Abstract
Curcumin is a non-toxic polyphenol with pleiotropic activities and limited bioavailability. We investigated whether a brief exposure to low doses of curcumin would induce in the myogenic C2C12 cell line an endoplasmic reticulum (ER) stress response and protect against oxidative stress. A 3-hr curcumin administration (5–10 μM) increased protein levels of the ER chaperone Grp94, without affecting those of Grp78, calreticulin and haeme-oxygenase-1 (HO-1). Exposure of cells to hydrogen peroxide 24 hrs after the curcumin treatment decreased caspase-12 activation, total protein oxidation and translocation of NF-κB to the nucleus, compared with untreated cells. Grp94 overexpression, achieved by means of either stable or transient trasfection, induced comparable cytoprotective effects to hydrogen peroxide. The delayed cytoprotection induced by curcumin acted through Grp94, because the curcumin-induced increase in Grp94 expression was hampered by either stable or transient transfection with antisense cDNA; in these latter cells, the extent of total protein oxidation, as well as the translocation of NF-κB to the nucleus, and the percentage of apoptotic cells were comparable to those observed in both curcumin-untreated wild-type and empty vector transfected cells. Defining the mechanism(s) by which Grp94 exerts its antioxidant defence, the determination of cytosolic calcium levels in C2C12 cells by fura-2 showed a significantly reduced amount of releasable calcium from intracellular stores, both in conditions of Grp94 overexpression and after curcumin pre-treatment. Therefore, a brief exposure to curcumin induces a delayed cytoprotection against oxidative stress in myogenic cells by increasing Grp94 protein level, which acts as a regulator of calcium homeostasis.
Keywords: curcumin, unfolded protein response, Grp94, calcium, reactive oxygen species, apoptosis, C2C12, NF-κB
Introduction
Curcumin (diferuloylmethane) is a low molecular weight polyphenol, derived from the dietary spice turmeric, Curcuma longa, which is currently used in clinical trials as an anti-neoplastic drug [1]. Nevertheless, curcumin apparently displays tissue-specific biological effects, in so far as it decreases proliferation and induces apoptosis of neoplastic cells, it protects non-neoplastic ones from oxidative stress, acting as a scavenger of superoxide anion and hydroxyl radicals (reactive oxygen species; ROS) [2, 3]. Furthermore, curcumin and other derivatives are strong inducers of haeme-oxygenase-1 (HO-1), a Phase 2 detoxification enzyme and a member of the HSP family, highly induced by hypoxia and ROS [3–5]. Curcumin also acts as a potent inhibitor of the sarco-/ endoplasmic reticulum Ca2+ ATPase (SERCA) and increases membrane permeability and cation leakage [6]. Both mechanisms may favour calcium depletion from intracellular stores, a condition known to induce the endoplasmic reticulum (ER) stress-response, which is relevantly involved in promoting either cytoprotection or cell death, the latter in the case of sustained induction [7, 8].
The aim of the present study was to investigate whether curcumin administration would induce a cytoprotective ER stress-response, which might contribute to the antioxidant defence. The rationale to explore whether the cytoprotection induced by curcumin acted through the ER stress-response was provided by previous reports from our and other laboratories. It has been shown that ER chaperones and stress proteins, such as Grp78, Grp94 and calreticulin, blocked calcium dyshomeostasis and cell death induced by exposure to either oxygen radicals or organic oxidants [9–11] or by conditions that potentially increase ROS production, such as ischaemia-reperfusion and calcium overload [12–14]. Here we analysed the presence and the extent of the antioxidant defence induced by curcumin preconditioning, namely the transient administration of the drug 24 hrs before exposure to oxidative stress [11, 15]. Curcumin-treated and untreated, proliferating C2C12 cells were challenged with hydrogen peroxide, and the effects on apoptosis, total protein oxidation and NF-κB activation were monitored. Moreover, the same experimental protocol was performed in C2C12 cells in which genetic manipulation of Grp94 protein level was achieved by specific expression of grp94 cDNAs (sense or antisense).
Although curcumin-induced antioxidant protection may be the result of the involvement of multiple executors, which are recruited by the activation of diverse signaling pathways [1], our results identify Grp94 as a prominent player.
Materials and methods
Cell culture
The skeletal myogenic murine cell line C2C12 ECACC1 was used between passages 14 and 18. Cells were grown in Dulbecco’s modified Eagle medium (DMEM, Sigma, Salisbury, UK) containing 10% foetal calf serum and L-glutamine.
Generation of stably transfected clones was performed with constructs and procedures as previously described [14, 16]. Clones were grown as described in Reference [16] and used between passages10 and 20.
Transient transfections were achieved using bicistronic vectors in order to identify transfected cells; a construct, which contained GFP and grp94 cDNAs (pT94), was used for overexpression, whereas the construct containing only the GFP cDNA (pT, Invitrogen, Groningen, The Netherlands) was used for control, as previously described [14]. A nuclear-targeted β-galactosidase cDNA and a portion of rabbit grp94 cDNA in antisense orientation were cloned into the pBK phagemid, under the control of the RSV promoter (Stratagene, Waldbronn, Germany), to achieve grp94 antisense transcription. The control phagemid contained a portion of rabbit grp94 cDNA lacking the translation initiation sequence.
Curcumin (Sigma) was dissolved in DMSO at a stock concentration of 20 mM, aliquoted in the dark and stored at –20°C. About 80,000 wild-type C2C12 cells were seeded in six-well plates, whereas about 10,000 cells were plated on gelatin-coated glass slides in 24-well plates. After 24 hrs, cells were exposed to fresh growth medium added with 5–10 μM curcumin in less than 0.1%DMSO for 3 hrs. Control plates were incubated with vehicle. Culture medium was then changed and cells used 24 hrs later. The same protocol was applied to C2C12 clones, since G418 was omitted from the culture medium during and after curcumin or vehicle treatment.
For transient transfections, about 16,000 cells were seeded on gelatin-coated glass slides in 24-well plates. Twenty-four hours later, 4 μg of cDNA, solubilized in 100 μl of 10 mM Tris-ethylenediaminetetraacetic acid (Tris-EDTA) buffer containing 0.125 M calcium and 0.75 mM phosphate, were added to 1 ml of culture medium in each well. The day after, cells were extensively washed with PBS and then returned to growth medium for curcumin treatment as described above.
After extensive rinse of cultures with DMEM, oxidative stress was achieved by exposing cells to 400–800 μM H2O2 in 1 ml/well of growth medium for six-well plates and in 0.5 ml/well of the same medium for 24-well plates. A number of wells, with any precedent curcumin treatment, were exposed to 20 μM curcumin 1 hr before and during H2O2 addition.
Evaluation of cell necrosis was performed on cover slips in parallel wells by adding propidium iodide (PI) to the incubation medium as detailed in Reference [14].
For each condition, cells were tested in triplicate and at least three independent experimental sets were repeated.
