Abstract
A highly heterogeneous population of stem and progenitor cells has been described by light immunohistochemistry in the mammalian adult heart, but the ultrastructural identity of cardiac stem cells remains unknown. Using electron microscopy, we demonstrate the presence of cells with stem features in the adult mouse heart. These putative cardiac stem cells are small (6–10 μm), round cells, with an irregular shaped nucleus, large nucleolus, few endoplasmic reticulum cisternae and mitochondria, but numerous ribosomes. Stem cells located in the epicardial stem cell niche undergo mitosis and apoptosis. Cells with intermediate features between stem cells and cardiomyocyte progenitors have also been seen. Moreover, electron microscopy showed that cardiomyocyte progenitors were added to the peripheral working cardiomyocytes. Telocytes make a supportive interstitial network for stem cells and progenitors in the stem cell niche. This study enhances the hypothesis of a unique type of cardiac stem cell and progenitors in different stages of differentiation. In our opinion, stem cells, cardiomyocyte progenitors and telocytes sustain a continuous cardiac renewal process in the adult mammalian heart.
Keywords: cardiac stem cells, cardiomyocytes progenitors, telocytes, cardiac stem cell niche, cardiac renewal, regenerative medicine, epicardium
Introduction
A highly heterogeneous population of stem and progenitor cells [1–11] has been described by light immunohistochemistry in the mammalian adult heart, but the ultrastructural identity of cardiac stem cells (CSC) remains unknown. All these resident stem cells seem to have regenerative potential and to be the most appropriate source for cardiac cellular therapy [8]. However, it is still not clear that the different putative adult cardiac stem and progenitor cells represent different developmental and/or physiological stages of a unique type of resident stem cell [3, 11].
In this paper, we try to identify by electron microscopy (EM) CSC in a cardiac stem cell niche (CSCN) which contains cardiomyocyte progenitors (CMP) already described in the peri-coronary epicardial space [12]. The identity of putative CSC is still indefinite and their ultrastructural phenotype(s) should be of particular interest for their precise location in the myocardial complex architecture in order to understand the regenerative physiology of the heart. We show here the ability of putative CSC to continuously self-renew and produce differentiated CMP.
Materials and methods
Small fragments from mouse atrioventricular myocardium with epicardium were processed for transmission electron microscopy (EM) according to routine procedures, as we previously described [12]. Hearts from six 4-, 6- and 12-month-old C57BL/6 mice have been examined after the institutional ethical committee approval.
Results
We have previously reported the existence of CMP, cells with immature features, in the adult mouse heart [12], exclusively located in the subepicardium surrounding coronary artery (Fig. 1). EM showed that in CSCN the connective tissue is loose and particularly rich in capillaries and interstitial cells: mast cells, adipocytes, macrophages, fibroblasts, nerves, telocytes [13], CMP and mononuclear cells (Fig. 2).
Fig 1.
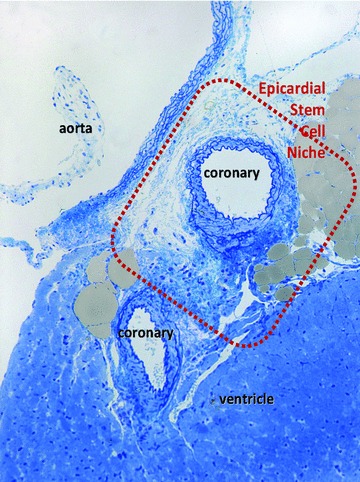
High resolution light microscopy on toluidine blue stained semithin section (∼1 μm thick ultramicrotome section) of Epon-embedded mouse heart (6 month old) shows the limited space where cardiomyocyte progenitors have been found by electron microscopy. Cardiac stem cell niche is located in the subepicardial area surrounding coronary artery next to the emergence from aorta (rectangle red mark).
Fig 2.
