Abstract
Human adipose-derived stem cells (ASCs) may differentiate into cardiomyocytes and this provides a source of donor cells for tissue engineering. In this study, we evaluated cardiomyogenic differentiation protocols using a DNA demethylating agent 5-azacytidine (5-aza), a modified cardiomyogenic medium (MCM), a histone deacetylase inhibitor trichostatin A (TSA) and co-culture with neonatal rat cardiomyocytes. 5-aza treatment reduced both cardiac actin and TropT mRNA expression. Incubation in MCM only slightly increased gene expression (1.5- to 1.9-fold) and the number of cells co-expressing nkx2.5/sarcomeric α-actin (27.2%versus 0.2% in control). TSA treatment increased cardiac actin mRNA expression 11-fold after 1 week, which could be sustained for 2 weeks by culturing cells in cardiomyocyte culture medium. TSA-treated cells also stained positively for cardiac myosin heavy chain, α-actin, TropI and connexin43; however, none of these treatments produced beating cells. ASCs in non-contact co-culture showed no cardiac differentiation; however, ASCs co-cultured in direct contact co-culture exhibited a time-dependent increase in cardiac actin mRNA expression (up to 33-fold) between days 3 and 14. Immunocytochemistry revealed co-expression of GATA4 and Nkx2.5, α-actin, TropI and cardiac myosin heavy chain in CM-DiI labelled ASCs. Most importantly, many of these cells showed spontaneous contractions accompanied by calcium transients in culture. Human ASC (hASC) showed synchronous Ca2+ transient and contraction synchronous with surrounding rat cardiomyocytes (106 beats/min.). Gap junctions also formed between them as observed by dye transfer. In conclusion, cell-to-cell interaction was identified as a key inducer for cardiomyogenic differentiation of hASCs. This method was optimized by co-culture with contracting cardiomyocytes and provides a potential cardiac differentiation system to progress applications for cardiac cell therapy or tissue engineering.
Keywords: adipose-derived stem cell, cardiomyocyte, cardiomyogenic differentiation, epigenetic modification, co-culture
Introduction
Despite all best efforts in scientific research and medical treatment, cardiovascular disease remains the number one cause of death globally and is projected to remain so. An estimated 17.5 million people died from cardiovascular disease in 2005, representing 30% of all worldwide deaths [1]. Recent advances in cardiac tissue engineering suggest exciting directions that are likely to lead to more effective therapies for cardiovascular disease [2, 3].
Tissue engineering is a rapidly growing technology that aims to create, repair or replace tissues and organs by using combinations of cells, biomaterials and biomolecules. Tissue engineering strategies promise to revolutionize current therapies for irreversible cardiovascular disease, and significantly improve the quality of life for millions of patients [4]. Cardiac tissue engineering approaches rely mainly on the use of donor cardiomyocytes but clinical application of these methods will be severely limited by cell supply. As a consequence, other cells that have the potential to differentiate into cardiomyocytes are being investigated [5].
Human adipose-derived stem cells (ASCs) may be a good candidate for cardiac tissue engineering as they can be harvested from patients by a simple, minimally invasive method and in large numbers (200,000 cells per ml of lipoaspirate tissue) [6].
Furthermore adipose tissue can be harvested in relatively large quantities (from 100 ml to several litres) using a low-morbidity liposuction procedure that was performed in 341,144 elective cosmetic procedures in the United States in 2004 [7]. Moreover, cultured populations of ASCs can be expanded rapidly (more than 10-fold within 1 week), and over long periods in culture [6].
A number of investigators have published data suggesting that ASCs have the ability to differentiate into cells that exhibit features of the cardiomyocyte in vivo. To understand the process of ASCs differentiating into cardiomyocytes, various in vitro experiments have been performed to determine which cell population has the potential to become cardiomyocytes, and to elucidate which factors and techniques influence this differentiation [8–10].
5-azacytidine (5-aza), a DNA methyltransferase inhibitor, was the first agent used for cardiomyogenic differentiation of bone marrow stem cells (BMSCs) as there have been many studies using this approach [11]. These studies have reported successful transformation of rabbit or mouse, but not human ASCs (hASCs) into contractile cardiomyocyte-like cells [8, 12, 13]. Repeated exposure to 5-aza inhibited human cell growth, and caused apoptosis, limiting clinical application [13]. Because of these limitations with 5-aza, researchers have sought other methods for cardiomyogenic differentiation, modified culture media (including growth factors), cardiomyocyte extracts and histone deacetylase inhibitor such as trichostatin A (TSA) used for that purpose in various experiments [9, 10, 14–16], as well as co-culture with cardiomyocytes [17]. BMSCs have shown clear evidence that direct cell–cell interaction with cardiomyocytes can induce stem cells to differentiate into a cardiac lineage [17–21]. Recently, ASCs were shown to differentiate towards a cardiac lineage expressing cardiac markers in co-culture with cardiomyocytes [22].
Although numerous methods including 5-aza, modified culture media, TSA and co-culture systems have been examined for their capacity to induce cardiomyogenic differentiation, the results are inconsistent and ineffective for cardiac tissue engineering. The aim of this study was to optimize the methods and culture timing for cardiomyogenic differentiation of ASCs. We compared the capacities of three different cardiomyogenic differentiation methods using ASC in vitro and developed a co-culture system.
Materials and methods
Primary culture of human adipose-derived stem cells
ASCs were isolated from freshly excised human subcutaneous adipose tissue (donor age between 43 to 52 years) according to the method described by Zuk et al. [23] with approval of the St Vincent’s Health Human Research Ethics Committee. In brief, fat (∼300 ml) was minced and washed extensively with equal volumes of phosphate-buffered saline (PBS), and then incubated at 37°C for 45 to 60 min. in 0.1% type I collagenase (Worthington Biochemical, Lakewood, NJ, USA). Enzyme activity was neutralized with Dulbecco’s modified Eagle’s medium-low glucose (DMEM-lg, Gibco, Grand Island, NY, USA), containing 10% FCS and cells were centrifuged at 1200 rpm for 10 min. to remove adipocytes. The pellet was resuspended in 0.16 M NH4Cl and incubated at room temperature for 5 min. to lyse red blood cells. The cells were collected by centrifugation at 1200 rpm for 5 min., filtered through a 100 μm nylon mesh to remove fissile debris, and incubated on culture plate overnight at 37°C in a humidified atmosphere containing 5% CO2 in control medium (DMEM-lg, 10% FCS, 1% antibiotic/antimyocotic solution). Following incubation, the plates were washed extensively with PBS to remove residual non-adherent cells.