Western blot analysis
Adherent cells in a six-well plate were solubilized in 25 μl of Cell Lysis buffer (Promega, Milano, Italy). Twenty-five micrograms of total protein were analysed by Western blotting, as previously described [14, 17]. The following primary antibodies were used: anti-Grp94 3C4 mouse monoclonal antibody [16], anti-Grp78 (SPA-826) and anti-calreticulin (SPA-600) rabbit polyclonal antibodies (Stressgen, Victoria, BC, Canada); anti HO-1 (OSA-111) mouse monoclonal antibody (Stressgen), anti-GADD153 (CHOP) rabbit polyclonal antibody (Santa Cruz Biotech.). After incubation with the appropriate secondary antibody conjugated with peroxidase (Santa Cruz Biotech., Heidelberg, Germany), blots were developed using an enhanced chemiluminescent detection system (ECL, GE Healthcare, Milano, Italy).
For procaspase-12 analysis, adherent cells were lysed together with floating cells, collected after a 5 min. supernatant centrifugation at 300 g, in a final volume of 40 μl. Protein concentration was determined in parallel wells, lysed after extensive rinsing with PBS, and equal volumes of lysate, corresponding to 20 μg of total protein amount, were used for assay with the anti caspase-12 rabbit polyclonal antibody (Santa Cruz Biotech.).
Protein levels were quantified via measurement of optical density using the NIH Image J analysis Software (Bethesda, MD, USA) and normalized to the densitometric value of the Ponceau red staining of the corresponding actin band.
Protein oxidation detection
To assess the formation of protein carbonyl groups, the OxyBlot protein oxidation detection kit (Chemicon, Hampshire, UK) was used according to the manufacturer’s detailed protocol. After extensive rinse with PBS, cells were solubilized as described in Dalla Libera et al.[18]. About 12 μg of total protein was then used for derivatization with DNPH and processed for Western blot analysis. A positive control included derivatization of 3 μg of oxidized bovine serum albumin (BSA), whereas the negative control was perfomed with an equal amount of total protein reacted in the absence of DNPH.
Levels of oxidated protein were quantified using the NIH ImageJ analysis software and normalized as described above.
Immunofluorescence
Cells, grown on glass cover slips in 24-well plates, were fixed and permeabilized as previously described [16]. Cover slips were incubated overnight at 4°C with either rabbit polyclonal or mouse monoclonal anti-NF-κB antibodies (Santa Cruz Biotech.), followed by incubation with the appropriate secondary antibodies conjugated with Texas Red (Santa Cruz Biotech.) for 2 hrs at room temperature (RT). Double labelling for β-galactosidase and NF-κB was performed by incubating fixed cells with a mixture of rabbit policlonal anti-NF-κB antibodies and mouse monoclonal anti-β-galactosidase antibody (Santa Cruz Biotech). Appropriate dilutions of the secondary goat antibodies, conjugated with Texas Red (Santa Cruz Biotech.) and FITC (Cappel, Eschwege, Germany) were mixed, centrifuged for 10 min. at 10,000 g to eliminate aggregates of cross-reacting immunoglobulins and incubated on cover slips for 2 hrs at RT. Controls were performed using non-immune immunoglobulins in the first step.
Cover slips were mounted with buffered glycerol added with 2 μg/ml of 4,8-diamine-2-phenylindol (DAPI; Sigma) and examined using an Axioplan epifluorescence microscope (Zeiss, Arese, Italy).
TUNEL assay
Nucleosomal DNA fragmentation was visualized by TdT-mediated dUTP Nick End Labeling (TUNEL). Cells were fixed and incubated with 5 U TdT and 0.5 nmol of either Tetramethyl-rhodamine-5dUTP or Fluorescein-12-dUTP (Roche, Monza, Italy) following manufacturer instructions. The former deoxynucleotide was used in combination with transfection with the pT construct, which contained GFP, whereas the latter one was used in combination with constructs containing the βgal cDNA. After rinsing with PBS, autofluorescence was quenched by incubation for 15 min. at RT with 50 mM ammonium chloride. For βgal immunoreactivity, cover slips were incubated overnight at 4°C with a rabbit polyclonal anti-βgal antibody (5’Prime-3’Prime Inc., Boulder, CO, USA) and subsequently with a Texas-Red conjugated secondary antibody (Santa Cruz Biotech.). After adequate rinsing, cover slips were mounted on glass slides with buffered glycerol added with DAPI.
More than 100–150 transfected cells were evaluated for each cover slip; each experiment was performed in triplicate; values correspond to at least three independent experiments.
Ca2+ measurements
Cells, plated on cover slips (24 mm diameter), were incubated for about 60 min. at RT with 2 μM fura-2/AM in DMEM containing 10% FCS, 0.04% pluronic and 250 μM sulfinpyrazone as previously described [19]. The cover slips were washed at 37°C with a modified Krebs–Ringer Buffer (mKRB, 140 mM NaCl, 2.8 mM KCl, 2 mM MgCl2, 1 mM CaCl2, 10 mM HEPES, 11 mM glucose pH 7.4), mounted on a thermostated chamber (Medical System Corp., Greenvale, NY, USA), placed on the stage of an inverted microscope (Zeiss, Axiovert) equipped for single cell fluorescence measurements and imaging analysis (TILL Photonics, Graefelfing, Germany). For presentation, the ratios (F340/F380) were off-line averaged (20–30 cells) and normalized to the resting value measured within the first minute of the experiment.
Statistical analysis
Data are expressed as mean ± standard error of mean (SEM). One-way anova and Student’s t-test were used to compare mean values. P < 0.05 was regarded as statistically significant.
Results
A single curcumin treatment increases Grp94 levels and induces delayed antioxidant protection in C2C12 myoblasts
C2C12 proliferating cells, treated or not with 5–10 μM curcumin for 3 hrs, were lysed after 24 hrs and analysed by Western blotting to monitor the expression level of ER stress-proteins, as putative effectors of delayed cytoprotection (second window of protection [15]). Figure 1A shows that curcumin treatment increased significantly the Grp94 relative amount (see histogram in Fig. 1B; P < 0.0001, n= 9), without affecting the levels of calreticulin and Grp78 (1.06 ± 0.05, 1.09 ± 0.14 and 1.01±0.18, for Grp78/actin density ratio of control, 5 μM and 10 μM curcumin-treated cells, respectively, mean values ± SEM, n= 6; 0.99 ± 0.09, 1.02 ± 0.28 and 0.98 ± 0.14, for calreticulin/actin density ratio of control, 5 μM and 10 μM curcumin-treated cells, respectively, mean values ± SEM, n= 5). Other proteins usually up-regulated by the ER stress response, such as the transcriptional regulator CHOP and procaspase-12, did not show any significant increase after the curcumin pre-treatment (Figs. 1A and 2A). A comparable lack of effect was observed on protein levels of HO-1, which was barely detectable under our conditions (Fig. 1A).
Fig 1.
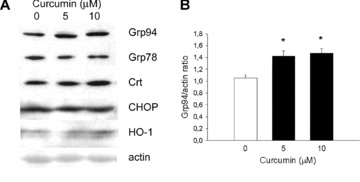
ER stress proteins level in curcumin pretreated C2C12 cells. (A) Representative Western blotting of 25 μg of whole lysate of cells grown for 3 hrs in the absence or in the presence of 5 and 10 μM curcumin and then lysed 24 hrs after replacement with fresh culture medium. Blot was stained with antibodies for Grp94, Grp78, calreticulin (Ctr), CHOP and haeme-oxygenase 1 (HO-1). Actin staining is shown as a reference for sample loading. (B) Histogram shows Grp94/actin density ratio of different untreated and treated cultures (n= 9). Each culture condition was tested in triplicate and at least three independent experimental sets were repeated. Mean values ± SEM. *P < 0.0001; anova test.
