Abstract
In the past years, cardiovascular progenitor cells have been isolated from the human heart and characterized. Up to date, no studies have been reported in which the developmental potential of foetal and adult cardiovascular progenitors was tested simultaneously. However, intrinsic differences will likely affect interpretations regarding progenitor cell potential and application for regenerative medicine. Here we report a direct comparison between human foetal and adult heart-derived cardiomyocyte progenitor cells (CMPCs). We show that foetal and adult CMPCs have distinct preferences to differentiate into mesodermal lineages. Under pro-angiogenic conditions, foetal CMPCs form more endothelial but less smooth muscle cells than adult CMPCs. Foetal CMPCs can also develop towards adipocytes, whereas neither foetal nor adult CMPCs show significant osteogenic differentiation. Interestingly, although both cell types differentiate into heart muscle cells, adult CMPCs give rise to electrophysiologically more mature cardiomyocytes than foetal CMPCs. Taken together, foetal CMPCs are suitable for molecular cell biology and developmental studies. The potential of adult CMPCs to form mature cardiomyocytes and smooth muscle cells may be essential for cardiac repair after transplantation into the injured heart.
Keywords: cardiac progenitor cell, foetal, adult, multipotency, differentiation
Introduction
For several decades, the adult heart was considered a post-mitotic organ, devoid of progenitor cells that contribute to homeostasis or restoration of damaged tissue after acute or chronic injury. However, this lack of intrinsic regeneration capacity has recently been contested by two studies that reported cardiomyocyte renewal in human beings [1] and replacement of adult cardiomyocytes by cells from a αMHC-negative cell source after cardiac injury in the mouse [2]. Despite these findings, endogenous repair remains limited and is not sufficient to fully restore cardiac function after myocardial infarction. Therefore, additional approaches like progenitor cell-based therapy are required to replace lost cells and improve perfusion of the heart. Ideally, these progenitor cells should have an inherent cardiovascular potential in order to optimally differentiate into cardiac cells. Differentiation into other mesenchymal lineages, e.g. cartilage or adipocytes, might lead to adverse side effects such as arrhythmia and could cause cardiac dysfunction.
The identification of small cells in the adult heart that expressed stem cell markers and had telomerase activity [3] led to the isolation and characterization of several human adult cardiovascular progenitor cell populations [4–6]. These cells have been proposed as an ideal source for cardiac stem cell-based therapy to repair the injured myocardium [7]. Recently, we have isolated cardiomyocyte progenitor cells (CMPCs) from human heart biopsies [8, 9]. Foetal and adult heart-derived CMPCs showed similar phenotypes and expression patterns of early cardiac transcription factors. Stimulation with 5-azacytidine and transforming growth factor beta (TGFβ) resulted in the formation of cardiomyocytes within 3–4 weeks with high efficiency (93–98%α-actinin-positive cardiomyocytes with foetal CMPCs compared to 84–93% when using adult CMPCs) [8]. Both of these cardiomyocyte populations expressed a striated pattern of sarcomeric proteins [8]. Electrophysiologically, CMPC-derived cardiomyocytes (CMPC-cm) have a rather mature phenotype [8, 10]. Similar to human cardiosphere-derived cells [4] and c-Kit-positive cardiovascular progenitor cells [6], CMPCs are able to form cells expressing endothelial and smooth muscle cell markers [8].
Possible differences between foetal and adult progenitor cell multipotency are important when deciding on the optimal cell population to investigate mechanisms regulating proliferation or differentiation, cellular behaviour in response to drug screening, or their clinical applicability. Therefore, we investigated the differentiation potential of foetal versus adult CMPCs towards cardiomyocytes, endothelial and smooth muscle cells, fat and bone.
Materials and methods
Cell isolation
Informed consent procedures were followed and prior approval of the ethics committee of the University Medical Center Utrecht was obtained. CMPCs from human foetal and adult hearts were isolated by magnetic-activated cell sorting (MACS) as described previously [8, 11]. Whole foetal hearts were obtained after elective abortion of 13–17 week-old foetuses. Adult heart tissue was obtained from the auricles from patients undergoing cardiac surgery. The biopsies were anonymously transported to the laboratory for further processing. Mesenchymal stem cells (MSCs) were obtained from bone marrow aspirates from the sternum of patients undergoing cardiac surgery. MSCs were isolated by density gradient centrifugation (Ficoll-paque, 1.077g/ml, GE Health Care Bio-Sciences AB, Uppsala, Sweden) and were subsequently plated on tissue culture plastic without coating.
Cell culture
Independent isolations of CMPC and MSC cultures were used for all experiments. To determine the cellular proliferation rate, 1000 CMPCs were plated in a gelatin-coated 24 wells plate on day 0. The cells were trypsinized at day 1, 3, 5, 7 or 10, resuspended and counted using a counting chamber.
Differentiation of CMPCs into cardiomyocytes with 5-azacytidine and TGFβ was performed as described previously [8, 11]. MSCs were maintained in M199 medium (Gibco) supplemented with 10% FCS (Gibco), penicillin-streptomycin (PenStrep, 100U/ml each, Gibco, Breda, the Netherlands), 20 mg/ml ECGF (Roche diagnostics, Almere, the Netherlands) and 8 IU/ml heparin (Leo Pharma, Breda, the Netherlands). Wild-type HEK 293 cells and HEK 293 cells stably overexpressing murine wild-type Kir2.1-GFP fusion protein (KWGF cells [12]) were maintained in DMEM medium (Gibco) containing 5% FCS and PenStrep.
For co-culture experiments with HEK293 cells or KWGF cells, CMPC-derived cardiomyocytes were first cultured in differentiation medium [8, 11] and KWGF cells were plated on top the next day.
