the exact immunophenotype of CEPCs, and to define other participating cell types in adult vessel repair and regeneration.
EPCs defined in vitro: the Achilles heel in EPC biology
EOCs and EC-like cells
Most investigators have focused on the study of ‘EPCs’ obtained after the ex vivo culture of unfractionated MNCs that contain putative CEPCs. This is because CEPCs appear to be present in the circulation at very low numbers, requiring ‘ex vivo culture’ to obtain sufficient numbers to characterize and study them in disease models. Numerous assays have been developed to plate MNCs in specific conditions to ‘make putative CEPCs differentiate into ECs in vitro’[2, 3, 37, 38, 55, 69–72], ‘to expand CEPCs ex-vivo’[73–76], or to ‘assay CEPCs for colony forming capacity’[17, 77–79]. Irrespective of the assay or the purposes of the EPC cultures reported so far, two major cell types have been shown to emerge out of these MNC cultures: (1) cells that display a mixed endothelial-monocytic/haematopoietic phenotype [47, 57, 58, 74, 76, 80–86] which we refer to as EC-like cells, and (2) cells with high proliferative potential that display typical endothelial characteristics, reminiscent to vessel wall ECs, which we refer to as endothelial outgrowth cells (EOCs) [37, 58, 75, 79, 81, 85–88].
The nomenclature used to describe each major cell type has been widely varying and often intermixed (Table 3), which has contributed to the confusion in this field. Also, in many of the early studies of EPC biology, it has been impossible to appreciate the exact cell type (EC-like cells versus EOCs) obtained in vitro, owing to the limited characterization of the cells described in the reports. Indeed, both EC-like cells and EOCs express several identical surface markers such as CD31, lectin binding, vWF and uptake of LDL [57]. However, recent studies have shown that EC-like cells do not represent true ECs and derive from heterogeneous CD45+haematopoietic cells, including CD34+CD45+(CD133+) HSCs/HPCs and CD45+CD14+ monocytic cells that both co-express a set of ‘endothelial’ surface markers [57, 58, 85]. Therefore, we encourage the use of extensive criteria, as depicted in Tables 2 and 3 that allows for an accurate discrimination between these two cell types in vitro. This discrimination is based on phenotypical, morphological and functional characteristics typical for cultured vessel wall ECs. However, it is important to note that the markers depicted in Tables 2 and 3 are not uniformly expressed by all ECs lining the blood vessels in vivo, and ECs may change their surface marker expression profile upon ex vivo culture [89, 90]. Nevertheless, ECs in vivo have many characteristics in common similar to the in vitro defined EOCs, as described in Tables 2 and 3[37, 79, 85, 91, 92]. Therefore, apart from their vessel forming ability, it has been stipulated that cells can be ascribed as ‘true EPCs’ only if they generate true ECs sharing most of these characteristics [57, 86, 87, 93–95]. Conversely, the circulating cells that generate EC-like cells in vitro cannot be considered as true CEPCs [57].
3.
Characteristics of human EC-like cells, EOCs and CECs
| EC-like cells | EOCs | CECs |
|---|---|---|
| (EPCs, ECs, CFU-ECs, CACs, ATs, early outgrowth CE-EPCs, CMMCs and early EPCs) | (EPCs, ECs, CFU-ECs, BOECs, ECFCs, EPDCs, EC-like, late EPCs, late endothelial outgrowth) | (Circulating endothelial cells) |
| 1. Generated after 4–21 days in culture | 1. Appear after > 7 days in culture | 1. Low proliferative ECs, shed from the vascular wall into the circulation |
| 2. Round (pancake) to spindle shaped appearance; no typical confluent monolayer | 2. Typical polygonal cells in a confluent cobblestone monolayer | 2. Have a similar phenotypical profile compared to EOCs |
| 3. Express endothelial and haematopoietic markers (e.g. CD45, CD14) | 3. Express CD31, CD34, CD105, CD146, VE-Cadherin, VEGFR-2, but not the haematopoietic surface markers CD133, CD14 or CD45 | 3. Do not express haematopoietic markers and have no apparent haematopoietic potential or function |
| 4. Bind UEA-1 lectin and take up LDL | 4. Bind UEA-1 lectin and take up LDL | |
| 5. Maintain haematopoietic potential and/or functions | 5. Have no apparent haematopoietic potential | |
| 6. Have low proliferative potential | 6. Bear high proliferative potential | |
| 7. Do not generate vascular tubes in vitro in matrigel | 7. Generate vascular tubes in vitro/in vivo in matrigel | |
| 8. Improve neovascularization in vivo | 8. Improve neovascularization in vivo | |
| 9. Originate from CD45+ haematopoietic lineage cells (CD34+CD45+, CD133+CD45+, CD34−CD45+, CD14+CD45+) | 9. Originate from CD45−CD133−CD34+ cells, bone marrow (*) and probably the vascular wall |
CFU-ECs [77, 159, 160]: colony forming unit of endothelial cells; ATs [161]: attaching cells; CACs [47]: circulating angiogenic cells; CE-EPCs [76]: culture expanded endothelial progenitor cells; CMMCs [162]: culture modified mononuclear cells; EOCs [58]: endothelial outgrowth cells; BOECs [163]: blood EOCs; ECFCs [79]: endothelial colony forming cells; EPDCs [37]: endothelial progenitor-derived cells; CECs [163]: circulating endothelial cells, (*) [94]: bone marrow multipotent adult progenitor cells (MAPCs) have been suggested to be the earliest EOC precursors.
In the murine system, similar EOCs and EC-like populations have been described in vitro, or at least the same nomenclature has been used to describe similar endothelial progenies. Similar to the human situation, early EPCs have been shown to derive from haematopoietic precursors [96]. So far, murine MNCs have been plated to obtain EOCs, but it is unknown what the exact phenotype is of the circulating cell (see the putative murine CEPCs phenotypes in Table 1) that gives rise to these murine EOCs in vitro (i.e. a precursor–product relationship is completely lacking) [97, 98].
With regard to the role in neovascularization in vivo of both in vitro generated EOCs and EC-like cells, conflicting schools of thought exist. There is evidence that injected (in vitro generated) EC-like and EOCs act in synergy during vascular repair. EOCs appear to structurally contribute to neovessels, whereas EC-like cells do not directly contribute to the neovessels, but rather, act in an indirect paracrine fashion by locally secreting angiogenic substances that promote structural healing by resident ECs and incorporated EOCs. Because the incorporation and direct structural contribution to neoendothelium has been claimed to be a major criterion for defining a cell as an EPC, some have redefined EC-like cells as ‘angiogenic cells’ instead of EPCs, because they do not directly participate in neovascularization, and therefore, do not act as true EPCs in the literal sense (i.e. structurally contribute to neovessels) [47, 57, 99]. In fact, this redefinition is in agreement with studies in the embryo [46] and adulthood [23, 25, 45], showing that (non-cultured) haematopoietic lineage cells (that give rise to EC-like cells in vitro) do not structurally contribute to growing vessels, but rather, act in a indirect, instructive manner to help in neovascularization. However, disagreement persists in the scientific community with respect to this redefinition and a consensus in the field is far from unanimous.
