Abstract
Environmental exposure to arsenic is known to cause various cancers. There are some potential relationships between cell malignant transformation and C-X-C chemokine receptor type 4 (CXCR4) expressions. Metastasis, one of the major characteristics of malignantly transformed cells, contributes to the high mortality of cells. CXCR4 and its natural chemokine ligand C-X-C motif ligand 12 (CXCL12) play a critical role in metastasis. Therefore, identification of nutritional factors which are able to inhibit CXCR4 is important for protection from environmental arsenic-induced carcinogenesis and for abolishing metastasis of malignantly transformed cells. The present study demonstrates that apigenin (4′, 5, 7-trihydroxyflavone), a natural dietary flavonoid, suppressed CXCR4 expression in arsenic-transformed Beas-2B cells (B-AsT) and several other type of transformed/cancer cells in a dose- and time-dependent manner. Neither proteasome nor lysosome inhibitor had any effect in reducing the apigenin-induced down-regulation of CXCR4, indicating that apigenin-induced down-regulation of CXCR4 is not due to proteolytic degradation. The down-regulation of CXCR4 is mainly due to the inhibition of nuclear factor κB (NF-κB) transcriptional activity. Apigenin also abolished migration and invasion of transformed cells induced by CXCL12. In a xenograft mouse model, apigenin down-regulated CXCR4 expression and suppressed tumor growth. Taken together, our results show that apigenin is a novel inhibitor of CXCR4 expression. This dietary flavonoid has the potential to suppress migration and invasion of transformed cells and prevent environmental arsenic-induced carcinogenesis.
Keywords: CXCR4, CXCL12, apigenin, transformed cell, metastasis
Introduction
Human environmental exposure to arsenic is a world-wide health concern because of its global existence in food, soil, and water (Hughes, 2002). This exposure to arsenic could cause cell malignant transformation (Chang et al., 2010; Escudero-Lourdes et al., 2012), leading to cancers of the lung, skin, kidney, urinary bladder, and liver (Haque et al., 2003; IARC, 2004; Kitchin and Conolly, 2010). There is a possible relationship between malignant cell transformation and C-X-C chemokine receptor type 4 (CXCR4). CXCR4 is over-expressed in chronically cadmium-exposed cells which had obtained malignant transformation characteristics (Qu et al., 2012). It has been reported that ectopically expressing CXCR4 in immortalized normal breast epithelial cells MCF-10A could induce transformation and enhance invasive phenotype (Su et al., 2011). The functions of CXCR4 suggest that CXCR4 may play a crucial role during cell malignant transformation and metastasis. CXCR4 is capable of transducing complex signalings, including up-regulation of receptor tyrosine kinases (RTKs), deregulation of p53/MDM2 axis, up-regulation of E-cadherin and c-myc, as well as modulation of cell cycle molecules to facilitate cell transformation.
Metastasis, anaplasia, and invasiveness are the major characters of malignant transformation. Tumor metastasis, which is responsible for more than 90% cancer-associated deaths, is a highly complex process that involves cancer cell adhesion, migration, invasion, and interaction between cancer cells and the host (Gupta et al., 2005). The mechanism of tumor metastasis is still not completely understood. Up to now, a wide variety of molecules have been related to tumor metastasis such as matrix metalloproteinases (MMPs), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), tumor growth factor-β (TGF-β) (Leivonen and Kahari, 2007), and chemokines and their receptors (Kruizinga et al., 2009).
Chemokines are a super family of small, cytokine-like proteins that regulate immunologic and inflammatory processes such as leukocyte trafficking, adhesion, hematopoiesis, and angiogenesis (Kruizinga et al., 2009). Chemokines, which are secreted from normal tissues and sometimes from cancer cells, attract cells by engaging receptor molecules located on the cell surface. Many types of cancer cells have been reported to express chemokine receptors (Coussens and Werb, 2002). The most extensively studied chemokine receptor/ligand signaling axis in tumor cell metastasis is CXCR4 and its natural ligand Chemokine (C-X-C motif) ligand 12 (CXCL12) (Marchesi et al., 2004; Raman et al., 2007). CXCR4 expression has been correlated with poor overall survival rate in patients with breast and colon cancer (Kim et al., 2006; Holm et al., 2007). At least 23 different types of human cancer cells express CXCR4, and this receptor-ligand pair appears to be involved in the directed migration of cancer cells to sites of metastasis (Balkwill, 2004). Moreover, the CXCR4/CXCL12 axis has also been related to leukocyte trafficking (Hernandez et al., 2003), B cell lymphopoiesis (Nagasawa et al., 1996), neuronal cell migration (Hurwitz et al., 2001), and HIV invasion of host cells (Akashi et al., 2008). Due to its functions in tumor metastasis, CXCR4 is not surprisingly considered as a probable cancer therapeutic target. Thus, agents that can interrupt the CXCR4/CXCL12 cell signaling pathway have a potential in preventing cancer progress, especially metastasis.
Certain natural products, especially nutritional factors, generally regarded as safe, have been shown to mediate anticancer activities against variety of cancers. Apigenin (4′, 5, 7-trihydroxyflavone) is a natural dietary flavonoid (Patel et al., 2007), which is widely contained in many fruits and vegetables such as orange, grapefruit, celery, onion, and wheat sprouts (Patel et al., 2007). Anticancer effects of apigenin has been shown on different types of cancers including breast (Choi and Kim, 2009), prostate (Shukla et al., 2005), lung (Li et al., 2007), and hematologic (Budhraja et al., 2012). Studies have revealed that apigenin inhibits pancreatic cancer cell growth in vitro by induction of cell cycle arrest (Ujiki et al., 2006) and induces apoptosis through different cellular signaling transduction pathways including nuclear factor κ B (NF-κB) (Helbig et al., 2003), p53 (Zheng et al., 2005), and PI3K/Akt (Way et al., 2004). Apigenin has also been reported to inhibit VEGF transcriptional activation through the hypoxia-inducible factor 1 (HIF-1) binding site and to specifically reduce HIF-1α expression (Li et al., 2007).
The present study was designed to investigate the effect of apigenin in transformed cells on the expression of CXCR4, which plays a critical role in tumor cell invasion and metastasis. The present study also investigated the effect of apigenin on CXCR4 in transformed cells in constitutive or inducible migration/invasion.