Angiogenesis assays
To determine their angiogenic potential, CMPCs were cultured in EGM2 medium or plated on Matrigel™ (Chemicon, Amsterdam, the Netherlands) and stimulated with 25 ng/ml vascular endothelial growth factor (VEGF) as described [8]. Following overnight culture, cells were isolated for RNA isolation, Western blot analysis, flow cytometry, or fixed in 4% paraformaldehyde (MP Biomedicals, Illkirch, France) for immunocytochemistry. The number and characteristics of the formed tube-like structures were analysed using Angioquant software [13].
Adipogenic differentiation
To induce adipogenic differentiation [14], CMPCs and MSCs were cultured in DMEM containing 10% FCS, 4.5 g/l glucose, 1 μM dexamethasone, 1 mM sodium pyruvate, 0.5 mM 3-Isobutyl-1-methylxanthine (IBMX), 10 μg/ml insuline, 0.2 mM indomethacin and PenStrep. Medium was refreshed twice weekly. Cells were cultured until fat deposits became visible under light microscopy (MSCs 2–3 days, CMPCs 5 days). As negative control, cells were cultured in normal growth medium containing 0.5% FCS. After differentiation, cells were lysed for RNA isolation or used for staining.
For Oil Red O staining, cells were fixed in 4% paraformaldehyde, washed with 60% isopropanol and dried. Oil Red O solution, prepared by dissolving 3.5 mg/ml Oil Red O (Sigma-Aldrich, Zwijndrecht, the Netherlands) in isopropanol and H2O, was added to the cells. For quantification of Oil Red O content, cells were lysed in isopropanol. Optical density was measured at 500 nm.
Osteogenic differentiation
For osteogenic differentiation [15], CMPCs and MSCs were cultured in DMEM containing 10% FCS, 2 mM L-Glutamine, 4.5 g/l glucose, 0.1 μM dexamethasone, 50 μg/ml ascorbic acid and PenStrep. After the first week, 5 mM β-Glycerophosphate was added to the medium. Negative controls were cultured in normal growth medium containing 0.5% FCS. CMPCs and MSCs were lysed for RNA isolation or stained for alkaline phosphatase or Alizarin Red S after 21 days.
For alkaline phosphatase staining, cells were washed once with phosphate buffer solution (PBS), fixed for 5 min. in 37% formalin (Klinipath, Duiven, the Netherlands) at room temperature and washed three times with PBS. Subsequently, fixed cells were incubated with staining solution (0.2 mg/ml Naphthol AS-MX Phosphate (Sigma), 0.6 mg/ml Fast Blue (Sigma), 0.1 M Tris-HCl (pH 8.8) and 0.01% (w/v) MgSO4), and washed twice with PBS before taking pictures. To stain for Alizarin Red S, cells were washed once with PBS, fixed for 1 hr in ice cold 70% ethanol and washed twice with H2O. Fixed cells were stained with 0.1% (w/v) Alizarin Red S (Fluka, Zwijndrecht, the Netherlands) for 30 min. in the dark at room temperature and washed four times before taking pictures.
RNA isolation and quantitative RT-PCR
Cells were lysed in TriPure (Roche). Total RNA was isolated and DNAse treated (Amersham Biosciences, Diegem, Belgium). Five hundred ng total RNA was used for cDNA synthesis with iScript cDNA synthesis kit (BioRad). For qRT-PCR, 10 μl cDNA (1:20 diluted) was mixed with 10 μl SYBR-Green mix (BioRad) and forward plus reverse primers (final concentration 0.5 μM each). The PCR was run on a MyiQ iCycler (BioRad, Veenendaal, the Netherlands). PCR conditions were: 2 min. at 94°C followed by 40 cycles of: 30 sec. at 94°C, 30 sec. at annealing temperature (see Table S1) and 30 sec. at 72°C. Specificity of PCR products was visually checked on polyacrylamide gels or melting curve analysis afterwards. Amplicon quantities were determined by comparison with known quantities of cloned PCR products and normalized to β-actin expression. Primers were designed with Beacon Designer 4.0 (Premier Biosoft International, Palo Alto, CA, USA). Primer sequences and annealing temperatures are provided in Table S1.
Western blot analysis
Western blot analysis was performed as described before [8]. Primary antibodies used were for platelet/endothelial cell adhesion molecule (PECAM; Santa Cruz), αSMA (Dako, Glostrup, Denmark) and GAPDH (Abcam, Cambridge, UK). As secondary antibodies HRP coupled anti-rabbit or anti-mouse antibodies were used, and signal was obtained using SuperSignal (Thermo Scientific, Breda, the Netherlands).
Flow cytometry
Flow cytometry was performed as described before [8]. Primary antibodies used were for FITC- or PE-conjugated mouse stem cell antigen (Sca)-1 and human CD105, c-Kit, MDR-1 or PECAM (all from Pharmingen BD, Erembodegem, Belgium). For αSMA (Dako), a two-step protocol was followed using a goat-anti-mouse secondary antibody.
Immunocytochemistry
Immunocytochemistry was performed as described previously [8]. Primary antibodies used were for human PECAM (Santa Cruz), αSMA (Dako), α-actinin (Sigma) and Connexin 43 (Zymed, Breda, the Netherlands). Secondary antibodies were Cy3 donkey-anti-goat (Dako), 488 nm goat-anti-mouse and 555 nm goat-anti-rabbit (both from Invitrogen, Breda, the Netherlands) or DaM-TR and GaR-FITC (both from Jackson ImmunoResearch, Suffolk, UK).
Telomerase activity
Telomerase activity was measured in proliferating CMPCs using a telomeric repeat amplification protocol (TRAPeze) according to the manufacturer’s protocol and analysed on polyacrylamide gels.