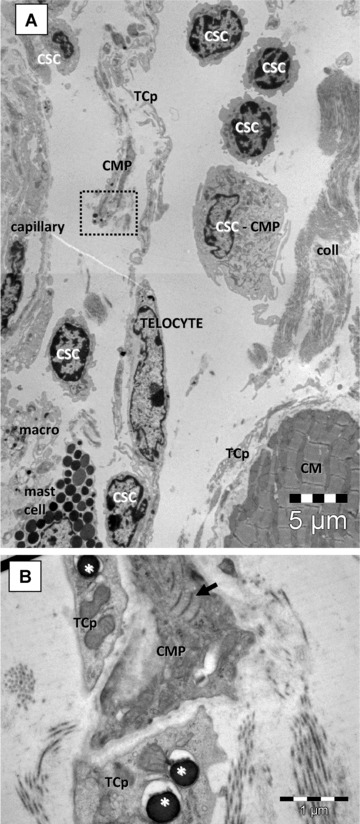
(A) Electron microscopy of the niche depicted in Fig. 1 shows the presence of putative cardiac stem cells (CSC), isolated or in small groups, cardiomyocyte progenitors (CMP) and cells with intermediate features (CSC-CMP). All these cells are placed in a loose extracellular matrix. (B) Details at higher magnification from area marked in (A) with a dotted square. The CMP have characteristic leptofibrils (arrow) and is closely assisted by telocytes processes (TCp) which contain few dense granules (asterisk) suggesting paracrine signalling. CM – cardiomyocyte; macro – macrophage; coll – collagen fibres.
Using EM and exclusion criteria we found that some mononuclear cells (Fig. 2A) present ultrastructural features of stem cells (Fig. 3). These putative CSC have been found in the loose connective tissue surrounding the epicardial coronary artery (Fig. 1). CSC are small, round-oval cells (6–10 μm), with large, irregular nuclei, scattered chromatin and large nucleolus, few mitochondria, few long endoplasmic reticulum cisternae and a large amount of free ribosomes (Fig. 3A and B).
Fig 3.
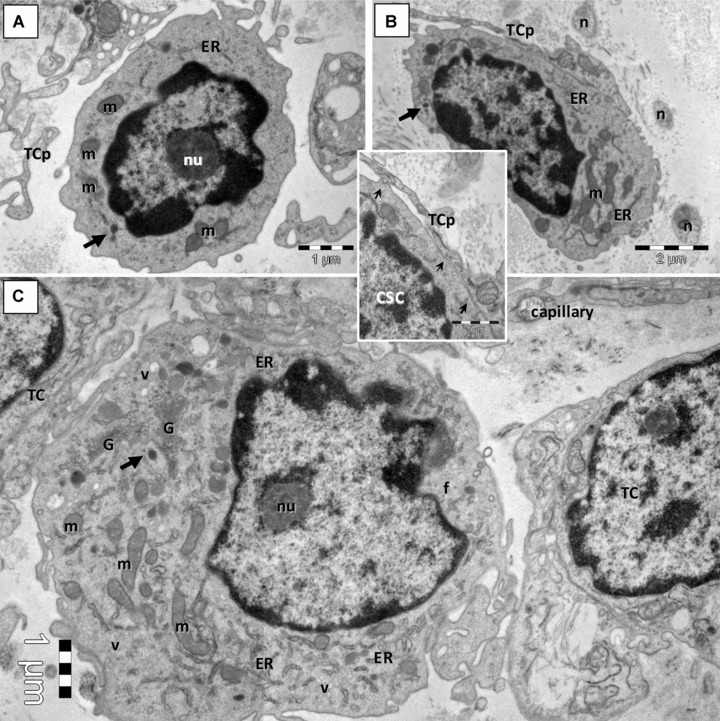
Electron microscopy images show putative cardiac stem cells (CSC) in mouse cardiac stem cell niche. (A, B) CSC are small, round cells, with discrete irregular shaped nucleus, large nucleolus (nu), few endoplasmic reticulum cisternae (ER) and mitochondria (m), dense granules (arrows) and numerous ribosomes. Telocyte processes (TCp) are in close proximity or applied on the plasma membrane of CSC (small arrows in Inset). n – nerve fibres. (C) Committed cell with more abundant ER and numerous long mitochondria (m), well-developed Golgi apparatus (G), clusters of vesicles (v) but few filaments (f) and no basal lamina.