Fig 2.
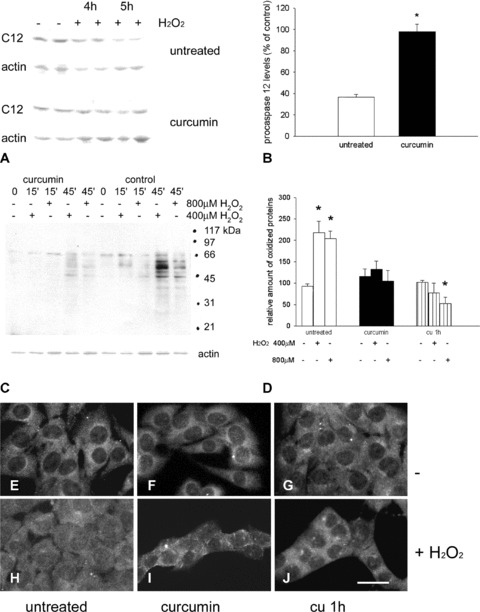
Procaspase-12 protein level, total protein oxidation and NF-κB nuclear translocation in curcumin-pre-treated C2C12 cells upon exposure to hydrogen peroxide. (A) Representative Western blot analysis showing immunoreactivity for procaspase-12 (C12) before (−) and after (+) exposure to 800 μM hydrogen peroxide for the indicated times (4 and 5 hrs) of cells untreated and pre-treated with curcumin. Actin staining is shown as a reference for gel loading. (B) Histogram shows the relative amount of procaspase-12 detected after 5 hrs of exposure to hydrogen peroxide (expressed as percentage of the amount detected in untreated and curcumin-treated cells before addition of H2O2). Mean values ± SEM; n= 6; *P < 0.001; Student’s t-test. (C) Representative Oxyblot analysis showing the presence of protein carbonyl groups in lysates obtained from untreated (control) and curcumin treated C2C12 cells before (−) and after (+) exposure to 400–800 μM hydrogen peroxide for the indicated times (15 and 45 min.). Actin staining is shown as a reference for gel loading. Migration of molecular weight standards (BioRad Broad range, Segrate, Italy) is indicated on the right. (D) Densitometric values of carbonylated signals was normalized to the corresponding actin level and expressed as percentage of wild-type C2C12 levels. Histogram shows the relative amount of oxidized proteins detected in C2C12 cells, untreated, treated for 3 hr with curcumin the day before (curcumin) or added with 20μM curcumin 1 hr before the experiment (cu 1h). Cells were maintained for 45 min. in the presence or in the absence of the indicated hydrogen peroxide concentration. Mean values ± SEM; n= 5; *P < 0.0001; anova test. (E)–(J) Representative micrographs showing C2C12 cultures, left untreated, treated with curcumin the day before (curcumin) or added with 20 μM curcumin 1 hr before the challenge with hydrogen peroxide (cu 1h). Cells were grown in the absence (E)–(G) or in the presence of 800 μM hydrogen peroxide for 2 hrs (H)–(J) and stained for NFκB. Bar: 30 μm.
Nevertheless, the brief curcumin pre-treatment was effective in increasing the antioxidant defence of C2C12 myoblasts, upon hydrogen peroxide addition to the culture medium, 24 hrs after the drug administration. Different hydrogen peroxide exposure times were required to explore relatively late effects, such as caspase activation and apoptosis, compared with earlier ones, such as protein carbonylation.
Exposure of untreated cells for 4–5 hrs to 800 μM H2O2 decreased procaspase-12 levels by about 60%, compared with control cells (Fig. 2A and B). Conversely, cells exposed to hydrogen peroxide 24 hrs after curcumin treatment showed less than 10% decrease in procaspase-12 levels (Fig. 2A and B; P < 0.001).
Total protein oxidation levels were then analysed by means of Oxyblot analysis (Fig. 2C). Exposure to 400–800 μM H2O2 for 30–45 min. significantly increased the degree of protein oxidation, whereas the pre-treatment with curcumin (5–10 μM) hampered this increase. Total protein oxidation also appeared to be significantly reduced when curcumin (20 μM) was added to the growth medium, 1 hr before and during exposure to hydrogen peroxide (Fig. 2D).
Because reactive oxygen intermediates are responsible for NF-κB activation [20], which, conversely, is inhibited by acute curcumin administration [21], we tested the presence of a delayed effect of the curcumin treatment on NF-κB nuclear translocation. Low levels of nuclear NF-κB immunoreactivity were detectable in control cultures of proliferating C2C12 cells, although the staining in the cytoplasm predominated (Fig. 2E–G). After 24 hrs, 5–10 μM curcumin-treated and untreated cells were exposed to 400–800 μM H2O2 for 1–2 hrs (Fig. 2H–J). As determined by PI staining, this protocol of exposure to hydrogen peroxide had no significant effect on cell viability, which was greater than 98%. Consistent with previous observations [20], a 2 hrs exposure to 800 μM H2O2 induced in curcumin-untreated cells a redistribution of NF-κB immunoreactivity, which accumulated in the nucleus, albeit remaining detectable in the cytoplasm (Compare Fig. 2H and E). Exposure of untreated cells to curcumin (20μM), 1 hr before and during hydrogen peroxide addition, blunted the increase in nuclear NF-κB immunoreactivity (Fig. 2J). Interestingly, the same effect was observed when the curcumin treatment was performed 24 hrs in advance, at a lower dose (5–10 μM) and for a limited time (3 hrs; Fig. 2I).
Stable or transient Grp94 overexpression induces antioxidant protection in C2C12 myoblasts
The data so far suggest that several aspects of the antioxidant defence induced by curcumin occur as a delayed protection response [15], where the increase in Grp94 protein levels may play an effector role. We then determined whether the selective increase of Grp94, obtained by either stable or transient transfection, would induce an antioxidant protection. Therefore, we analysed two C2C12 clones, clone 82A11 [14], which stably overexpressed Grp94 without displaying any increase in Grp78 and calreticulin protein levels, and clone S1C8 [14, 16], as a control (Fig. S1A). The two clones were repeatedly checked for Grp94, Grp78 and calreticulin expression throughout the passages performed during the study.
Exposure to H2O2 (800 μM) for 4–5 hrs caused a 75% decrease in procaspase-12 levels in control S1C8 clone, whereas procaspase-12 levels of the Grp94 overexpressing clone 82A11 showed less than 10% relative decrease (Fig. 3A and B; P < 0.05). Total protein oxidation of S1C8 cells significantly increased after 45–90 min. exposure to hydrogen peroxide, compared with unexposed ones (Fig. 3C and D; P < 0.01); on the other hand, the degree of protein oxidation of Grp94 overexpressing 82A11 cells was not significantly affected by exposure to hydrogen peroxide (Fig. 3C and D).
Fig 3.