Materials and Methods
Materials
Chloroquine and MG132 were purchased from Sigma-Aldrich (St. Louis, MO). Matrigel was purchased from BD Biosciences (Billerica, MA). CXCR4 antibody was purchased from Abcam (Cambridge, MA). GAPDH, ubiquitin, and NF-κB subunit p65 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and IκBα and IKKβ antibodies from Cell Signaling Technology Inc. (Beverly, MA).
Cell culture and plasmid transfection
Colon cancer cell lines SW480, DLD-1, HCT116, HT29, RKO, lung cancer cell line A549, prostate cancer cell line PC3, and normal human bronchial epithelial cell line Beas-2B were obtained from the American Type Culture Collection (ATCC; Rockville, MD). Arsenic-, chromium-, cadmium-, and nickel-induced transform Beas-2B cells (B-AsT, B-CdT, B-CrT, and B-NiT) were developed and maintained in our lab (Chang et al., 2010; Pan et al, 2011.; Son et al., 2012). HCT116, A549, and PC3 cells were cultured in RPMI 1640 medium with penicillin (100 IU/mL), streptomycin (100 μg/mL) and 10% fetal bovine serum (FBS) and incubated at 37°C with 5% CO2. DLD-1, HT29, RKO, Beas-2B, and transformed cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS at 37°C under a humidified 95%:5% (v/v) mixture of air and CO2. SW480 cells were maintained in Leibovitz’s L-15 medium. B-AsT cells were transfected with p-IκBα or p-IKKβ vector using lipofectamine 2000 (Invitrigon) as recommended by the manufacturer.
Cell viability assays
Cell viability was evaluated by MTT assay. Briefly, cells were seeded in sextuplet into 96-well plates at a density of 1×103 cells/well. The treatments were started at indicated concentrations 24 hours after seeding. At the indicated time, cells were incubated in 3-(4, 5-dimethylthiszol-2-yl)-2, 5-diphenytetrazolium bromide (MTT) (Sigma-Aldrich) solution for 4 hours, then supplemented with 100 μl of DMSO and shaken for 15 minutes. The absorbance of cultures was measured using a multi-well spectrophotometer at a test wavelength of 560 nm. Results were calculated as percentage of absorbance in control cultures.
Western blot analysis
Whole-cell extracts were prepared by adding RIPA buffer (Sigma-Aldrich) containing protease inhibitor cocktail. Protein concentrations of all samples were determined by using Coomassie (Bradford) protein assay reagent (Thermo, Rockford, IL). Proteins were separated on SDS-PAGE gels and transferred to nitrocellulose membranes. The membranes were probed with primary antibodies followed by incubation with horseradish peroxidase (HRP) conjugated secondary antibodies (Pierce, Rockford, IL). After that proteins of interest were visualized using a Chemiluminescent Detection Kit (Pierce). The blots were exposed to Hyperfilm (Amersham Pharmacia Biotech, Piscataway, NJ). The density of western blot bands was quantified with the software Image-Pro Plus V6.0.
Immunoprecipitation assay
Cells were lysed with RIPA buffer. Lysates were incubated with the CXCR4 antibodies for overnight at 4°C. Immunocomplexes were collected through Protein G Agarose (Millipore, Billerica, MA), washed four times in lysis buffer. The samples were resolved by western blot assay probed with anti- ubiquitin antibody
Wound healing migration assay
Cell motility was measured by a wound healing assay as described previously with modification (Kuang et al., 2011). B-AsT cells were allowed to grow to full confluency in 6-well plates and then starved overnight with serum free medium to inactivate cell proliferation. Cells were then scraped with pipette tips and washed with PBS. DMEM media containing indicated concentrations of apigenin were added. 100 ng/mL CXCL12 was used to stimulate cell migration. After 24 hours, cells were imaged and migrated cells were quantified by manual counting.
Boyden chamber invasion assay
Invasion assay was carried out using modified Boyden chambers consisting of 24-well cell culture insert membrane filter (8 μM pore size) (BD Biosciences). Briefly, the top surface of chamber was coated with 100 μL Matrigel (100 μg/mL). Then upper chambers were seeded with 1 × 105 cells/well in 100 μL medium (serum free), the bottom chambers were added medium (10% serum) with or without 100 ng/mL CXCL12. Both media contained indicated concentrations of apigenin. After 24 hours, the cells on the top surface of the filter were scraped using a cotton swab while cells spreading on the bottom side (invasive cells) were fixed with cold 4% paraformaldehyde and stained with crystal violate. Invasive cells were imaged under inverted microscope and quantified by manual counting.
Immunofluoresence microscope
Cells cultured on chamber slides were washed with PBS and fixed in 4% paraformaldehyde for 10 minutes. A 1% glycine/0.5% Triton X-100 solution was used to permeabilize cells at room temperature for 15 minutes. After blocked with 5% bovine serum albumin (BSA) for 1 hour, cells were incubated in PBS containing 0.2% Triton X-100, 1% BSA, and anti-NFκB p65 antibody overnight at 4°C. Then, cells were washed with PBST (PBS containing 0.1% Tween-20) followed by an incubation with secondary antibody (in PBS containing 1% BSA) for 45 minutes. At last, cells were washed with PBS containing 0.1% Tween-20 and then PBS alone. The slides were dried and mounted with Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI).
Luciferase reporter assay
Luciferase reporter assay was carried out as described previously (Wang et al., 2010). Transformed Beas-2B cells (1 × 106 cells/dish) were seeded into 10 cm cell culture dish. When cells achieve 60% confluence, reporter gene constructs were transfected using the lipofectamine 2000, with 8 μg luciferase vector per plate. After transfection, cells were reseeded in 24-well plates, and consequently pretreated with the indicated concentrations of apigenin for 24 hours before being washed and lysed with luciferase lysis buffer (Promega, Madison, WI). Renilla luciferase reporter was used as a transfection efficiency control. Luciferase activity of lysates was measured following the manufacturer’s protocol (Luciferase Assay System, Promega) with GloMax® 96 Microplate Luminometer (Promega).
Mouse xenograft model and immunohistochemistry
The 4 week-old male athymic nude mice (Nu/Nu mice), which purchased from Jackson Laboratories (Bar Harbor, ME), were randomized into the 2 treatment groups with 5 mice in each group. B-AsT cells (5×106 cells/100 μL in medium plus 50% Matrigel) were subcutaneously injected into the right flanks of nude mice. Tumors were allowed to grow for one week and apigenin (0 and 40 mg/kg body weight) was administered intraperitoneally in 100 μL of dimethyl sulfoxide/0.9% physiologic saline (1:0.5) daily for 5 days a week for 3 weeks. Mice body weight and tumor mass were recorded every 5 days. Tumor volume was calculated using the formula volume = (length × width2) × 0.5. Solid tumors were removed, fixed with 10% formaldehyde, and embedded in paraffin. Immunohistochemical staining for CXCR4 were carried out. Staining results were imaged under microscope. All animals were handled according to the Institutional Animal Care and Use, University of Kentucky.