Electrophysiology
To measure resting membrane potentials (RMPs) in CMPCs, cells were cultured in media appropriate for undifferentiated or differentiated cells [8, 11]. Determination of membrane potential was performed as described earlier [8]. Briefly, patch clamp microelectrodes were used to measure the membrane potential of CMPCs and CMPC-cm at 37°C. For BaCl2 experiments, the RMP was first measured in control medium and then in medium containing 1 mM BaCl2. In addition, sharp microelectrodes were used to measure CMPC-cm action potentials in response to bipolar field stimulation at ∼1 Hz.
Statistics
All data are presented as mean ± SEM. Number of replicates is indicated in the figure legends. Data was analysed by Student’s t-test for direct comparisons. Kruskal–Wallis non-parametric test followed by ANOVA with Tukey post hoc analysis was used for group comparisons (SPSS, Chicago, IL, USA). Significance was assumed when P < 0.05.
Results
Proliferation and cardiomyogenic differentiation of CMPCs
CMPCs derived from both foetal and adult human heart tissue show a similar spindle-shaped morphology (Fig. 1A). Cultured foetal CMPCs (fCMPCs) revealed a significantly higher proliferation rate than adult CMPCs (aCMPCs) up to 10 days after seeding (Fig. 1B). Foetal and adult CMPCs both expressed telomerase (Fig. S1) and were positive for the stem cell markers Sca-1, c-Kit and MDR-1, as well as CD105 and CD90 (Fig. S2). When induced to differentiate into cardiomyocytes by 5-azacytidine and TGFβ, both foetal and adult CMPC-cm showed organized sarcomeric structures and robust staining for the gap junction protein Connexin 43 (Cx43, Fig. 1C), which is in line with the previously observed high degree of intercellular coupling [8]. Interestingly, in aCMPC-cm, Cx43 labelling was not only found as intense staining all around the cells but also in a polarized fashion in aCMPC-cm that presented an elongated phenotype. In these cells, labelling was most intense at the longitudinal cell border (Fig. 1C). Foetal CMPC-cm more frequently showed spontaneous beating than adult CMPC-cm, although both fCMPC-cm and aCMPC-cm are quiescent in the absence of foetal calf serum and field stimulation. Adult CMPC-cm have a more negative RMP than fCMPC-cm: –82.8 ± 0.9 mV versus–73.4 ± 1.8 mV, respectively (Figs 1D and 2A). When stimulated, fCMPC-cm and aCMPC-cm show action potentials of comparable overshoot. Compared to their foetal counterparts, aCMPC-cm have a more mature action potential shape with a spike and dome morphology accompanied by a longer lasting plateau phase (Fig. 1D).
Fig 1.
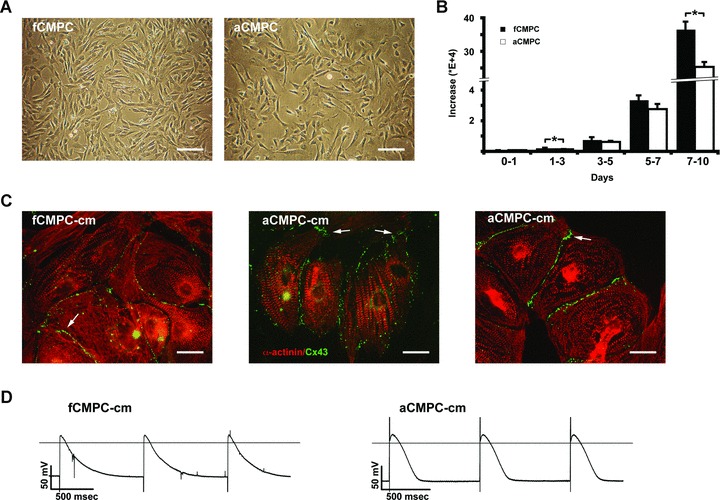
Proliferation and differentiation of CMPCs. (A) Bright field images showing foetal (fCMPC, left) and adult CMPCs (aCMPC, right) under normal culture conditions. Note the similarities in spindle-shaped phenotype. Scale bars: 200 μm. (B) Growth differences between fCMPCs and aCMPCs. Cells were counted at day 1, 3, 5, 7 and 10 after plating. A significantly higher increase in cell number was observed in fCMPCs versus aCMPCs between day 1 and 3 (P= 0.007, n= 3) and day 7 and 10 (P= 0.013). (C) Immunostaining for α-actinin (red) and Connexin 43 (green) in foetal (fCMPC-cm, left) and adult CMPC-derived cardiomyocytes (aCMPC-cm, middle and right). White arrows indicate the presence of gap junctions at the cell membrane borders. Scale bars: 50 μm. (D) Action potentials recorded from fCMPC-cm (left) and aCMPC-cm (right) using sharp microelectrodes. Monolayers were field stimulated at ∼1 Hz. Field stimulation artefacts (seen as a sharp downward peak at the onset of the fCMPC-cm action potentials and a sharp upward peak at the aCMPC-cm action potentials) did not affect the overall phenotype of CMPC action potential.
Fig 2.
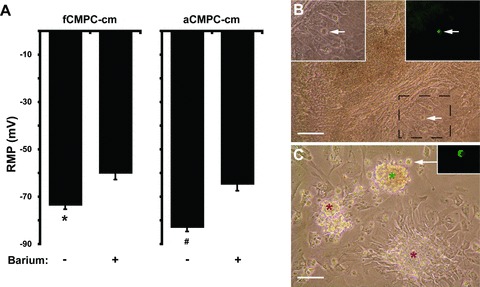
Membrane potential stability of fCMPC-cm and aCMPC-cm. (A) Resting membrane potential of fCMPC-cm and aCMPC-cm with (+) or without (–) 1 mM BaCl2. Barium-induced significant depolarizations in fCMPC-cm (min barium n= 6, plus n= 4, *P < 0.01) and aCMPC-cm (min barium n= 7, plus n= 7, #P < 0.01). (B) Bright field image of fCMPC-cm cocultured with KWGF cells (white arrow). Insets show magnification of boxed area (left: bright field, right: GFP fluorescence). Scale bar: 200 μm. (C) Bright field image of beating clusters (red asterisks) and a quiescent cluster (green asterisk) of fCMPC-cm. White arrow indicates a KWGF cell adjacent to the quiescent cluster (GFP fluorescence is shown in inset). Same areas are shown in Movies S1–S3. Scale bar: 200 μm.