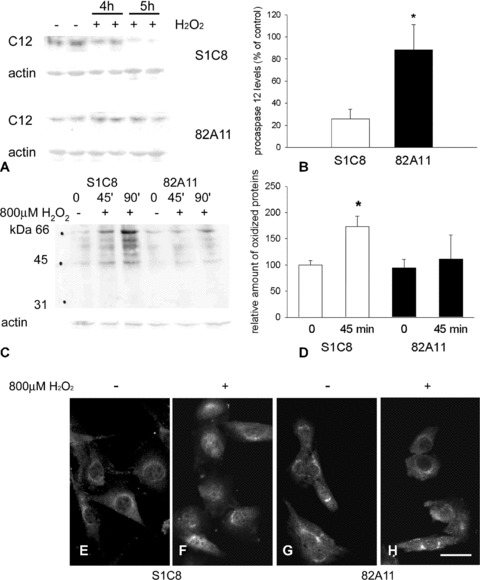
Procaspase-12 protein level, total protein oxidation and NF-κB nuclear translocation in stable Grp94 overexpressing cells upon exposure to hydrogen peroxide. (A) Representative Western blot analysis showing immunoreactivity of control S1C8 and Grp94 overexpressing 82A11 clones for procaspase-12 (C12) before (−) and after (+) 4 and 5 hrs exposure to 800 μM hydrogen peroxide. Actin staining is shown as a reference for gel loading. (B) Histogram shows the relative amount of procaspase-12 levels detected in S1C8 and 82A11 cells after 5 hrs of exposure to hydrogen peroxide and expressed as percentage of the procaspase-12 amount detected before addition of H2O2. Mean values ± SEM; n= 5; *P < 0.05; Student’s t-test. (C) Representative Oxyblot analysis showing the presence of protein carbonyl groups in lysates obtained from control clone S1C8 cells and Grp94 overexpressing 82A11 cells before (−) and after (+) exposure to 800 μM hydrogen peroxide for the indicated times (45 and 90 min.). Actin staining is shown as a reference for gel loading. (D) Histogram shows the relative amount of oxidized proteins detected in control clone S1C8 cells and Grp94 overexpressing 82A11 cells maintained for 45 min. in the presence or in the absence of 800 μM hydrogen peroxide. Densitometric values of carbonylated signals detected in each lane was normalized to the corresponding actin level and expressed as percentage of control clone levels. Mean values ± SEM; n= 5; *P < 0.003; anova test. (E)–(H) Micrographs show cultures of control clone S1C8 cells and Grp94 overexpressing 82A11 cells grown in the absence (E and G) or in the presence (F and H) of 800 μM hydrogen peroxide for 2 hrs and stained for NF-κB. Bar: 30 μm.
Like wild-type C2C12 cells, both the control S1C8 and the overexpressing Grp94 82A11 clone showed low levels of nuclear NF-κB immunoreactivity, most of the immunoreactivity being detectable in the cytoplasm (Fig. 3E and G). Exposure of control clone cells to H2O2 (800 μM) for 2 hrs redistributed NF-κB immunoreactivity, which appeared more concentrated in the nucleus compared with the cytosol (Fig. 3F), whereas hydrogen peroxide did not induce any appreciable increase in nuclear NF-κB immunoreactivity of Grp94 overexpressing cells (Fig. 3H).
In order to validate further the cytoprotective role of Grp94 against oxidative injury, the protein was transiently overexpressed in C2C12 cells, and the protection against apoptosis was evaluated using a single-cell approach. Transiently transfected cells were visualized by means of GFP expression (Fig. 4B and D). The percentage of apoptotic nuclei, evaluated on the total number of GFP-positive cells, was about 1% in cultures maintained in growth medium. Cells were then exposed to H2O2 (800 μM) for 5 hrs, and apoptosis was detected by TUNEL assay (red fluorescence in Fig. 4B and D). The percentage of apoptotic nuclei was significantly reduced in cultures transfected with grp94 cDNA (pT94), compared with those transfected with the empty vector (pT) (yellow fluorescence in Fig. 4B and D; 6.23%± 0.74% and 20.4%± 0.40%, mean ± SEM of TUNEL and GFP positive cells transfected with pT94 and pT, respectively; P < 0.0001; n= 6).
Fig 4.
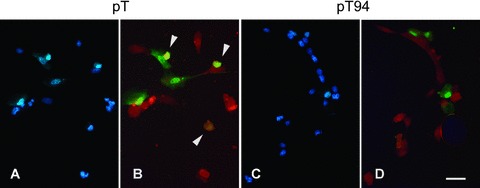
Apoptosis induced by exposure to hydrogen peroxide in transient Grp94 overexpressing C2C12 cells. Representative micrographs of C2C12 cell cultures transiently transfected with either empty vector (pT; A and B) or grp94 cDNA (pT94; C and D). (A) and (C) show nuclei counterstained with DAPI. (B) and (D) show merged pictures of transfected cells, identified by the presence of GFP (green fluorescence) and of apoptotic nuclei (red fluorescence). Arrowheads indicate the presence of apoptosis in transfected cells (yellow fluorescence). Bar: 30 μm.
Down-regulation of Grp94 expression blunts the antioxidant defence induced by a single curcumin treatment
We then investigated whether the transfection with antisense grp94 cDNA blunted the increase in Grp94 protein level induced by curcumin treatment and the antioxidant protection.
C2C12 cells from the AS3A12 clone (stably transfected with antisense grp94 cDNA [16]) displayed a 50% decrease in Grp94 protein levels, compared with S1C8 control clone (see Table 1 in Reference [16] and Fig. 5A). The Grp94 expression level of these cells did not vary 24 hrs after curcumin exposure (Fig. 5A), leading to a ratio between Grp94 and actin relative amount of 0.55 ± 0.06 and 0.60 ± 0.07, for untreated and curcumin-treated cultures, respectively (mean values ± SEM, n= 8). Also Grp78 protein level remained unchanged upon treatment (Fig. 5A; Grp78/actin relative amount of 1.05 ± 0.09 and 0.84 ± 0.06, for untreated and curcumin-treated cultures, respectively; mean values ± SEM, n= 6).
Fig 5.
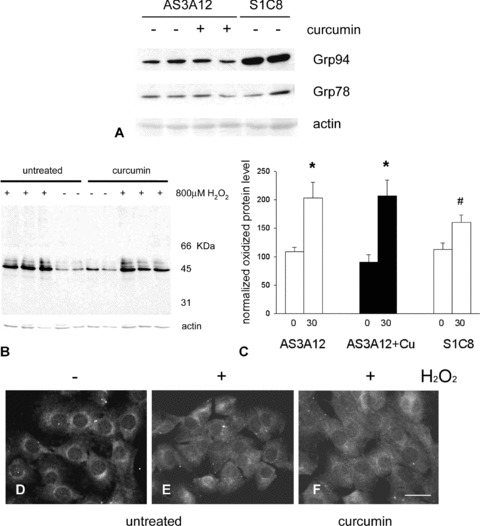
Total protein oxidation and NF-κB nuclear translocation in C2C12 cells stably transfected with antisense grp94 cDNA upon exposure to hydrogen peroxide. (A) Representative Western blot of 25 μg of whole lysates of untreated S1C8 control clone cells and AS3A12 clone cells grown for 3 hrs in the absence or in the presence of curcumin and then lysed 24 hrs after replacement with fresh culture medium. Blot was stained with antibodies for Grp94 and Grp78. Actin staining is shown as a reference for sample loading. (B) Representative Oxyblot analysis showing the presence of protein carbonyl groups in lysates obtained from antisense grp94 clone AS3A12 cells before (−) and after (+) exposure to 800 μM hydrogen peroxide for 30 min. Actin staining is shown as a reference for gel loading. (C) Histogram shows the relative amount of oxidized proteins detected in antisense grp94 clone AS3A12 cells, pre-treated or untreated with curcumin and in S1C8 control clone cells, maintained for 30 min. in the presence or in the absence of 800 μM hydrogen peroxide. Densitometric values of carbonylated signals detected in each lane was normalized to the corresponding actin level and expressed as percentage of control clone S1C8 cells levels. Mean values ± SEM; n= 6; *P < 0.0001; # P < 0.04; anova test. (D)–(F). Micrographs show cultures of antisense grp94 clone AS3A12 cells maintained in the absence (D) or in the presence (E and F) of 800 μM hydrogen peroxide for 2 hrs and stained for NFκB. Cells in (F) were pre-treated with curcumin, 24 hrs before addition with hydrogen peroxide. Bar: 30 μm.