What are potential caveats with in vitro defined cells?
An important drawback in the study of cultured cells is that ‘EC-like cells’ and ‘EOCs’ may have acquired or lost properties during culture that influence experimental outcomes (in a positive or negative way) compared to their non-cultured counterpart (i.e. the circulating cells from which they originate, which are in fact the cells of true interest, the EPC). In other words, the function (e.g. vasculogenic and incorporation properties) and phenotype (e.g. surface marker profile) of in vitro propagated endothelial lineage cells may be culture artefacts compared to their in vivo counterpart. In fact, this question is relevant to any in vitro defined cell population and should cause one to be careful in directly comparing the role, phenotype and function of cultured cells with respect to their original in vivo (non-passaged) precursors [90, 100]. More specific to the field of EPC biology, two reports showed that injected, non-cultured CD14+ monocytes did not enhance or incorporate into neovessels, whereas their in vitro derivates did [76, 84]. This might indicate that native monocytes are already present abundantly at sites of tissue healing [25, 63] or that monocyte-derived EC-like cells are culture artefacts with altered properties (not normally present in vivo) induced by the culture conditions, and might not exist as such in vivo.
The search for the EOC precursor: lessons from embryonic development
The close developmental association, surface marker, and molecular overlap between haematopoietic cells and ECs supports the concept of the haemangioblast as being the bipotent predecessor for both haematopoietic cells and ECs (see Fig. 2) [101]. Although many studies support the haemangioblast concept in vitro, whether this cell exists as a distinct entity during embryonic development in vivo and whether it persists into adulthood remain a subject of great debate [102–105]. In fact, some groups have shown evidence for the existence of ‘haemogenic endothelial cells’ (HECs) (that express the markers CD31, CD34, VEGFR-2 and VE-Cadherin) [106], which provides an alternative explanation for the developmental overlap between haematopoietic and ECs (i.e. these ‘unipotent’ transitional ECs instead of haemangioblasts differentiate into haematopoietic progenitor cells (HPCs) in the embryo, see Fig. 2) [107].
2.
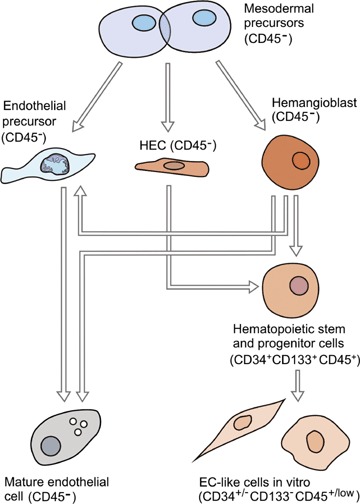
Haemato-endothelial developmental pathways and their relation to the expression of CD45. In the embryo, CD45 −mesodermal precursors give rise to CD45− endothelial precursors (EPCs), haemangioblasts and/or haemogenic endothelial cells (HECs). The CD45− EPCs differentiate into functional and mature ECs. Embryonic haemangioblasts are CD45− and differentiate to both CD45− endothelial lineage cells and CD45+ HSCs/HPCs in vitro. Alternatively, or in addition to haemangioblasts, CD45− HECs give rise to CD45+ haematopoietic stem/progenitor cells. HSCs/HPCs can give rise to EC-like cells in vitro and retain expression of CD45, whereas expression of the CD133 antigen is downregulated.
We have used some of the strategies previously utilized to define the embryonic ‘haemangioblast’ and ‘HECs’ to address the phenotype of the cell(s) that give rise to EOCs in the human adult, with specific emphasis on the common leucocyte marker CD45, as a defining marker [58]. Both the embryonic haemangioblast and HECs express VEGFR-2 and CD34, explaining why these markers have been popular to identify CEPCs in the human adult [106, 108, 109]. Importantly, studies in haemato-endothelial development have shown that CD34+ embryonic haemangioblasts or HECs do not express the common leucocyte antigen CD45, but acquire this marker only during differentiation into HPCs (and thus become CD34+CD45+), but not during commitment into the endothelial lineage (Fig. 2) [106, 108, 109]. Therefore, CD45 expression marks haematopoietic specification from foetal life into adulthood, while it is not expressed on endothelial lineage cells (Table 2). The developmental overlap, the common surface marker expression on HSCs/HPCs and ECs, and the findings that CD34+ cells can generate both haematopoietic and endothelial progeny in the adult [37] has led investigators to believe that CD34+CD45+ HSCs/HPCs display a ‘haemangioblastic’ or ‘EPC capacity’ in human postnatal life [3, 110]. However, analysis of the marker CD45 was not performed in the original description of CD34+ CEPCs [2, 3, 38, 111, 112]. This is a major limitation because CD34+ cells in the adult not only contain CD34+CD45+ HPCs but also a small CD34+CD45− cell fraction, similar to the embryonic situation. Importantly, we showed that the CD34+CD45− cell fraction, but not CD34+CD45+ haematopoietic cells within CD34+ cells generates EOCs [41, 58]. Therefore, if EOCs are considered to be derived from true CEPCs, these cells should be contained within the CD34+CD45− cell fraction, but not within the CD34+CD45+ haematopoietic cell fraction. Indeed, we showed that the CD34+CD45+ HSC/HPC cell fraction did not generate EOCs, but differentiated into EC-like cells through a CD14+ monocytic pathway [58].
We also detected the marker VEGFR-2 in the CD34+CD45− cell population, a tyrosine kinase receptor indispensable for both endothelial and haematopoietic lineages [113, 114]. However, CD34+VEGFR-2+ cells within the CD34+CD45∼ cell fraction may represent ‘mature’ ECs [39], or CD34+VEGFR-2+ cells that co-express CD133 may represent the pool of CEPCs [38, 39]. Indeed, because the CD34+VEGFR-2+ phenotype may also represent mature ECs, Peichev and co-workers have proposed that CD34+VEGFR-2+ cells co-expressing CD133 represent true immature CEPCs. Upon differentiation into mature ECs, CD34+VEGFR-2+CD133+ CEPCs are believed to lose expression of CD133. This is corroborated by the fact that mature ECs lining the blood vessels in the adult organism neither express the CD133 protein [38]. However, we showed that the EOC-generating CD34+CD45− cell fraction does not express CD133 [58]. In fact, several other investigators have been unable to detect the CD133 antigen on CD34+CD45− cells, which confirms our findings [42, 115, 116]. As such, the surface antigen CD133 cannot be used to identify putative circulating EOC-precursors. In fact, CD34+VEGFR-2+CD133+ cells were recently shown to be CD45+ haematopoietic progenitors instead of true CEPCs and do not generate EOCs [41, 58]. Therefore, previous reports showing that CD34+ (CD45+) HPCs contain EPCs or haemangioblasts, and that CD133+ cells can differentiate into ‘ECs’ can be challenged with respect to the specific CD34+ subpopulation studied, and/or the criteria used to identify endothelial progeny as bona fide ECs (see Table 3).