Another type of mononuclear cell in CSCN is a cell which is slightly larger (10–15 μm) than CSC. These cells have large and indented nuclei with less scattered chromatin and prominent nucleoli, more numerous endoplasmic reticulum cisternae, few mitochondria, clusters of small and clear vacuoles generating a multilocular appearance, few dense granules and lysosomes and well-developed Golgi apparatus (Fig. 3C). These cells could be considered an early committed cell, an intermediate stage between CSC and CMP, because some of their ultrastructural features (e.g. clusters of cytoplasmic vesicles, well-developed Golgi apparatus) are still visible in the early differentiated CMP (Fig. 4).
Fig 4.
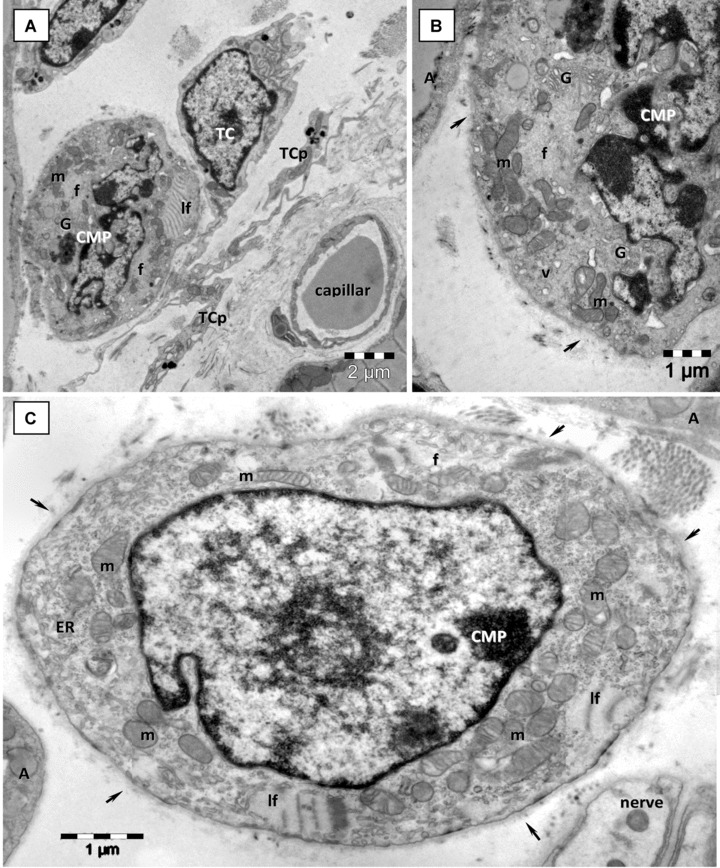
Electron microscopy images from epicardial stem cell niche show more differentiated CMP with characteristic leptofibrils (lf). (A) Telocyte (TC) chaperone a low differentiated CMP with distinctive leptofibrils (lf), unorganized myofibrils (f), Golgi apparatus and clusters of mitochondria (m). (B) Higher magnification of CMP from A on a successive ultrathin section demonstrates the presence of well-developed Golgi apparatus (G), cytoplasmic vesicles (v), mitochondria (m), myofibrils (f) and incomplete basal lamina (arrows). (C) Low differentiated CMP with leptofibrils (lf), mitochondria (m), numerous free ribosomes, myofilaments (f) and a continuous basal lamina (arrows). TCp – telocyte processes; A – adipocytes.
Unlike CSC, the CMP [12] are recognizable without difficulty (Figs 2 and 4). These cells display typical ultrastructural features of immature cardiomyocytes including: high nucleo-cytoplasmic ratio, unorganized bundles of filaments, lipid droplets, intracytoplasmatic dense bodies (similar to primordial Z-lines), intracytoplasmatic desmosome-like structures (primordial intercalated discs) and cortical leptofibrils. Moreover, these cells have large mitochondria, numerous caveolae and a continuous basal lamina. A central element of the niche is represented by telocytes, stromal supporting cells. EM showed that telocytes (Figs 2B, 3 and 4A) are real candidates for this role, offering physical and informational support for CMP [12].