Primary culture of neonatal ventricular myocytes
Neonatal rat cardiomyocytes were isolated from the ventricles according to the manufacture’s procedure (Neonatal cardiomyocyte isolation system, Worthington Biochemical). The investigation was performed under the Australian code of practice for the care and use of animals for scientific purposes (National Health and Medical Research Council of Australia, 2004). In brief, ventricles from Sprague-Dawley rats (1 to 3 days old; Experimental Medical and Surgical Unit, St. Vincent’s Hospital, Melbourne, Australia) were minced into 1 to 3 mm3 fragments and incubated in 50 μg/ml trypsin solution overnight. The next day, the sample was transferred into trypsin inhibitor and warmed in 30–37°C in a water bath, and then digested with collagenase at 37°C for 45 to 60 min. The collagenase-digested sample was centrifuged at 1200 rpm for 5 min. and filtered through a 100 um mesh, and allowed to remain undistributed for 20 min. at room temperature. Cardiomyocytes were suspended with cardiomyocyte culture medium (CCM; DMEM/F12 with HEPES, 5% horse serum, 1% antibiotic/antimycotic solution supplemented by 3 mM pyruvic acid, 2 g/l bovine serum albumin, 100 μg/ml ampicillin, 4 μg/ml transferring, 0.7 ng/ml sodium selenite, 5 μg/ml linoleic acid, 100 μM ascorbic acid) and plated on 10 μg/ml fibronectin coated flask, and then cultured at 37°C in a humidified atmosphere containing 5% CO2[24].
5-azacytidine treatment
ASCs (between passages 4 and 7) were seeded in 6-well plates at 50,000 cells/well. Twenty-four hours after seeding, the cells were washed with PBS twice. The cells were incubated in media containing 5-aza (Sigma) at 10 μmol/l for 24 hrs, then washed three times with PBS and cultured in control medium for 3 weeks. The cells were observed daily, and the medium changed two to three times per week until the experiment was terminated [8].
Modified cardiomyogenic medium
For cardiomyogenic differentiation we modified culture medium from Shim et al. [19] and Planat-Bénard et al. [10]. ASCs (between passages 4 and 7) were seeded in 6-well plates at 50,000 cells/well. Twenty-four hours after seeding, the cells were washed with PBS twice. Then, cells were cultured in modified cardiomyogenic medium (MCM: DMEM-lg, 10% FCS, 1% antibiotics, 1.0 mg/ml insulin, 0.55 mg/ml transferrin, 0.5 μg/ml sodium selenite, 50 mg/ml bovine serum albumin, 0.47 μg/ml linoleic acid, 10−4 M ascorbate, 10−9 M dexamethasone) for 3 weeks.
Trichostatin A treatment
ASCs (between passages 4 and 7) were seeded in 6-well plates at 50,000 cells/well. Twenty-four hours after seeding, the cells were washed with PBS twice. In first group, ASCs were cultured for 1, 2 and 3 weeks after 24 hr exposure of TSA at the beginning or with continuous exposure. In second group, the cells were exposed to 100 ng/ml TSA (Sigma) for 1 week, then washed three times with PBS and cultured in control medium or CCM for 2 weeks.
Co-culture system with neonatal rat cardiomyocytes
Using inserts – ASCs and neonatal rat cardiomyocytes were co-cultured at the ratio of 1:10 in different wells of 24-well plate separated by Falcon Cell Culture Inserts (BD Biosciences, San Jose, CA, USA). Semi-permeable membranes of these inserts (pore size 3.0 μm) allows the diffusion of secreted factors but prevents cell migration or transport between the two cell populations. Neonatal cardiomyocytes were plated on the upper insert and ASCs in the lower bottom or vice versa. ASCs co-cultured with ASCs served as a control group [20].
Direct co-culture – ASCs and neonatal rat cardiomyocytes were co-cultured at the ratio of 1:5 or 1:10 in 24-well plates allowing direct cell–cell interactions between the two cell populations. Neonatal cardiomyocytes were seeded 1 day before ASCs (ASCs on the top and cardiomyocytes at the bottom or vice versa) and co-cultured in CCM. ASCs cultured in CCM served as control group [25].
Calcium imaging
Free intracellular Ca2+ imaging recordings were obtained in cells loaded with the fluorescent Ca2+ indicator, fluo-4 AM (2 μg/ml, Invitrogen, Carlsbad, CA, USA). The cells were incubated with 1 ml of loading dye solution for 10 min. and then in DMEM/F12 for 10 min. at 37°C. Video images were made by a digital camera (Canon G10, Tokyo, Japan) through a fluorescent microscope with a mounted black/white camera (Olympus IX81 inverted microscope with F-view).
Real-time reverse transcriptase polymerase chain reaction
Real-time reverse transcriptase polymerase chain reaction (RT-PCR) was conducted with Taqman technology to assess gene expression of candidate genes, human specific cardiac α-actin (Applied Biosystems, Foster City, CA, USA, assay-on-demand primer/probe HS01109515_m1) and cardiac troponinT (TropT, HS00165960_m1), relative to GAPDH endogenous control. Reactions were carried out in triplicate for each sample, and included at least one reverse transcriptase negative control and no template control per run. The reaction was carried out in 96-well plates with the ABI Prism 7300 Real-Time PCR System (Applied Biosystems). The machine ran at 50°C for 2 min. for activation of AmpErase UNG, 95°C for 10 min. for activation of AmpliTaq Gold polymerase, then 50 cycles of 95°C for 15 sec. followed by 60°C for 1 min. for primer/probe dissociation and annealing/elongation, respectively. Relative fold change for controls (non-treated ASCs) were normalized to 1 and all results were expressed as relative fold change in candidate gene expression for each treatment group (5-aza/MCM/TSA/co-culture) as compared to the experimental control (non-treated ASCs). To confirm human specificity of the primers we determined that rat cardiomyocyte cultures did not produce a measurable number of amplicons for actin, troponin or GAPDH. One set of co-cultures were sorted (into hASCs and rat cardiomyocytes) by fluorescence-activated cell sorting (FACS) using DiI fluorescence intensity to separate labelled stem cells from unlabelled cardiomyocyte populations. The human cells were evaluated for actin mRNA as above.
Immunocytochemistry
Cells were cultured in 8-well chamber slide for immunocytochemistry. Cells were washed in PBS and fixed with cold methanol (100%) for 10 min., and then blocked by a serum-free blocking solution (Dako, Glostrup, Denmark). Primary antibodies were diluted in commercial diluents (Dako) and incubated with the cells for 60 min. at room temperature or overnight at 4°C. After PBS washings, biotinylated secondary antibodies (Dako) were added to the cells for 30 min. at room temperature. Streptavidin-FITC (Dako) was conjugated with secondary antibodies for 30 min. followed by PBS washings. For double staining, the cells (which are already stained by FITC) were treated by other primary antibodies and processed with secondary antibodies, and then conjugated with avidin-Texas Red (Dako). To characterize cardiomyogenic differentiation, we used cardiac transcriptional factors: GATA4, Nkx2.5 (Santa Cruz Biotech, Santa Cruz, CA, USA), cardiac filamentous proteins: sarcomeric α-actin (α-actin, Dako), troponinI (TropI, Santa Cruz Biotech), cardiac troponinI (cTropI), connexin 43 (Cx43), cardiac myosin heavy chain (cMHC, Abcam), myoD (Santa Cruz Biotech) and smooth muscle actin (SMA, Dako) as negative control. The cells were observed and taken images using fluorescence microscope (Olympus BX-61 microscope)
Statistical analysis
Data are expressed as mean ± S.E.M. (n= 3). Statistical analysis was performed with one-way ANOVA on Graphpad Prism software. Values of P < 0.05 were considered to indicate statistical significance.