Statistical analysis
All arrays were performed at least three independent experiments. The data were presented as mean ± SD, and statistical comparisons between groups were performed using one-way ANOVA followed by Newman-Keuls test. P value ≤0.05 was considered statistically significant.
Results
Apigenin suppresses CXCR4 expression in B-AsT cells and SW480 cells
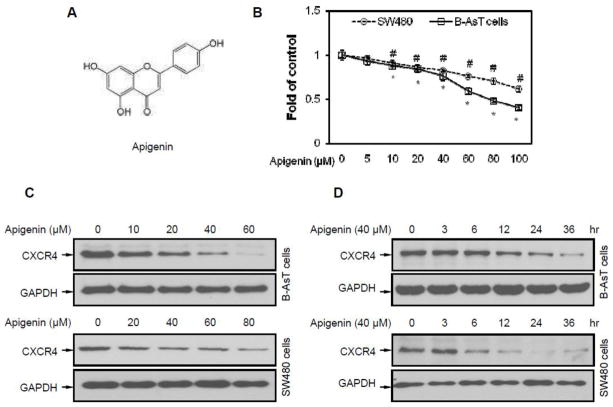
Apigenin is a flavonoid with a molecular weight of 270.25 g/mol (Figure 1A). To investigate the influence of apigenin on cell viability, an MTT assay was performed at first. The results show that the half maximal inhibitory concentration (IC50) of apigenin was between 60 μM and 80 μM on B-AsT cells and more than 100 μM on SW480 cells (Figure 1B) for a 24 hour exposure. When B-AsT or SW480 cells were incubated either with different concentrations of apigenin for 24 hours or with 40 μM apigenin for different times, apigenin suppressed the expression of CXCR4 in a dose- (Figure 1C) and time-dependent manner (Figure 1D). Apigenin induced CXCR4 suppression could be observed with treatment for as short as 6 hours and with a concentration as low as 20 μM. This down-regulation was not due to the decrease in cell viability (Figure 1B).
Fig. 1. Apigenin suppresses CXCR4 in B-AsT and SW480 cells.
(A) The chemical structure of apigenin with a molecular weight 270.25 g/mol. (B) Cell viability was quantified by MTT assay. B-AsT and SW480 cells were treated with different concentrations of apigenin for 24 hours. Columns, mean from six duplicates; bars, SE (*, P<0.05 versus control on B-AsT cells, #, P<0.05 versus control on SW480 cells). (C) Apigenin suppressed CXCR4 levels in a dose-dependent manner. Cells were treated with the indicated concentrations of apigenin for 24 hours. CXCR4 expressions were indicated by anti-CXCR4 and anti-GAPDH western blotting analyses. (D) Apigenin suppressed CXCR4 levels in a time-dependent manner. Cells were treated with 40 μM apigenin for the indicated times, after which western blotting was performed as described above.
Apigenin-induced CXCR4 down-regulation in various cells
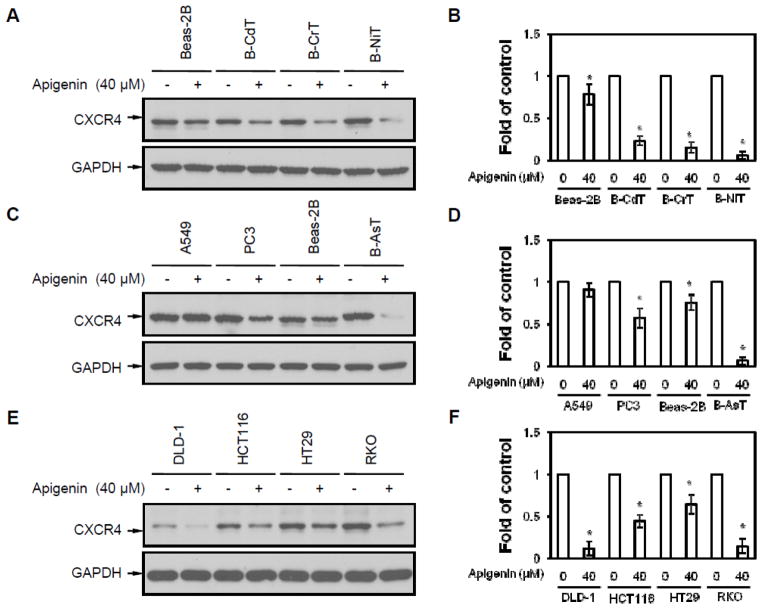
CXCR4 is expressed in a wide variety of cancer and transformed cells. Thus, different cell lines, including transformed cells (B-AsT, B-CdT, B-CrT, and B-NiT), and their parent cell line (normal human bronchial epithelial cells, Beas-2B), colon cancer cell lines (DLD-1, HCT116, HT29, and RKO), lung cancer cell line (A549), and prostate cancer cell line (PC3), were used to investigate whether apigenin could down-regulate expression of CXCR4. Cells were treated with 40 μM apigenin for 24 hours. The results show that apigenin down-regulated CXCR4 in transformed cells (Figures 2A and 2C), prostate cancer cells (Figure 2C), and colon cancer cells (Figure 2E). In A549 cells, the inhibitory effect of apigenin was not obvious. The effect of apigenin on CXCR4 expression was not significant in normal Beas-2B cells while dramatically in transformed cells (Figures 2A and 2C). The molecular weight of the CXCR4 bands in BAsT are different when compared with that in other cells. It may be due to the different post-translational modification. It is worthy to do more investigation on possible post-translational modifications. These results indicate that apigenin has a down-regulating effect on CXCR4 in most of transformed/cancer cell lines. The differential inhibition on CXCR4 expression by apigenin in Beas-2B and transformed cells suggest that the transformation of Beas-2B cells has changed its sensitivity to apigenin.
Fig. 2. Apigenin-induced CXCR4 down-regulation is not cell type specific.
