Abstract
Rats transgenic for HLA-B27 and human β2microglobulin (B27TR) develop a multi-systemic disease resembling inflammatory bowel disease (IBD) and spondyloarthritis. TNFα has a crucial role in chronic inflammation. Our objective was to evaluate the effect of anti-TNFα treatment on spontaneous IBD in B27TR. Nine-week-old B27TR received monoclonal anti-TNFα or an isotypic IgG2a,k up to age of 18 weeks. A second group was monitored up to 18 weeks and then randomly assigned to anti-TNFα or IgG2 a,k treatment. Each rat was monitored for clinical IBD manifestations. After sacrifice, the colon was examined for pathological changes. TNFα receptors (TNF-R1, TNF-R2), Fas/Fas-L expression and apoptosis were evaluated. IgG2a,k-treated and untreated B27TR presented signs of IBD at 11 weeks, whereas in anti-TNFα-treated B27TR no IBD signs were detected. In the late treatment, IBD signs improved after 1 week. Histopathological analysis of IgG2a,k-treated B27TR colon showed inflammatory signs that were widely prevented by early anti-TNFα treatment. Late treatment did not significantly reduce inflammation. TNF-R1 was weakly expressed in intestinal epithelial cells of IgG2a,k-treated B27TR, while it was comparable to controls in anti-TNFα-treated animals. TNF-R2 immunopositivity was strongly evident in IgG2a,k-treated B27TR, whereas was absent in anti-TNFα-treated rats. RT-PCR confirmed these results. IgG2a,k-treated B27TR showed, at 18 weeks, few Fas-positive cells and an increase of Fas-L-positive cells. At 27 weeks, Fas-/Fas-L-positive cell number was significantly low. Anti-TNFα treatment increased Fas-L expression, whereas Fas increased only with the early treatment. TNFα blockade is effective in preventing inflammation in early phase of IBD, maintaining the homeostatic balance of apoptosis.
Keywords: HLA-B27 transgenic rats, IBD, TNFα, apoptosis, Fas/Fas-L
Introduction
The major histocompatibility complex (MHC) class I gene HLA-B27 has a striking association with a group of human inflammatory disorders that affect the bowel, the joints and the axial skeleton, known as spondyloarthritis (SpA). Extensive endoscopic and histological studies have demonstrated that 5–10% of patients with ankylosing spondylitis (AS, the prototype of these disorders) have inflammatory bowel disease (IBD). Moreover, about two-thirds of SpA patients have microscopic signs of gut inflammation without clinical symptoms, but about 6–13% of them develop IBD during the disease course [1, 2].
In an attempt to create an animal model of B27-associated disease, inbred rats expressing both HLA-B27 and its associated light chain human β2-microglobulin (HLA-B27/hβ2 m) were developed [3]. HLA-B27 transgenic rats (B27TR) on LEW background as well as commercially available B27TR on Fisher F344 background (33–3 line), with high expression of HLA-B27, develop a multi-systemic inflammatory disease resembling human SpA [4, 5], including colitis and arthritis [3, 6]. A progressive chronic gastrointestinal inflammation spontaneously develops in these rats, showing a predominant Th1 inflammatory cytokine pattern [7]. B27TR are well recognized as an animal model of IBD [7].
The susceptibility to disease and the level of expression of the HLA-B27 allele (but not hβ2m) are strictly associated, and a threshold of HLA-B27 transgene copies exists, above which disease predictably arises [6, 8].
In B27TR, both CD4+ T cells and antigen-presenting cells (APCs) expressing high levels of HLA-B27 seem to be of critical importance in the pathogenesis of the disease [6, 9]. APCs have relatively poor efficacy in stimulating T cells [10], and this could result in loss of tolerance toward self-antigens. Alternatively (or additionally), impaired T-cell stimulation could result in altered control of gut bacteria, thereby sustaining stimulation of immune defence by macrophages [9]. Indeed, IBD and arthritis symptoms can be prevented in ‘germ-free’ conditions, while bacterial flora reconstitution fosters disease development [11].
Several mediators of inflammation were detected in B27TR colonic mucosa [9, 11, 12]. An increase of IFNγ and IL-2 was found in the early phase, suggesting a predominantly Th1-mediated response, while in advanced disease IL-1 and TNFα are expressed [9, 11, 12]. TNFα stimulates the development of the socalled ‘semi-mature’ dendritic cells (DCs), which are critical for tolerance induction [13]. Administration of recombinant murine IL-10, which down-regulates TNFα, to B27TR with established disease, despite the inhibition of the production of this cytokine in the gut mucosa, had no effect on the disease course arguing a critical role of TNFα in initiating the process [14].
Anti-TNFα therapies are interesting because of TNFα's role in sustaining chronic mucosal inflammation. Various anti-TNFα trials suggest that although infliximab, etanercept and adalimumab are similarly effective for the symptoms related to the spine, joints and skin of AS patients, infliximab appears to be effective in treating concomitant IBD. In contrast, the patients treated with etanercept and adalimumab had more flares and even some new onset of IBD [1, 15]. TNFα is thought to play a central and polymodal role in the host response to infections. Its action is mediated by two functionally distinct receptors, TNFα receptor 1 and receptor 2 (TNF-R1 and TNF-R2, respectively), which are co-expressed on the surface of most cell types, in particular neutrophils, monocytes and T lymphocytes [16].
TNFα plays an essential role in regulating intestinal epithelial cell (IEC) apoptosis and/or survival during chronic inflammation [17, 18]. Fas/CD95/APO-1 is a death receptor of the TNFα receptor family, and engagement of its ligand (Fas-L) initiates a series of intracellular events leading to programmed cell death [17, 19–21]. Activated T cells, which produce TNFα, can trigger the expression of Fas-L on non-lymphoid tissue, such as IEC, and this in turn can induce apoptosis in the T cells [17, 19–21]. This model is an example of ‘inducible immune privilege’ proposed to contribute to limiting the extent of infiltration by activated T cells into certain peripheral tissues [17]. TNF-R1, which is constitutively expressed by the IEC, mediates this mechanism.
The aim of our work was to study the effect of TNFα blockade on IBD in HLA-B27 transgenic rats. The study was designed to target two different phases of the disease, early and late, in order to evaluate the role of TNFα in initiating and sustaining gut inflammation.
Materials and methods
Experimental design
Male HLA-B27/hβ2 m transgenic rats (B27TR, n = 24) and male non-transgenic control rats (Fisher F344, n= 16) of the same breed were purchased from Taconic Farms (Taconic Farms, Inc., Germantown, WI, USA). All rats were bred and housed under conventional conditions. Study procedures were approved by the animal care committee of the local government.