Spontaneous beating in CMPC-derived cardiomyocytes is determined by resting membrane potential
Potassium inward rectifier (Kir) channels are involved in repolarization and stabilization of the RMP of a cell, and differential expression of Kir channels could underlie the different RMP and occurrence of spontaneous beating in foetal versus adult CMPC-cm. We therefore investigated the presence of Kir 2.1 and 2.2, which are the most prominent Kir channel isoforms in the heart [16]. We found that both fCMPC-cm and aCMPC-cm expressed Kir 2.1 and 2.2. However, expression was not significantly different (Fig. S3). Since Kir channel activity can be regulated post-translationally by phosphorylation [17, 18], we tested these channels functionally by blocking them with barium in fCMPC-cm and aCMPC-cm (Fig. 2A). Barium treatment resulted in a less negative RMP in both fCMPC-cm and aCMPC-cm, with a more pronounced effect in aCMPC-cm (+18.2 mV versus+13.5 mV in fCMPC-cm), implying a higher functionality of Kir channels in aCMPC-cm.
A lack of spontaneous beating was associated with a low and stable RMP in aCMPC-cm. Lowering the RMP of fCMPC-cm to the level seen in aCMPC-cm could therefore decrease spontaneous beating in fCMPC-cm as well. To investigate this, we determined the effect of coculturing fCMPC-cm with a Kir2.1GFP-overexpressing HEK 293 cell line (KWGF cells), which were shown to decrease spontaneously beating in neonatal rat cardiomyocytes via gap junctional coupling [12]. KWGF cells induced an RMP of –82.3 (±2.4) mV in fCMPC-cm (P < 0.05 versus fCMPC-cm alone, n= 5), which is identical to the RMP seen in aCMPC-cm single culture (–82.8 ± 0.9 mV, Fig. 2A). The KWGF cell-induced lower RMP subsequently resulted in inhibition of spontaneous beating in fCMPC-cm (Fig. 2B–C and Movies S1–S3). Foetal CMPC-cm cocultured with wild-type HEK 293 cells continued to beat spontaneously (Movie S4), indicating that stabilization of the RMP and inhibition of spontaneous beating in fCMPC-cm by KWGF cells was not due to random coculture effects.
Angiogenic properties of foetal versus adult CMPCs
To compare the angiogenic potential of foetal and adult CMPCs, fCMPCs and aCMPCs were cultured on Matrigel and stimulated with VEGF. Both populations formed a capillary-like network (Fig. 3A). Quantification of the tube-like structures revealed that aCMPC formed longer and thicker structures with less junctions (Fig. 3C). Immunostaining for PECAM and α-smooth muscle actin (αSMA) revealed the presence of endothelial- and smooth muscle-like cells within the cords (Fig. 3B). Interestingly, pronounced staining for PECAM was observed in fCMPC cultures, although aCMPC cultures showed a stronger reactivity for αSMA. Adult CMPCs also showed higher mRNA expression of smooth muscle myosin heavy chain (SM-MHC), and lower expression of Tie-2 and VE-Cadherin, compared to fCMPCs (Fig. 3D). Consistently, the protein level of PECAM was lower in aCMPCs than fCMPCs, while SMA protein was higher in aCMPCs when cultured under angiogenic conditions (Fig. 3E) and in an angiogenesis assay (Fig. S4). This confirms that, following angiogenic induction, aCMPCs seem to form mostly smooth muscle-like cells although fCMPCs tend to differentiate into endothelial-like cells.
Fig 3.
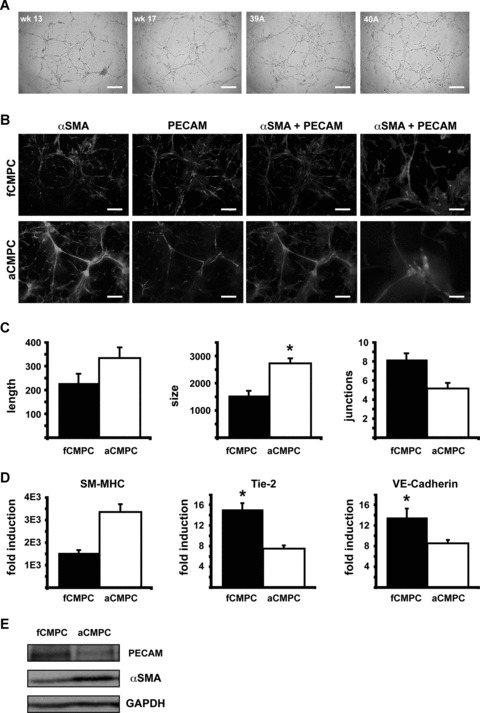
Angiogenic potential of foetal and adult CMPCs. (A) Representative bright field images of Matrigel assays showing foetal (week 13 and 17) and adult (clones 39A and 40A) CMPC-derived tube-like structures. Scale bars: 500 μm. (B) Immunofluorescence images of the same assay as in A with staining for αSMA (green) and PECAM (red). Scale bars: 500 μm. Scale bars in images most right are 200 μm. (C) Angioquant quantification from images in (B) showing average length and size of the tubes (arbitrary units) and the number of junctions. Data are from one of three separate experiments performed in triplo (*P= 0.02). (D) Quantitative RT-PCR analyses indicating fold induction of SM-MHC, Tie-2 and VE-Cadherin expression in foetal and adult CMPCs from angiogenesis assays compared to respective controls cultured in normal culture medium (SM-MHC: not significant, P= 0.08, n= 2. Tie-2: P= 0.01, n= 5. VE-Cadherin: P= 0.04, n= 5). (E) Western blot analysis of CMPCs cultured under angiogenic conditions. FCMPCs show high PECAM expression, but low αSMA expression. In contrast, aCMPCs show low PECAM expression, but high αSMA expression. GAPDH was used as loading control.