Exposure of the antisense AS3A12 clone to H2O2 (400–800 μM, 30 min.) significantly increased total protein oxidation levels, compared with non-exposed cells (Fig. 5B and C; P < 0.0001, n= 6). Similarly, the exposure to hydrogen peroxide of curcumin-treated AS3A12 cells led to a significant increase in total protein oxidation compared with curcumin-treated not-exposed cells (Fig. 5B and C; P < 0.0001, n= 6). Interestingly, the degree of protein oxidation induced by hydrogen peroxide in both curcumin-treated and untreated AS3A12 cells was significantly higher than that observed in parallel cultures of control S1C8 clone cells (P≤ 0.04, n= 6; Fig. 5C). We next investigated the effects of antisense grp94 cDNA expression on NF-κB nuclear translocation. As described for control S1C8 clone, NF-κB immunoreactivity of proliferating antisense AS3A12 cells was primarily localized in the cytosol, and the low levels of staining detectable in the nucleus increased after exposure to H2O2 (800 μM, 2 hrs; Fig. 5D and E). Treatment with curcumin did not, however, prevent the hydrogen peroxide-induced increase in nuclear immunoreactivity, which occurred in grp94 antisense AS3A12 cells (Fig. 5F), as shown above for control S1C8 and untreated wild-type C2C12 cells.
Transient transfection with antisense grp94 cDNA was then performed to validate further the involvement of Grp94 in the antioxidant protection induced by curcumin. The rationale for these additional experiments was that the phenotype observed in stable transfected cells might reveal intrinsic differences and not the specific contribution of the protein of interest. Transiently transfected cells were visualized for the expression of β-galactosidase, whose cDNA was contained in the same phagemid, together with antisense grp94 cDNA (ASGrp94/βgal). The control construct contained, in sense orientation, the same segment of cDNA, which did not originate a translatable grp94 mRNA (control/βgal). After transfection, cells were either treated or not with curcumin and exposed 24 hrs later to H2O2 (800 μM for 2 hrs). Hydrogen peroxide treatment increased the nuclear immunoreactivity for NF-κB in untransfected cells (arrowheads in Fig. 6B), as well as in cells transfected with either antisense (arrows in Fig. 6B) or control constructs (not shown). Conversely, pre-treatment with curcumin hampered the hydrogen peroxide-induced increase in nuclear NF-κB immunoreactivity in untransfected (arrowheads in Fig. 6C and D) and control/βgal-transfected cells (arrows in Fig. 6D), whereas antisense-transfected cells displayed positive nuclear immunoreactivity (arrows in Fig. 6C).
Fig 6.
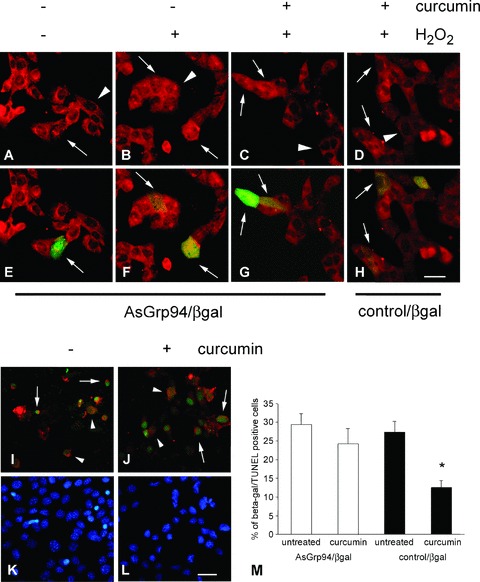
NF-κB nuclear translocation and apoptosis in C2C12 cells transiently transfected with antisense grp94 cDNA upon exposure to hydrogen peroxide. (A)–(H) Representative micrographs of C2C12 cell cultures transiently transfected with antisense grp94 cDNA vector containing β-galactosidase cDNA (AsGrp94/βgal) or with control/βgal vector, showing both single labelling for NFκB (A–D) and merging with staining for β-galactosidase (green fluorescence; E–H). Distribution of NF-κB immunoreactivity is shown after growth in the absence (A and B and E and F) or in the presence (C and D and G and H) of curcumin treatment, followed by the exposure to 800 μM hydrogen peroxide for 2 hrs (B–D and F–H). Arrows indicate transfected cells and arrowheads untrasfected ones. (I)–(L) Micrographs of C2C12 cell cultures transiently transfected with AsGrp94/βgal, showing merging of β-galactosidase labelling (red fluorescence) and TUNEL (green fluorescence). Apoptosis was evaluated after exposure to 800 μM hydrogen peroxide for 6 hrs, either in the absence (I) or in the presence (J) of curcumin pre-treatment. Arrows indicate apoptotic transfected cells, whereas arrowheads indicate TUNEL-negative transfected ones. (K) and (L) show counterstain of nuclei with DAPI. Bar: 30 μm. (M) Histogram shows the percentage of apoptotic nuclei observed among β-galactosidase-positive cells of C2C12 cultures transiently transfected with either ASGrp94/βgal (white bars) or with control/βgal vector (black bars), pre-treated with curcumin or left untreated and exposed to 800 μM hydrogen peroxide for 5–6 hrs. Mean values ± SEM; n= 8; *P < 0.003; anova test.
This experimental protocol was also used to evaluate the effect of antisense grp94 mRNA expression on curcumin-dependent anti-apoptotic protection. TUNEL assay was used to show apoptosis in curcumin-treated and untreated transfected cells after a 6-hr-exposure to H2O2 (800 μM). No significant difference was observed in the percentage of apoptotic β-galactosidase-positive cells evaluated on the amount of transfected cells between these two culture conditions (arrows, Fig. 6I, J and M). Conversely, the percentage of β-galactosidase-positive cells displaying apoptotic nuclei appeared significantly reduced in cells transfected with the control vector and pre-treated with curcumin, compared with parallel cultures either transfected with control vector but left untreated or transfected with antisense grp94 cDNA and pre-treated with curcumin (P < 0.003; Fig. 6M).