Results
Primary ASC culture
ASCs were able to adhere to tissue culture flasks whereas non-adherent cells, such as red blood cells, were removed by media change. Processing of 100 ml of fat tissue routinely yielded 2 to 5 × 106 ASCs. Cells proliferated rapidly and were passaged by trypsin-ethylenediaminetetraacetic acid twice a week (approximate doubling time, 24 hrs). The initial adherent cells grew into spindle-, triangular- or stellate-shaped cells. After the second passage, ASCs appeared to adopt a more uniform fibroblast-like shape (Fig. S1A).
Undifferentiated ASC were characterized by their expression of CD markers using flow cytometry. ASC expressed CD73, CD90 and CD 105, but not haemopoietic lineage markers CD34 and CD45. Representative histograms are shown in Fig. S2. This CD marker profile is consistent with previous reports for ASC [23].
The cells were characterized by colony forming assay and stro-1 staining. After 2 weeks of culture in 20% FCS, about 8% of ASCs formed colony. ASCs cultured in normal condition (about 70% confluence in 10% FCS) showed stro-1 positive staining (Fig. S3).
Cardiomyogenic treatment of ASC with 5-azacytidine or modified culture medium
For cardiomyogenic differentiation, ASCs were treated with 5-aza for 24 hrs or continuously with MCM. Both 5-aza and MCM treated cells were cultured for 3 weeks. Non-treated ASCs acted as a control (at the same passage). Cells treated by 5-aza for 24 hrs were not really changed in their shape (Fig. S1B). Few cells died after 5-aza treatment because of their cytotoxicity (data not shown). But cells proliferated afterward. In contrary, the shapes of cells cultured in MCM were changed to polygonal or rod shape (Fig. S1C).
Cardiac actin and TropT mRNA were detected in ASCs at 1, 2 and 3 weeks by real-time RT-PCR (Fig. 1). 5-aza caused a time-dependent decrease in cardiac actin mRNA from 0.2 to 0.01 at 1 and 2 weeks, respectively. In contrast, cardiac actin mRNA levels of MCM treated cells were significantly increased by 1.5-fold at 1 week and by 1.9-fold at 2 weeks, before returning to baseline at 3 weeks (Fig. 1A). TropT mRNA also increased following MCM treatment, 1.8-fold at 2 weeks (statistically not significant). TropT mRNA expression was not detected in 5-aza treated cells at 2 or 3 weeks (Fig. 1B).
Fig 1.
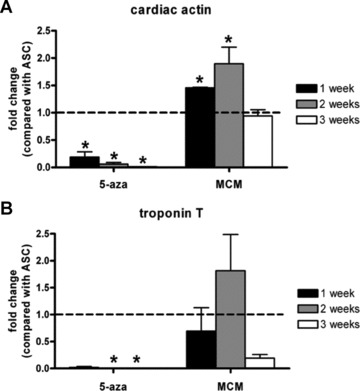
Real-time RT-PCR analysis of cardiac actin and TropT mRNA in hASCs 1, 2, 3 weeks after 5-aza treatment or culture in MCM. (A) Cardiac actin, (B) TropT. GAPDH was used as internal control. Bar graphs indicate fold change of mRNA compared with untreated ASCs (dotted line). Results are shown as mean ± S.E.M. (n= 3). *P < 0.05 versus untreated ASCs as control.
5-aza and MCM treated ASCs were double stained (Fig. 2) with Nkx2.5 (cardiac transcription factor; nuclei) and α-actin (cardiac contractile protein; cytoplasm) (Fig. 2A–C), or with Nkx2.5 and TropI (troponin-inhibitory subunit; cytoplasm) (Fig. 2D–F). In the control group, Nkx2.5 was detected in some ASCs but double staining for Nkx2.5 and α-actin or TropI was rare (Fig. 2A, D). The number of cells expressing both Nkx2.5 and α-actin or TropI was significantly increased following MCM treatment (27.2%versus 0.2% in control) whereas 5-aza treatment only slightly increased the number of cells expressing two cardiac markers (9.2%) (Fig. 2B, C, E and F). We did not observe contractile cells in either treatment despite extensive searching.
Fig 2.

Immunocytochemistry for ASCs (A, D), 5-aza treated ASCs (B, E), ASCs cultured in MCM (C, F) after 3 weeks. Images show expression of Nkx2.5 (green) and α-actin (red) (A–C); Nkx2.5 (green) and TropI (red) (D–F). Scale bar = 100 μm.
Differences in proliferation (Ki67 expression) were also examined between treated groups (data not shown). ASCs cultured in control or MCM maintained their proliferation capacities (80.4% and 63.4% Ki67 positive cells, respectively); however, growth of 5-aza treated ASCs was significantly reduced at 3 weeks (44.2% proliferating cells).
Cardiomyogenic treatment of ASC with trichostatin A
ASCs were treated with TSA for 24 hrs then cultured in either control medium or treated continuously with TSA for 1, 2, or 3 weeks. Twenty-four hours treatment showed no significant effects on cardiac actin mRNA expression; however, continuous exposure for 1 week caused an 11-fold increase of cardiac actin mRNA compared to control. The mRNA level decreased to 2.9- and 1.3-fold after 2 or 3 weeks of continuous TSA treatment (Fig. 3A).
Fig 3.
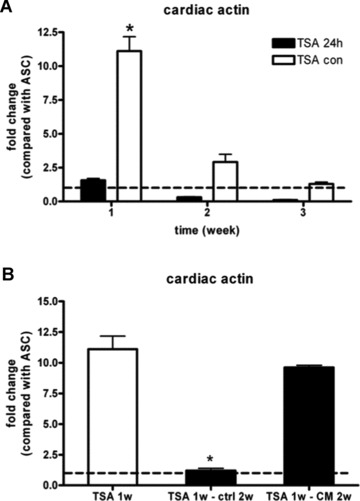
Real-time RT-PCR analysis of cardiac actin mRNA in hASCs after trichostatin A treatment for 24 hrs, and cultured in control medium or continuously for 1, 2 and 3 weeks (A). ASCs also treated by TSA continuously for 1 week were cultured in control medium or CCM for 2 weeks (B). GAPDH was used as internal control. Bar graphs indicate fold change of mRNA compared with ASCs (dotted line). Results are shown as mean ± S.E.M. (n= 3). *P < 0.05 versus other groups
To determine if the increased cardiac gene expression could be maintained in culture conditions more suited to cardiomyocytes, ASCs treated by TSA continuously for 1 week were cultured in control medium or CCM for 2 weeks. Real time RT-PCR revealed a significant difference between the two groups in maintenance of cardiac actin mRNA expression. Cardiac actin mRNA level was 11.1-fold increase after 1 week TSA treatment, however, dropped dramatically to 1.2-fold after 2 weeks culture in control medium. In contrast, the cells cultured in CCM for 2 weeks tended to maintain cardiac actin mRNA level at higher levels (9.6-fold, Fig. 3B).