Adipogenic potential of foetal versus adult CMPCs
To assess their potential to differentiate into other cell types of mesodermal origin, CMPCs were subjected to an adipogenic differentiation protocol. Albeit less efficiently than MSCs, fCMPCs formed significantly more lipoprotein-containing vacuoles than aCMPCs and non-stimulated controls (Fig. 4A and B). Adult CMPC cultures hardly showed any vacuoles positive for Oil Red O (Fig. 4A and B). We further quantified the degree of adipogenesis on a marker gene expression level using qRT-PCR (Fig. 4C and Fig. S5). After adipogenic induction, a significantly larger increase of leptin, adipsin and PPARγ2 expression, and decrease of the inhibitor CCN1 were observed in fCMPCs compared to aCMPCs (Fig. 4C).
Fig 4.
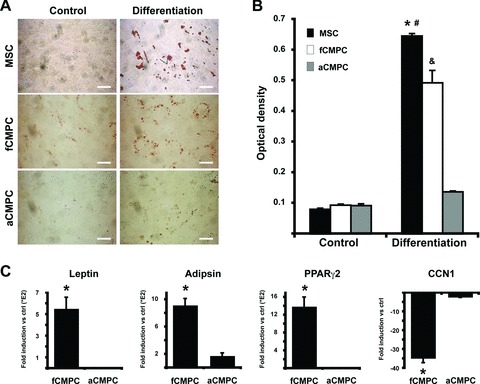
Adipogenic potential of foetal and adult CMPCs. (A) MSCs and CMPCs cultured in 0.5% culture medium (control) or adipogenic medium (Differentiation) and stained for Oil Red O. Scale bars: 500 μm. (B) Quantification of A indicated a larger increase of Oil Red O in fCMPC cultures than aCMPC cultures (n= 4, *P < 0.001 versus all controls and aCMPC Differentiation, #P= 0.005 versus fCMPC Differentiation, &P < 0.001 versus all controls and aCMPC differentiation). (C) Quantitative RT-PCR analyses showing fold induction of leptin, adipsin, PPARγ2 and CCN1 expression in fCMPCs and aCMPCs cultured in adipogenic medium compared to respective controls cultured in normal culture medium (n= 3, leptin: P= 0.035, adipsin: P= 0.006, PPARγ2: P= 0.022, CCN1: P= 0.004).
Osteogenic potential of foetal versus adult CMPCs
After induction of osteogenesis, only MSCs, and not foetal or adult CMPCs, showed osteogenic differentiation, as shown by positive staining for alkaline phosphatase and Alizarin Red S (Fig. 5A). Although the expression of the osteogenic transcription factor Runx2 and growth factor CTGF were up-regulated in fCMPCs (Fig. 5B), the expression levels remained very low, especially when compared to differentiated MSCs (Fig. S6). Therefore, substantial osteogenic differentiation remained absent in both fCMPCs and aCMPCs.
Fig 5.
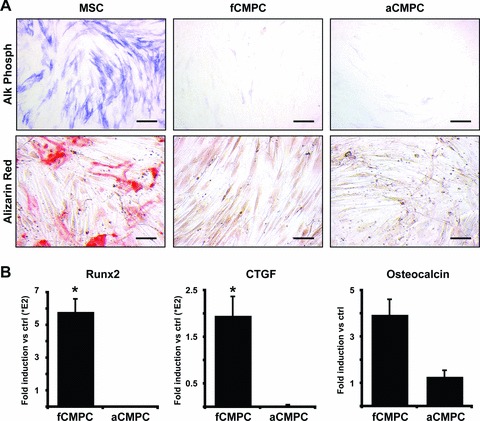
Osteogenic potential of foetal and adult CMPCs. (A) MSCs and CMPCs cultured in osteogenic differentiation medium and stained for alkaline phosphatase or Alizarin Red S. Scale bars: 500 μm. (B) Quantitative RT-PCR analyses showing fold induction of Runx2, CTGF and osteocalcin expression in fCMPCs and aCMPCs cultured in osteogenic medium compared to respective controls cultured in normal culture medium (n= 3, Runx2: P= 0.042, CTGF: P= 0.013, osteocalcin: not significant, P= 0.061).
Discussion
Progenitor cells are abundantly present in the embryo, but their number decreases substantially during development to foetal, neonatal and adult stages [19–21]. Additionally, progenitor cell ageing has been associated with a decline in function and plasticity [22, 23]. Because the developmental potential of progenitor cells influences their applicability in experimental and clinical settings, we compared the growth and differentiation potential of CMPCs from foetal and adult human hearts.
Proliferation and differentiation potential of foetal versus adult progenitor cells
Human foetal and adult heart-derived CMPCs harbour telomerase activity, which is characteristic for highly cycling cells, and can be expanded substantially. Notably, fCMPCs showed a significantly higher proliferation. This is consistent with the observed higher proliferation of human foetal MSCs compared to adult MSCs [24], which indicates that age and origin-based effects on proliferative capacity are not limited to CMPCs.