(A) Beas-2B cells and transformed cells (B-CdT, B-CrT, and B-NiT) were exposed to 40 μM apigenin for 24 hours. CXCR4 expressions were measured by anti-CXCR4 and anti-GAPDH western blotting analyses. (B) Densitometry analysis of expression level from western blot as in A. (C) Lung cancer cell line A549, prostate cancer cell line PC3, human bronchial epithelial cell line Beas-2B and transformed Beas-2B (B-AsT) were exposed to 40 μM apigenin for 24 hours, after which western blotting was performed. (D) Densitometry analysis of expression level from western blot as in C. (E) Different colon cancer cell lines (DLD-1, HCT116, HT29 and RKO) were exposed to 40 μM apigenin for 24 hours, after which western blotting was done. (F) Densitometry analysis of expression level from western blot as in E. Columns, mean from 3 different experiments; bars, SE (*, P<0.05 versus without apigenin treatment).
Apigenin suppresses migration and invasion in B-AsT cells
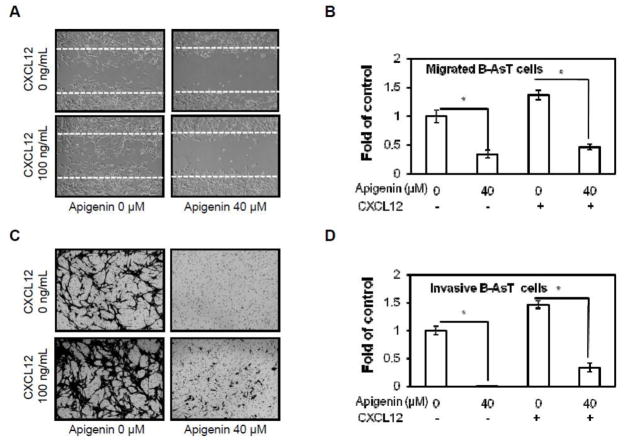
The CXCL12/CXCR4 signaling has been shown to play a critical role in cancer metastasis (Marchesi et al., 2004). Breast cancer cell motility could be induced by exposure to CXCL12 (Muller et al., 2001). Knock down of CXCR4 could inhibit breast cancer metastasis (Smith et al., 2004). The effect of apigenin on migration of B-AsT cells was measured by wound healing migration assay. After treatment with apigenin at indicated concentrations for 24 hours, migratory cells were counted. As shown in Figures 3A and 3B, apigenin significantly inhibited cell migration in both CXCL12 stimulated and non-stimulated B-AsT cells. The effect of apigenin on cell invasion was analyzed by Boyden chamber invasion assay. The results indicate that apigenin markedly suppressed the constitutive or CXCL12-induced cell invasion through Matrigel (Figures 3C and 3D). These results suggest that apigenin is highly effective on inhibiting constitutive or CXCL12-induced migration and invasion of B-AsT cells.
Fig. 3. Apigenin suppresses CXCL12 induced migration and invasion in B-AsT cells.
(A) B-AsT cells were scratched with a pipette tip and then treated with 40 μM apigenin followed by 0 or 100 ng/mL CXCL12 stimulation. Cells were photographed under microscope (magnification, ×200). (B) Migrated cells were quantified by manual counting, quantitative analysis of migrated cells as in A was shown. (C) The invasion ability of B-AsT cells were determined by Boyden chamber invasion assay. Cells in lower surface of the chamber were stained and photographed under microscope at ×200 magnification. (D) Invaded cells were quantified by manual counting and quantitative analysis of invasive cells as in C was shown. Columns, mean from 3 different experiments with 3 duplicates; bars, SE (*, p < 0.05 versus control).
Down-regulation of CXCR4 by apigenin is not mediated through protein degradation
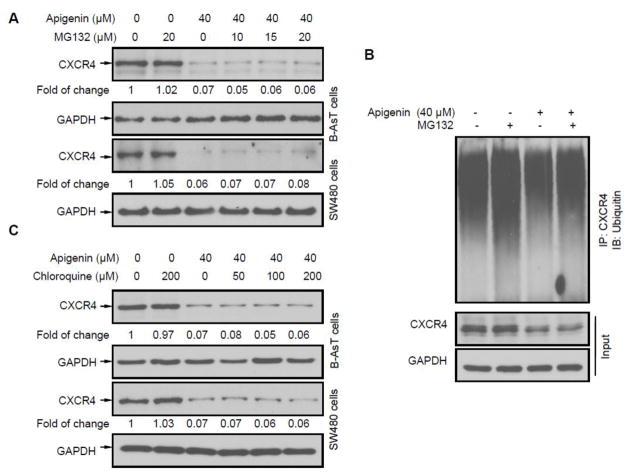
CXCR4 has been shown to be ubiquitinated at its lysine residue, which leads to CXCR4 degradation (Bhandari et al., 2007). To investigate whether apigenin down-regulated CXCR4 through proteasomal degradation, a proteasome inhibitor MG132 was used to inhibit proteasomal degradation of CXCR4 in B-AsT and SW480 cells. These cells were treated with MG132 for 1 hour followed by exposed to apigenin for 24 hours (Sung et al., 2008). As shown in Figure 4A, MG132 had no effect on apigenin induced reduction of CXCR4 in these types of cells, suggesting that apigenin did not affect CXCR4 by a proteasomal degradation mechanism. MG132 treatment could increase the accumulation of ubiquitinated CXCR4 with or without apigenin treatment. Apigenin treatment did not cause an additional accumulation on ubiquitinated CXCR4 compared with control group, indicating apigenin did not have effects on ubiquitin-mediated protein degradation (Figures 4B). On the other hand, since CXCR4 could undergo ligand-dependent lysosomal degradation (Bhandari et al., 2007), chloroquine, a lysosomal inhibitor, was used to inhibit lysosomal degradation of CXCR4. As shown in Figure 4C, chloroquine did not prevent the down-regulation of CXCR4. These results indicate that protein degradation was not the primary mechanism of CXCR4 suppression by apigenin.
Fig. 4. Down-regulation of CXCR4 by apigenin is not mediated through protein degradation.
(A) Cells were treated with indicated concentration of MG132 for 1 h at 37°C, followed by treatment with 40 μM apigenin for 24 hours. Western blot analysis with antibodies against CXCR4 and GAPDH were performed. (B) Cells lysates were incubating with CXCR4 antibodies overnight at 4°C. Immunocomplexes were collected through Protein G Agarose. Western blot assay were applied probed with anti- ubiquitin antibody. (C) Cells were treated with indicated concentration of chloroquine for 1 hour at 37°C, followed by treatment with 40 μM apigenin for 24 hours, and then western blotting was performed as described above.