Do EOCs derive from an immature CEPC?
The developmental and maturation from ‘stem cells’ or ‘progenitors’ into ‘mature cells’ is well established in many developing systems, and is characterized by a stepwise loss and gain of specific markers, and/or cell functions. However, because of the lack of a marker that discriminates circulating EOC-precursors from in vitro cultured EOCs, the exact identity of circulating EOC-precursors remains uncertain. As a consequence, it is unknown whether CD34+CD45− EOC-precursors are distinct from EOCs generated in vitro with respect to phenotypical and functional characteristics. In other words, it is unknown whether the EOCs ‘generated’ or ‘expanded’ex vivo represent the differentiated progeny of a distinct, undifferentiated precursor and whether EOCs are just circulating mature ECs.
Nevertheless, it has been claimed that EOCs are a CEPC-derived population based on functional differences between EOCs and ‘control’ mature vessel wall ECs such as human umbilical vein endothelial cells (HUVECs). In these studies, EOCs displayed a higher proliferative potential, angiogenic cytokine release and stress resistance compared to HUVEC and other vessel wall ECs [37, 88, 117]. However, conflicting results have been reported on these characteristics and these findings do not allow us to conclude that EOCs are an EPC-derived cell population [118]. Indeed, to address EPCness of EOCs, the most straightforward approach would be the direct assessment of EOCs (head-to-head with mature vessel wall ECs) in in vivo vasculogenic assays in mice such as experimental myocardial infarction, hind limb ischaemia and other clinical relevant conditions.
To date however, only one study has reported enhanced vessel forming ability of EOCs compared to ‘mature’ vessel wall ECs in an ischaemic mouse model [119]. All other studies have categorized EOCs as EPCs or an EPC-derived population based on vasculogenic properties assayed in in vivo and in vitro matrigel assays [57, 120]. In the matrigel assay, endothelial lineage cells are incubated in an extracellular matrix derived from murine sarcoma cells that supports vascular tube formation. Vascular tube formation in matrigel is thought to recapitulate in vivo vasculogensis and angiogenesis. However, the relevance of matrigel assays for addressing EPCness of EOCs is cumbersome, because ‘mature ECs’ such as HUVECs were also shown to robustly incorporate and generate vascular tubes using a matrigel assay in vivo[57, 120].
Therefore, more experimental data are required to document incorporation and regeneration of durable blood vessels by injected EOCs (and compared with mature vessel wall ECs such as HUVECs) in several relevant in vivo models. Moreover, because in vitro cultured EOCs may have decreased vasculogenic properties (‘decrease of EPCness’) compared to their in vivo EOC-precursors, the identification of a specific EOC-precursor marker would allow the isolation of EOC-precursors and test them in these assays and directly compare them with cultured EOCs and mature vessel wall ECs.
Do EOCs derive from high proliferative vessel wall ECs?
Because EOCs display striking similarities with ‘mature vessel wall ECs’, one may speculate whether blood-derived EOCs are just ‘culture-expanded’ mature ECs that have dislodged from the vessel wall (e.g. at angiogenic sites, during increased shear stress, vein-puncture related etc. [121, 122]), rather than being the differentiated progeny from a CD34+CD45− BM-derived immature CEPC. Indeed, it is known that ECs can detach from the vessel wall into the circulation in normal physiology and during several clinical disorders [123]. These vessel wall dislodged ECs, designated as circulating ECs (‘CECs’), have been claimed to be ‘mature ECs’, but the general view is that these CECs do not give rise to EOCs in vitro because CECs bear no or low proliferative potential, whereas EOCs bear high proliferative potential [123]. In fact, in a seminal report studying sex-mismatched BM transplant patients, Lin et al. claimed that EOCs originate from putative BM precursors, whereas CECs with low proliferative potential originate from the vessel wall [87].
However, it is possible that the myeloablative procedure in this study might have attenuated the proliferative capacity of the recipient's vessel wall ECs, therefore neglecting other sources for EOCs than the BM, such as the vessel wall. Indeed, vessel wall ECs such as HUVECs have been shown to expand to similar levels compared to blood-derived EOCs, indicating that ‘mature’ vessel wall ECs do have a robust proliferative potential and may be at the origin of EOCs [37, 124–126]. This has been corroborated by a recent single cell study showing that ECs of the adult vessel wall display a proliferative hierarchy ranging from non-replicating ECs, to very high proliferative ECs [127]. Notably, we also found that 2% of freshly isolated HUVECs have a similar expansion potential compared to UCB-derived EOCs at the single cell level (F. Timmermans, unpublished observation). Although these findings suggest that EOCs cultured from circulating blood derive from high proliferative vessel wall ECs or true resident vessel wall EPCs (that both can enter into the bloodstream), this issue needs to be clarified. Furthermore, it remains to be seen whether high proliferative vessel wall ECs originally derive from incorporated BM precursors or not. Finally, it would be interesting to know whether BM EOC-precursors are located in the haematopoietic niche of the BM, or reside with the BM vessel wall.
CEPCs and CECs: functionally different cells having the same identity?
It is common use to indicate CECs as vessel wall ECs with low proliferative potential that preferentially slough off from the vessel wall during normal and pathological conditions [123]. Although several studies have suggested that the number of CECs is increased in some disorders [123], there has been a parallel debate on how ‘CECs’ should be defined from an immunophenotypic point of view (see reference [128] versus [129]), an obstacle reminiscent to the field of CEPCs. Moreover, our data showing that putative CEPCs (EOC-precursors) reside in the CD45− cell fractions, and do not express CD133, further complicates the immunophenotypic discrimination between ‘CECs’ and ‘CEPCs’, because to date, the markers CD133 and CD45 were thought to discriminate putative CEPCs from CECs [39, 130, 131]. Also, the claim that CEPCs tend to be smaller cells compared to CECs, has not yet been validated given the lack of a clear precursor–product relationship that included this parameter in vitro as well as in vivo, as discussed in the first section of this review.
To date, the literature in the field of EPCs supports the marker combination CD34+VEGFR-2+ to identify CEPCs in humans [112]. However, circulating CD34+VEGFR-2+ cells may also represent haematopoietic progenitors [132] and mature circulating ECs [39]. Moreover, to date, it has not been carefully addressed whether the CD34+VEGFR-2+ phenotype contains the CEPCs that generate EOCs in vitro. Therefore, the search for a novel marker or unique combination of markers and parameters that accurately discriminates CECs from CEPCs or high proliferative vessel wall ECs with flow cytometry is mandatory.