These results were confirmed by immunostaining using cardiac protein expression. ASCs cultured in CCM for 2 weeks followed by 1-week continuous TSA treatment were stained positively with cMHC, α-actin, cTropI and Cx43 (Fig. 4).
Fig 4.
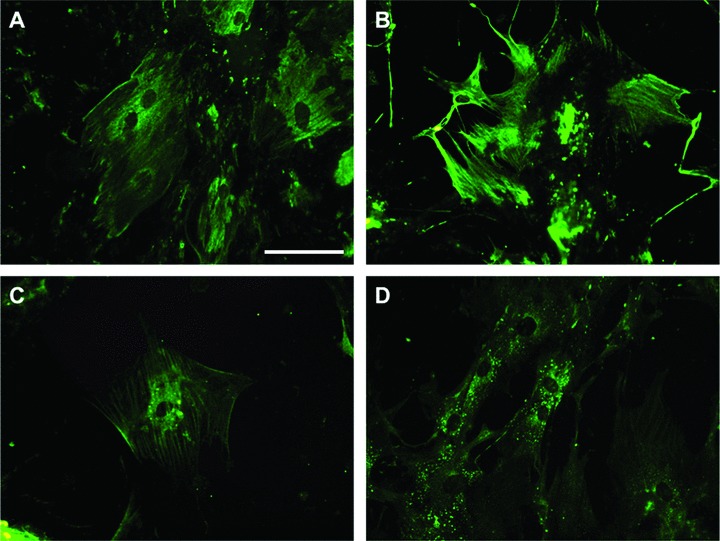
Immunocytochemistry for 1 week TSA treated ASCs after culturing in CCM for 2 weeks. Cells were positively stained by cMHC (A), α-actin (B), cTropI (C) and Cx43 (D). Primary antibodies were conjugated FITC. Scale bar = 100 μm.
Primary neonatal rat cardiomyocyte culture
Neonatal rat cardiomyocytes were harvested and seeded at 5 × 105 cells per well in 6-well plate. The average cell yield was 3.6 × 106 cells per neonatal rat heart. Cardiomyocytes began to beat 24 hrs after seeding and contractility was maintained up to 26 days. Their contractile capacity relied on cell density, plated at less than 5 × 104 cardiomyocytes per well in a 6-well plate, the distance between cells was too great to allow cell-to-cell contact and spontaneous contractility was rapidly lost. At concentrations higher than 1 × 106 cells per well, the cells peeled away from the flask surface within 10 days.
Co-culture system with insert
ASCs were subjected to indirect co-culture with neonatal rat cardiomyocytes suspended in a culture well insert for up to 3 weeks (Fig. S4). Human cardiac actin mRNA detected up to 21 days by real-time RT-PCR was not significantly affected (Fig. S4A) by co-culture. TropT mRNA expression showed an initial decline at 1 week (P < 0.05) then time-dependent increases to control levels at 2 and 3 weeks (Fig. S4B). Immunocytochemical staining of these cells showed no significant difference compared to control (data not shown).
Co-culture with direct cell-to-cell contact
ASCs were cultured in direct contact with neonatal rat cardiomyocytes for 2 weeks. ASC were plated either on top or beneath cardiomyocytes (ASCs-T and ASCs-B, respectively) in either 1:5 or 1:10 ratios (ASCs to cardiomyocytes; 1 × 105 ASCs: 5 × 105 cardiomyocytes or 5 × 104 ASCs: 5 × 105 cardiomyocytes in 6-well plates) (Fig. S5). Co-culture at a 1:10 ratio showed a 2- to 3-fold increase in cardiac actin mRNA compared to a 1:5 ratio. ASCs-B showed increased cardiac actin mRNA expression compared to ASCs-T at a 1:10 ratio (32-fold versus 24-fold) (Fig. S5). Remaining experiments were conducted with the optimal conditions of ASC below cardiomyocytes (ASCs-B) at 1:10 ratio.
ASCs were affected by rat cardiomyocytes contraction from day 1 of co-culture. ASCs were pulled by underneath rat cardiomyocytes in first week; however, they started to act like pacemaker cells and beat spontaneously after 1 week (Movie S1A). ASCs had direct contact with adjacent rat cardiomyocytes and their beating rhythm was synchronized with rat cardiomyocytes’. To confirm origin of beating cells, cells were observed under fluorescence microscope with Texas Red filter (CM-DiI label). CM-DiI labelled hASCs showed spontaneous contractile capacity (Movie S1B).
Human specific cardiac actin mRNA expression was then investigated at day 3, 5, 7, 14, 21 days (Fig. 5). Between days 3 and 7, cardiac actin mRNA expression increased in a time-dependent fashion (3.6-fold to 33.2-fold compared to control). There was no further increase at day 14 (33.9-fold), but at day 21 cardiac actin mRNA expression was significantly decreased to 2.3-fold. The primer species specificity was confirmed by lack of cross-species amplification (human primer tested on rat RNA) and maintenance of signal level after FACS sorting human cells out of co-cultures at day 14 of culture.
Fig 5.

Cardiac actin mRNA level of ASCs in co-culture at day 3, 5, 7, 14, 21 by real-time RT-PCR. GAPDH was used as internal control. Stem cells sorted from co-cultures by FACS at day 14 retained expression levels, confirming human probe specificity. Bar graphs indicated fold change of mRNA compared with ASCs (dotted line). Results are shown as mean ± S.E.M. (n= 3). *P < 0.05 versus untreated ASCs as control.
Sorted ASCs were stained by GATA4, Nkx2.5, α-actin, TropI, cMHC and MyoD (as negative control) (Fig. 6). Immunocytochemistry revealed expression of cardiac transcription factors, GATA4 and Nkx2.5, and contractile proteins, α-actin, TropI and cMHC in CM-DiI labelled ASCs (Fig. 6A–E). MyoD was not expressed in ASCs (Fig. 6F).
Fig 6.

Immunocytochemistry of ASCs after sorting from co-culture with cardiomyocytes by CM-DiI labelling. Cells were stained by GATA4 (A), Nkx2.5 (B), α-actin (C), TropI (D), cMHC (E) and MyoD (F) conjugated by FITC (ASCs were pre-labelled by CM-DiI which shown in red). MyoD was used as negative control. Scale bar = 100 μm.