Down-regulation of CXCR4 by apigenin is through suppressing NF-κB activation
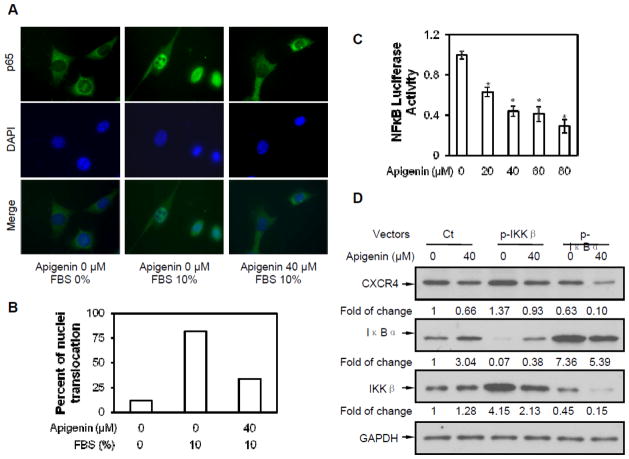
The promoter of CXCR4 is known to contain several NF-κB binding sites (Pandey et al., 2007). In addition, apigenin has been shown to inhibit NF-κB activation in various cancer cell lines. It is possible that apigenin affects CXCR4 by suppressing NF-κB activation. NF-κB subunit p65 translocation assay was used to explore whether apigenin affects NF-κB activation in B-AsT cells. As shown in Figure 5A, p65 translocated into nuclei after FBS stimulation for 15 minutes and apigenin inhibited p65 translocation. These results suggest that apigenin may down-regulate CXCR4 expression by suppressing NF-κB activation. To confirm the above results, a NF-κB luciferase reporter assay was performed. Results showed that the transcriptional activities of NF-κB were strongly inhibited by treatment with 20 μmol/L or higher concentration of apigenin and that the inhibitory effect was in a dose-dependent manner (Figure 5C).
Fig. 5. Down-regulation of CXCR4 by apigenin through suppressing NF-κB activity.
(A) Cells were starved overnight then treated with 40 μM apigenin for 24 hours, following stimulated with 10% FBS. NF-κB subunit p65 was probed with p65 antibody. (B) Quantitative analysis of p65 translocation. Each group consisted of 50 cells were been counted. (C) Apigenin inhibition of NF-κB activation was measured with luciferase reporter. pNF-κB-luciferase vector transfected cells were treated with different concentrations of apigenin for 24 hours. The relative transcriptional activity of NF-κB was measured by luciferase assay as described in Material and Methods. Columns, mean of luciferase activities calculated from 3 independent experiments; bars, SE (*, p < 0.05 versus control). (D) p-IKKβ or p-IκBα vector transfected cells were treated with 40 μM apigenin for 24 hours. Western blot analysis with antibodies against CXCR4, IKKβ, IκBα and GAPDH were performed.
NF-κB is present in the cytosol in an inactive state, complexed with the inhibitor of κB (IκB) protein. Most agents activate NF-κB through degradation of IκB. The key regulatory steps in this pathway involve activation of IκB kinase (IKK), which switches on IκB degradation. To determine the relationship between CXCR4 down-regulation and NF-κB activation in apigenin treatment, B-AsT cells were transfected with p-IKKβ or p-IκBα vector respectively, and then treated with apigenin. As shown in Figure 5D, without apigenin treatment, CXCR4 increased in IKKβ over-expressed cells and decreased in IκBα over-expressed cells. Apigenin also suppressed CXCR4 expression in IKKβ or IκBα overexpressed cells. Apigenin did increase IκBα but had a slight effect on IKKβ. These results suggest that apigenin may down-regulate CXCR4 via suppressing NF-κB signaling.
Apigenin suppresses CXCR4 expression and tumor growth in vivo
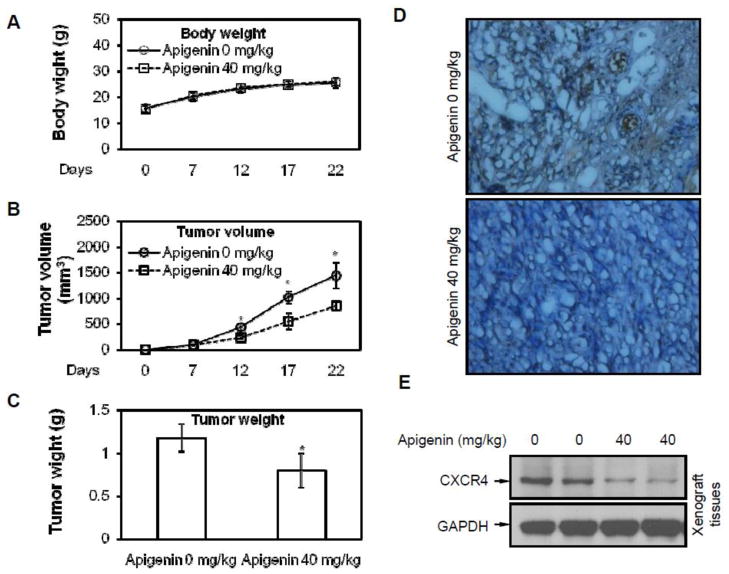
B-AsT cells were implanted in the right flanks of nude mice. One week later, animals were randomized into two groups and treatments were started according to the experimental protocol. As shown in Figure 6A, the body weight of mice had no observable difference between treated with 0 mg/kg and 40 mg/kg apigenin, suggesting that apigenin had little or no toxicity to the mice at the concentration of 40 mg/kg. After 22 days of growth, the tumor volume and tumor weight were measured. Apigenin reduced both tumor volume and weight (Figures 6B and 6C). The tumor weight was reduced by 32.3% in treated group. The results of immunohistochemical analysis show that apigenin suppressed the expression of CXCR4 in tumor tissues (Figure 6D), which was further confirmed by western blotting assay (Figure 6E). These results suggest that apigenin inhibited tumor growth in vivo.
Fig. 6. Apigenin suppresses CXCR4 expression and tumor growth in vivo.