Twelve B27TR at the age of 9 weeks, which is prior to the onset of colitis, were randomly assigned to treatment with a mouse anti-rat TNFα monoclonal antibody (mAb), which neutralizes rat TNFαin vivo, or an isotype-matched negative control immunoglobulin IgG2a,k (n= 6 each group), both kindly supplied by Centocor (Centocor, Inc., Malvern, PA, USA). Each rat weekly received an intraperitoneal injection of anti-TNFα or isotype IgG2 a,k (15 mg/kg) up to the age of 18 weeks.
Twelve B27TR were monitored up to the age of 18 weeks and then randomly assigned to treatment with anti-TNFα mAb (n= 6) or with isotypic IgG2 a,k (n= 6) up to the age of 27 weeks. Non-transgenic F344 littermates were used as control (n= 4 in each protocol arm).
Each rat was weighted weekly and monitored for clinical manifestation of gastrointestinal inflammation, assigning numerical scores to the stool character (normal stool = 1, soft stool = 2, watery stool = 3).
Sample collection
At the end of the experiments, the animals were killed by ether anaesthesia. Blood samples were collected and aliquots of serum were stored at −70 °C until assayed.
The dissected colon was prepared for routine histology or snap-frozen in liquid nitrogen for biomolecular analysis.
Paraffin sections of the colon (5-μm thick) were cut across the longitudinal axis, and stained with haematoxylin-eosin. The sections were scored blindly by two investigators for histological evidence of inflammation and structural damage, in accordance with a previously defined scoring scheme [22, 23]. In brief, the severity of the colonic lesions was evaluated for degree of inflammation (none = 0, mild = 1, moderate = 2, severe = 3), ulcer size (none = 0, small = 1, large = 2), elongation and or distortion of crypts (none = 0, mild = 1, severe = 2), reduction in goblet cell number (none = 0, moderate = 1, severe = 2), depth of lesions (none = 0, epithelial = 1, lamina propria = 2, submucosa = 3). The total histological score for the colon specimens ranged from 0 to 12.
Immunohistochemistry
The sections were de-waxed and heated in 10 mM citrate buffer (pH 6.0) for antigen retrieval. After blocking endogenous peroxidase activity, immunohistochemistry was performed using the Ultravision Detection System (Lab Vision Corporation, Fremont, CA, USA), according to the manufacturer's protocol, using rabbit polyclonal antibodies raised against rat (see Table 1). The immunopositive products were detected using 3,3′-diaminobenzidine tetrahydrochloride substrate (DAB kit; Vector Laboratories, Burlingame, CA, USA) or 3-amino-9-ethylcarbazole substrate (AEC kit; Vector Laboratories). The sections were counterstained with haematoxylin, observed under a light microscope and photographed with a digital camera. Negative controls were obtained by omitting the primary antibodies.
1.
Details of antibodies used for immunohistochemistry
| Antibodies | Source | Dilution |
|---|---|---|
| TNF-R1 | Abcam Ltd., Cambridge, UK | 1:100 |
| TNF-R2 | Abcam Ltd., Cambridge, UK | 1:50 |
| Fas | Santa Cruz Biotechnology, Santa Cruz, CA, USA | 1:100 |
| Fas-L | Santa Cruz Biotechnology, Santa Cruz, CA, USA | 1:100 No antigen retrieval |
For in situ detection of apoptosis in the colon mucosa, we used the terminal deoxynucleotidyl transferase-mediated triphosphate end-labeling (TUNEL) method (Klenow FragEL DNA Fragmentation Detection kit; Calbiochem, Nottingham, UK).
Evaluation of Fas/Fas-L-positive and apoptotic cells
Quantitative analysis on tissue sections (400× magnification) was performed, considering as positive, for Fas/Fas-L, any epithelial and lamina propria cells exhibiting identifiable reactivity distinct from background. Morphologically preserved TUNEL-positive cells were referred to as apoptotic cells. For each animal, 10 random fields, with fully longitudinally sectioned crypts, from 2 sections of 4 different slides of the colon were examined, for a total of 80 fields and at least 1000 cells/section (immunoreactive and non-immunoreactive cells) counted. The apoptotic index (AI) was defined as the ratio of TUNEL-positive to total nuclei counted multiplied by 100.
Semiquantitative reverse-transcription polymerase chain reaction (RT-PCR) analysis
For RT-PCR analysis, total RNA was isolated from the colon of the rats using the PureLink Micro-to-Midi Total RNA Purification system (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. One microgram of total RNA was reverse transcribed and amplified using the SuperScrip III One-Step RT-PCR System with Platinum Taq DNA Polymerase (Invitrogen). TNF-R1 and TNF-R2 primer sequences were designed on the basis of the published gene sequences (see Table 2). The amounts of RT-PCR products were determined by densitometric analysis using NIH image analysis software. Within the linear range of amplification, at least three values of each amplification product were normalized to the starting mRNA volume and compared to the corresponding GAPDH values (P <0.05, anova and Tukey's w test).
2.
Primers used for semiquantitative RT-PCR
| Gene name | Primer sequences | Product size | GenBank accession number |
|---|---|---|---|
| TNF-R1 | Forward 5′-ACCAAGTGCCACAAAGGAAC-3′ | 249 bp | NM_013091 |
| Reverse 5′-CTGGAAATGCGTCTCACTCA-3′ | |||
| TNF-R2 | Forward 5′-AAATGCAAGCACAGATGCAG-3′ | 244 bp | NM_130426 |
| Reverse 5′-CAGCAGACCCAGAGTTGTCA-3′ | |||
| GAPDH | Forward 5′-AGACAGCCGCATCTTCTTGT-3′ | 300 bp | NM_017008 |
| Reverse 5′-CTTGCCGTGGGTAGAGTCAT-3′ | |||
Serum cytokine levels
Serum levels of IL-1 and IL-2 were measured by ELISA assay (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
Statistical analysis
Data obtained from individual rats in the different experimental groups were summarized by calculating group means and standard deviation (S.D.), or standard error (S.E.M.). Statistical differences among the experimental groups were evaluated by anova. The level of significance was set at P < 0.05.
Results
Clinical evaluation
There were no significant differences in body weight gain between anti-TNFα-treated and IgG2a,k-treated B27TR during the experimental period. At 11 weeks of age, both IgG2a,k-treated and untreated B27TR developed clinical signs of bowel inflammation (diarrhoea). In contrast, B27TR early-treated with anti-TNFα mAb manifested no signs of inflammation and the stool character remained normal for the duration of the experiment (Fig. 1 A).