Summary
We have highlighted several areas of controversy that persist in the study of EPC biology. While significant progress has been made in our understanding that neovasculogenesis is a multicellular event, and is unlikely to occur from a single recruited progenitor cell type, further progress will require the development of novel assays and conscientious read-out criteria that specifically identify the functional ability of putative EPCs to directly participate in postnatal vasculogenesis. Without a standard set of in vitro and in vivo assays that allow one to allocate specific cell phenotypic profiles with specific activities, the field of EPC biology may stagnate. One can only look at the tremendous clinical advancements made in the field of HSC transplantation that occurred once both in vitro and in vivo assays were established, that strictly defined the hierarchical staged development of haematopoietic precursors, as a paradigm for the progress in clinical applications of EPCs to cardiovascular disorders that may follow the development of similar assays to define neoangiogenesis.
Acknowledgments
This work was supported by grant G.0096.05 of the Fund for Scientific Research, Flanders (FWO Vlaanderen). Frank Timmermans is a doctoral research fellow of the Fund for Scientific Research, Flanders (FWO Vlaanderen). National Institute of Neurological Disorders and Stroke P50 NS052606 (D.A.I.), NF043019 Department of Defense (D.A.I.), W81XWH-05-1-0161, Riley Children's Foundation (D.A.I. and M.C.Y.), P30 CA82709 (D.A.I.), National Institutes of Health 1 P01 HL085036 (M.C.Y. and D.A.I.), R21 HL088885 National Institutes of Health/National Heart, Lung, and Blood Institute (D.A.I.) and National Institutes of Health 2 P01 HL053586-11A1 (D.A.I.). We thank Christiaan De Boever for artwork. F.T., J.P., M.C.Y., D.A.I., B.V. and J.C. have no competing financial interests to disclose.
References
- 1.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–95. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, Silver M, Van Der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–6. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–7. [PubMed] [Google Scholar]
- 4.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 5.Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi JS, Bambino K, Gupta S, Heikamp E, McDevitt MR, Scheinberg DA, Benezra R, Mittal V. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007;21:1546–58. doi: 10.1101/gad.436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 7.Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, Gurtner GC. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood. 2005;105:1068–77. doi: 10.1182/blood-2004-03-1051. [DOI] [PubMed] [Google Scholar]
- 8.Ii M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, Maruyama K, Yoon YS, Wecker A, Luedemann C, Eaton E, Silver M, Thorne T, Losordo DW. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res. 2006;98:697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
- 9.Urao N, Okigaki M, Yamada H, Aadachi Y, Matsuno K, Matsui A, Matsunaga S, Tateishi K, Nomura T, Takahashi T, Tatsumi T, Matsubara H. Erythropoietin-Mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006;98:1405–13. doi: 10.1161/01.RES.0000224117.59417.f3. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 11.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothe-lium by adult stem cells. J Clin Invest. 2001;107:1395–402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang S, Walker L, Afentoulis M, Anderson DA, Jauron-Mills L, Corless CL, Fleming WH. Transplanted human bone marrow contributes to vascular endothelium. Proc Natl Acad Sci USA. 2004;101:16891–6. doi: 10.1073/pnas.0404398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki T, Nishida M, Futami S, Fukino K, Amaki T, Aizawa K, Chiba S, Hirai H, Maekawa K, Nagai R. Neoendo-thelialization after peripheral blood stem cell transplantation in humans: a case report of a Tokaimura nuclear accident victim. Cardiovasc Res. 2003;58:487–92. doi: 10.1016/s0008-6363(02)00780-0. [DOI] [PubMed] [Google Scholar]
- 14.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–6. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 15.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:e1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–7. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 17.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 18.Chironi G, Walch L, Pernollet M-G, Gariepy J, Levenson J, Rendu F, Simon A. Decreased number of circulating CD34+KDR+ cells in asymptomatic subjects with preclinical atherosclerosis. Atherosclerosis. 2007;191:115–20. doi: 10.1016/j.atherosclerosis.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 19.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–6. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 20.Loomans CJM, De Koning EJP, Staal FJT, Rookmaaker MB, Verseyden C, De Boer HC, Verhaar MC, Braam B, Rabelink TJ, Van Zonneveld A-J. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–9. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 21.Sorrentino SA, Bahlmann FH, Besler C, Muller M, Schulz S, Kirchhoff N, Doerries C, Horvath T, Limbourg A, Limbourg F, Fliser D, Haller H, Drexler H, Landmesser U. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-{gamma} agonist rosiglitazone. Circulation. 2007;116:163–73. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- 22.De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9:789–95. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 23.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–73. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 24.Rajantie I, Llmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for peri-endothelial vascular mural cells. Blood. 2004;104:2084–6. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vas-culature. Circ Res. 2004;94:230–8. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 26.Larrivee B, Niessen K, Pollet I, Corbel SY, Long M, Rossi FM, Olive PL, Karsan A. Minimal contribution of marrow-derived endothelial precursors to tumor vasculature. J Immunol. 2005;175:2890–9. doi: 10.4049/jimmunol.175.5.2890. [DOI] [PubMed] [Google Scholar]
- 27.Gothert JR, Gustin SE, Van Eekelen JAM, Schmidt U, Hall MA, Jane SM, Green AR, Gottgens B, Izon DJ, Begley CG. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood. 2004;104:1769–77. doi: 10.1182/blood-2003-11-3952. [DOI] [PubMed] [Google Scholar]
- 28.Galimi F, Summers RG, Van Praag H, Verma IM, Gage FH. A role for bone marrow-derived cells in the vasculature of noninjured CNS. Blood. 2005;105:2400–2. doi: 10.1182/blood-2004-02-0612. [DOI] [PubMed] [Google Scholar]
- 29.O'Neill TJIV, Wamhoff BR, Owens GK, Skalak TC. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ Res. 2005;97:1027–35. doi: 10.1161/01.RES.0000189259.69645.25. [DOI] [PubMed] [Google Scholar]