Although both foetal and adult CMPCs can differentiate into cardiomyocytes with well-organized sarcomeres, fCMPCs differentiate into electrophysiologically less mature cardiomyocytes than aCMPCs. Foetal CMPC-cm beat spontaneously and have a less negative RMP when compared to aCMPC-cm. We were able to inhibit this spontaneous beating by lowering the RMP to the level seen in aCMPC-cm, which was equal to the potassium equilibrium potential (around –82 mV). This RMP is comparable to that of cardiomyocytes in the adult human heart (–74 ± 1 and –77 ± 3 mV for atrial and ventricular cardiomyocytes respectively) [25, 26], confirming the more mature status of adult CMPC-cm.
Next to a distinct cardiomyogenic potential, foetal and adult CMPCs also show differences with regard to angiogenesis; in vitro, foetal CMPCs form intricate endothelial tube-like structures, although adult CMPCs form longer tubes with fewer junctions that contain mainly smooth muscle-like cells. This difference in angiogenic potential is consistent with an earlier study that showed that foetal and adult MSCs respectively form endothelial and smooth muscle cells in vivo after transplantation into damaged rat hearts [27]. Additionally, foetal endothelial progenitor cells (EPCs) were shown to form functional vascular networks in vivo, containing smaller tubes with more junctions, indicating enhanced neoangiogenesis, when compared to adult EPCs [28]. Differentiation towards other mesodermal cell types can also be affected by a foetal versus adult origin. For instance, foetal MSCs showed higher osteogenic gene expression and calcium deposition than adult MSCs upon differentiation [29]. Consistently, foetal CMPCs have more adipogenic potential than aCMPCs. However, neither foetal nor adult CMPCs showed substantial osteogenic differentiation.
Our results indicated that a foetal versus adult origin not only influenced CMPC proliferation and differentiation, but also their multipotency. Differences in multipotency were also found in MSCs: even though adult bone marrow-derived MSCs did proliferate and could differentiate towards osteogenic, chondrogenic and adipogenic lineages, foetal MSC proliferation efficiency was higher and they could additionally differentiate into neurons, skeletal muscle and even blood cells [30]. Together, our observations and those of others [23] suggest that the effect of a foetal versus adult origin on the developmental potential of stem/progenitor cells may be a general biological phenomenon that should be taken into account when deciding on the optimal cell source for experimental and clinical purposes [31].
Comparisons with other cell types
Both fCMPCs and aCMPCs were positive for Sca-1, c-Kit, CD105 and CD90. This suggests that human CMPCs have much in common with human cardiosphere-derived cells [5] and c-Kit+ cardiovascular progenitor cells [6]. However, although CMPCs also express MDR-1, human cardiosphere-derived cells do not, and, to our knowledge, human c-Kit+ progenitor cells were not tested for this specific marker. This suggests that although these different populations may have similar properties, they still remain distinct from each other. To determine if these differences were caused by the different methods for isolation and culture, a direct comparison will be required. Furthermore, small batch-to-batch variabilities were described for cardiosphere-derived cells [4], which, together with genomic instability, may affect the interpretation of differences in proliferation and differentiation of foetal versus adult progenitor cells. However, in our experiments, we did not observe substantial batch-to-batch variability, nor were CMPCs genomically unstable [8]. This supports our conclusion that foetal and adult CMPCs truly have a distinct developmental potential.
CMPCs were isolated from the human heart with an antibody raised against mouse Sca-1, which was described earlier to recognize cardiovascular progenitor cells in mouse hearts [32–34]. The human antigen recognized by this antibody is yet unknown and may or may not be related to the murine antigen, since no human Sca-1 homologue has been identified to date [35]. Identification of the protein bound by the antibody will allow generation of more specific antibodies for CMPC isolation and help to unravel the developmental origin of CMPCs.
Implications of developmental plasticity
The inherent differences of foetal and adult progenitor cells could have substantial consequences for their use in regenerative medicine. We have recently shown that, after transplantation into infarcted hearts, foetal CMPCs are able to differentiate into cardiomyocytes and vascular cells in vivo and restore long-term cardiac function [36]. In the present study, we have demonstrated that adult CMPCs show enhanced cardiomyogenic differentiation and smooth muscle cell formation compared to foetal CMPCs in vitro. Future research may reveal if the observed differences between fCMPCs and aCMPCs in vitro persist in vivo. Possibly, the more mature aCMPC-derived cardiomyocytes may integrate better (resulting in a lower risk for arrhythmogenicity) and restore lost tissue in the infarcted heart more efficiently than fCMPC-derived cardiomyocytes. Additionally, aCMPC-derived smooth muscle cells could help to stabilize newly formed vessels, leading to long-term enhanced perfusion. Ultimately, this could help to improve cardiac repair.
In summary, we have demonstrated that human foetal and adult CMPCs have distinct proliferation rates and differentiation potential. Foetal CMPCs seem more versatile and may be very suitable for cardiomyogenic and angiogenic development studies. Adult CMPCs form more mature cardiomyocytes and smooth muscle cells, although lacking adipo- and osteogenic potential. This suggests a decreased risk for arrhythmogenesis after transplantation into the heart. Adult CMPCs may therefore be useful to replace lost cardiac tissue in clinical settings.
Acknowledgments
We thank Alain van Mil for the Angioquant analysis. This work was supported by a VIDI grant (016.056.319) and VENI grant (916.36.012) from the Netherlands Organization for Scientific Research (NWO), the Van Ruyven foundation, the BSIK program ‘Dutch Program for Tissue Engineering’ (UGT-6746), the Netherlands Heart Foundation (2003B07304 and 2005T102) the Bekalis Foundation and the Center for Biomedical Genetics. This research forms part of the Project P1.04 SMARTCARE of the research program of the BioMedical Materials institute, co-funded by the Dutch Ministry of Economic Affairs.
Supporting Information
Fig S1 Telomerase activity. Representative example oftelomerase activity (lanes –) in fCMPCs and aCMPCs. Embryonicstem cells were used as positive control (Pos). Products oftelomerase activity start at 50 bp and display 6 bp periodicity.Preincubation of the samples at 85°C (lanes +)inactivated telomerase.