1.
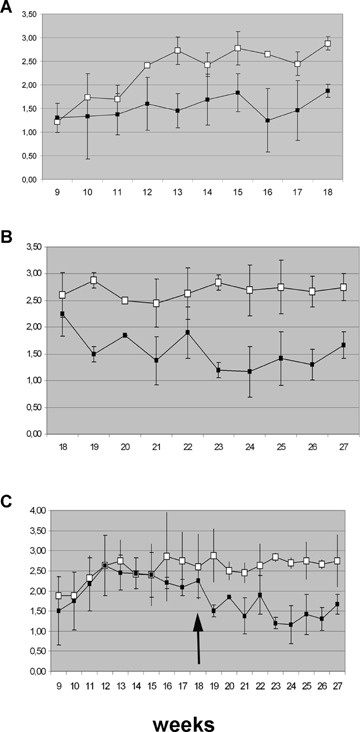
Effect of anti-TNFα mAb treatment on diarrhoea in HLA-B27 transgenic rats. (A) Early-treated group; (B) late-treated group; (C) follow-up of the late-treated group. Stool score was determined weekly (normal stool = 1, soft stool = 2, watery stool = 3). (A) At 11 weeks of age, both IgG2 a,k-treated and untreated B27TR reached the stool score of 3 at the 13th week. (B, C) Stool character decreased from 3 to 2, after the first week of anti-TNFα mAb treatment, and then normalized in the following weeks. Data are shown as means ± S.E.M. Filled squares, anti-TNFα treated group; open squares, IgG2a,k-treated group.
In the late-treated group, clinical signs of inflammation improved after the first week of anti-TNFα mAb treatment, and stool score decreased and then normalized (Fig. 1B and C).
Histological analysis
During necropsy, the colon was first visually examined. Numerous IgG2a,k-treated B27TR showed macroscopic signs of colon inflammation, such as oedematous and hyperemic colonic wall with a prominent vascular network. Representative histological pictures for this group of rats are shown in Fig. 2B, C and E. The mucosal folds were not preserved, and enlarged tubular glands displaying loss of mucus-containing cells and a loss of epithelial cells were observed. In the lamina propria, a prominent inflammatory infiltration was detectable between the glands and around the glandular crypts, as well as a relevant oedema and perivascular infiltrate in the submucosa (Fig. 2B and C). At 27 weeks of age, IgG2a,k-treated rats showed more severe damage than at 18 weeks (Fig. 2E).
2.
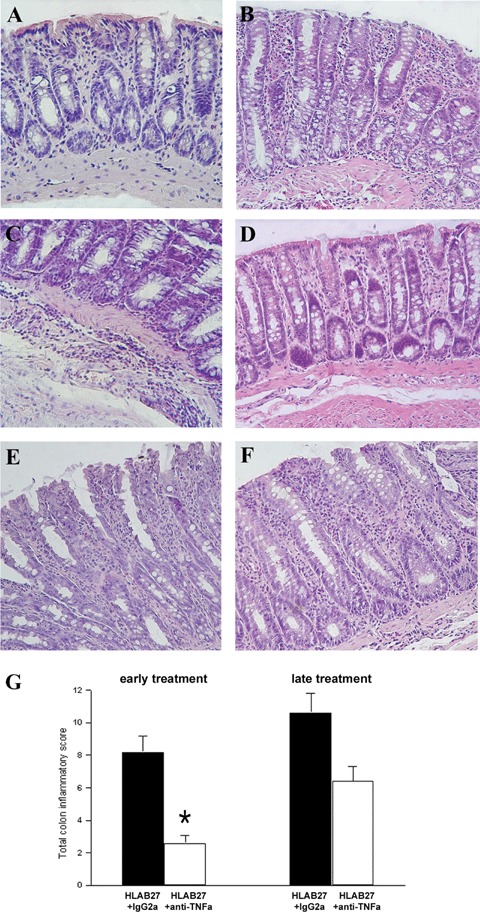
Representative histological sections of colon. (A) Normal non-transgenic F344 rats. (B, C) 18-week-old IgG2a,k-treated B27TR. (D) Early treatment with anti-TNFα mAb. (E) 27-weekold IgG2a,k-treated B27TR. (F) Late treatment with mAb anti-TNFα. Haematoxylin-eosin staining. Original magnification ×20. (G) Histological score of colonic inflammation (range 0–12). (*P < 0.05 anti-TNFα-treated B27TR versus IgG2a,k-treated B27TR).
Early treatment with anti-TNFα mAb prevented the extensive inflammatory infiltrate within the mucosa and submucosa, as well as the architectural damage of the colon observed in the IgG2a, k-treated rats (Fig. 2D). The mucosal folds and mucus-containing cells were preserved, showing closely packed straight tubular glands; epithelial cells lining the surface were well preserved, and a slight inflammatory infiltrate was observed in the lamina propria under the crypts. The submucosa was spared and no signs of inflammatory infiltrate were detectable (Fig. 2D).
The late treatment with anti-TNFα antibody did not induce a significant remission of the pathological signs of inflammation, despite clear clinical improvement (Fig. 2 F).
The histological score of colonic inflammation is reported in Fig. 2G.
Expression of TNFα receptors
Immunohistochemistry
Colonic IEC of F344 rats showed a strong immunopositivity for TNF-R1 (Fig. 3 A). Specimens of IgG2a,k-treated B27TR were weakly stained or negative for TNF-R1 (Fig. 3B). IEC of B27TR early-treated with anti-TNFα mAb showed a strong immunopositivity for TNF-R1, as F344 specimens (Fig. 3 C).
3.