The mononuclear cells presented in this paper as putative stem cells have been found to undergo mitosis (Fig. 5A and B) and apoptosis (Fig. 5C and D) in the CSCN. Because we have found mitosis and apoptosis in cells with ‘stemness’ features with EM, sometimes in the same section, we appreciate that this behaviour is quite common.
Fig 5.
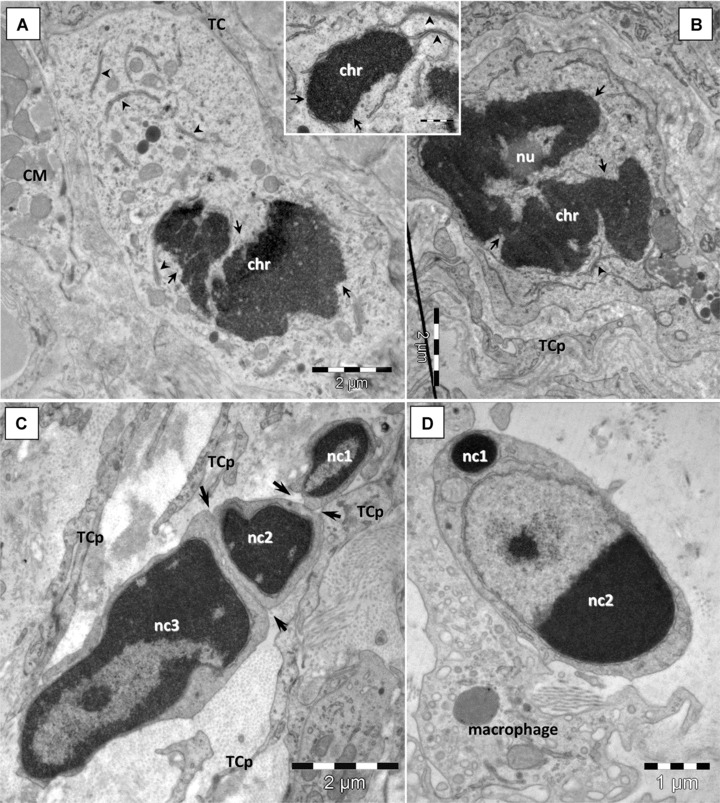
Cardiac stem cells in epicardial stem cell niche maintain their dynamic equilibrium through mitosis (A, B) and apoptosis (C, D). (A, B) CSC in prophase of mitosis: condensed chromatin (chr), nuclear envelope breaks (small arrows). Detached nuclear envelope fragments make parallel pairs with ER cisternae in the cytoplasm (arrowhead). Nucleolus (nu) is still visible in (B). (C, D) Apoptosis of stem cells: hypercondensation of chromatin and nuclear fragmentation (nc1–3). Plasma membrane scission (arrows) generates apoptotic bodies containing nuclear fragments. Note the chromatin clumping in a nuclear fragment (nc2 in D) and preserved nuclear envelope. CM – cardiomyocyte; TCp – telocyte processes.
The integration of newly formed cardiomyocytes into the cardiac muscle is of particular interest. In Fig. 6, we illustrate a possible integration mechanism: committed cells connect with adult cardiomyocytes in the presence of telocytes (Fig. 6A), gradually differentiate into cardiomyocytes and develop their particular junctional complexes at the same time (Fig. 6B and C).
Fig 6.
