Abstract
Background
Screening tests are available to determine immunity to vaccine‐preventable diseases, such as mumps and rubella. We aimed to define better assay for detecting immune status of health care personnel to vaccine‐preventable diseases.
Methods
Mumps and rubella antibodies of health care personnel at Shimane University Hospital were examined by hemagglutination inhibition assay (HI), comparing with those by enzyme immunoassay (EIA).
Results
A total of 910 sera from health care personnel were tested. There was poor correlation between HI and EIA in detecting mumps antibodies with correlation coefficient values (r) = 0.190 (P < 0.001), but in rubella antibodies HI and EIA were relatively well correlated (r = 0.930, P < 0.001). Seropositivity rate of HI versus EIA was found to be 65.7 versus 93.2, and 89.5 versus 86.5% for mumps and rubella, respectively. As compared with EIA, HI identified sixfold larger seronegative subjects in mumps. Moreover, in mumps, 88.8% of seronegative subjects detected by HI were seropositive by EIA, while 3.7% of seropositive subjects detected by HI were seronegative by EIA. In rubella, 2.1% of seronegative subjects detected by HI were seropositive by EIA, and 1.7% of seropositive by HI was seronegative by EIA.
Conclusion
Considerable difference between HI and EIA in determining immune status of health care personnel to mumps and rubella suggests beneficial use of EIA for the identification of accurate susceptible personnel who subsequently undergo an effective vaccination programs. Seroprevalence survey of health care personnel by using appropriate assay is essential for prevention and infection control strategies in health care settings.
Keywords: hemagglutination inhibition assay, enzyme immunoassay, mumps, rubella, seroprevalence survey, health care personnel
INTRODUCTION
Vaccine‐preventable diseases, such as measles, mumps, rubella, and varicella, are viral infections, known to be transmittable in health care settings. Health care personnel are at risk for exposure to and acquiring these diseases, and maintenance of immunity is therefore essential for prevention and infection control programs for health care personnel 1, 2.
There are several assays that determine the serologic evidence of immunity to vaccine‐preventable diseases, and include hemagglutination inhibition assay (HI), compliment fixation assay (CF), and enzyme immunoassay (EIA) (enzyme‐linked immunosorbent assay, ELISA). Specificity and sensitivity, as well as cost, differ among the assays. EIA is superior in specificity and sensitivity 3, but its cost is much higher (approximately threefold expensive) than HI or CF. Choice of assays may be clinically crucial, since identification of susceptible health care personnel, who should be recommended vaccination, is dependent on these laboratory results. However, comparative data between HI, CF, and EIA in a large series of health care personnel are lacking. In this study, to better define the assay for immune status of health care personnel against vaccine‐preventable diseases, antibody titers of 910 health care personnel against mumps and rubella viruses were simultaneously analyzed by HI and EIA.
SUBJECTS AND METHODS
Study Population
Subjects included in this study were health care personnel at Shimane University Hospital. Since 2005, the infection control committee of Shimane University Hospital in Japan has introduced serologic screening of immune status of health care personnel against vaccine‐preventable diseases. After informed consent, sera were collected. The present study was carried out in the form of an audit as part of the hospital's safety and clinical service development.
Comparison Between HI and EIA in Detecting Virus Antibodies
Collected sera underwent a serologic screening for mumps and rubella. Antibodies against mumps and rubella were tested by HI assay (Japan Clinical Laboratories, Inc.). The antibody levels were defined as negative as <×4 for mumps and <×8 for rubella on HI assay. EIA tests were performed by staff of the immunoserology unit of the central clinical laboratory in Shimane University Hospital. In EIA, anti‐mumps and anti‐rubella IgG were investigated by commercially available VIDAS assay kit; Mumps‐IgG and RUB‐IgG (BioMerieux, Marcy‐l'Étoile France). In this method, the quantitative cut‐off value for seronegative was <0.35 for mumps and <10 IU/l for rubella, and a titer at 0.35–0.50 for mumps and 10–15 IU/l for rubella was defined as equivocal value. Seropositive cut‐off was ≥0.5 for mumps and 15 IU/l for rubella.
Statistical Analysis
Spearman's rank correlation was employed for the relationship between EIA and HI, and a correlation coefficient was calculated for mumps and rubella, respectively.
RESULTS
Nine hundred and ten health care personnel were studied, comprising 253 physicians, 394 nurses, 103 laboratory technicians, 115 administrative staff, and 45 teaching staff. Three hundred and four were males and 606 females. The age ranged from 21 to 65 years (mean, 37.8 years). The titers of antibodies against mumps and rubella tested by HI were simultaneously compared with those done by EIA.
Scattergrams that compare mumps and rubella antibody titers obtained by HI to those by EIA for individuals of 910 health care personnel are shown in Figures 1 and 2, respectively. In mumps, the titers of HI were poorly correlated with those of EIA, and correlation coefficient values (r) were calculated at 0.190 (P < 0.001; Fig. 1) whereas, rubella titers correlated relatively well with HI and EIA (r = 0.930, P < 0.001; Fig. 2).
Figure 1.