Increased Grp94 levels affect ER calcium release
We previously showed that, in the presence of stable Grp94 overexpression, C2C12 cells showed a reduced rate of cytosolic Ca2+ ([Ca2+]i) increase after exposure to a calcium ionophore [14]. Here we investigated in more detail the effects of Grp94 overexpression at the level of Ca2+ content of intracellular stores. Changes in [Ca2+]i were monitored with the Ca2+ indicator fura-2 (see Materials and methods) by applying a previously described protocol [19], which is here briefly summarized and shown in Figure 7A. Store depletion was induced by adding the SERCA inhibitor cyclopiazonic acid (CPA) in a Ca2+-free medium; the SERCA inhibitor, by inducing the passive release of stored Ca2+, caused a transient increase in [Ca2+]i, which is proportional to the Ca2+ content within the stores. Figure 7A shows [Ca2+]i changes in a representative experiment carried out with control S1C8 cells and three Grp94-overexpressing clones (82A11, 82E3, 83D2). The two latter clones (82E3 and 83D2) overexpressed Grp94 with unchanged level of Grp78 and calreticulin (mean values ± SEM of protein normalized to actin level were 2.20 ± 0.40 and 1.39 ± 0.03 for Grp94; 1.04 ± 0.14 and 0.84 ± 0.01 for Grp78; 0.81 ± 0.07 and 1.03 ± 0.33 for calreticulin, respectively, n= 4; Fig. S1B). The transient increase in [Ca2+]i elicited by CPA was significantly reduced in cells overexpressing Grp94 compared with controls, as well as the integral of the [Ca2+]i rise caused by CPA addition, an indirect measurement of the total Ca2+ content of intracellular stores. This latter parameter showed a reduction in all the three Grp94 overexpressing clones examined, ranging from 80% to 20% of the value detected in the control clone (80%, 40% and 17% for 83D2, 82E3 and 82A11 clones, respectively).
Fig 7.
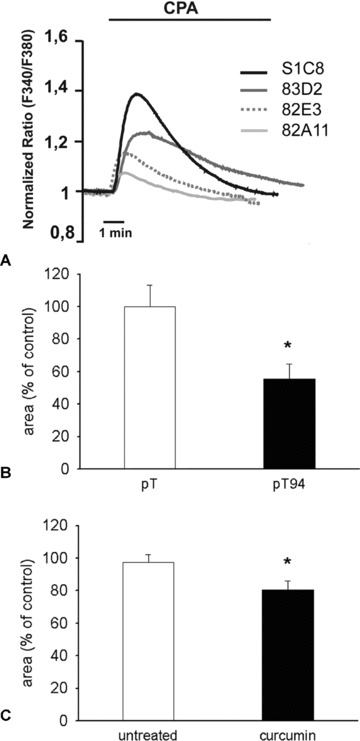
Intracellular Ca2+ content in either Grp94 overexpressing or curcumin-treated C2C12 cells. (A) Representative traces of C2C12 clones, either control (S1C8) or Grp94 overexpressing (82A11, 82E3 and 83D2) cells, loaded with fura-2, bathed in Ca2+-free, EGTA-containing medium and challenged with CPA (20 μM). [Ca2+]i changes are expressed as the ratio (F340/F380) normalized to the average value obtained within the first minute of the experiment. (B) Average Ca2+ content of intracellular stores measured, as described in (A), in transiently overexpressing Gpr94 (pT94) or control (pT) C2C12 cells. Values are expressed as the integral of the normalized ratio traces measured above the resting value upon CPA addition. Mean ± SEM; *P < 0.01; n= 8, Student’s t-test. (C) Average Ca2+ content of intracellular stores measured, as described in (A), in C2C12 cells left untreated and 24 hrs after curcumin treatment. Values are expressed as the integral of the normalized ratio traces measured above the resting value upon CPA addition. Mean ± SEM; *P= 0.03; n= 16; Student’s t-test.
Comparable results were observed after inducing selective Grp94 overexpression by means of transient transfection with pT94, with respect to C2C12 cells transfected with the empty vector (pT; Fig. 7B). In this experimental condition, Grp94 overexpression induced a marked mean reduction in the amount of Ca2+ released by CPA (45%), a strong effect usually found upon high level of protein expression by transient cDNA transfection. A similar, although less extensive, decrease in releasable Ca2+ from the intracellular stores was observed in wild-type C2C12 cells examined 24 hrs after the treatment with curcumin (5–10 μM; Fig. 7C; 18% of reduction compared with untreated cells; P= 0.03; n= 16).
Discussion
The induction of delayed cytoprotection represents a promising tool to circumvent cell and tissue damage secondary to acute exposure to stressing stimuli of high intensity [15]. A major limit to its widespread adoption is represented by the rather scanty availability of relatively safe ‘conditioning’ stimuli. The present study shows that the spice derivative curcumin may act as an inducer of a very mild ER stress response, characterized by about 40% increase in Grp94 protein levels. Despite its fair increase, Grp94 appears to be a key player in curcumin-induced delayed cytoprotection, reducing cellular protein oxidation, NF-κB nuclear translocation and apoptotic death, upon hydrogen-peroxide exposure of myogenic C2C12 cells. The participation of the Grp94 chaperone relies on the fact that the protective effect is present according to the selective modulation of its protein level (by means of sense or antisense grp94 cDNA transfection) and despite of curcumin treatment. Furthermore, we demonstrate that increased Grp94 levels significantly reduce Ca2+ release from intracellular stores in C2C12 cells, probably modulating the Ca2+ content within the ER.
Curcumin induces a cytoprotective ER-stress response
It has been shown that a sustained exposure to curcumin induces a pro-apoptotic ER stress response in neoplastic cell lines [22, 23]. Phosphorylation of PERK and its substrate, eIF2a, initiates after 3 hrs of exposure to 5–10 μM curcumin [22]. Here we report that a transient exposure to the drug induces a cytoprotective response in myogenic C2C12 cells, characterized by the selective increase of the ER chaperone Grp94. The precise mechanism through which curcumin evokes the ER stress response remains to be determined; a role might be played either by its reported ability to disrupt disulfide bond formation by the electrophilic dienone on its own chemical structure [22], to inhibit cellular proteasome activity [24] or to induce ER Ca2+ depletion, due to increased ion permeability of cellular membranes and inhibition of the SERCA pump [6, 25].
The cytoprotection obtained by a brief curcumin exposure appears effective against protein oxidation, NF-κB nuclear translocation and apoptosis induced by oxidative stress, even operating in the absence of the drug. In addition, to be evoked by oxidative stress itself [26], the ER stress-response protects against loss of viability due to oxidants exposure. An effector so far identified is Grp78. The protection, in fact, was observed when Grp78 protein levels were increased upon exposure to reducing agents (DTTox), SERCA inhibitors (thapsigargin), glycosylation inhibitors (tunicamycin) or calcium ionophores (A23187) [9–11], but it was lacking when the increase in Grp78 level was abolished by means of stable transfection with antisense grp78 cDNA [9, 10]. However, this body of evidence did not exclude the possibility that other Grp proteins participated to the cytoprotective effect. Among them is Grp94, whose expression levels are influenced by the modulation of those of Grp78 [9, 27]. We showed that a short exposure of C2C12 cells to curcumin increased Grp94 protein levels in a selective manner, at variance with other stimuli such as thapsigargin, much more effective in up-regulating Grp78 than Grp94 (our unpublished observations). Furthermore, this curcumin pulse did not induce expression of HO-1 nor did it increase the transcription and translation of Hsp70 or other small Hsps (31 and our unpublished observations), different from what was observed in other cell types [3, 28–30] or when administered concomitantly to Hsps inductors, i.e. heat stress [31].