Athymic nude mice were inoculated with B-AsT cells (5×106 cells per mouse, subcutaneously) and randomly divided into 2 groups (n = 5 per group) for treatment with vehicle/apigenin. (A) Apigenin had no influence in mice body weight during treatment. (B) Apigenin inhibited tumor growth as measured by tumor volume. (C) Solid tumors weight in the apigenin treated mice was significantly light than those in untreated mice. (D) Tissue sections subjected to staining revealed that apigenin inhibited CXCR4 expression. (E) Tissues subjected to western blotting indicated that apigenin inhibited CXCR4 expression. Columns, mean of luciferase activities calculated from 3 independent experiments; bars, SE (*, p < 0.05 versus control).
Discussion
Apigenin is a natural dietary flavonoid which has been show to exhibit anticancer activities. CXCR4, a chemokine receptor, has been closely linked with cancer cell growth, invasion, angiogenesis, metastasis, and transformation. The present study shows that apigenin suppressed the expression and function of CXCR4. Our results reveal that apigenin inhibited the expression of CXCR4 in arsenic-transformed Beas-2B cells. Apigenin also inhibited CXCR4 expression in other transformed cells and other type of cancer cells, indicating that this inhibition is not cell type specific. Apigenin decreased CXCR4 through reducing the activity of transcriptional factor NF-κB, but not via protein degradation. In vivo study also shows that apigenin down-regulated CXCR4 expression and reduced tumor size/weight.
The CXCR4 chemokine receptor has been found to be overexpressed in more than 20 different tumors, including lung, prostate, kidney, breast, ovarian, and colon carcinoma (Uchida et al., 2003; Fernandis et al., 2004). Clinical study shows that the level of CXCR4 in oral squamous cell carcinoma was significantly different from normal tissues, indicating that CXCL12/CXCR4 may be important in early steps of oral transformation and in the progress of oral carcinogenesis (Xia et al., 2012). The consequence of over-expression of CXCR4 in tumor cells is still unclear. Gene fusion of PAX3- and PAX7-FKHR (Libura et al., 2002), gene mutations of von Hippel Lindau tumor suppressor (Staller et al., 2003), hypoxia of tumor microenvironment (Schioppa et al., 2003), activation of NF-κB (Helbig et al., 2003), and expression of cytokines such as VEGF (Bachelder et al., 2002) and TNFα (Kulbe et al., 2005), have all been implicated in CXCR4 overexpression. CXCR4 appears as an ideal therapeutic target for the development of novel therapeutic agent for the prevention of metastatic cancer.
The results of present study indicate that apigenin suppressed CXCR4 expression in BAsT and SW480 cells in a dose- and time-dependent manner. Our results also show that apigenin suppressed CXCR4 expression in different kinds of transformed cells and other types of cancer cells, indicating that the effect of apigenin on CXCR4 is not cell type specific. Protein down-regulation has two probable pathways, i.e, protein degradation or gene transcription decrease. A previous study has show that CXCR4 could be degradated through an ubiquitin-depended pathway (Bhandari et al., 2007). Our results, however, show that apigenin does not down-regulate CXCR4 through protein degradation. Since down-regulation of CXCR4 by apigenin did not occur at the post-translational level, we postulate that the inhibition of CXCR4 expression by apigenin could occur at the transcriptional level. The transcription factors HIF-1α (Schioppa et al., 2003; Staller et al., 2003), PPARγ (Richard and Blay, 2007), and NF-κB (Helbig et al., 2003) have been implicated in the regulation of CXCR4. Interestingly, NF-κB binding site has also been identified in the proximal region of the CXCR4 promoter and postulated to play an important role in CXCR4 expression in human breast cancer cells (Helbig et al., 2003). Apigenin has been reported to down-regulate NF-κB activation in various tumor cells (Pandey et al., 2007). Therefore, it is possible that down-regulation of CXCR4 by apigenin is associated with the inhibition of NF-κB activation. Indeed, we found that repression of NF-κB by apigenin resulted in down-regulation of CXCR4. Besides CXCR4, the activation of NF-κB induces expression of various adhesion molecules including VEGF-C, MMP-2/MMP-9, and Cox-2 which are also related to cancer cell metastasis. Because apigenin can inhibit NF-κB activation (Kang et al., 2012), it is possible that apigenin may suppress the functions of these molecules as well. We have also found that apigenin inhibited the ligand-induced migration/invasion in B-AsT cells. These results show a critical role of the CXCR4 in migration/invasion of the tumor and the potentiality of apigenin to down-regulate the expression or the activity of CXCR4.
Malignant transformation is the process by which cells acquire the properties of cancer. Although malignant transformation may occur because of changes within the cell, it can be induced by inorganic toxic substances such as arsenic or cadmium and organic compounds such as tobacco-specific nitrosamines. Despite extensive efforts in determining how transformation of the normal cells occurs in a number of experimental systems (Chang et al., 2010; Pan et al., 2011; Wang et al., 2011), the carcinogenic mechanism remains poorly understood. It is interesting that over-expression of CXCR4 could induce the transformation of immortalized normal breast epithelial cells MCF-10A and enhance invasive phenotype of transformed cells (Su et al., 2011). Sequential genetic change has been reported at the TP53 and CXCR4 locus during transformation of human ovarian surface epithelium (Archibald et al., 2012). CXCR4 has been reported to be up-regulated in chronic cadmium exposed cells, which acquire the transformation phenotype (Qu et al., 2012). It is possible that CXCR4 could be important in the mechanism of cell malignant transformation.
Taken together, the results of present study demonstrate that apigenin is able to down-regulate the expression of CXCR4, which is a key receptor involved in the cross talk between tumor cells and their microenvironments, and to subsequentially suppresses the metastatic activity of transformed cells. Apigenin can also inhibit tumorigenesis, indicating that this compound has the potential to be developed as a cancer preventive and therapeutic agent.
Highlights.
Apigenin, a diet nutritional factor, has a potential in preventing environmental arsenic-induced carcinogenesis.
Apigenin suppresses the expression and functions of CXCR4 in malignant transformed cells in vitro and in vivo.
The down-regulation of CXCR4 is mainly due to inhibition of NF-κB activity.
Acknowledgments
We thank Dr. Zhigang Wang (University of Kentucky) for his helpful suggestions and thank Hong Lin for her technical help. This work was supported by the National Institutes of Health [R01ES015518, R01ES015375, R01CA116697 and R01 ES020870].