Fig S2 Stem cell marker expression. Flow cytometricanalysis for mouse Sca-1 and human c-Kit, MDR-1, CD105 and CD90,indicating that both fCMPCs (A) and aCMPCs (B) werepositive for these markers. Insets in the upper-right cornerindicate the percentages of positive cells.
Fig S3 Potassium inward rectifier channel expression.Quantitative RT-PCR analysis for Kir 2.1 (A) and Kir 2.2(B) in foetal and adult CMPC-derived cardiomyocytes.Expression levels were not significantly different (n = 3).
Fig S4 Angiogenic gene expression in foetal and adultCMPCs. Flow cytometric analysis confirmed that, after angiogenesis,more aCMPCs than fCMPCs were positive for the smooth muscle markerαSMA (A–B, fCMPC n = 2,aCMPC n = 3, P < 0.001), while morefCMPCs than aCMPCs express endothelial markers PECAM(C–D, P = 0.056).
Fig S5 Adipogenic gene expression during MSCdifferentiation. Quantitative RT-PCR analyses for leptin, adipsin,PPARγ2 and CCN1 in MSCs cultured in normal culture medium(control) versus adipogenic medium (differentiation,n = 3, leptin: P = 0.005,adipsin: P = 0.003, PPARγ2: P= 0.005, CCN1: P = 0.002).
Fig S6 Osteogenic gene expression during MSCdifferentiation. Quantitative RT-PCR analyses for Runx2, CTGF andosteocalcin in MSCs cultured in normal culture medium (control)versus osteogenic medium (differentiation, n= 2, Runx2: P = 0.004, CTGF: notsignificant, P = 0.116, osteocalcin: P = 0.045).
Table S1: Primer sequences and annealing temperatures
Movie S1: Cluster of contracting, foetal CMPC-derived cardiomyocytes shown in Fig. 3B the day before coculture with KWGF cells.
Movie S2: Same cluster of foetal CMPC-derived cardiomyocytes as in Movie S1, the day after coculture with KWGF cells, leading to inhibition of spontaneous contractions in fCMPC-cm.
Movie S3: Several clusters of replated foetal CMPC-derived cardiomyocytes shown in Fig. 3C the day after coculture with KWGF cells. Note the lack of contractions in the cluster adjacent to a KWGF cell and continued contractions in clusters not adjacent to KWGF cells. After 41 sec., filter is switched from bright field to FITC channel. GFP fluorescence from the KWGF cell is visible after 50 sec.; channel is switched to TRITC after 57 sec., switched back to FITC after 62 sec. and turned back to bright field after 95 sec.
Movie S4: Cluster of contracting foetal CMPC-derived cardiomyocytes the day after coculture with normal HEK 293 cells. The same relative number of cocultured cells was used as in Movie S2 (5%).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsieh PC, Segers VF, Davis ME, et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–4. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–7. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 4.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 5.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 6.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Vliet P, Sluijter JP, Doevendans PA, et al. Isolation and expansion of resident cardiac progenitor cells. Expert Rev Cardiovasc Ther. 2007;5:33–43. doi: 10.1586/14779072.5.1.33. [DOI] [PubMed] [Google Scholar]
- 8.Goumans MJ, De Boer TP, Smits AM, et al. TGF-beta1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res. 2007;1:138–49. doi: 10.1016/j.scr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Van Vliet P, Roccio M, Smits AM, et al. Progenitor cells isolated from the human heart: a potential cell source for regenerative therapy. Neth Heart J. 2008;16:163–9. doi: 10.1007/BF03086138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Boer TP, Van Veen TA, Jonsson MK, et al. Human cardiomyocyte progenitor cell-derived cardiomyocytes display a maturated electrical phenotype. J Mol Cell Cardiol. 2010;48:254–60. doi: 10.1016/j.yjmcc.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Smits AM, Van Vliet P, Metz CH, et al. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: an in vitro model for studying human cardiac physiology and pathophysiology. Nat Protoc. 2009;4:232–43. doi: 10.1038/nprot.2008.229. [DOI] [PubMed] [Google Scholar]
- 12.De Boer TP, Van Veen TA, Houtman MJ, et al. Inhibition of cardiomyocyte automaticity by electrotonic application of inward rectifier current from Kir2.1 expressing cells. Med Biol Eng Comput. 2006;44:537–42. doi: 10.1007/s11517-006-0059-8. [DOI] [PubMed] [Google Scholar]
- 13.Niemisto A, Dunmire V, Yli-Harja O, et al. Robust quantification of in vitro angiogenesis through image analysis. IEEE Trans Med Imaging. 2005;24:549–53. doi: 10.1109/tmi.2004.837339. [DOI] [PubMed] [Google Scholar]
- 14.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 15.Noort WA, Kruisselbrink AB, In’t Anker PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002;30:870–8. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- 16.Schram G, Pourrier M, Melnyk P, et al. Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circ Res. 2002;90:939–50. doi: 10.1161/01.res.0000018627.89528.6f. [DOI] [PubMed] [Google Scholar]
- 17.Fakler B, Brandle U, Glowatzki E, et al. Kir2.1 inward rectifier K+ channels are regulated independently by protein kinases and ATP hydrolysis. Neuron. 1994;13:1413–20. doi: 10.1016/0896-6273(94)90426-x. [DOI] [PubMed] [Google Scholar]