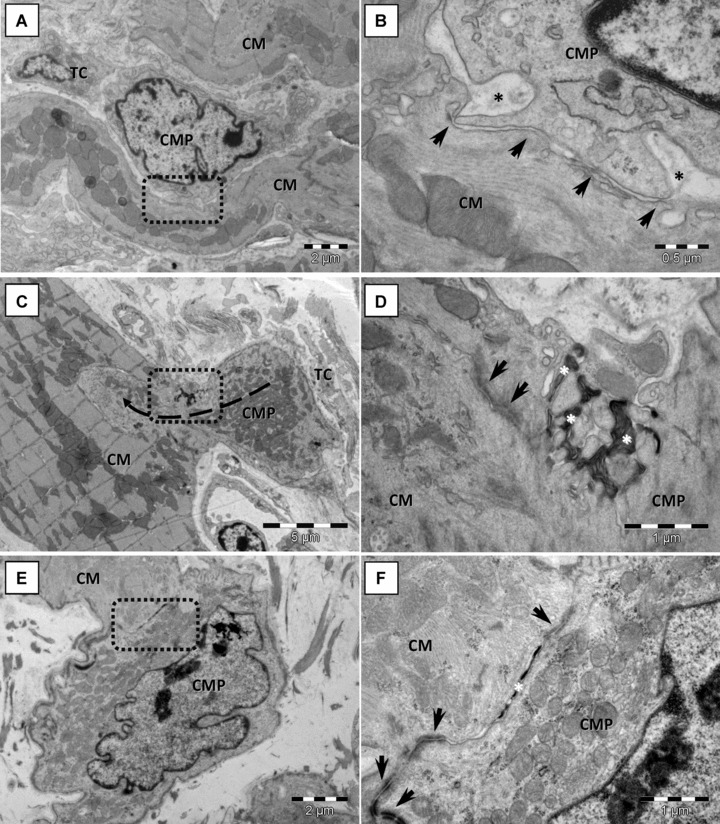
Cardiomyocyte progenitors (CMP) merge with adult mouse cardiomyocytes (CM). Rectangular marked areas in A, C, E are enlarged in B, D and F, respectively. (A, B) Direct cellular contact (arrows) between a committed cell and a cardiomyocyte shows none distinctive junctional structure. Basal lamina of cardiomyocytes encloses the CMP cellular projection (asterisks in B). (C, D) Finger-like cellular projections (dashed arrow) of CMP interacts with neighbouring cardiomyocytes to form adhesive contacts. Asymmetric reinforced contacts (immature desmosomes – arrows) and electron-dense lamellar material (asterisks) in between adjoining cells. (E, F) Completely differentiated desmosomes (arrows) and reduced electron-dense material (asterisk) in gap between contacting membranes of CMP and cardiomyocytes.
Discussion
This EM study supports the idea of a unique type of cardiac stem cell in different stages of differentiation and maturation [11] and demonstrates the ability of putative CSC to continuously self-renew and produce differentiated cardiomyocyte progenitors. The mitotic and apoptotic CSC (Fig. 5), the presence of CMP in different stages of development (Figs 4 and 6) and the integration of newly formed cardiomyocytes suggest that there is a dynamic pool of stem cells in a continuous process of commitment and differentiation. In our opinion, stem cells, progenitors and telocytes sustain a continuous cardiac renewal process in the adult mammalian heart [12].
The next step would be to identify surface molecules (markers) which accurately differentiate stem cells from progenitors. Another important aspect to be clarified is telocyte involvement (Figs 1–6) [12–24] in stem cell homeostasis and cardiac renewal.