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akashi T, Koizumi K, Tsuneyama K, Saiki I, Takano Y, Fuse H. Chemokine receptor CXCR4 expression and prognosis in patients with metastatic prostate cancer. Cancer Sci. 2008;99:539–542. doi: 10.1111/j.1349-7006.2007.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald KM, Kulbe H, Kwong J, Chakravarty P, Temple J, Chaplin T, Flak MB, McNeish IA, Deen S, Brenton JD, Young BD, Balkwill F. Sequential genetic change at the TP53 and chemokine receptor CXCR4 locus during transformation of human ovarian surface epithelium. Oncogene. 2012 doi: 10.1038/onc.2011.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelder RE, Wendt MA, Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 2002;62:7203–7206. [PubMed] [Google Scholar]
- Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14:171–179. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Bhandari D, Trejo J, Benovic JL, Marchese A. Arrestin-2 interacts with the ubiquitin-protein isopeptide ligase atrophin-interacting protein 4 and mediates endosomal sorting of the chemokine receptor CXCR4. J Biol Chem. 2007;282:36971–36979. doi: 10.1074/jbc.M705085200. [DOI] [PubMed] [Google Scholar]
- Budhraja A, Gao N, Zhang Z, Son YO, Cheng S, Wang X, Ding S, Hitron A, Chen G, Luo J, Shi X. Apigenin induces apoptosis in human leukemia cells and exhibits anti-leukemic activity in vivo. Mol Cancer Ther. 2012;11:132–142. doi: 10.1158/1535-7163.MCT-11-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Pan J, Wang X, Zhang Z, Chen F, Shi X. Reduced reactive oxygen species-generating capacity contributes to the enhanced cell growth of arsenic-transformed epithelial cells. Cancer Res. 2010;70:5127–5135. doi: 10.1158/0008-5472.CAN-10-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EJ, Kim GH. Apigenin Induces Apoptosis through a Mitochondria/Caspase-Pathway in Human Breast Cancer MDA-MB-453 Cells. J Clin Biochem Nutr. 2009;44:260–265. doi: 10.3164/jcbn.08-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero-Lourdes C, Wu T, Camarillo JM, Gandolfi AJ. Interleukin-8 (IL-8) over-production and autocrine cell activation are key factors in monomethylarsonous acid [MMA(III)]-induced malignant transformation of urothelial cells. Toxicol Appl Pharmacol. 2012;258:10–18. doi: 10.1016/j.taap.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandis AZ, Prasad A, Band H, Klosel R, Ganju RK. Regulation of CXCR4-mediated chemotaxis and chemoinvasion of breast cancer cells. Oncogene. 2004;23:157–167. doi: 10.1038/sj.onc.1206910. [DOI] [PubMed] [Google Scholar]
- Gupta PB, Mani S, Yang J, Hartwell K, Weinberg RA. The evolving portrait of cancer metastasis. Cold Spring Harb Symp Quant Biol. 2005;70:291–297. doi: 10.1101/sqb.2005.70.033. [DOI] [PubMed] [Google Scholar]
- Haque R, Mazumder DN, Samanta S, Ghosh N, Kalman D, Smith MM, Mitra S, Santra A, Lahiri S, Das S, De BK, Smith AH. Arsenic in drinking water and skin lesions: dose-response data from West Bengal, India. Epidemiology. 2003;14:174–182. doi: 10.1097/01.EDE.0000040361.55051.54. [DOI] [PubMed] [Google Scholar]
- Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278:21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, Diaz GA. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34:70–74. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- Holm NT, Byrnes K, Li BD, Turnage RH, Abreo F, Mathis JM, Chu QD. Elevated levels of chemokine receptor CXCR4 in HER-2 negative breast cancer specimens predict recurrence. J Surg Res. 2007;141:53–59. doi: 10.1016/j.jss.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol Lett. 2002;133:1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- Hurwitz AA, Foster BA, Allison JP, Greenberg NM, Kwon ED. The TRAMP mouse as a model for prostate cancer. Curr Protoc Immunol. 2001;Chapter 20(Unit 20):25. doi: 10.1002/0471142735.im2005s45. [DOI] [PubMed] [Google Scholar]
- IARC. Some drinking-water disinfectants and contaminants, including arsenic. Monographs on chloramine, chloral and chloral hydrate, dichloroacetic acid, trichloroacetic acid and 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone. IARC Monogr Eval Carcinog Risks Hum. 2004;84:269–477. [PMC free article] [PubMed] [Google Scholar]
- Kang OH, Lee JH, Kwon DY. Apigenin inhibits release of inflammatory mediators by blocking the NF-kappaB activation pathways in the HMC-1 cells. Immunopharmacol Immunotoxicol. 2012;33:473–479. doi: 10.3109/08923973.2010.538851. [DOI] [PubMed] [Google Scholar]
- Kim J, Mori T, Chen SL, Amersi FF, Martinez SR, Kuo C, Turner RR, Ye X, Bilchik AJ, Morton DL, Hoon DS. Chemokine receptor CXCR4 expression in patients with melanoma and colorectal cancer liver metastases and the association with disease outcome. Ann Surg. 2006;244:113–120. doi: 10.1097/01.sla.0000217690.65909.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchin KT, Conolly R. Arsenic-induced carcinogenesis--oxidative stress as a possible mode of action and future research needs for more biologically based risk assessment. Chem Res Toxicol. 2010;23:327–335. doi: 10.1021/tx900343d. [DOI] [PubMed] [Google Scholar]
- Kruizinga RC, Bestebroer J, Berghuis P, de Haas CJ, Links TP, de Vries EG, Walenkamp AM. Role of chemokines and their receptors in cancer. Curr Pharm Des. 2009;15:3396–3416. doi: 10.2174/138161209789105081. [DOI] [PubMed] [Google Scholar]
- Kuang L, Wang L, Wang Q, Zhao Q, Du B, Li D, Luo J, Liu M, Hou A, Qian M. Cudratricusxanthone G inhibits human colorectal carcinoma cell invasion by MMP-2 down-regulation through suppressing activator protein-1 activity. Biochem Pharmacol. 2011;81:1192–1200. doi: 10.1016/j.bcp.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Kulbe H, Hagemann T, Szlosarek PW, Balkwill FR, Wilson JL. The inflammatory cytokine tumor necrosis factor-alpha regulates chemokine receptor expression on ovarian cancer cells. Cancer Res. 2005;65:10355–10362. doi: 10.1158/0008-5472.CAN-05-0957. [DOI] [PubMed] [Google Scholar]