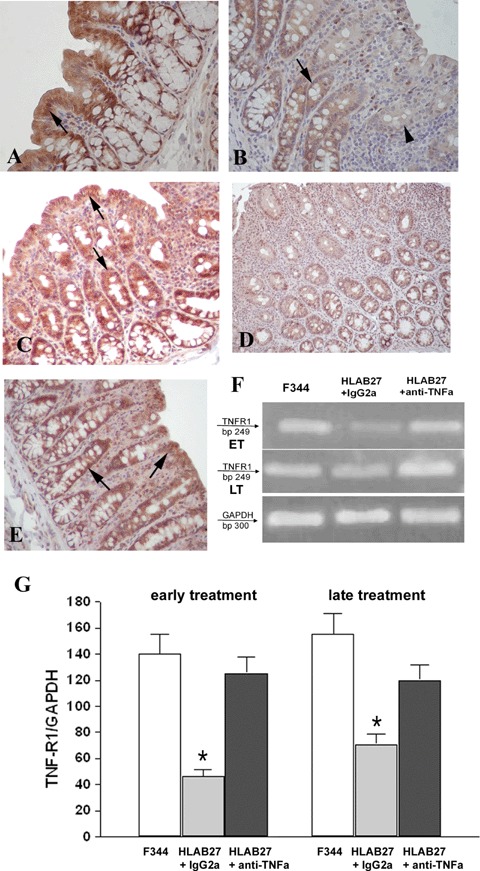
Detection of TNFα receptor 1 (TNF-R1) in colonic mucosal tissue. (A) Positive colonic intestinal epithelial cells (IEC) for TNF-R1 in non-transgenic F344 rats (arrow). (B) IgG2 a, k-treated B27TR colon with weakly stained (arrow) or negative (arrowhead) IEC. (C) The early treatment with anti-TNFα mAb preserves the expression of TNF-R1 in IEC (arrows). (D) 27-week-old IgG2a,k-treated B27TR showed negative immunoreactivity for TNF-R1 in IEC. (E) The late treatment with anti-TNFα mAb restored the expression of TNF-R1 in IEC (arrows). Brown colour: DAB immunohistochemical developing; Red colour: AEC immunohistochemical developing; Blue colour: haematoxylin counterstain. Original magnification ×40. (F, G) RT-PCR results for TNF-R1 are shown. TNF-R1 was well expressed in non-transgenic F344 rats, whereas it was down-regulated in IgG2a, k-treated B27TR, both at 18 and 27 weeks. Early treatment with anti-TNFα mAb maintained the expression of TNF-R1 mRNA at control levels. The late treatment increased the mRNA to control levels. Data are representative of at least three separate experiments. *P < 0.05 IgG2a,k-treated B27TR versus F344 and anti-TNFα-treated B27TR. ET: early treatment; LT: late treatment.
At 27 weeks of age, the colon of IgG2a,k-treated B27TR displayed negative immunostaining for TNF-R1 (Fig. 3D). In the IEC, the late treatment increased the expression of TNF-R1 to levels similar to controls (Fig. 3E).
TNF-R2 positivity was absent from the colon of F344 rats (Fig. 4 A), whereas a strong positivity was observed in the IgG2a,k-treated B27TR, both at 18 and 27 weeks of age (Fig. 4B and C). In particular, some cells of the inflammatory infiltrate were strongly positive (Fig. 4B). In the specimens from anti-TNFα-treated B27TR, no staining for TNF-R2 was observed, both in early- and late-treated rats (Fig. 4D).
4.
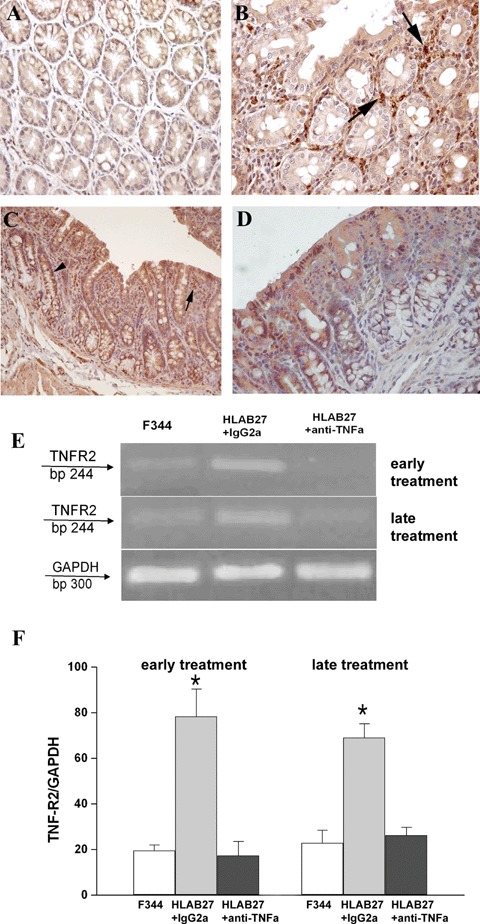
Detection of TNFα receptor 2 (TNF-R2) in colonic mucosal tissue. (A) Immunoreactivity for TNF-R2 was absent in the colon of non-transgenic F344. (B, C) Strong immunopositivity in the colon of IgG2a, k-treated B27TR, both at 18 and 27 weeks of age. Macrophages and mast cells (arrows) and intestinal epithelial cells (arrowhead) were strongly positive. (D) The treatment with anti-TNFα mAb prevented the increased positivity for TNF-R2. Brown colour: DAB immunohistochemical developing; Red colour: AEC immunohistochemical developing; Blue colour: haematoxylin counterstain. Original magnification A, B ×40; C, D ×20. (E, F) RT-PCR results for TNF-R2. Low TNF-R2 mRNA expression was detected in F344 colon specimens, while up-regulation was observed in IgG2a, k-treated B27TR, both at 18 and 27 weeks. Both early and late anti-TNFα-treated rats showed low levels of TNF-R2 mRNA. Data are representative of at least three separate experiments. *P < 0.05 IgG2a, k-treated B27TR versus F344 and anti-TNFα-treated B27TR.
Semiquantitative RT-PCR
There was a significant down-regulation of TNF-R1 mRNA levels in the colon of IgG2a,k-treated B27TR compared to F344 rats, both at 18 and 27 weeks of age. Early treatment with anti-TNFα antibody maintained the levels of TNF-R1 mRNA similar to control rats, while the late treatment restored TNF-R1 mRNA to normal levels (Fig. 3 F and G).
TNF-R2 mRNA was expressed at a low level in the colon of F344 rats, while it was up-regulated in IgG2a,k-treated B27TR, both at 18 and 27 weeks of age. The early anti-TNFα-treatment maintained mRNA levels of TNF-R2 similar to controls. The late treatment significantly down-regulated the expression of TNF-R2 (Fig. 4E and F).