Calcium imaging
To confirm that ASC-derived cells after differentiation showed functional characteristics of cardiomyocytes, calcium imaging was performed with the fluorescent Ca2+ indicator Fluo-4 AM. CM-DiI labelled hASCs showed Fluo-4 fluorescence intensity oscillations and their contraction was confirmed under bright field (Movie S2). The rhythm of the Ca2+ transients and contractions was coupled with the surrounding rat cardiomyocytes. These paired hASCs were observed from 2 days after co-culture. The synchronicity of Ca2+ transients in CM-DiI labelled ASCs and the surrounding rat cardiomyocytes suggested the existence of cell-to-cell communications, probably gap junctions.
Gap junctions between ASC and cardiomyocyte
To confirm the presence of functional gap junction, ASCs labelled by CM-DiI were co-cultured with rat cardiomyocytes labelled by calcein AM (Fig. 7), at the ratio of 1:10. After 24 hrs of co-culture, cells were observed under fluorescence microscope showed calcein AM and DiI staining were co-localized in same cells (Fig. 7). Most hASCs were stained only by CM-DiI, but some cells were also labelled green which means calcein AM molecules were transferred to these cells via gap junctions from adjacent rat cardiomyocytes.
Fig 7.

Gap junction between ASC and cardiomyocyte. ASCs were labelled by CM-DiI (Red, not able to transfer to adjacent cells through gap junction) and rat cardiomyocytes were labelled by calcein AM (Green, able to transfer to adjacent cells through gap junction). Red cells are hASCs under Texas Red filter (A, D) and Green cells were rat cardiomyocytes or hASC with calcein AM transferred from cardiomyocytes under FITC filter (B, E) and two pictures were merged (C, F). Gap junction between two cell types by direct contact allowed calcein AM transfer from rat cardiomyocyte to hASC. White arrow showed hASCs with calcein AM transferred from beating rat cardiomyocytes in merged images. Scale bar = 100 μm.
Discussion
We have shown here how to induce cardiomyogenic differentiation of hASCs by treatment with epigenetic modifiers (5-aza, TSA), by an inducing culture medium formulation and by a co-culture system using neonatal rat cardiomyocytes.
The ability of 5-aza to induce cardiomyogenic gene and protein expression in BMSCs (also frequently referred to as mesenchymal stem cells) [11, 26] and rabbit ASCs has been reported previously [8], however, we were not able to succeed with this method for hASCs. Others have also failed to reproduce cardiac differentiation from MSC using 5-aza. Only passage 4 (P4) but not P1 and P8 rat BMSCs showed expression of cardiac-specific markers including Nkx2.5 and TropI [27]. Rat BMSCs could not be expanded extensively in vitro nor could they be induced to differentiate into cardiomyocyte-like cells using various concentrations of 5-aza with single or repeated treatment [28]. Moreover, spontaneously and synchronously pulsating cardiomyocyte-like cells were not observed after 8 weeks in culture. In addition, repeated exposure of 5-aza to ASCs significantly inhibited cell growth [13]. Lee et al. also failed to observe the conversion of hASCs into beating cardiomyocyte-like cells (they neither formed myotubes nor produced spontaneously beating cells after 8 weeks) [13]. In our study, expression of cardiac actin and TropT mRNA were actually reduced more than 5-fold from control levels (Fig. 1). Although some ASCs treated by 5-aza expressed Nkx2.5, TropI and α-actin, the number of cells expressing both Nkx2.5 and α-actin or TropI was only slightly increased (the number of cells expressing two cardiac markers 9.2%versus 0.2% in controls, Fig. 2B, E) and we did not find any contracting cells after 5-aza treatment. Furthermore, the proliferation capacity of 5-aza-treated ASCs was decreased after 3 weeks of treatment (44.2%versus 80.4% in control).
The reason for resistance of ASC to induction of differentiation by 5-aza was further investigated by examining the DNA methylation profile of a 2.5-kb region in the promoter of eight cardiac markers in ASCs, as part of a larger methylated DNA immunoprecipitation microarray-based study (A.L. Sørensen, A.H. Reiner and PC; manuscript in preparation) (Fig. S6). The promoters examined included GATA4, NKX2.5, cardiac actin, TropI, TropT, MHC and CX43. In these ASC populations, most cardiac-related genes were not methylated, implying that cardiomyogenic differentiation of ASCs could not be hampered by promoter DNA methylation. Therefore, it is not surprising that these promoters were not directly responsive to demethylation by 5-aza.
As an alternative epigenetic modification, the histone deacetylase inhibitor TSA has been used for cardiomyogenic differentiation of embryonic stem cells [14, 15, 29]. Histone deacetylases regulate gene expression patterns by affecting chromatin structure [14]. In addition, they deacetylate lysine residues and induce compaction of chromatin, reducing the access of transcription factors to nearby promoters and thereby resulting in gene silencing [30]. TSA promotes the differentiation of embryonic stem cells into cardiac myocytes [14] but inhibits the differentiation of adipocytes [31] or oligodendrocytes [32]. In the skeletal muscle cell lineage, TSA either augments or inhibits the differentiation depending on the developmental stage [33]. We have now differentiated ASCs into cardiomyocyte-like cells using TSA, showing high levels of cardiac actin mRNA (11-fold increase) and cardiac proteins (cMHC, α-actin, cTrop I). Clearly this finding was markedly different from 5-aza treatment suggesting that chromatin structural opening is more effective than DNA demethylation for cardiomyogenic differentiation of hASCs.
Several groups have defined cardiomyogenic differentiation media. We developed a novel MCM based on data from Shim et al. (human BMSCs expressed cardiac markers including GATA4 after their treatment) [19] and Planat-Bénard et al. (rare beating cells which expressed cardiac markers from ASCs) [10]. Contractile cardiomyocytes were not detected in ASC after 3 weeks MCM treatment, but in contrast to 5-aza treatment, the mRNA levels of myocardial contractile proteins were significantly increased. Cardiac actin and TropT mRNA almost doubled at 2 weeks and the number of cells expressing both Nkx2.5 and α-actin or TropI by immunocytochemistry was increased more than 10-fold. In contrast to the antiproliferative effects of 5-aza treatment, cell proliferation capacity in MCM was maintained (63% cells in MCM were Ki67 positive), although a loss of proliferation was observed on differentiation (5% cells were Ki67/TropI positive).