- 30.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525–31. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 31.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–77. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–89. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 33.Gagner JP, Shamamian P. Antigen expression profile in circulating endothelial progenitor cells. Nat Rev Cancer. 2007;7(1) [Google Scholar]
- 34.Leor J, Marber M. Endothelial progenitors: a new tower of Babel? J Am Coll Cardiol. 2006;48:1588–90. doi: 10.1016/j.jacc.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 35.Goon PKY, Lip GYH. Endothelial progenitor cells, endothelial cell dysfunction and much more: observations from cardiac syndrome X. Heart. 2007;93:1020–1. doi: 10.1136/hrt.2006.112664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, Alitalo K, Weissman IL, Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA. 2008;105:6620–5. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bompais H, Chagraoui J, Canron X, Crisan M, Liu XH, Anjo A, Tolla-Le Port C, Leboeuf M, Charbord P, Bikfalvi A, Uzan G. Human endothelial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood. 2004;103:2577–84. doi: 10.1182/blood-2003-08-2770. [DOI] [PubMed] [Google Scholar]
- 38.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MAS, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8. [PubMed] [Google Scholar]
- 39.Bertolini F, Shaked Y, Mancuso P, Kerbel R. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–45. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 40.Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Lino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24:1442–7. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 41.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–18. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Delorme BBA, Gentile C, Sabatier F, Monsonis F, Desouches C, Blot-Chabaud M, Uzan G, Sampol J, Dignat-George F. Presence of endothelial progenitor cells, distinct from mature endothelial cells, within human CD146+ blood cells. Thromb Haemost. 2005;94:1270–9. doi: 10.1160/TH05-07-0499. [DOI] [PubMed] [Google Scholar]
- 43.Grant MB, May WS, Caballero S, Brown GAJ, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–12. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 44.Bailey AS, Jiang S, Afentoulis M, Baumann CI, Schroeder DA, Olson SB, Wong MH, Fleming WH. Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood. 2004;103:13–9. doi: 10.1182/blood-2003-05-1684. [DOI] [PubMed] [Google Scholar]
- 45.Stadtfeld M, Graf T. Assessing the role of hematopoietic plasticity for endothelial and hepatocyte development by non-invasive lineage tracing. Development. 2005;132:203–13. doi: 10.1242/dev.01558. [DOI] [PubMed] [Google Scholar]
- 46.Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, Satake M, Suda T. A role for hematopoietic stem cells in promoting angiogenesis. Cell. 2000;102:199–209. doi: 10.1016/s0092-8674(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 47.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/ macro-phages and secrete angiogenic growth factors. Circulation. 2003;107:1164–9. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 48.Heil M, Eitenmiiller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, Hicklin DJ, Chaplin D, Foster FS, Benezra R, Kerbel RS. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–7. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 50.Ciarrocchi A, Jankovic V, Shaked Y, Nolan DJ, Mittal V, Kerbel RS, Nimer SD, Benezra R. Id1 restrains p21 expression to control endothelial progenitor cell formation. PLoS One. 2007;2:e1338. doi: 10.1371/journal.pone.0001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–8. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 52.Liao F, Doody JF, Overholser J, Finnerty B, Bassi R, Wu Y, Dejana E, Kussie P, Bohlen P, Hicklin DJ. Selective targeting of angiogenic tumor vasculature by vascular endothelial-cadherin antibody inhibits tumor growth without affecting vascular permeability. Cancer Res. 2002;62:2567–75. [PubMed] [Google Scholar]
- 53.Perry SS, Zhao Y, Nie L, Cochrane SW, Huang Z, Sun X-H. Id1 but not Id3 directs long-term repopulating hematopoietic stem-cell maintenance. Blood. 2007;110:2351–60. doi: 10.1182/blood-2007-01-069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow–derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–6. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 55.Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N. CD34-/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasore-generative capacities. Circ Res. 2006;98:e20–5. doi: 10.1161/01.RES.0000205765.28940.93. [DOI] [PubMed] [Google Scholar]
- 56.Chang EI, Loh SA, Ceradini DJ, Chang EI, Lin S-e, Bastidas N, Aarabi S, Chan DA, Freedman ML, Giaccia AJ, Gurtner GC. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1{alpha} stabilization during ischemia. Circulation. 2007;116:2818–29. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- 57.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, Gillebert TC, Plum J, Vandekerckhove B. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27:1572–9. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 59.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFR[beta]+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–9. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conejo-Garcia JR, Buckanovich RJ, Benencia F, Courreges MC, Rubin SC, Carroll RG, Coukos G. Vascular leukocytes contribute to tumor vascularization. Blood. 2005;105:679–81. doi: 10.1182/blood-2004-05-1906. [DOI] [PubMed] [Google Scholar]
- 61.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis, 2006, 103, 12493, 8. [DOI] [PMC free article] [PubMed]
- 62.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–65. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J-L, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–47. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Langer H, May AE, Daub K, Heinzmann U, Lang P, Schumm M, Vestweber D, Massberg S, Schonberger T, Pfisterer I, Hatzopoulos AK, Gawaz M. Adherent platelets recruit and induce differentiation of murine embryonic endothelial progenitor cells to mature endothelial cells in vitro. Circ Res. 2006;98:e2–10. doi: 10.1161/01.RES.0000201285.87524.9e. [DOI] [PubMed] [Google Scholar]
- 65.Schlaeger TM, Bartunkova S, Lawitts JA, Teichmann G, Risau W, Deutsch U, Sato TN. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adulttransgenicmice. Proc Natl Acad Sci USA. 1997;94:3058–63. doi: 10.1073/pnas.94.7.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gothert JR, Gustin SE, Hall MA, Green AR, Gottgens B, Izon DJ, Begley CG. In vivo fate-tracing studies using the Scl stem cell enhancer: embryonic hematopoietic stem cells significantly contribute to adult hematopoiesis. Blood. 2005;105:2724–32. doi: 10.1182/blood-2004-08-3037. [DOI] [PubMed] [Google Scholar]