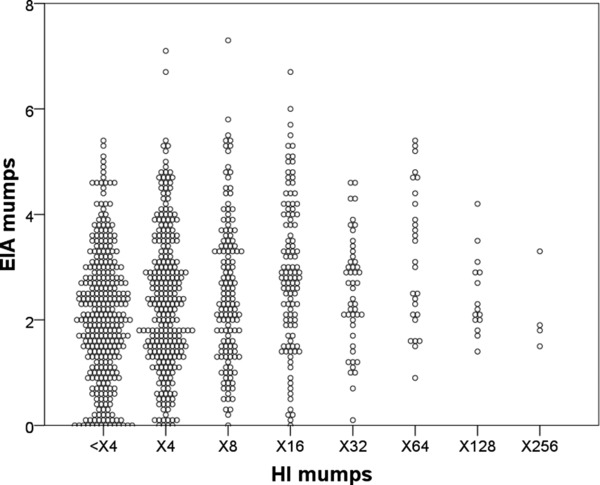
Scattergrams of mumps titers detected by HI versus EIA.
The antibody titers against mumps of health care personnel (n = 910) detected by HI were compared with those by EIA.
Figure 2.
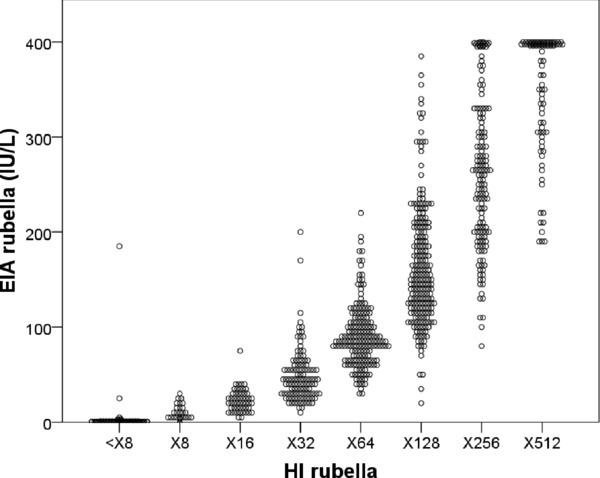
Scattergrams of rubella titers detected by HI versus EIA.
The antibody titers against rubella of health care personnel (n = 910) detected by HI were compared with those by EIA.
Serologic results of health care personnel against mumps and rubella detected by HI and EIA are shown in Table 1. The percentages of seropositive subjects against mumps detected by HI versus EIA were 65.7 (n = 598) versus 93.2 (n = 848), respectively. On the other hand, HI identified sixfold larger number of seronegative subjects against mumps, as compared to EIA (n = 51 versus 312). Moreover, in mumps, 277 of 312 seronegative subjects (88.8%) detected by HI were seropositive by EIA, whereas 22 of 598 seropositive subjects (3.7%) detected by HI were seronegative by EIA. In rubella, seropositive subjects detected by HI versus EIA were 89.5% (n = 814) versus 86.5% (n = 787), respectively. Seronegative subjects detected by HI versus EIA were 10.5% (n = 96) versus 11.9% (n = 108), respectively, however, 2 of 96 seronegative subjects (2.1%) by HI were seropositive by EIA. Moreover, 14 of 814 seropositive subjects (1.7%) by HI were seronegative by EIA.
Table 1.
Serologic Results Against Mumps and Rubella Antibodies Detected by HI Versus EIA in Health Care Personnel (n = 910)
| n (%) | |||
|---|---|---|---|
| HI | EIA | Mumps | Rubella |
| Negative | Negative | 29 (3.2) | 94 (10.3) |
| Equivocal | 6 (0.7) | 0 (0) | |
| Positive | 277 (30.4) | 2 (0.2) | |
| Total | 312 (34.3) | 96 (10.5) | |
| Positive | Negative | 22 (2.4) | 14 (1.5) |
| Equivocal | 5 (0.6) | 15 (1.6) | |
| Positive | 571 (62.7) | 785 (86.3) | |
| Total | 598 (65.7) | 814 (89.5) | |
| n (%) | |||
|---|---|---|---|
| EIA | HI | Mumps | Rubella |
| Negative | Negative | 29 (3.2) | 94 (10.3) |
| Positive | 22 (2.4) | 14 (1.5) | |
| Total | 51 (5.6) | 108 (11.9) | |
| Equivocal | Negative | 6 (0.7) | 0 (0) |
| Positive | 5 (0.6) | 15 (1.6) | |
| Total | 11 (1.2) | 15 (1.6) | |
| Positive | Negative | 277 (30.4) | 2 (0.2) |
| Positive | 571 (62.7) | 785 (86.3) | |
| Total | 848 (93.2) | 787 (86.5) | |
DISCUSSION
Vaccine‐preventable diseases, such as mumps and rubella, can be transmitted from patients to health care personnel and from personnel to patients in health care settings, and therefore all health care personnel need to be immune to vaccine‐preventable diseases for prevention and infection control programs. Personnel are considered immune when they have documentation of physician‐diagnosed diseases, documentation of vaccine on or after their first birthday, or serologic evidence of immunity 2. Ensuring serologic evidence of immunity to vaccine‐preventable diseases should be based on an adequate and reliable assay method. Serologic screening using a certain assay with less specificity and sensitivity may create considerable number of false‐negative susceptible personnel.