The third and most effective approach (Fig. S7) taken to cardiomyogenic differentiation was to co-culture ASCs with an inducing population of neonatal rat cardiomyocytes. Adult human BMSCs have the potential to differentiate into cardiomyocytes using a direct co-culture system [18]. Cell-to-cell interaction acts as a key inducer for cardiomyogenic differentiation as soluble factors secreted into medium were not alone sufficient to induce differentiation of hBMSCs into cardiomyocytes. Similar results have been obtained with rat BMSCs [17]. Physical contact between the cardiomyocytes and hBMSCs was required, potentially transmitting physical or electrical stimuli. One important pathway may involve Jagged 1 protein, which can activate Notch signal and enhance cardiac differentiation of rat BMSCs, and this effect can be inhibited by secretase inhibitor (DAPT) [21]. To clarify whether physical contact or soluble chemical factors are important in the differentiation of ASCs, we used a semi-diffusible membrane culture system (using insert, 3 μm pore size), which permits diffusion of molecules, but not cells, to reach the lower chamber. We found that there were no significant changes to mRNA expression of cardiac actin and TropT and no cardiac specific protein expression. In agreement with other studies, contractile cells were, however, observed in a direct co-culture system. Furthermore, cells so treated maintained their contractile ability for at least 3 weeks in vitro. The reason why only the direct co-culture system induced ASCs to contract spontaneously is not known. However, direct contact between cells provides (1) mechanical stretch from adjacent rat cardiomyocytes; (2) potentially electrical signal conduction through direct cell membrane contact or (3) soluble factor transferred from rat cardiomyocytes to ASC through gap junctions, or all three.
In this study, we attempted to optimize the ratio of cell numbers and the relative position of the co-cultured cells. A 1:10 ratio caused 2- to 3-fold increase in cardiac actin compared with a 1:5 ratio, which was used in another study [22]. Once the ratio and the position was optimized, ASCs were co-cultured with cardiomyocytes for 3, 5, 7, 14, 21 days to examine expression of cardiac actin mRNA (Fig. 5). Surprisingly, increased cardiac actin expression was evident as early as day 3 and increased in a time-dependent fashion by day 7, was maintained to 14 days by about 33-fold, then dropped to 2-fold at day 21. ASCs were optimally induced to cardiomyogenic lineage between 7 days and 14 days. The reason for the loss of cardiac actin mRNA expression after day 14 was not clear. However, after 2 weeks of co-culture, cell density was very high and sometimes cells made multilayered 3-D structures that usually detached from the flask, reducing their viability.
The finding of differentiation by co-culture was supported by immunocytostaining using cardiac specific markers (Fig. 6). Because human cardiac protein specific antibodies also recognized rat cardiomyocytes, cells were sorted by FACS using DiI labelling after 2 weeks of co-culture (the level of cardiac actin mRNA peaked at 2 weeks). Contrary to 5-aza or MCM treatment, GATA4 was detected from contact co-cultured ASCs. GATA4 is known as a key transcription factor for commitment to cardiac lineage and these cells were also strongly positive for Nkx2.5, α-actin, TropI and cMHC. It was confirmed that these cardiac marker positive cells had originated from ASCs by merging with DiI-labelled (red) images.
We showed the synchronicity of Ca2+ transients and the existence of gap junction connections between hASCs and rat cardiomyocytes using Fluo-4 AM or CM-DiI and calcein AM (Fig. 7). hASCs and rat cardiomyocytes formed gap junctions and effective transfer of small molecules from day 1 of co-culture. They showed synchronous Ca2+ transients and contractions from day 2 of co-culture. However, it remains unclear how the junctions might actually stimulate hASC to contract spontaneously. Gap junctions allow electrical coupling between cardiac myocytes, forming the cell-to-cell pathways for orderly spread of the wave of electrical excitation responsible for synchronous contraction [33], so electrical stimuli from contractile rat cardiomyocytes may have a critical role in cardiomyogenic differentiation of ASCs.
Though it suggests potential mechanisms for successful differentiation of hASCs to cardiomyocytes, this co-culture method is not likely to be directly applicable to differentiation of cells for clinical use for several reasons. Firstly, rat cardiomyocytes cannot be used in human beings due to immune rejection. Therefore human donor cells would be required for the clinical application of this method. Autologous heart cells would be an ideal source for a co-culture system, but the harvesting of these cells would be unacceptable in patients suffering heart failure. Secondly, using rat cardiomyocytes or commercially available human cardiomyocytes will require a sorting procedure, a process that can compromise cell viability. Lastly, the sheer number of cells required will be a significant limitation for clinical application. For cell therapy or tissue engineering solutions to heart failure, more than 109 cells may be required to transplant or an even greater number may be required due to the relatively low survival rate of the grafted cells from current protocols. Clearly other approaches are needed, but now we have shown it is possible to differentiate hASCs down the cardiac line.
In conclusion, we have examined four approaches of cardiac differentiation methods in this study. 5-aza treatment was not found to be useful for cardiac induction because of poor reproducibility. MCM and TSA induced partial differentiation to cardiomyocyte-like cells but these treatments were insufficient to induce spontaneous contraction. Finally, we showed that ASCs can be differentiated into beating cardiomyocytes using direct cell–cell interaction with rat cardiomyocytes. ASCs were found to express cardiac mRNA, transcription factors and contractile proteins. This study also reports that some hASC, in co-culture with neonatal rat CM, form gap junctions with CM and exhibit spontaneous Ca2+ oscillations from the sarcoplasmic reticulum. This appears to be the first demonstration of functional cardiomyogenic differentiation of stem cells derived from human fat with clear evidence of mRNA, protein, Ca2+ imaging and gap junction. This method was optimized to produce contraction of the donor cells and potentially provides an approach for developing to a clinically applicable method for cell therapy or tissue engineering.
Acknowledgments
Anita L. Sørensen is thanked for expert help with methylated DNA immunoprecipitation. These studies were supported by grants from National Health and Medical Research Council of Australia 400303;509271 and the Cass Foundation.
Supporting Information
CM-DiI labelling Calcein-AM labelling
Flow cytometry
Fluorescence-activated cell sorting analysis
Fig. S1 Morphology of ASCs (A), 5-aza treated ASCs(B) and ASCs cultured in MCM (C). Human primary ASCswere isolated and plated on tissue culture flask (A). ASCsappeared to assume a more uniform fibroblast-like shape. 5-azatreated ASCs were spindle-shaped (B), whereas ASCs culturedin MCM were more polygonal shape (C). Scale bar ∇ 100μm.
Fig. S2 Characterization of hASCs by flow cytometry. Representative histograms of ASC incubated with monoclonal antibodies against CD34, CD45, CD73, CD90 and CD105. ASC were positive for CD73 (92.3%), CD90 (96.2%) and CD105 (91.5%) and negative for CD34 (0.55%) and CD45 (0.17%). Thin black lines represent isotype controls and bold black lines represent cells incubated with antibody.
Fig. S3 Characterization of hASCs by colony forming assayand stro-1 staining. ASCs were plated at the density of 100 to 400in 60 mm dishes and cultured in 20% FCS for 2 weeks.Cells were stained by crystal violet (A). Around8% colony forming cells were counted in heterogeneousstromal vascular fraction (B). ASCs were stained by stro-1conjugated by FITC. Scale bar ∇ 100 μm.