- 67.Madeddu P, Emanueli C, Spillmann F, Meloni M, Bouby N, Richer C, Alhenc-Gelas F, Van Weel V, Eefting D, Quax PHA, Hu Y, Xu Q, Hemdahl AL, Van Golde J, Huijberts M, De Lussanet Q, Boudier HS, Couffinhal T, Duplaa C, Chimenti S, Staszewsky L, Latini R, Baumans V, Levy BI. Murine models of myocardial and limb ischemia: diagnostic end-points and relevance to clinical problems. Vasc Pharmacol. 2006;45:281–301. doi: 10.1016/j.vph.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 68.Heil M, Eitenmiiller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–12. [PubMed] [Google Scholar]
- 70.Romagnani P, Annunziato F, Liotta F, Lazzeri E, Mazzinghi B, Frosali F, Cosmi L, Maggi L, Lasagni L, Scheffold A, Kruger M, Dimmeler S, Marra F, Gensini G, Maggi E, Romagnani S. CD14+CD34 low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res. 2005;97:314–22. doi: 10.1161/01.RES.0000177670.72216.9b. [DOI] [PubMed] [Google Scholar]
- 71.Elsheikh E, Uzunel M, He Z, Holgersson J, Nowak G, Sumitran-Holgersson S. Only a specific subset of human peripheral-blood monocytes has endothelial-like functional capacity. Blood. 2005;106:2347–55. doi: 10.1182/blood-2005-04-1407. [DOI] [PubMed] [Google Scholar]
- 72.Davies WR, Wang S, Oi K, Bailey KR, Tazelaar HD, Caplice NM, McGregor CGA. Cyclosporine decreases vascular progenitor cell numbers after cardiac transplantation and attenuates progenitor cell growth in vitro. J Heart Lung Transplant. 2005;24:1868–77. doi: 10.1016/j.healun.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 73.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106:3009–17. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 74.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–7. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003;108:2710–5. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 76.Sharpe EE3rd, Teleron AA, Li B, Price J, Sands MS, Alford K, Young PP. The origin and in vivo significance of murine and human culture-expanded endothelial progenitor cells. Am J Pathol. 2006;168:1710–21. doi: 10.2353/ajpath.2006.050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hill JM, Zalos G, Halcox JPJ, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 78.Guven H, Shepherd RM, Bach RG, Capoccia BJ, Link DC. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J Am Coll Cardiol. 2006;48:1579–87. doi: 10.1016/j.jacc.2006.04.101. [DOI] [PubMed] [Google Scholar]
- 79.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 80.Harraz M, Jiao C, Hanlon HD, Hartley RS, Schatteman GC. CD34- blood-derived human endothelial cell progenitors. Stem Cells. 2001;19:304–12. doi: 10.1634/stemcells.19-4-304. [DOI] [PubMed] [Google Scholar]
- 81.Quirici N, Soligo D, Caneva L, Servida F, Bossolasco P, Deliliers GL. Differentiation and expansion of endothelial cells from human bone marrow CD133(+) cells. Br J Haematol. 2001;115:186–94. doi: 10.1046/j.1365-2141.2001.03077.x. [DOI] [PubMed] [Google Scholar]
- 82.Rohde E, Bartmann C, Schallmoser K, Reinisch A, Lanzer G, Linkesch W, Guelly C, Strunk D. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells. 2007;25:1746–52. doi: 10.1634/stemcells.2006-0833. [DOI] [PubMed] [Google Scholar]
- 83.Aranguren XL, Luttun A, Clavel C, Moreno C, Abizanda G, Barajas MA, Pelacho B, Uriz M, Arana M, Echavarri A, Soriano M, Andreu EJ, Merino J, Garcia-Verdugo JM, Verfaillie CM, Prosper F. In vitro and in vivo arterial differentiation of human multipotent adult progenitor cells. Blood. 2007;109:2634–42. doi: 10.1182/blood-2006-06-030411. [DOI] [PubMed] [Google Scholar]
- 84.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neo-vascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–6. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 85.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–27. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 86.Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, Simari RD. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023–5. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 87.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–7. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He T, Peterson TE, Holmuhamedov EL, Terzic A, Caplice NM, Oberley LW, Katusic ZS. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol. 2004;24:2021–7. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- 89.Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54:385–95. doi: 10.1369/jhc.4A6514.2005. [DOI] [PubMed] [Google Scholar]
- 90.Amatschek S, Kriehuber E, Bauer W, Reininger B, Meraner P, Wolpl A, Schweifer N, Haslinger C, Stingl G, Maurer D. Blood and lymphatic endothelial cell-specific differentiation programs are stringently controlled by the tissue environment. Blood. 2007;109:4777–85. doi: 10.1182/blood-2006-10-053280. [DOI] [PubMed] [Google Scholar]
- 91.Conway E, Carmeliet P. The diversity of endothelial cells: a challenge for therapeutic angiogenesis. Genome Biol. 2004;5:207. doi: 10.1186/gb-2004-5-2-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 93.Gulati R, Simari RD. Cell therapy for angiogenesis: embracing diversity. Circulation. 2005;112:1522–4. doi: 10.1161/CIRCULATIONAHA.105.566497. [DOI] [PubMed] [Google Scholar]
- 94.Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–46. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luttun A, Verfaillie CM. Will the real EPC please stand up? Blood. 2007;109:1795–6. [Google Scholar]
- 96.Loomans CJM, Wan H, De Crom R, Van Haperen R, De Boer HC, Leenen PJM, Drexhage HA, Rabelink TJ, Van Zonneveld AJ, Staal FJT. Angiogenic murine endothelial progenitor cells are derived from a myeloid bone marrow fraction and can be identified by endothelial NO synthase expression. Arterioscler Thromb Vasc Biol. 2006;26:1760–7. doi: 10.1161/01.ATV.0000229243.49320.c9. [DOI] [PubMed] [Google Scholar]
- 97.Somani A, Nguyen J, Milbauer LC, Solovey A, Sajja S, Hebbel RP. The establishment of murine blood outgrowth endothelial cells and observations relevant to gene therapy. Transl Res. 2007;150:30–9. doi: 10.1016/j.trsl.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 98.Chakroborty D, Chowdhury UR, Sarkar C, Baral R, Dasgupta PS, Basu S. Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. J Clin Invest. 2008;118:1380–9. doi: 10.1172/JCI33125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, Jeong JO, Curry C, Qin G, Yoon YS. Role of host tissues for sustained humoral effects after endothe-lial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007 doi: 10.1084/jem.20070166. jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chien KR. Alchemy and the new age of cardiac muscle cell biology. PLoS Biol. 2005;3:e131. doi: 10.1371/journal.pbio.0030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marshall CJ, Thrasher AJ. The embryonic origins of human haematopoiesis. Br J Haematol. 2001;112:838–50. doi: 10.1046/j.1365-2141.2001.02537.x. [DOI] [PubMed] [Google Scholar]
- 102.Vogeli KM, Jin S-W, Martin GR, Stainier DYR. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature. 2006;443:337–9. doi: 10.1038/nature05045. [DOI] [PubMed] [Google Scholar]
- 103.Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. 2006;11:519–33. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 104.Yoder MC. Hemangioblasts: of mice and men. Blood. 2007;109:2667–8. [Google Scholar]