Fas and Fas-L expression
Immunohistochemistry
IEC and cells of the lamina propria showed immunopositivity for both Fas and Fas-L. In F344 rats, Fas was mainly expressed by cells of the lamina propria and Fas-L mainly by IEC (Fig. 5 A and D). In IgG2a,k-treated B27TR, the number of Fas-positive cells in the lamina propria was significantly decreased, while the number of Fas-L-positive cells increased (Fig. 5B and E and Table 3). In particular, a higher number of positive cells were observed around the crypts close to the muscularis mucosae. In IEC, Fas expression increased, while positivity for Fas-L decreased (Fig. 5B and E and Table 3). The same pattern was observed at 18 and 27 weeks of age, with a slight decrease in both Fas and Fas-L positivity of IEC at the later time. The treatment with anti-TNFα mAb significantly increased the number of Fas-positive cells in the lamina propria at 18 and 27 weeks of age. IEC Fas-positivity decreased significantly with anti-TNFα treatment (Fig. 5 C and Table 3). Anti-TNFα treatment did not affect Fas-L expression in the lamina propria at weeks 18 and 27. In IEC, the treatment caused a slight decrease at 18 weeks, but a slight increase at 27 weeks (Fig. 5 F and Table 3).
5.
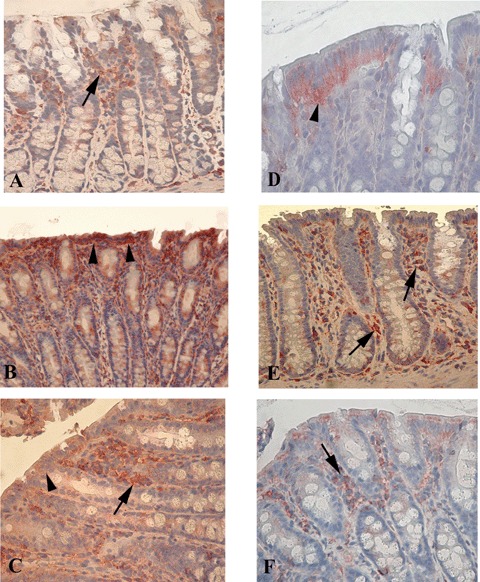
Immunohistochemistry for Fas and Fas-ligand (Fas-L) on colonic mucosal tissue. (A–C) Fas expression. (A) Fas-positive cells (arrow) in the lamina propria of non-transgenic F344 rats. (B) In IgG2a, k-treated B27TR, Fas-positive cells of the lamina propria significantly decreased, whereas positive intestinal epithelial cells (IEC) increased (arrowheads). (C) Anti-TNFα treatment increased significantly the number of Fas positive cells in the lamina propria (arrow), while the positive IEC decreased (arrowhead). (D, E) Fas-L expression. (D) In control non-transgenic F344 rats, Fas-L was expressed mainly by the IEC (arrowhead). (E) IgG2 a,k-treated B27TR showed a higher number of positive cells in the lamina propria (arrows). (F) Anti-TNFα treatment had no effect on Fas-L expression in the lamina propria (arrow) compared to IgG2a,k treatment. Red colour: AEC immunohistochemical developing; Blue colour: haematoxylin counterstain. Original magnification A, B, C, E× 20; D, F × 40.
3.
Statistical analysis for Fas and Fas-L positive cells
| F344 | HLA-B27 + IgG 18ws | HLA-B27 + Ab 18ws | HLA-B27 + IgG 27ws | HLA-B27 + Ab 27ws | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fas | IEC | 9.14 ± 5.06 | 18.01 ± 0.54* | 5.30 ± 2.43§ | 15.69 ± 5.72* | 5.64 ± 1.28§ | ||||||||||||||||||||
| LP | 33.87 ± 7.59 | 19.85 ± 2.25* | 26.07 ± 3.05§ | 21.33 ± 2.3* | 27.57 ± 6.9 | |||||||||||||||||||||
| Fas-L | IEC | 12.21 ± 3.49 | 5.59 ± 3.46* | 3.77 ± 3.64* | 3.94 ± 2.74* | 7.31 ± 4.93* | ||||||||||||||||||||
| LP | 5.23 ± 1.36 | 13.48 ± 1.86** | 14.2 ± 2.8 | 11.39 ± 2.14** | 11.87 ± 2.23 | |||||||||||||||||||||
Data are shown as means ± S.D. IEC = intestinal epithelial cells, LP = lamina propria. *P < 0.05, **P < 0.01 versus F344; §P < 0.05 versus IgG-treated HLA-B27 transgenic rats.
TUNEL assay
Apoptotic cells were rare and limited to the surface epithelium in F344 rats. B27TR showed a significantly increased AI within the epithelium and in the lamina propria. In IgG2a,k-treated rats, a greater number of apoptotic cells was identified in IEC at 18 and 27 weeks, whereas anti-TNFα treatment induced apoptosis in the cells of the lamina propria (Fig. 6 and Table 4).
6.
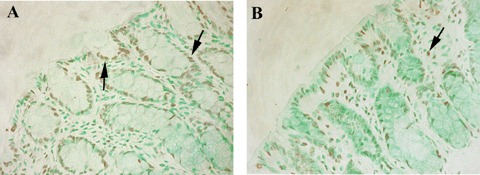
TUNEL assay. (A) TUNEL-positive cells were found in the surface epithelium and crypts (arrows) of the colon in IgG2a,k-treated HLA-B27 transgenic rats. (B) In anti-TNFα-treated HLA-B27 transgenic rats, apoptotic cells are found mainly in the lamina propria (arrow) of the colon. Brown colour: DAB developing; Green colour: Fast Green counterstain. Original magnification × 20.
4.
Statistical analysis for TUNEL-positive cells
| F344 | HLA-B27 + IgG 18ws | HLA-B27 + Ab 18ws | HLA-B27+ IgG 27ws | HLA-B27 + Ab 27ws | |
|---|---|---|---|---|---|
| IEC | 0.36 ± 0.26 | 68 ± 10.9** | 35.4 ± 7.2**§§ | 56.8 ± 13.2** | 47 ± 7.9** |
| LP | 0.14 ± 0.2 | 6.3 ± 1.5** | 42.2 ± 4.1**§§ | 4.7 ± 2.7* | 38.3 ± 7.3**§§ |
Data are shown as means ± S.D. IEC = intestinal epithelial cells, LP = lamina propria. *P < 0.05, **P < 0.01 versus F344; §§P < 0.01 versus IgG-treated HLA-B27 transgenic rats.
Serum cytokine levels
IL-1 levels were higher in IgG2a,k-treated B27TR, at 18 and 27 weeks of age, compared to F344 rats. The early anti-TNFα treatment inhibited the increase of IL-1, while the late treatment was not able to reduce the serum levels of IL-1 (Table 5). IL-2 was significantly higher in B27TR compared to F344, and anti-TNFα treatment did not affect the cytokine levels (Table 5).
5.