- Leivonen SK, Kahari VM. Transforming growth factor-beta signaling in cancer invasion and metastasis. Int J Cancer. 2007;121:2119–2124. doi: 10.1002/ijc.23113. [DOI] [PubMed] [Google Scholar]
- Li ZD, Liu LZ, Shi X, Fang J, Jiang BH. Benzo[a]pyrene-3,6-dione inhibited VEGF expression through inducing HIF-1alpha degradation. Biochem Biophys Res Commun. 2007;357:517–523. doi: 10.1016/j.bbrc.2007.03.178. [DOI] [PubMed] [Google Scholar]
- Libura J, Drukala J, Majka M, Tomescu O, Navenot JM, Kucia M, Marquez L, Peiper SC, Barr FG, Janowska-Wieczorek A, Ratajczak MZ. CXCR4-SDF-1 signaling is active in rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and adhesion. Blood. 2002;100:2597–2606. doi: 10.1182/blood-2002-01-0031. [DOI] [PubMed] [Google Scholar]
- Marchesi F, Monti P, Leone BE, Zerbi A, Vecchi A, Piemonti L, Mantovani A, Allavena P. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004;64:8420–8427. doi: 10.1158/0008-5472.CAN-04-1343. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Nakajima T, Tachibana K, Iizasa H, Bleul CC, Yoshie O, Matsushima K, Yoshida N, Springer TA, Kishimoto T. Molecular cloning and characterization of a murine pre-B-cell growth-stimulating factor/stromal cell-derived factor 1 receptor, a murine homolog of the human immunodeficiency virus 1 entry coreceptor fusin. Proc Natl Acad Sci U S A. 1996;93:14726–14729. doi: 10.1073/pnas.93.25.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JJ, Chang QS, Wang X, Son YO, Liu J, Zhang Z, Bi YY, Shi X. Activation of Akt/GSK3beta and Akt/Bcl-2 signaling pathways in nickel-transformed BEAS-2B cells. Int J Oncol. 2011;39:1285–1294. doi: 10.3892/ijo.2011.1157. [DOI] [PubMed] [Google Scholar]
- Pandey PK, Yadav S, Pandey M. Human arsenic poisoning issues in central-east Indian locations: biomarkers and biochemical monitoring. Int J Environ Res Public Health. 2007;4:15–22. doi: 10.3390/ijerph2007010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise (review) Int J Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- Qu W, Tokar EJ, Kim AJ, Bell MW, Waalkes MP. Chronic Cadmium Exposure In Vitro Causes Acquisition of Multiple Tumor Cell Characteristics in Human Pancreatic Epithelial Cells. Environ Health Perspect. 2012 doi: 10.1289/ehp.1205082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard CL, Blay J. Thiazolidinedione drugs down-regulate CXCR4 expression on human colorectal cancer cells in a peroxisome proliferator activated receptor gamma-dependent manner. Int J Oncol. 2007;30:1215–1222. [PubMed] [Google Scholar]
- Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S, Mishra A, Fu P, MacLennan GT, Resnick MI, Gupta S. Up-regulation of insulin-like growth factor binding protein-3 by apigenin leads to growth inhibition and apoptosis of 22Rv1 xenograft in athymic nude mice. FASEB J. 2005;19:2042–2044. doi: 10.1096/fj.05-3740fje. [DOI] [PubMed] [Google Scholar]
- Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- Son YO, Wang L, Poyil P, Budhraja A, Hitron JA, Zhang Z, Lee JC, Shi X. Cadmium induces carcinogenesis in BEAS-2B cells through ROS-dependent activation of PI3K/AKT/GSK-3beta/beta-catenin signaling. Toxicol Appl Pharmacol. 2012 doi: 10.1016/j.taap.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- Su H, Sobrino Najul EJ, Toth TA, Ng CM, Lelievre SA, Fred M, Tang CK. Chemokine receptor CXCR4-mediated transformation of mammary epithelial cells by enhancing multiple RTKs expression and deregulation of the p53/MDM2 axis. Cancer Lett. 2011;307:132–140. doi: 10.1016/j.canlet.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Sung B, Jhurani S, Ahn KS, Mastuo Y, Yi T, Guha S, Liu M, Aggarwal BB. Zerumbone down-regulates chemokine receptor CXCR4 expression leading to inhibition of CXCL12-induced invasion of breast and pancreatic tumor cells. Cancer Res. 2008;68(21):8938–44. doi: 10.1158/0008-5472.CAN-08-2155. [DOI] [PubMed] [Google Scholar]
- Uchida D, Begum NM, Almofti A, Nakashiro K, Kawamata H, Tateishi Y, Hamakawa H, Yoshida H, Sato M. Possible role of stromal-cell-derived factor-1/CXCR4 signaling on lymph node metastasis of oral squamous cell carcinoma. Exp Cell Res. 2003;290:289–302. doi: 10.1016/s0014-4827(03)00344-6. [DOI] [PubMed] [Google Scholar]
- Ujiki MB, Ding XZ, Salabat MR, Bentrem DJ, Golkar L, Milam B, Talamonti MS, Bell RH, Jr, Iwamura T, Adrian TE. Apigenin inhibits pancreatic cancer cell proliferation through G2/M cell cycle arrest. Mol Cancer. 2006;5:76. doi: 10.1186/1476-4598-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Kuang L, Pan X, Liu J, Wang Q, Du B, Li D, Luo J, Liu M, Hou A, Qian M. Isoalvaxanthone inhibits colon cancer cell proliferation, migration and invasion through inactivating Rac1 and AP-1. Int J Cancer. 2010;127:1220–1229. doi: 10.1002/ijc.25119. [DOI] [PubMed] [Google Scholar]
- Wang X, Son YO, Chang Q, Sun L, Hitron JA, Budhraja A, Zhang Z, Ke Z, Chen F, Luo J, Shi X. NADPH oxidase activation is required in reactive oxygen species generation and cell transformation induced by hexavalent chromium. Toxicol Sci. 2011;123:399–410. doi: 10.1093/toxsci/kfr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way TD, Kao MC, Lin JK. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem. 2004;279:4479–4489. doi: 10.1074/jbc.M305529200. [DOI] [PubMed] [Google Scholar]
- Xia J, Chen N, Hong Y, Chen X, Tao X, Cheng B, Huang Y. Expressions of CXCL12/CXCR4 in oral premalignant and malignant lesions. Mediators Inflamm. 2012;2012:516395. doi: 10.1155/2012/516395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng PW, Chiang LC, Lin CC. Apigenin induced apoptosis through p53-dependent pathway in human cervical carcinoma cells. Life Sci. 2005;76:1367–1379. doi: 10.1016/j.lfs.2004.08.023. [DOI] [PubMed] [Google Scholar]