- 18.Zitron E, Kiesecker C, Luck S, et al. Human cardiac inwardly rectifying current IKir2.2 is upregulated by activation of protein kinase A. Cardiovasc Res. 2004;63:520–7. doi: 10.1016/j.cardiores.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Laugwitz KL, Moretti A, Lam J, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amir G, Ma X, Reddy VM, et al. Dynamics of human myocardial progenitor cell populations in the neonatal period. Ann Thorac Surg. 2008;86:1311–9. doi: 10.1016/j.athoracsur.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 21.Campagnoli C, Roberts IA, Kumar S, et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 22.Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080–6. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- 23.Roobrouck VD, Ulloa-Montoya F, Verfaillie CM. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008;314:1937–44. doi: 10.1016/j.yexcr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Gotherstrom C, Ringden O, Westgren M, et al. Immunomodulatory effects of human foetal liver-derived mesenchymal stem cells. Bone Marrow Transplant. 2003;32:265–72. doi: 10.1038/sj.bmt.1704111. [DOI] [PubMed] [Google Scholar]
- 25.Drouin E, Charpentier F, Gauthier C, et al. Electrophysiologic characteristics of cells spanning the left ventricular wall of human heart: evidence for presence of M cells. J Am Coll Cardiol. 1995;26:185–92. doi: 10.1016/0735-1097(95)00167-x. [DOI] [PubMed] [Google Scholar]
- 26.Nemtsas P, Wettwer E, Christ T, et al. Adult zebrafish heart as a model for human heart? An electrophysiological study. J Mol Cell Cardiol. 2010;48:161–71. doi: 10.1016/j.yjmcc.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 27.Iop L, Chiavegato A, Callegari A, et al. Different cardiovascular potential of adult- and fetal-type mesenchymal stem cells in a rat model of heart cryoinjury. Cell Transplant. 2008;17:679–94. doi: 10.3727/096368908786092739. [DOI] [PubMed] [Google Scholar]
- 28.Au P, Daheron LM, Duda DG, et al. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008;111:1302–5. doi: 10.1182/blood-2007-06-094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guillot PV, De Bari C, Dell’Accio F, et al. Comparative osteogenic transcription profiling of various fetal and adult mesenchymal stem cell sources. Differentiation. 2008;76:946–57. doi: 10.1111/j.1432-0436.2008.00279.x. [DOI] [PubMed] [Google Scholar]
- 30.O’Donoghue K, Fisk NM. Fetal stem cells. Best Pract Res Clin Obstet Gynaecol. 2004;18:853–75. doi: 10.1016/j.bpobgyn.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–13. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 32.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuura K, Nagai T, Nishigaki N, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–91. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 34.Pfister O, Mouquet F, Jain M, et al. CD31- but not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 35.Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339–47. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 36.Smits AM, Van Laake LW, Den Ouden K, et al. Human cardiomyocyte progenitor cell transplantation preserves long-term function of the infarcted mouse myocardium. Cardiovasc Res. 2009;83:527–35. doi: 10.1093/cvr/cvp146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1 Telomerase activity. Representative example oftelomerase activity (lanes –) in fCMPCs and aCMPCs. Embryonicstem cells were used as positive control (Pos). Products oftelomerase activity start at 50 bp and display 6 bp periodicity.Preincubation of the samples at 85°C (lanes +)inactivated telomerase.
Fig S2 Stem cell marker expression. Flow cytometricanalysis for mouse Sca-1 and human c-Kit, MDR-1, CD105 and CD90,indicating that both fCMPCs (A) and aCMPCs (B) werepositive for these markers. Insets in the upper-right cornerindicate the percentages of positive cells.
Fig S3 Potassium inward rectifier channel expression.Quantitative RT-PCR analysis for Kir 2.1 (A) and Kir 2.2(B) in foetal and adult CMPC-derived cardiomyocytes.Expression levels were not significantly different (n = 3).
Fig S4 Angiogenic gene expression in foetal and adultCMPCs. Flow cytometric analysis confirmed that, after angiogenesis,more aCMPCs than fCMPCs were positive for the smooth muscle markerαSMA (A–B, fCMPC n = 2,aCMPC n = 3, P < 0.001), while morefCMPCs than aCMPCs express endothelial markers PECAM(C–D, P = 0.056).
Fig S5 Adipogenic gene expression during MSCdifferentiation. Quantitative RT-PCR analyses for leptin, adipsin,PPARγ2 and CCN1 in MSCs cultured in normal culture medium(control) versus adipogenic medium (differentiation,n = 3, leptin: P = 0.005,adipsin: P = 0.003, PPARγ2: P= 0.005, CCN1: P = 0.002).
Fig S6 Osteogenic gene expression during MSCdifferentiation. Quantitative RT-PCR analyses for Runx2, CTGF andosteocalcin in MSCs cultured in normal culture medium (control)versus osteogenic medium (differentiation, n= 2, Runx2: P = 0.004, CTGF: notsignificant, P = 0.116, osteocalcin: P = 0.045).
Table S1: Primer sequences and annealing temperatures
Movie S1: Cluster of contracting, foetal CMPC-derived cardiomyocytes shown in Fig. 3B the day before coculture with KWGF cells.
Movie S2: Same cluster of foetal CMPC-derived cardiomyocytes as in Movie S1, the day after coculture with KWGF cells, leading to inhibition of spontaneous contractions in fCMPC-cm.
Movie S3: Several clusters of replated foetal CMPC-derived cardiomyocytes shown in Fig. 3C the day after coculture with KWGF cells. Note the lack of contractions in the cluster adjacent to a KWGF cell and continued contractions in clusters not adjacent to KWGF cells. After 41 sec., filter is switched from bright field to FITC channel. GFP fluorescence from the KWGF cell is visible after 50 sec.; channel is switched to TRITC after 57 sec., switched back to FITC after 62 sec. and turned back to bright field after 95 sec.
Movie S4: Cluster of contracting foetal CMPC-derived cardiomyocytes the day after coculture with normal HEK 293 cells. The same relative number of cocultured cells was used as in Movie S2 (5%).