Serum levels of IL-1 and IL-2 by ELISA
| F344 | HLA-B27 + IgG 18ws | HLA-B27 + Ab 18ws | HLA-B27+ IgG 27ws | HLA-B27 + Ab 27ws | |
|---|---|---|---|---|---|
| IL-1 | 26.8 ± 4.29 | 48.46 ± 13.78 | 21.77 ± 6.95 | 39.25 ± 15.51 | 38.83 ± 7.8 |
| IL-2 | 141.48 ± 10.1 | 159.89 ± 1.46 | 162.9 ± 2.83 | 163.23 ± 2.87 | 158.8 ± 2.39 |
Data are shown as means (pg/ml) ± S.E.M. IL-1 = Interleukin 1, IL-2 = Interleukin 2.
Discussion
Our data clearly show that TNFα blockade, used before the development of any clinical IBD manifestations, markedly inhibits the onset of colon inflammation in disease-prone HLA-B27 transgenic rats (B27TR). In contrast, when the disease symptoms are established, the treatment is significantly less efficacious, improving only clinical signs but not significantly reducing the pathological features.
The intestinal epithelium, which is part of the innate immune system, plays an active role in the maintenance of mucosal homeostasis [24]. Aberrant secretion of proinflammatory chemokines and cytokines, such as TNFα, IL-1 and IL-6, by epithelial cells is an integral part of the dysregulated immune response that initiates or perpetuates intestinal inflammation [24].
B27TR is an experimental model of colitis, which has been used for several years to evaluate the activity and mechanisms of actions of anti-inflammatory molecules [22, 23–26]. Our study was focused on the effect of TNFα blockade on intestinal inflammation, both as preventive and rescue treatment. When used early, TNFα blockade significantly prevented the onset of clinical manifestations and pathological manifestations while, in the advanced phase of IBD, it was able to ameliorate but not resolve the symptoms. The histopathological damage observed at 18 weeks was effectively prevented by TNFα blockade. Several experimental models suggest that the aetiology of IBD is not only related to immune abnormalities but also to a defect of the epithelial cell layer [27], which forms a physiological barrier against antigens and potential pathogenic microorganism in the gut lumen [28, 29].
The protective effect shown by the integrity of the epithelial cell layer in the specimens of the early-treated rats is an important finding, corroborating the positive effect of anti-TNFα on the course of mucosal inflammation. The striking efficacy of the early treatment might be also due to a relatively low or absent inflammation in the animals at the beginning of the experiment, considering the absence of clinical signs. After 18 weeks, the observed pathological modifications are features of a chronic inflammatory state, and starting TNFα blockade later, in the advanced phase, seems not to be effective in obtaining healing of the colonic mucosa. This was in contrast with a recent study on a murine model of chronic colitis [30], where a delayed anti-TNFα treatment significantly improved the clinical signs and histological features. In B27TR, beside TNFα, other cytokines are probably involved and it is likely that a dysregulated cellular immune mechanism fosters and sustains the inflammatory state. The high levels of IL-1 and IL-2 found in 27-week-old treated or untreated B27TR may partially account for the inefficacy of the late treatment.
The effect obtained by the preventive treatment may be useful in those AS patients who show microscopic intestinal injuries but subclinical signs of gut inflammation. The efficacy of infliximab therapy in IBD concomitant to AS and the inhibition of flares and new onset of IBD [15] may be partially due to the preservation of the integrity of the intestinal mucosa.
The chronic inflammation in B27TR is considered to be the result of HLA-B27 transgene expression-induced alterations in antigen processing and subsequent immune responses to the microbial environment in the lumen of gastrointestinal tract [31, 32]. Many animal models of experimental colitis support the concept that IBD are caused by an aggressive immune response to commensal non-pathogenic bacteria in a genetically susceptible host [27]. In this context, the role of TNFα and its receptors is somewhat controversial and polymodal, depending on the tissue type and cell target [17, 33–36].
In IEC, TNF-R1 seems to be involved in immune privilege, a mechanism that may limit the extent of lymphocyte infiltration tolerated within tissues, such as the colon [17, 19, 33, 34]. In IgG2 a,k-treated B27TR, the significant reduction in the expression of TNF-R1 by IEC may be involved in the loss of this mechanism, favouring dysregulated proliferation and infiltration by inflammatory cells. This hypothesis seems to be supported by the decreased expression of Fas-L in IEC as well as by the decreased number of Fas-positive cells in the lamina propria. The infiltrating cells, which are responsive to this defence mechanism, are reduced in IgG2a,k-treated rats, and this reduction seems to be prevented by anti-TNFα treatment. Vice versa, IgG2 a,k-treated rats showed increased Fas-L expression in the infiltrating cells, while IEC expressed more Fas than control and anti-TNFα-treated rats. This suggests a higher sensitivity of IEC to pro-apoptotic stimuli that may account for the increased epithelial cell loss observed in IgG2a,k-treated rats. Fas-L is expressed on the surface of activated lymphocytes, targeting apoptosis on cells expressing Fas, such as the colon's epithelial cells [37]. In human ulcerative colitis (UC), extensive infiltration by activated lymphocytes has been associated, through Fas-L expression, to mucosal damage [37]. In B27TR, TNFα blockade increased the number of Fas-positive cells in the lamina propria and significantly decreased Fas-positive IEC, but had no effect on Fas-L expressing cells except for a slight increase of Fas-L-positive IEC at 27 weeks of age. Other cytokines may be responsible for the increased Fas-L expression by these cells. IL- 2-induced T-cell activation and proliferation may increase T-cell expression of Fas-L [38]; we observed high levels of IL-2 in B27TR, and this may partially account for the increased Fas-L expression. Our results confirm previous data on TNFα neutralization in SAMP/Yitc ileitis mice, where the authors showed a protection of the IEC apoptosis and induction of programmed cell death of the mononuclear cells in the lamina propria. In contrast with our data, they did not find a Fas-mediated mechanism [35].
In the colonic mucosa, the TUNEL assay showed an increase of the apoptotic cells in B27TR compared with normal colon. The increase was higher in areas corresponding to those with high number of Fas-positive cells. In particular, in IgG2a,k-treated rats, apoptosis increased in the superficial epithelial layer, suggesting a Fas-induced mechanism of IEC depletion. Moreover, few apoptotic cells were found in the lamina propria favouring the massive infiltrate observed in the colon of these rats. Indeed, rats treated with anti-TNFα antibody showed a significant increase of TUNELpositive cells in the lamina propria, thus indicating the activation and/or maintenance of defence mechanism against a massive infiltrate.