- 105.Huber TL, Kouskoff V, Joerg Fehling H, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–30. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 106.Wang L, Li L, Shojaei F, Levac K, Cerdan C, Menendez P, Martin T, Rouleau A, Bhatia M. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21:31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 107.Dieterlen-Lievre F, Pouget C, Bollerot K, Jaffredo T. Are intra-aortic hemopoietic cells derived from endothelial cells during ontogeny? Trends Cardiovasc Med. 2006;16:128–39. doi: 10.1016/j.tcm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 108.Oberlin E, Tavian M, Blazsek I, Peault B. Blood-forming potential of vascular endothelium in the human embryo. Development. 2002;129:4147–57. doi: 10.1242/dev.129.17.4147. [DOI] [PubMed] [Google Scholar]
- 109.Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108:2095–105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cogle CR, Wainman DA, Jorgensen ML, Guthrie SM, Mames RN, Scott EW. Adult human hematopoietic cells provide functional hemangioblast activity. Blood. 2004;103:133–5. doi: 10.1182/blood-2003-06-2101. [DOI] [PubMed] [Google Scholar]
- 111.Burger PE, Coetzee S, McKeehan WL, Kan M, Cook P, Fan Y, Suda T, Hebbel RP, Novitzky N, Muller WA, Wilson EL. Fibroblast growth factor receptor-1 is expressed by endothelial progenitor cells. Blood. 2002;100:3527–35. doi: 10.1182/blood.V100.10.3527. [DOI] [PubMed] [Google Scholar]
- 112.Pelosi E, Valtieri M, Coppola S, Botta R, Gabbianelli M, Lulli V, Marziali G, Masella B, Muller R, Sgadari C, Testa U, Bonanno G, Peschle C. Identification of the hemangioblast in postnatal life. Blood. 2002;100:3203–8. doi: 10.1182/blood-2002-05-1511. [DOI] [PubMed] [Google Scholar]
- 113.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu X-F, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–6. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 114.Shalaby F, Ho J, Stanford WL, Fischer K-D, Schuh AC, Schwartz L, Bernstein A, Rossant J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–90. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 115.Duda DG, Cohen KS, Scadden DT, Jain RK. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc. 2007;2:805–10. doi: 10.1038/nprot.2007.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vroling L, Yuana Y, Schuurhuis GJ, Van Hinsbergh VW, Gundy C, De Haas R, Van Cruijsen H, Boven E, Hoekman K, Broxterman HJ. VEGFR2 expressing circulating (progenitor) cell populations in volunteers and cancer patients. Thromb Haemost. 2007;98:440–50. [PubMed] [Google Scholar]
- 117.He T, Peterson TE, Katusic ZS. Paracrine mitogenic effect of human endothelial progenitor cells: role of interleukin-8. Am J Physiol Heart Circ Physiol. 2005;289:H968–72. doi: 10.1152/ajpheart.01166.2004. [DOI] [PubMed] [Google Scholar]
- 118.Ingram DA, Krier TR, Mead LE, McGuire C, Prater DN, Bhavsar J, Saadatzadeh MR, Bijangi-Vishehsaraei K, Li F, Yoder MC, Haneline LS. Clonogenic endothelial progenitor cells are sensitive to oxidative stress. Stem Cells. 2007;25:297–304. doi: 10.1634/stemcells.2006-0340. [DOI] [PubMed] [Google Scholar]
- 119.Nagano M, Yamashita T, Hamada H, Ohneda K, Kimura K-i, Nakagawa T, Shibuya M, Yoshikawa H, Ohneda O. Identification of functional endothelial progenitor cells suitable for the treatment of ischemic tissue using human umbilical cord blood. Blood. 2007;110:151–60. doi: 10.1182/blood-2006-10-047092. [DOI] [PubMed] [Google Scholar]
- 120.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–8. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 121.Boos C, Lane D, Kang D, Goon P, Blann A, Lip G. Temporal and venepuncture-related decline in circulating endothelial cell capture from mixed venous blood. J Thromb Thrombolysis. 2006;22:125–31. doi: 10.1007/s11239-006-8422-z. [DOI] [PubMed] [Google Scholar]
- 122.Rowand JLMG, Doyle GV, Miller MC, Pierce MS, Connelly MC, Rao C, Terstappen LW. Endothelial cells in peripheral blood of healthy subjects and patients with metastatic carcinomas. Cytometry A. 2007;71:105–13. doi: 10.1002/cyto.a.20364. [DOI] [PubMed] [Google Scholar]
- 123.Blann AD, Woywodt A, Bertolini F, Bull TM, Buyon JP, Clancy RM, Haubitz M, Hebbel RP, Lip GY, Mancuso P, Sampol J, Solovey A, Dignat-George F. Circulating endothelial cells. Biomarker of vascular disease. Thromb Haemost. 2005;93:228–35. doi: 10.1160/TH04-09-0578. [DOI] [PubMed] [Google Scholar]
- 124.Hastings R, Qureshi M, Verma R, Lacy PS, Williams B. Telomere attrition and accumulation of senescent cells in cultured human endothelial cells. Cell Prolif. 2004;37:317–24. doi: 10.1111/j.1365-2184.2004.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thornton SC, Mueller SN, Levine EM. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983;222:623–5. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- 126.Augustin-Voss HGVA, Pauli BU. Senescence of aortic endothelial cells in culture: effects of basic fibroblast growth factor expression on cell phenotype, migration, and proliferation. J Cell Physiol. 1993;157:279–88. doi: 10.1002/jcp.1041570210. [DOI] [PubMed] [Google Scholar]
- 127.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–6. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 128.Strijbos MH, Kraan J, Den Bakker MA, Lambrecht BN, Sleijfer S, Gratama JW. Cells meeting our immunophenotypic criteria of endothelial cells are large platelets. Cytometry Part B: Clin Cytom. 2007;72:86–93. doi: 10.1002/cyto.b.20156. [DOI] [PubMed] [Google Scholar]
- 129.Mancuso P, Burlini A, Pruneri G, Goldhirsch A, Martinelli G, Bertolini F. Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood. 2001;97:3658–61. doi: 10.1182/blood.v97.11.3658. [DOI] [PubMed] [Google Scholar]
- 130.Goon CJB PatrickKY, Stonelake PaulS, Blann AndrewD, Lip GregoryYH. Detection and quantification of mature circulating endothelial cells using flow cytometry and immunomagnetic beads: a methodological comparison. Thromb Haemost. 2006;96:45–52. doi: 10.1160/TH06-04-0185. [DOI] [PubMed] [Google Scholar]
- 131.Khan SS, Solomon MA, McCoy JP., Jr Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytometry Part B: Clin Cytom. 2005;64B:1–8. doi: 10.1002/cyto.b.20040. [DOI] [PubMed] [Google Scholar]
- 132.Ziegler BL, Valtieri M, Porada GA, Maria RD, Uuml , Her R, Masella B, Gabbianelli M, Casella I, Pelosi E, Bock T, Zanjani ED, Peschle C. KDR receptor: a key marker defining hematopoietic stem cells. Science. 1999;285:1553–8. doi: 10.1126/science.285.5433.1553. [DOI] [PubMed] [Google Scholar]
- 133.Aird WC. Phenotypic heterogeneity of the endothelium: I. structure, function, and mechanisms. Circ Res. 2007;100:158–73. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 134.Galic Z, Kitchen SG, Kacena A, Subramanian A, Burke B, Cortado R, Zack JA. T lineage differentiation from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:11742–7. doi: 10.1073/pnas.0604244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yip HK, Chang LT, Chang WN, Lu CH, Liou CW, Lan MY, Liu JS, Youssef AA, Chang HW. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2007;39:69–74. doi: 10.1161/STROKEAHA.107.489401. [DOI] [PubMed] [Google Scholar]