Fig. S4 Real-time RT-PCR analysis of cardiac actin(A) and TropT mRNA (B) in hASCs 1, 2, 3 weeks afterco-culture with an insert. GAPDH was used as internal control. Bargraphs indicated fold change of mRNA compared with ASCs (dottedline). Results are shown as mean ± S.E.M. (n ∇ 3).
Fig. S5 Human cardiac actin mRNA levels in co-culturesystem with ASCs at different ratios and positions at day 14. Theratio of ASCs to cardiomyocytes was 1:5 or 1:10 and the positionwas ASCs on the top and cardiomyocyte at the bottom or viceversa. GAPDH was used as internal control. Bar graphs indicatedfold change of mRNA compared with ASCs (dotted line). Results areshown as mean ± S.E.M. (n ∇ 3).*P < 0.05 versus other groups.
Fig. S6 DNA demethylation profile of indicated cardiacpromoters in undifferentiated ASCs. ASC DNA was subjected tomethylated DNA immunoprecipitation (MeDIP), and methyl-DNA enrichedand input fractions were subjected to hybridization to a Nimblegenhuman promoter microarray. A 2.5-kb region was examined in eachpromoter, covering −2 kb upstream of the transcription startsite to +0.5 kb upstream of the transcription start site.The five tracks show for each promoter, from top to bottom (1, top)genomic localization (ENSEMBL nucleotide number is indicated), (2)log2 MeDIP/Input ratios centered to zero (no enrichment overinput), for each probe, (3) any detected methylation peak at aP level of <0.01 (no significant peak were noted for any of the genes examined), (4) position of any CpG island within the tiled region examined (green bars) and (5) position and orientation of the transcript (blue bar). The data indicate that no significant CpG methylation is detected by the MeDIP approach in ASCs, for any of the promoters examined.
Fig. S7 Comparison of cardiomyogenic differentiation using four different methods. Based on cardiac actin mRNA expression, there was increasing efficacy from one-week treatment of 5-aza, two-week culture in MCM to one-week treatment with TSA. Co-culture for 2 weeks maximized cardiac differentiation at 34-fold increase in cardiac actin mRNA expression.
Movie S1 Video clip of beating cells derived from ASCs inco-culture. Co-culture of rat cardiomyocytes and hASCs show in leftside under bright field (A), CM-DiI labelled hASCs show inright side under Texas Red filter from same field of A (B).Scale bar ∇ 100 μm.
Movie S2 Calcium imaging of beating cells derived fromASCs in co-culture. Video shows CM-DiI labelled hASCs under Texasred filter at first, Ca2+ transient under GFPfilter, and then beating cells in bright field. Scale bar ∇100 μm.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.World Health Organization . Available at. http://www.who.int/mediacentre/factsheets/fs317/en/index.html. Accessed November 1, 2008.
- 2.Eschenhagen T, Zimmermann WH. Engineering myocardial tissue. Circ Res. 2005;97:1220–31. doi: 10.1161/01.RES.0000196562.73231.7d. [DOI] [PubMed] [Google Scholar]
- 3.Ott HC, Matthiesen TS, Goh SK, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 4.Zammaretti P, Jaconi M. Cardiac tissue engineering: regeneration of the wounded heart. Curr Opin Biotechnol. 2004;15:430–4. doi: 10.1016/j.copbio.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Leor J, Amsalem Y, Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol Ther. 2005;105:151–63. doi: 10.1016/j.pharmthera.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Nakagami H, Morishita R, Maeda K, et al. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 2006;13:77–81. doi: 10.5551/jat.13.77. [DOI] [PubMed] [Google Scholar]
- 7.Surgery TASfAP . Available at. http://www.surgery.org/download/2008Qfacts.pdf. Accessed August 14, 2009.
- 8.Rangappa S, Fen C, Lee EH, et al. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg. 2003;75:775–9. doi: 10.1016/s0003-4975(02)04568-x. [DOI] [PubMed] [Google Scholar]
- 9.Gaustad KG, Boquest AC, Anderson BE, et al. Differentiation of human adipose tissue stem cells using extracts of rat cardiomyocytes. Biochem Biophys Res Commun. 2004;314:420–7. doi: 10.1016/j.bbrc.2003.12.109. [DOI] [PubMed] [Google Scholar]
- 10.Planat-Benard V, Menard C, Andre M, et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–9. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 11.Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strem BM, Zhu M, Alfonso Z, et al. Expression of cardiomyocytic markers on adipose tissue-derived cells in a murine model of acute myocardial injury. Cytotherapy. 2005;7:282–91. doi: 10.1080/14653240510027226. [DOI] [PubMed] [Google Scholar]
- 13.Lee WC, Sepulveda JL, Rubin JP, et al. Cardiomyogenic differentiation potential of human adipose precursor cells. Int J Cardiol. 2009;133:399–401. doi: 10.1016/j.ijcard.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 14.Kawamura T, Ono K, Morimoto T, et al. Acetylation of GATA-4 is involved in the differentiation of embryonic stem cells into cardiac myocytes. J Biol Chem. 2005;280:19682–8. doi: 10.1074/jbc.M412428200. [DOI] [PubMed] [Google Scholar]
- 15.Oyama T, Nagai T, Wada H, et al. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176:329–41. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gwak SJ, Bhang SH, Yang HS, et al. In vitro cardiomyogenic differentiation of adipose-derived stromal cells using transforming growth factor-beta1. Cell Biochem Funct. 2009;27:148–54. doi: 10.1002/cbf.1547. [DOI] [PubMed] [Google Scholar]
- 17.Wang T, Xu Z, Jiang W, et al. Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol. 2006;109:74–81. doi: 10.1016/j.ijcard.2005.05.072. [DOI] [PubMed] [Google Scholar]
- 18.Rangappa S, Entwistle JW, Wechsler AS, et al. Cardiomyocyte-mediated contact programs human mesenchymal stem cells to express cardiogenic phenotype. J Thorac Cardiovasc Surg. 2003;126:124–32. doi: 10.1016/s0022-5223(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 19.Shim WS, Jiang S, Wong P, et al. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun. 2004;324:481–8. doi: 10.1016/j.bbrc.2004.09.087. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Yu X, Lin Q, et al. Bone marrow mesenchymal stem cells differentiate into functional cardiac phenotypes by cardiac microenvironment. J Mol Cell Cardiol. 2007;42:295–303. doi: 10.1016/j.yjmcc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Yu B, Zhang Y, et al. Jagged1 protein enhances the differentiation of mesenchymal stem cells into cardiomyocytes. Biochem Biophys Res Commun. 2006;341:320–5. doi: 10.1016/j.bbrc.2005.12.182. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Y, Liu T, Song K, et al. ADSCs differentiated into cardiomyocytes in cardiac microenvironment. Mol Cell Biochem. 2009;324:117–29. doi: 10.1007/s11010-008-9990-3. [DOI] [PubMed] [Google Scholar]