Thus, TNFα blockade in B27TR contributes, through the Fas- FasL axis and controlled apoptosis, to the maintenance of mucosal integrity and functionality. These results confirm and support the mechanism hypothesized for the anti-TNFα blocker infliximab in Crohn's Disease (CD), which reverses the inappropriate T-cell accumulation by inducing apoptosis [39].
A role for TNF-R2 in this scenario cannot be clearly found and explained. TNF-R2 is not constitutively expressed and has no effect in normal conditions, but plays an important role in abnormal conditions such as CD and UC, as well as in animal models of colitis [40]. Our results seem to confirm previous data showing an up-regulation of TNF-R2 expression in several forms of intestinal inflammation [40]. Therefore, TNF-R2 up-regulation may be involved in the perpetuation of colonic inflammation and in altered epithelial cell function.
In conclusion, these data indicate that TNFα blockade plays a pivotal tool in the prevention of the spreading of inflammation into intestinal mucosa, maintaining the homeostatic balance of cell apoptosis. Studies are needed to further elucidate the roles of the various cells of the immune system involved and the mechanisms of action of TNFα in colonic inflammation.
Acknowledgments
This study was supported by an unrestricted grant from the Schering Plough, Italy.
References
- 1.De Keyser F, Van den Bosch F, Mielants H. Anti-TNFα therapy in ankylosing spondylitis. Cytokine. 2006;33:294–8. doi: 10.1016/j.cyto.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Jacques P, Mielants H, Coppieters K, De Vos M, Elewaut D. The intimate relationship between gut and joint in spondyloarthropathies. Curr Opin Rheumatol. 2007;19:353–7. doi: 10.1097/BOR.0b013e328133f59f. [DOI] [PubMed] [Google Scholar]
- 3.Hammer RE, Maika SD, Richardson JA, Tang JP, Taurog JD. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human β2 m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63:1099–112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- 4.Tran TM, Dorris ML, Satumtira N, Richardson JA, Hammer R, Shang J, Taurog JD. Additional human b2- microglobulin curbs HLA-B27 misfolding and promotes arthritis and spondylitis without colitis in male HLA-B27 transgenic rats. Arthritis Rheum. 2006;54:1317–27. doi: 10.1002/art.21740. [DOI] [PubMed] [Google Scholar]
- 5.Breban M. Animal models and in vitro models for the study of aetiopathogenesis of spondyloarthropathies. Clin Rheumatol. 1998;12:611–26. doi: 10.1016/s0950-3579(98)80040-x. [DOI] [PubMed] [Google Scholar]
- 6.Breban M, Hacquard-Bouder C, Falgarone G. Animal model of HLA-B27 associated disease. Curr Mol Med. 2004;4:31–40. doi: 10.2174/1566524043479347. [DOI] [PubMed] [Google Scholar]
- 7.Faure M. The chronic colitis developed by HLA-B27 transgenic rats is associated with altered in vivo mucin synthesis. Dig Dis Sci. 2004;49:339–46. doi: 10.1023/b:ddas.0000017462.75257.70. [DOI] [PubMed] [Google Scholar]
- 8.Taurog JD, Maika SD, Simmons WA, Breban M, Hammert RE. Susceptibility to inflammatory disease in HLA-B27 transgenic rat lines correlates with the level of B27 expression. J Immunol. 1993;150:4168–78. [PubMed] [Google Scholar]
- 9.Hacquard-Bouder C, Falgarone G, Bosquet A, Smaoui F, Monnet D, Ittah M, Breban M. Defective costimulatory function is a striking feature of antigen-presenting cells in an HLA-B27-transgenic rat model of spondyloarthropathy. Arthritis Rheum. 2004;50:1624–35. doi: 10.1002/art.20211. [DOI] [PubMed] [Google Scholar]
- 10.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 11.Hacquard-Bouder C, Ittah M, Breban M. Animal models of HLA-B27-associated diseases: new outcomes. Joint Bone Spine. 2006;73:132–8. doi: 10.1016/j.jbspin.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Taurog JD, Maika SD, Satumtira N, Dorris ML, McLean IL, Yanagisawa H, Sayad A, Stagg AJ, Fox GM, Lê O'Brien A, Rehman M, Zhou M, Weiner AL, Splawski JB, Richardson JA, Hammer RE. Inflammatory disease in HLA-B27 transgenic rats. Immunol Rev. 1999;169:209–23. doi: 10.1111/j.1600-065x.1999.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 13.Van Lieshout AW, Barrera P, Smeets RL, Pesman GJ, Van Riel PL, Van Den Berg WB, Radstake TR. Inhibition of TNF alpha during maturation of dendritic cells results in the development of semi-mature cells: a potential mechanism for the beneficial effects of TNF alpha blockade in rheumatoid arthritis. Ann Rheum Dis. 2005;64:408–14. doi: 10.1136/ard.2004.023259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertrand V, Quéré S, Guimbaud R, Sogni P, Chauvelot-Moachon L, Tulliez M, Lamarque D, Charreire J, Giroud JP, Couturier D, Chaussade S, Breban M. Effects of murine recombinant interleukin- 10 on the inflammatory disease of rats transgenic for HLA-B27 and human beta 2- microglobulin. Eur Cytokines Netw. 1998;9:161–70. [PubMed] [Google Scholar]
- 15.Braun J, Baraliakos X, Listing J, Davis J, Van der Heijde D, Haibel H. Differences in the incidence of flares or new onset of inflammatory bowel disease in patients with ankylosing spondylitis exposed to therapy with anti-tumor necrosis factor alpha agents. Arthritis Rheum. 2007;57:639–47. doi: 10.1002/art.22669. [DOI] [PubMed] [Google Scholar]
- 16.Hanai H, Watanabe F, Yamada M, Sato Y, Takeuchi K, Iida T, Tozawa K, Tanaka T, Maruyama Y, Matsushita I, Iwaoka Y, Saniabadi A. Correlation of serum soluble TNF-alpha receptors I and II levels with disease activity in patients with ulcerative colitis. Am J Gastroenterol. 2004;99:1532–8. doi: 10.1111/j.1572-0241.2004.30432.x. [DOI] [PubMed] [Google Scholar]
- 17.Pinkoski MJ, Green DR. Apoptosis in the Regulation of Immune Responses. J Rheumatol. 2005;32:19–25. [PubMed] [Google Scholar]