In this study, we showed the comparison between HI and EIA in detecting immunity to mumps and rubella, by using 910 sera from health care personnel. Although scattergrams showed relatively close relationship between HI and EIA in detecting rubella antibodies, our results indicate that there were considerable differences in the number of health care personnel between HI and EIA in detecting their immune status to mumps and rubella. Of 910 personnel, seronegative subjects for mumps identified by HI and EIA were 312 (34.3%) and 51 (5.6%), respectively, which indicates sixfold difference between HI and EIA. For rubella seronegative subjects identified by HI and EIA were 96 (10.5%) and 108 (11.9%), respectively. Moreover, 88.8% of seronegative subjects for mumps by HI were seropositive by EIA, and 2% of seronegative subjects for rubella by HI were seropositive by EIA. HI appears to reflect the protective antibody level, but it can result to detect excessive false‐negative subjects because of less sensitivity. Our results clearly show the evidence that, when serologic evidence of immunity is based on HI, a large number of personnel, who are seropositive by EIA, can be identified susceptible, especially in the case of mumps. EIA is in fact not the “gold standard” method for detecting virus antibodies, however, it is a more specific and sensitive method than HI. Thus, HI may not be sufficient for the promotion of adequate seroprevalence survey of health care personnel, followed by a vaccine program.
Moreover, our results showed that, 3.7% and 1.7% of seropositive personnel for mumps and rubella who were identified by HI were seronegative by EIA, respectively. HI seems unable to find the appropriate susceptible personnel who should be recommended for vaccination. To identify appropriate susceptible personnel and to promote adequate and effective immunization programs in hospital setting, EIA may be therefore crucial. On the other hand, seroprevalence survey of health care personnel may be cost‐effective. EIA is much more expensive than HI; however, seroprevalence survey using EIA may be more cost‐effective—as, prevention of illness through immunization for adequate susceptible personnel is far more cost‐effective than case management and outbreak control.
On the other hand, our result showed that the percentage of seropositive health care personnel analyzed by EIA was 93.2% and 86.5% for mumps and rubella, respectively. Similar result has been described by other hospitals of Japanese University 4, however seropositive rate of health care personnel for rubella has been reported to be higher in other countries, such as Italy and Turkey 5, 6, 7. This difference may be dependent on vaccine programs in each country. In Japan, routine vaccination program was introduced in 1977. Measles, mumps, and rubella (MMR) vaccination started in 1989, but since 1994 the monovalent rubella vaccine was recommended for infants because of the adverse effect of MMR vaccination. Therefore, in Japan the frequency of personnel who have immunity to rubella might be low as compared to other countries.
In conclusion, we found the considerable differences between HI and EIA in detecting immunity of health care personnel to mumps and rubella. Best understanding of immunity of health care personnel, followed by promotion of vaccination for susceptible personnel, might allow qualifications and safety in patient care. Our analysis presented here strongly suggests that EIA is beneficial to better define the immune status of health care personnel against vaccine‐preventable diseases.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1. Centers for Disease Control and Prevention . Immunization of health‐care workers: recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Hospital Infection Control Practices Advisory Committee (HICPAC). Morb Mortal Wkly Rep 1997;46:1–42. [PubMed] [Google Scholar]
- 2. Bolyard EA, Tablan OC, Williams WW, Pearson ML, Shapiro CN, Deitchmann SD. Guideline for infection control in healthcare personnel, 1998. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 1998;19:407–463. [DOI] [PubMed] [Google Scholar]
- 3. Forghani B, Schmidt NJ. Antigen requirements, sensitivity, and specificity of enzyme immunoassays for measles and rubella viral antibodies. J Clin Microbiol 1979;9:657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hatakeyama S, Moriya K, Itoyama S, et al. Prevalence of measles, rubella, mumps, and varicella antibodies among healthcare workers in Japan. Infect Control Hosp Epidemiol 2004;25:591–594. [DOI] [PubMed] [Google Scholar]
- 5. Fedeli U, Zanetti C, Saia B. Susceptibility of healthcare workers to measles, mumps rubella and varicella. J Hosp Infect 2002;51:133–135. [DOI] [PubMed] [Google Scholar]
- 6. Celikbas A, Ergonul O, Aksaray S, et al. Measles, rubella, mumps, and varicella seroprevalence among health care workers in Turkey: Is prevaccination screening cost‐effective? Am J Infect Control 2006;34:583–587. [DOI] [PubMed] [Google Scholar]
- 7. Alp E, Cevahir F, Gökahmetoglu S, Demiraslan H, Doganay M. 2012. Prevaccination screening of health‐care workers for immunity to measles, rubella, mumps, and varicella in a developing country: What do we save? J Infect Public Health 5:127–132. [DOI] [PubMed] [Google Scholar]