References
- 1.Urbanek K, Cesselli D, Rota M, et al. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci USA. 2006;103:9226–31. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torella D, Ellison GM, Karakikes I, et al. Resident cardiac stem cells. Cell Mol Life Sci. 2007;64:661–73. doi: 10.1007/s00018-007-6519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barile L, Messina E, Giacomello A, et al. Endogenous cardiac stem cells. Prog Cardiovasc Dis. 2007;50:31–48. doi: 10.1016/j.pcad.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Zhou B, Pu WT. More than a cover: epicardium as a novel source of cardiac progenitor cells. Regen Med. 2008;3:633–5. doi: 10.2217/17460751.3.5.633. [DOI] [PubMed] [Google Scholar]
- 6.Wu SM, Chien KR, Mummery C. Origins and fates of cardiovascular progenitor cells. Cell. 2008;132:537–43. doi: 10.1016/j.cell.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roccio M, Goumans MJ, Sluijter JP, et al. Stem cell sources for cardiac regeneration. Panminerva Med. 2008;50:19–30. [PubMed] [Google Scholar]
- 8.Winter EM, Van Oorschot AA, Hogers B, et al. A new direction for cardiac regeneration therapy: application of synergistically acting epicardium-derived cells and cardiomyocyte progenitor cells. Circ Heart Fail. 2009;2:643–53. doi: 10.1161/CIRCHEARTFAILURE.108.843722. [DOI] [PubMed] [Google Scholar]
- 9.Stamm C, Choi YH, Nasseri B, et al. A heart full of stem cells: the spectrum of myocardial progenitor cells in the postnatal heart. Ther Adv Cardiovasc Dis. 2009;3:215–29. doi: 10.1177/1753944709336190. [DOI] [PubMed] [Google Scholar]
- 10.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellison GM, Galuppo V, Vicinanza C, et al. Cardiac stem and progenitor cell identification: different markers for the same cell? Front Biosci. 2010;2:641–52. doi: 10.2741/s91. [DOI] [PubMed] [Google Scholar]
- 12.Popescu LM, Gherghiceanu M, Manole CG, et al. Cardiac renewing: interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches. J Cell Mol Med. 2009;13:866–86. doi: 10.1111/j.1582-4934.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popescu LM, Faussone-Pellegrini MS. Telocytes – A case of serendipity: the winding way from interstitial cells of Cajal (ICC), via interstitial Cajal-like cells (ICLC) to telocytes. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01059.x. Doi: 10.1111/j.1582-4934.2010.001059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinescu ME, Popescu LM. Interstitial Cajal-like cells (ICLC) in human atrial myocardium. J Cell Mol Med. 2005;9:972–5. doi: 10.1111/j.1582-4934.2005.tb00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popescu LM, Gherghiceanu M, Cretoiu D, et al. The connective connection: interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells. Electron microscope study in situ. J Cell Mol Med. 2005;9:714–30. doi: 10.1111/j.1582-4934.2005.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinescu ME, Gherghiceanu M, Mandache E, et al. Interstitial Cajal-like cells (ICLC) in atrial myocardium: ultrastructural and immunohistochemical characterization. J Cell Mol Med. 2006;10:243–57. doi: 10.1111/j.1582-4934.2006.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popescu LM, Gherghiceanu M, Hinescu ME, et al. Insights into the interstitium of ventricular myocardium: interstitial Cajal-like cells (ICLC) J Cell Mol Med. 2006;10:429–58. doi: 10.1111/j.1582-4934.2006.tb00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandache E, Popescu LM, Gherghiceanu M. Myocardial interstitial Cajal-like cells (ICLC) and their nanostructural relationships with intercalated discs: shed vesicles as intermediates. J Cell Mol Med. 2007;11:1175–84. doi: 10.1111/j.1582-4934.2007.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gherghiceanu M, Hinescu ME, Andrei F, et al. Interstitial Cajal-like cells (ICLC) in myocardial sleeves of human pulmonary veins. J Cell Mol Med. 2008;12:1777–81. doi: 10.1111/j.1582-4934.2008.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morel E, Meyronet D, Thivolet-Bejuy F, et al. Identification and distribution of interstitial Cajal cells in human pulmonary veins. Heart Rhythm. 2008;5:1063–7. doi: 10.1016/j.hrthm.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 21.Suciu L, Popescu LM, Regalia T, et al. Epicardium: interstitial Cajal-like cells (ICLC) highlighted by immunofluorescence. J Cell Mol Med. 2009;13:771–7. doi: 10.1111/j.1582-4934.2009.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popescu LM, Gherghiceanu M, Manole CG, et al. Cardiac renewing: interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches. J Cell Mol Med. 2009;13:866–86. doi: 10.1111/j.1582-4934.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gherghiceanu M, Popescu LM. Human epicardium: ultrastructural ancestry of mesothelium and mesenchymal cells. J Cell Mol Med. 2009;13:2949–51. doi: 10.1111/j.1582-4934.2009.00869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostin S, Popescu LM. A distinct type of cell in myocardium: interstitial Cajal-like cells (ICLCs) J Cell Mol Med. 2009;13:295–308. doi: 10.1111/j.1582-4934.2008.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