This mild ER response observed in C2C12 cells upon a brief curcumin treatment not only significantly reduced procaspase-12 activation but also was effective in blunting protein oxidation and NF-κB nuclear translocation, similar to what was observed in the presence of the drug ([21] and this work).
Grp94 as a mediator of curcumin cytoprotection
Our results indicate that Grp94 represents a relevant mediator of the delayed cytoprotection induced by curcumin, regardless of the presence of other stress-proteins. Antisense grp94-transfected cells upon hydrogen peroxide exposure show impaired increase of Grp94 levels after a brief curcumin pre-treatment, as well as the lack of cytoprotective effects, i.e. high degree of protein oxidation, nuclear NF-κB translocation and apoptosis. Similarly to calreticulin, Grp94 binds high amounts of Ca2+[8, 32, 33], but, different from calreticulin [34, 35], its cellular levels inversely correlate to the rise in [Ca2+]i evoked by exposure either to calcium ionophore [14, 36] or to ischaemia-reperfusion [12]. This relationship deeply influences cell survival, in so far as increased levels of Grp94 appear cytoprotective, decreased or absent levels of the protein enhance cell susceptibility to death ([12, 14, 32–33, 36] and this work).
Oxidative stress causes a rapid increase in [Ca2+]i, which can result in enhanced Ca2+ influx into mitochondria, disrupting mitochondrial metabolism and leading to cell death [37–38]. Here we show that both stable and transient Grp94 overexpression reduces the release of Ca2+ from intracellular stores acutely induced by CPA. The possibility that Grp94 acted as cytosolic Ca2+ buffer due to the fact that part of the molecules could display a transmembrane localization [39], appears remote because in C2C12 cells, only a small fraction (12%) of Grp94 protein is detectable under such conformation [40]. Consistent with the increase of Grp94 protein levels, the present work shows that a simple brief exposure to curcumin reduced the intracellular CPA-releasable Ca2+ pool, measured 24 hrs after treatment, suggesting that Grp94 may modify intracellular Ca2+ content.
Conclusions
Although the precise molecular mechanism through which Grp94 affects cellular Ca2+ handling remains to be determined, its positive effects on the antioxidant cytoprotection of myogenic C2C12 cells are evident. In this context, the role of Grp94 in reducing the levels of releasable Ca2+ from intracellular stores reveals the major contribution of the Ca2+ signal in the potentiation of the injury induced by oxidative stress [37], in particular on protein oxidation and NF-κB nuclear translocation. A previous study investigating the protective role played by the ER stress response against oxidative damage showed that oxidant-induced cell death was prevented whereas lipid peroxidation was not [10]. Based on our results, we speculate that a reduced [Ca2+]i, due to increased Grp94 protein levels, restrains the effects of hydrogen peroxide on protein oxidation and on the pathways involved in NF-κB nuclear translocation [20].
In conclusion, our study enlightens a major mechanism through which curcumin exerts delayed cytoprotection. Considering the low oral bioavailability of curcumin and its quick catabolism by liver, it is possible to suggest that several systemic cytoprotective effects, observed after oral curcumin assumption in both animal experimental studies and human trials [1–3], might be explained by its ability to evoke, at a very low dose and in few hours, a mild ER stress response in distal tissues.
Acknowledgments
The financial support of Ministero dell’Istruzione Università e Ricerca (FIRB grant no. RBIN042Z2Y to L.G. and P.P.) and Agenzia Spaziale Italiana (grant OSMA-WP1B51–2 to L.G.) are gratefully acknowledged.
Supporting Information
Fig. S1 Representative Western blots of 15 μg of wholelysates of S1C8 control clone and Grp94 overexpressing 81A11(A), 83D2 and 82E3 (B) cell clones. Blots werestained with antibodies for Grp94, Grp78 and calreticulin (CRT).Actin staining is shown as a reference for sample loading.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Hatcher H, Planalp R, Cho J, et al. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–52. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma RA, Gesher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41:1955–68. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Calabrese V, Bates TE, Mancuso C, et al. Curcumin and the cellular stress response in free radical-related diseases. Mol Nutr Food Res. 2008;52:1062–73. doi: 10.1002/mnfr.200700316. [DOI] [PubMed] [Google Scholar]
- 4.Motterlini R, Foresti R, Bassi R, et al. Curcumin, an antioxidant and antiinflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–12. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 5.Scapagnini G, Foresti R, Calabrese V, et al. Caffeic acid phenethyl ester and curcumin: a novel class of heme oxygenase-1 inducers. Mol Pharmacol. 2002;61:554–61. doi: 10.1124/mol.61.3.554. [DOI] [PubMed] [Google Scholar]
- 6.Bilmen JG, Zafar Khan S, Javed M, et al. Inhibition of the SERCA Ca2+ pumps by curcumin. Curcumin putatively stabilizes the interaction between the nucleotide-binding and phosphorylation domains in the absence of ATP. Eur J Biochem. 2001;268:6318–27. doi: 10.1046/j.0014-2956.2001.02589.x. [DOI] [PubMed] [Google Scholar]
- 7.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y, Hendershot LM. ER chaperone functions during normal and stress conditions. J Chem Neuroanat. 2004;28:51–65. doi: 10.1016/j.jchemneu.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Bowes RC III, Van De Water B, et al. Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances and cell death in renal epithelial cells. J Biol Chem. 1977;272:21751–9. doi: 10.1074/jbc.272.35.21751. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Miller E, Van De Water B, et al. Endoplasmic reticulum stress protein block oxidant-induced Ca2+ increase and cell death. J Biol Chem. 1998;273:12858–62. doi: 10.1074/jbc.273.21.12858. [DOI] [PubMed] [Google Scholar]
- 11.Hung CC, Ichimura T, Stevens JL, et al. Protection of renal epitelial cells against oxidative injury by endoplasmic reticulum stress preconditioning is mediated by ERK1/2 activation. J Biol Chem. 2003;278:29317–26. doi: 10.1074/jbc.M302368200. [DOI] [PubMed] [Google Scholar]
- 12.Bando Y, Katayama T, Kasai K, et al. GRP94 (94 kDa glucose-regulated protein) suppresses ischemic neuronal cell death against ischemia/reperfusion injury. Eur J Neurosci. 2003;18:829–40. doi: 10.1046/j.1460-9568.2003.02818.x. [DOI] [PubMed] [Google Scholar]