- 18.Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS. A unified model for apical caspase activation. Mol Cell. 2003;1:529–41. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 19.Bonfoco E, Stuart PM, Brunner T, Lin T, Griffith TS, Gao Y, Nakajima H, Henkart PA, Ferguson TA, Green DR. Inducible nonlymphoid expression of Fas ligand is responsible for superantigen-induced peripheral deletion of T cells. Immunity. 1998;9:711–20. doi: 10.1016/s1074-7613(00)80668-8. [DOI] [PubMed] [Google Scholar]
- 20.Pinkoski MJ, Droin NM, Green DR. Tumor necrosis factor alpha up-regulates nonlymphoid Fas-ligand following superantigen- induced peripheral lymphocyte activation. J Biol Chem. 2002;277:42380–5. doi: 10.1074/jbc.M208167200. [DOI] [PubMed] [Google Scholar]
- 21.Pinkoski MJ, Droin NM, Lin T, Genestier L, Ferguson TA, Green DR. Nonlymphoid Fas ligand in peptide-induced peripheral lymphocyte deletion. Proc Natl Acad Sci USA. 2002;99:16174–9. doi: 10.1073/pnas.262660999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keith JC, Jr, Sainz IM, Isordia-Salas I, Pixley RA, Leathurby Y, Albert LM, Colman RW. A monoclonal antibody against kininogen reduces inflammation in the HLA-B27 transgenic rat. Arthritis Res Ther. 2005;7:R769–76. doi: 10.1186/ar1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson RL, Wang L, Albert L, Keith JC, Jr, Dorner AJ. Molecular effects of recombinant human interleukin-11 in the HLAB27 rat model of inflammatory bowel disease. Lab Invest. 1998;78:1503–12. [PubMed] [Google Scholar]
- 24.Bamias G, Nyce MR, De La Rue SA, Cominelli F. New concepts in the pathophysiology of inflammatory bowel disease. Ann Intern Med. 2005;143:895–904. doi: 10.7326/0003-4819-143-12-200512200-00007. [DOI] [PubMed] [Google Scholar]
- 25.Makino J, Andoh A, Hata K, Yotsuya S, Shikama H, Imamura M, Fujiyama Y, Bamba T. Inhibitory effects of the new anti-inflammatory agent, IS-741, on spontaneous colitis in HLA-B27(β2- microglobulin transgenic rats. J Gastroenterol Hepatol. 2002;17:854–60. doi: 10.1046/j.1440-1746.2002.02815.x. [DOI] [PubMed] [Google Scholar]
- 26.Harnish DC, Albert LM, Leathurby Y, Eckert AM, Ciarletta A, Kasaian M, Keith JC., Jr Beneficial effects of estrogen treatment in the HLA-B27 transgenic rat model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G118–25. doi: 10.1152/ajpgi.00024.2003. [DOI] [PubMed] [Google Scholar]
- 27.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 28.Tidball CS. The nature of the intestinal epithelial barrier. Am J Dig Dis. 1971;16:745–67. doi: 10.1007/BF02239605. [DOI] [PubMed] [Google Scholar]
- 29.Podolsky DK. Regulation of intestinal epithelial proliferation: a few answers, many questions. Am J Physiol. 1993;264:G179–86. doi: 10.1152/ajpgi.1993.264.2.G179. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Jiu J, Liu S, Fa X, Li F, Du Y. Blockage of tumor necrosis factor prevents intestinal mucosal inflammation through down-regulation of interleukin-23 secretion. J Autoimmun. 2007;29:187–194. doi: 10.1016/j.jaut.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Dieleman LA, Hoentjen F, Qian BF, Sprengers D, Tjwa E, Torres MF, Torrice CD, Sartor RB, Tonkonogy SL. Reduced ratio of protective versus proinflammatory cytokine responses to commensal bacteria in HLA-B27 transgenic rats. Clin Exp Immunol. 2004;136:30–9. doi: 10.1111/j.1365-2249.2004.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dieleman LA, Goerres MS, Arends A, Sprengers D, Torrice C, Hoentjen F, Grenther WB, Sartor RB. Lactobacillus GG prevents recurrence of colitis in HLA-B27 transgenic rats after antibiotic treatment. Gut. 2003;52:370–6. doi: 10.1136/gut.52.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green DR, Ferguson TA. The role of Fas ligand in immune privilege. Nat Rev Mol Cell Biol. 2001;2:917–24. doi: 10.1038/35103104. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson TA, Green DR, Griffith TS. Cell death and immune privilege. Int Rev Immunol. 2002;21:153–72. doi: 10.1080/08830180212058. [DOI] [PubMed] [Google Scholar]
- 35.Marini M, Bamias G, Rivera-Nieves J, Moskaluk CA, Hoang SB, Ross WG, Pizarro TT, Cominelli F. TNFα neutralization ameliorates the severity of murine Crohn's-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc Natl Acad Sci USA. 2003;100:8367–71. doi: 10.1073/pnas.1432897100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corredor J, Yan F, Shen CC, Tong W, John SK, Wilson G, Whitehead R, Polk DB. Tumor necrosis factor regulates intestinal epithelial cell migration by receptor-dependent mechanisms. Am J Physiol Cell Physiol. 2003;284:C953–61. doi: 10.1152/ajpcell.00309.2002. [DOI] [PubMed] [Google Scholar]
- 37.Ueyama H, Kiyohara T, Sawada N, Isozaki K, Kitamura S, Kondo S, Miyagawa J, Kanayama S, Shinomura Y, Ishikawa H, Ohtani T, Nezu R, Nagata S, Matsuzawa Y. High Fas ligand expression on lymphocytes in lesions of ulcerative colitis. Gut. 1998;43:48–55. doi: 10.1136/gut.43.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boone DL, Dassopoulos T, Chai S, Chien M, Lodolce J, Ma A. Fas is not essential for lamina propria T lymphocytes homeostasis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G382–8. doi: 10.1152/ajpgi.00373.2002. [DOI] [PubMed] [Google Scholar]
- 39.Van den Brande JM, Koehler TC, Zelinkova Z, Bennink RJ, Te Velde AA, Ten Cate FJ, Van Deventer SJ, Peppelenbosch MP, Hommes DW. Prediction of antitumour necrosis factor clinical efficacy by real-time visualisation of apoptosis in patients with Crohn's disease. Gut. 2007;56:509–17. doi: 10.1136/gut.2006.105379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizoguchi E, Mizoguchi A, Takedatsu H, Cario E, De Jong YP, Ooi CJ, Xavier RJ, Terhorst C, Podolsky DK, Bhan AK. Role of tumor necrosis factor receptor 2 (TNFR2) in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology. 2002;122:134–44. doi: 10.1053/gast.2002.30347. [DOI] [PubMed] [Google Scholar]