- 136.Allanore Y, Batteux F, Avouac J, Assous N, Weill B, Kahan A. Levels of circulating endothelial progenitor cells in systemic sclerosis. Clin Exp Rheumatol. 2007;25:60–6. [PubMed] [Google Scholar]
- 137.Hildbrand P, Cirulli V, Prinsen RC, Smith KA, Torbett BE, Salomon DR, Crisa L. The role of angiopoietins in the development of endothelial cells from cord blood CD34+ progenitors. Blood. 2004;104:2010–9. doi: 10.1182/blood-2003-12-4219. [DOI] [PubMed] [Google Scholar]
- 138.Martin K, Stanchina M, Kouttab N, Harrington E, Rounds S. Circulating endothelial cells and endothelial progenitor cells in obstructive sleep apnea. Lung. 2008;186:145–50. doi: 10.1007/s00408-008-9073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Retlontlo S, Hristov M, Gordillo-Moscoso AA, Ruiz E, Weber C, Tejerina T. High-reproducible flow cytometric endothelial progenitor cell determination in human peripheral blood as CD34+/CD144+/CD3-lymphocyte sub-population. J Immunol Meth. 2008;335:21–7. doi: 10.1016/j.jim.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 140.Lipsic E, Van Der Meer P, Voors A, Westenbrink B, Van Den Heuvel A, De Boer H, Van Zonneveld A, Schoemaker R, Van Gilst W, Zijlstra F, Van Veldhuisen D. A single bolus of a long-acting erythropoietin analogue darbepoetin alfa in patients with acute myocardial infarction: a randomized feasibility and safety study. Cardiovasc Drugs Ther. 2006;20:135–41. doi: 10.1007/s10557-006-7680-5. [DOI] [PubMed] [Google Scholar]
- 141.Schomig K, Busch G, Steppich B, Sepp D, Kaufmann J, Stein A, Schomig A, Ott I. Interleukin-8 is associated with circulating CD133+ progenitor cells in acute myocardial infarction. Eur Heart J. 2006;27:1032–7. doi: 10.1093/eurheartj/ehi761. [DOI] [PubMed] [Google Scholar]
- 142.Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S. Vascular trauma induces rapid but transient mobilization of VEGFR2+AC133+ endothelial precursor cells. Circ Res. 2001;88:167–74. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 143.Povsic TJ, Zavodni KL, Kelly FL, Zhu S, Golclschmitlt-Clermont PJ, Dong C, Peterson ED. Circulating progenitor cells can be reliably identified on the basis of aldehyde dehydrogenase activity. J Am Coll Cardiol. 2007;50:2243–8. doi: 10.1016/j.jacc.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 144.Prunier F, Pfister O, Hadri L, Liang L, Del Monte F, Liao R, Hajjar RJ. Delayed ery-thropoietin therapy reduces post-MI cardiac remodeling only at a dose that mobilizes endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2007;292:H522–9. doi: 10.1152/ajpheart.00357.2006. [DOI] [PubMed] [Google Scholar]
- 145.Zhu S, Liu X, Li Y, Goldschmidt-Clermont PJ, Dong C. Aging in the Atherosclerosis milieu may accelerate the consumption of bone marrow endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2007;27:113–9. doi: 10.1161/01.ATV.0000252035.12881.d0. [DOI] [PubMed] [Google Scholar]
- 146.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 147.Cheng XW, Kuzuya M, Nakamura K, Maeda K, Tsuzuki M, Kim W, Sasaki T, Liu Z, Inoue N, Kondo T, Jin H, Numaguchi Y, Okumura K, Yokota M, Iguchi A, Murohara T. Mechanisms underlying the impairment of ischemia-induced neovascularization in matrix metalloproteinase 2-deficient mice. Circ Res. 2007;100:904–13. doi: 10.1161/01.RES.0000260801.12916.b5. [DOI] [PubMed] [Google Scholar]
- 148.Zhang W, Zhang G, Jin H, Hu R. Characteristics of bone marrow-derived endothelial progenitor cells in aged mice. Biochem Biophys Res Comm. 2006;348:1018–23. doi: 10.1016/j.bbrc.2006.07.161. [DOI] [PubMed] [Google Scholar]
- 149.Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, Kerbel RS. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342–6. [PubMed] [Google Scholar]
- 150.Tavian M, Peault B. The changing cellular environments of hematopoiesis in human development in utero. Exp Hematol. 2005;33:1062–9. doi: 10.1016/j.exphem.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 151.Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–26. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 152.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Nat Acad Sci USA. 2001;98:10716–21. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–12. [PubMed] [Google Scholar]
- 154.Arkin S, Naprstek B, Guarini L, Ferrone S, Lipton JM. Expression of intercellular adhesion molecule-1 (CD54) on hematopoietic progenitors. Blood. 1991;77:948–53. [PubMed] [Google Scholar]
- 155.Rokhlin OW, Cohen MB, Kubagawa H, Letarte M, Cooper MD. Differential expression of endoglin on fetal and adult hematopoietic cells in human bone marrow. J Immunol. 1995;154:4456–65. [PubMed] [Google Scholar]
- 156.Jokubaitis VJ, Sinka L, Driessen R, Whitty G, Haylock DN, Bertoncello I, Smith I, Peault B, Tavian M, Simmons PJ. Angiotensin-converting enzyme (CD143) marks hematopoietic stem cells in human embryonic, fetal and adult hematopoietic tissues. Blood. 2007;111:4055–63. doi: 10.1182/blood-2007-05-091710. [DOI] [PubMed] [Google Scholar]
- 157.Watt SM, Butler LH, Tavian M, Buhring H-J, Rappold I, Simmons PJ, Zannettino ACW, Buck D, Fuchs A, Doyonnas R, Chan JY-H, Levesque J-P, Peault B, Roxanis I. Functionally defined CD164 epitopes are expressed on CD34+ cells throughout ontogeny but display distinct distribution patterns in adult hematopoietic and nonhematopoietic tissues. Blood. 2000;95:3113–24. [PubMed] [Google Scholar]
- 158.Kukk E, Wartiovaara U, Gunji Y, Kaukonen J, Bühring HJ, Rappold I, Matikainen MT, Vihko P, Partanen J, Palotie A, Alitalo K, Alitalo R. Analysis of tie receptor tyrosine kinase in haemopoietic progenitor and leukaemia cells. Br J Haematol. 1997;98:195–203. doi: 10.1046/j.1365-2141.1997.1732989.x. [DOI] [PubMed] [Google Scholar]
- 159.Silvestre JS, Gojova A, Brun V, Potteaux S, Esposito B, Duriez M, Clergue M, Le Ricousse-Roussanne S, Barateau V, Merval R, Groux H, Tobelem G, Levy B, Tedgui A, Mallat Z. Transplantation of bone marrow-derived mononuclear cells in ischemic apolipoprotein E-knockout mice accelerates atherosclerosis without altering plaque composition. Circulation. 2003;108:2839–42. doi: 10.1161/01.CIR.0000106161.43954.DF. [DOI] [PubMed] [Google Scholar]
- 160.Gehling UM, Ergun S, Fiedler W. CFU-EC: how they were originally defined. Blood. 2007;110:1073. doi: 10.1182/blood-2007-03-081638. [DOI] [PubMed] [Google Scholar]
- 161.Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K-i, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–36. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gulati R, Jevremovic D, Peterson TE, Witt TA, Kleppe LS, Mueske CS, Lerman A, Vile RG, Simari RD. Autologous culture-modified mononuclear cells confer vascular protection after arterial injury. Circulation. 2003;108:1520–6. doi: 10.1161/01.CIR.0000089084.48655.49. [DOI] [PubMed] [Google Scholar]
- 163.Lin Y, Chang L, Solovey A, Healey JF, Lollar P, Hebbel RP. Use of blood outgrowth endothelial cells for gene therapy for hemophilia A. Blood. 2002;99:457–62. doi: 10.1182/blood.v99.2.457. [DOI] [PubMed] [Google Scholar]