- 13.Miyake H, Hara I, Arakawa S, et al. Stress protein GRP78 prevents apoptosis induced by calcium ionophore, ionomycin, but not by glycosylation inhibitor, tunicamycin, in human prostate cancer cells. J Cell Biochem. 2000;77:396–408. doi: 10.1002/(sici)1097-4644(20000601)77:3<396::aid-jcb5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Vitadello M, Penzo D, Petronilli V, et al. Overexpression of the stress-protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J. 2003;17:923–5. doi: 10.1096/fj.02-0644fje. [DOI] [PubMed] [Google Scholar]
- 15.Das M, Das DK. Molecular mechanism of preconditioning. IUBMB Life. 2008;60:199–203. doi: 10.1002/iub.31. [DOI] [PubMed] [Google Scholar]
- 16.Gorza L, Vitadello M. Reduced amount of the glucose-regulated protein GRP94 in skeletal myoblasts results in loss of fusion competence. FASEB J. 2000;14:461–475. doi: 10.1096/fasebj.14.3.461. [DOI] [PubMed] [Google Scholar]
- 17.Tarricone E, Scapin C, Vitadello M, et al. Cellular distribution of Hsp70 expression in rat skeletal muscles. Effects of moderate exercise training and chronic hypoxia. Cell Stress Chaper. 2008;13:483–95. doi: 10.1007/s12192-008-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalla Libera L, Ravara B, Gobbo V, et al. Skeletal muscle myofibrillar protein oxidation in heart failure and the protective effect of carvedilol. J Mol Cell Cardiol. 2005;38:803–7. doi: 10.1016/j.yjmcc.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Zatti G, Ghidoni R, Barbiero L, et al. The presenilin 2 M239I mutation associated with familial Alzheimer’s disease reduces Ca2+ release from intracellular stores. Neurobiol Dis. 2004;15:269–78. doi: 10.1016/j.nbd.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Takada Y, Mukhopadhyay A, Kundu GC, et al. Hydrogen peroxide activates NF-κB through tyrosine phosphorylation of IκBα and serine phosphorylation of p65. Evidence for the involvement of IκBα kinase and Syk protein-tyrosine kinase. J Biol Chem. 2003;278:24233–41. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 21.Singh S, Aggarwal BB. Activation of transcription factor NF-κB is suppressed by curcumin (Diferulolylmethane) J Biol Chem. 1995;270:24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 22.Pae H-O, Jeong S-O, Jeong G-S, et al. Curcumin induces pro-apoptotic endoplasmic reticulum stress in human leukemia HL-60 cells. Biochem Biophys Res Comm. 2007;353:1040–5. doi: 10.1016/j.bbrc.2006.12.133. [DOI] [PubMed] [Google Scholar]
- 23.Bakhshi J, Weinstein L, Poksay KS, et al. Coupling endoplasmic reticulum stress to the cell death program in mouse melanoma cells: effect of curcumin. Apoptosis. 2008;13:904–14. doi: 10.1007/s10495-008-0221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jana NR, Dikshit P, Goswami A, et al. Inhibition of proteasomal function by curcumin induces apoptosis through mitochondrial pathway. J Biol Chem. 2004;279:11680–5. doi: 10.1074/jbc.M310369200. [DOI] [PubMed] [Google Scholar]
- 25.Wootton LL, Michelangeli F. The effects of the phenylalanine 256 to valine mutation on the sensitivity of sarcoplasmic/ endoplasmic reticulum Ca2+ ATPase (SERCA) Ca2+ pump isoforms 1, 2, and 3 to thapsigargin and other inhibitors. J Biol Chem. 2006;281:6970–6. doi: 10.1074/jbc.M510978200. [DOI] [PubMed] [Google Scholar]
- 26.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–93. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 27.Li L-I, Li X, Ferrario A, et al. Establishment of a Chinese hamster ovary cell line that expresses grp78 antisense transcripts and suppresses A23187 induction of both GRP78 and GRP94. J Cell Physiol. 1992;153:575–82. doi: 10.1002/jcp.1041530319. [DOI] [PubMed] [Google Scholar]
- 28.Scapagnini G, Colombrita C, Amadio M, et al. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid Redox Signal. 2006;8:395–403. doi: 10.1089/ars.2006.8.395. [DOI] [PubMed] [Google Scholar]
- 29.Pugazhenthi S, Akhov L, Selvaraj G, et al. Regulation of Heme oxygenase-1 expression by demethoxy curcuminoids through Nrf2 by a PI 3-kinase/Akt-mediated pathway in mouse beta cells. Am J Physiol Endocrinol Metab. 2007;293:E645–55. doi: 10.1152/ajpendo.00111.2007. [DOI] [PubMed] [Google Scholar]
- 30.Scharstuhl A, Mutsaers HAM, Pennings SWC, et al. Curcumin-induced fibroblast apoptosis and in vitro wound contraction are regulated by antioxidants and heme oxygenase: implications for scar formation. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00339.x. April 3, DOI: 10.1111/j.1582--4934.2008.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato K, Ito H, Kamei K, et al. Stimulation of the stress-induced expression of stress proteins by curcumin in cultured cells and in rat tissues in vivo. Cell Stress Chaper. 1998;3:152–60. doi: 10.1379/1466-1268(1998)003<0152:sotsie>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biswas C, Ostrovsky O, Makarewich CA, et al. The peptide binding of GRP94 is regulated by calcium. Biochem J. 2007;405:233–41. doi: 10.1042/BJ20061867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Little E, Ramakrishnan M, Roy B, et al. The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit Rev Eukaryot Gene Expr. 1994;4:1–18. doi: 10.1615/critreveukargeneexpr.v4.i1.10. [DOI] [PubMed] [Google Scholar]
- 34.Groenendick J, Lynch J, Michalak M. Calreticulin, Ca2+ and calcineurin. Signalling from the endoplasmic reticulum. Mol Cells. 2004;17:383–38. [PubMed] [Google Scholar]
- 35.Ihara Y, Urata Y, Goto S, et al. Role of calreticulin in the sensitivity of myocardial H9c2 cells to oxidative stress caused by hydrogen peroxide. Am J Physiol Cell Physiol. 2006;290:C208–21. doi: 10.1152/ajpcell.00075.2005. [DOI] [PubMed] [Google Scholar]
- 36.Bando Y, Katayama T, Aleshin AN, et al. GRP94 reduces cell death in SH-SY5Y cells perturbated calcium homeostasis. Apoptosis. 2004;9:501–8. doi: 10.1023/B:APPT.0000031446.95532.ad. [DOI] [PubMed] [Google Scholar]
- 37.Davidson SM, Duchen MR. Calcium microdomains and oxidative stress. Cell Calcium. 2006;40:561–74. doi: 10.1016/j.ceca.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 38.Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol. 2007;17:511–7. doi: 10.1016/j.tcb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Reddy RK, Lu J, Lee AS. The endoplasmic reticulum chaperone glycoprotein GRP94 with Ca(2+)-binding and antiapoptotic properties is a novel proteolytic target of calpain during etoposide-induced apoptosis. J Biol Chem. 1999;274:28476–83. doi: 10.1074/jbc.274.40.28476. [DOI] [PubMed] [Google Scholar]
- 40.Frasson M, Vitadello M, Brunati AM, et al. Grp94 is Tyr-phosphorylated by Fyn in the lumen of the endoplasmic reticulum and translocates to Golgi in differentiating myoblasts. Biochim Biophys Acta – Mol Cell Res. 2009;1793:239–52. doi: 10.1016/j.bbamcr.2008.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Representative Western blots of 15 μg of wholelysates of S1C8 control clone and Grp94 overexpressing 81A11(A), 83D2 and 82E3 (B) cell clones. Blots werestained with antibodies for Grp94, Grp78 and calreticulin (CRT).Actin staining is shown as a reference for sample loading.