- 23.Choi YS, Cha SM, Lee YY, et al. Adipogenic differentiation of adipose tissue derived adult stem cells in nude mouse. Biochem Biophys Res Commun. 2006;345:631–7. doi: 10.1016/j.bbrc.2006.04.128. [DOI] [PubMed] [Google Scholar]
- 24.Morritt AN, Bortolotto SK, Dilley RJ, et al. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation. 2007;115:353–60. doi: 10.1161/CIRCULATIONAHA.106.657379. [DOI] [PubMed] [Google Scholar]
- 25.Gruh I, Beilner J, Blomer U, et al. No evidence of transdifferentiation of human endothelial progenitor cells into cardiomyocytes after coculture with neonatal rat cardiomyocytes. Circulation. 2006;113:1326–34. doi: 10.1161/CIRCULATIONAHA.105.559005. [DOI] [PubMed] [Google Scholar]
- 26.Tomita S, Li RK, Weisel RD, et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:II247–56. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 27.Zhang FB, Li L, Fang B, et al. Passage-restricted differentiation potential of mesenchymal stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun. 2005;336:784–92. doi: 10.1016/j.bbrc.2005.08.177. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Song J, Liu W, et al. Growth and differentiation of rat bone marrow stromal cells: does 5-azacytidine trigger their cardiomyogenic differentiation. Cardiovasc Res. 2003;58:460–8. doi: 10.1016/s0008-6363(03)00265-7. [DOI] [PubMed] [Google Scholar]
- 29.Hosseinkhani M, Hasegawa K, Ono K, et al. Trichostatin A induces myocardial differentiation of monkey ES cells. Biochem Biophys Res Commun. 2007;356:386–91. doi: 10.1016/j.bbrc.2007.02.151. [DOI] [PubMed] [Google Scholar]
- 30.Johnson CA, Turner BM. Histone deacetylases: complex transducers of nuclear signals. Semin Cell Dev Biol. 1999;10:179–88. doi: 10.1006/scdb.1999.0299. [DOI] [PubMed] [Google Scholar]
- 31.Lagace DC, Nachtigal MW. Inhibition of histone deacetylase activity by valproic acid blocks adipogenesis. J Biol Chem. 2004;279:18851–60. doi: 10.1074/jbc.M312795200. [DOI] [PubMed] [Google Scholar]
- 32.Marin-Husstege M, Muggironi M, Liu A, et al. Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J Neurosci. 2002;22:10333–45. doi: 10.1523/JNEUROSCI.22-23-10333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iezzi S, Cossu G, Nervi C, et al. Stage-specific modulation of skeletal myogenesis by inhibitors of nuclear deacetylases. Proc Natl Acad Sci USA. 2002;99:7757–62. doi: 10.1073/pnas.112218599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CM-DiI labelling Calcein-AM labelling
Flow cytometry
Fluorescence-activated cell sorting analysis
Fig. S1 Morphology of ASCs (A), 5-aza treated ASCs(B) and ASCs cultured in MCM (C). Human primary ASCswere isolated and plated on tissue culture flask (A). ASCsappeared to assume a more uniform fibroblast-like shape. 5-azatreated ASCs were spindle-shaped (B), whereas ASCs culturedin MCM were more polygonal shape (C). Scale bar ∇ 100μm.
Fig. S2 Characterization of hASCs by flow cytometry. Representative histograms of ASC incubated with monoclonal antibodies against CD34, CD45, CD73, CD90 and CD105. ASC were positive for CD73 (92.3%), CD90 (96.2%) and CD105 (91.5%) and negative for CD34 (0.55%) and CD45 (0.17%). Thin black lines represent isotype controls and bold black lines represent cells incubated with antibody.
Fig. S3 Characterization of hASCs by colony forming assayand stro-1 staining. ASCs were plated at the density of 100 to 400in 60 mm dishes and cultured in 20% FCS for 2 weeks.Cells were stained by crystal violet (A). Around8% colony forming cells were counted in heterogeneousstromal vascular fraction (B). ASCs were stained by stro-1conjugated by FITC. Scale bar ∇ 100 μm.
Fig. S4 Real-time RT-PCR analysis of cardiac actin(A) and TropT mRNA (B) in hASCs 1, 2, 3 weeks afterco-culture with an insert. GAPDH was used as internal control. Bargraphs indicated fold change of mRNA compared with ASCs (dottedline). Results are shown as mean ± S.E.M. (n ∇ 3).
Fig. S5 Human cardiac actin mRNA levels in co-culturesystem with ASCs at different ratios and positions at day 14. Theratio of ASCs to cardiomyocytes was 1:5 or 1:10 and the positionwas ASCs on the top and cardiomyocyte at the bottom or viceversa. GAPDH was used as internal control. Bar graphs indicatedfold change of mRNA compared with ASCs (dotted line). Results areshown as mean ± S.E.M. (n ∇ 3).*P < 0.05 versus other groups.
Fig. S6 DNA demethylation profile of indicated cardiacpromoters in undifferentiated ASCs. ASC DNA was subjected tomethylated DNA immunoprecipitation (MeDIP), and methyl-DNA enrichedand input fractions were subjected to hybridization to a Nimblegenhuman promoter microarray. A 2.5-kb region was examined in eachpromoter, covering −2 kb upstream of the transcription startsite to +0.5 kb upstream of the transcription start site.The five tracks show for each promoter, from top to bottom (1, top)genomic localization (ENSEMBL nucleotide number is indicated), (2)log2 MeDIP/Input ratios centered to zero (no enrichment overinput), for each probe, (3) any detected methylation peak at aP level of <0.01 (no significant peak were noted for any of the genes examined), (4) position of any CpG island within the tiled region examined (green bars) and (5) position and orientation of the transcript (blue bar). The data indicate that no significant CpG methylation is detected by the MeDIP approach in ASCs, for any of the promoters examined.
Fig. S7 Comparison of cardiomyogenic differentiation using four different methods. Based on cardiac actin mRNA expression, there was increasing efficacy from one-week treatment of 5-aza, two-week culture in MCM to one-week treatment with TSA. Co-culture for 2 weeks maximized cardiac differentiation at 34-fold increase in cardiac actin mRNA expression.
Movie S1 Video clip of beating cells derived from ASCs inco-culture. Co-culture of rat cardiomyocytes and hASCs show in leftside under bright field (A), CM-DiI labelled hASCs show inright side under Texas Red filter from same field of A (B).Scale bar ∇ 100 μm.
Movie S2 Calcium imaging of beating cells derived fromASCs in co-culture. Video shows CM-DiI labelled hASCs under Texasred filter at first, Ca2+ transient under GFPfilter, and then beating cells in bright field. Scale bar ∇100 μm.


