Abstract
The fields of tissue engineering (TE) and regenerative medicine (RegMed) are yet to bring about the anticipated therapeutic revolution. After two decades of extremely high expectations and often disappointing returns both in the medical as well as in the financial arena, this scientific field reflects the sense of a new era and suggests the feeling of making a fresh start although many scientists are probably seeking reorientation. Much of research was industry driven, so that especially in the aftermath of the recent financial meltdown in the last 2 years we have witnessed a biotech asset yard sale. Despite any monetary shortcomings, from a technological point of view there have been great leaps that are yet to find their way to the patient. RegMed is definitely bound to play a major role in our life because it embodies one of the primordial dreams of mankind, such as: everlasting youth, flying, remote communication and setting foot on the moon. The Journal of Cellular and Molecular Medicine has been at the frontier of these developments in TE and RegMed from its beginning and reflects recent scientific advances in both fields. Therefore this review tries to look at RegMed through the keyhole of history which might just be like looking ‘back to the future’.
Keywords: regenerative medicine, tissue engineering, genetical engineering, stem cells, biomaterials, cell therapies, history, economics
‘Those who cannot learn from history are doomed to repeat it’. G. Santayana, ‘The life of reason’[1].
Introduction
Regenerative medicine (RegMed) is an interdisciplinary field of research and clinical applications focused on the repair, replacement or regeneration of cells, tissues or organs to restore impaired function resulting from any cause, including congenital defects, disease, trauma and ageing. It uses a combination of several converging technological approaches, both existing and newly emerging, that moves it beyond traditional transplantation and replacement therapies. These approaches may include, but are not limited to, the use of soluble molecules, gene therapy, stem and progenitor cell therapy, tissue engineering (TE) and the reprogramming of cell and tissue types [2]. Tremendous research developments and advantages in the stem cell arena have shed new lights on future therapeutic possibilities and it seems logical that actively modulating or guiding repair processes holds the promise of regenerating damaged tissues and organs in the body by stimulating previously irreparable organs to heal themselves [3–6]. The prospect of transplanting in vitro grown organs and tissues has been widely investigated in the field of TE [7]. Rapid success seen with early TE results have stimulated scientists and clinicians to further pursue regenerative ideas when dealing with tissue failure or loss [8–18].
A brief review in the recent bibliography concerning advances in TE and RegMed would raise the impression that ‘we are almost there’, being able to produce tissue replacement ‘off the shelf’, in the near future for everyone in need [11, 14, 17, 19–24]. Interestingly enough, that was exactly the spirit some 10 years ago [25, 26]. It seems – literally spoken – that we are able to fabricate constructs that ‘look like tissue, smell like tissue and taste like tissue’ but not some that also function equally like one. An ever perpetuating evolution is yet to bring the long awaited revolution in the health sector. More critically spoken, others have proclaimed that the time must come to ‘stop tissue engineering and start engineering tissues’[27]. A brief historical tour of regeneration and RegMed constitutes along with a critical analysis of the present, the filter through which we put together the puzzle of its future.
Regeneration in the ancient world
The myth of the great Titan Prometheus, as introduced by Hesiod in his Theogony (8th century B.C.) provides an icon for RegMed. When he stole fire, a symbol for civilization and technology, to give it to mankind he received a cruel punishment [28]. Jupiter had him chained to the Carpathian Mountains, where an eagle by the name of Ethon would pick at his liver every day, which would in turn regenerate during the night (Fig. 1). His torture lasted 30,000 years until he was freed by Hercules [29]. The ancient Greeks were aware of the regenerating capacity of liver, hence they named it ‘hepar’ (Greek: 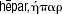 ) after
) after 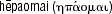 , meaning to ‘repair oneself’.
, meaning to ‘repair oneself’.
Fig 1.

The myth of the Titan Prometheus provides an icon for RegMed. Prometheus was known as the benefactor of mankind for his desire to assist mortals and give them many beneficial gifts that helped them to survive and live prosperous lives. His duty as a god was to form man from water and earth, and in doing so, gave them each a gift of strength or speed, craftiness or wisdom, and many other benefactors that improved their ways of living. The most well-known gift of Prometheus was the gift of fire which Prometheus stole from Zeus’ lightning bolts. Prometheus’ defiance and betrayal provoked Zeus to have him chained to Mount Caucasus, where an eagle ate daily from his ever-regenerating liver. As an immortal Titan, Prometheus’ liver grew by night what the eagle had eaten the day before. His torture lasted 30,000 years until he was freed by Hercules.
Three of Aristotle’s works on natural history have survived. These are History of Animals, Generation of Animals and Parts of Animals. He stated that animals in the early developmental stages have a higher potential for regeneration. He made detailed descriptions about regeneration on the limbs of salamanders and deer antlers [30]. He propagated that biological form originates from undifferentiated matter and clearly favoured what would later be described as ‘epigenesis’.
In the second chapter of Genesis, providing another example of epigenetic regeneration, ‘the Lord God built up into a woman the rib that he had taken from the man’[31]. Several thousand years later, the first transplantation of a moor’s leg in replacement of a patient’s diseased lower extremity was attributed to the physician – saints Cosmas and Damian, demonstrating the everlasting quest for tissue replacement [32]. Finally, data from a survey of more than 10,000 mummies from prehistoric Peru demonstrated that cranial trepanation was a routine procedure as early as 3000 B.C. This procedure was sometimes followed by alloplastic reconstruction, as demonstrated by shells, gourds and silver or gold plates found next to trepanated skulls [33].
Hence, it is evident that mankind has been following the endeavour of regeneration since the prehistoric ages. The dream to master regeneration is one primordial ambition like flying or communicating over distances and man cannot rest until he has mastered this quest too.
Regeneration in early research
Descartes (1596–1650) in his ‘L’ Homme’, was the first to defy a divine meaning in biological phenomena and stated that the function of the human body can be interpreted by means of its physical and chemical properties. However, it was not until the middle of the 18th century that the idea of an abstract motive power of biological organisms was dismissed by the general public and the scientific elite, when Lavoisier (1743–1794) postulated that the chemical processes governing life could be reproduced and studied in the laboratory. During the same time naturalists were taking sides on the phenomena of generation and regeneration: Pre-formationists supported that appendages to be regenerated and organisms to be born pre-existed as miniatures at the site of interest. So, at the base of a severed lizard tail, in their conception a miniature tail was preformed and waited to be ‘activated’ by an amputation. Likewise, in the sperm or in the ovum of the human there existed a miniature ‘homunculus’ that grew into a newborn infant. This theory was in accordance with the Christian beliefs of creation and prevailed until the middle of the 18th century. On the contrasting end, came the Aristotelean thesis that undifferentiated matter was able to give rise to life. This theory had been actually named ‘epigenesis’ by William Harvey (1578–1657) in his work ‘on the generation of animals’ grossly repeating on Aristotle’s works. Abraham Trembley (1710–1784) produced several publications on the regenerative phenomena on freshwater polyps. He managed to obtain a clone of 50 polyps from one organism that he had quartered. He performed sections at every conceivable plane, contradicting pre-formational beliefs of the time [34]. The question was posed: If the animal soul was the organizing and unifying element of life, how could a newly regenerated form arise? Until the end of the 18th century philosophical and religious debate linked to the science of regeneration was set aside, and epigenesis gained acceptance with the eventual ascendancy of epigenetic and developmental embryology bringing about a revolution in natural sciences.
Once the religious and social burdens had fallen, the 19th century was marked by a rapid succession of developments. Schleiden and Schwann described in 1838–1839 the cell theory. In 1858, Rudolf L. K. Virchow stated the famous omnis cellula ex cellula and through microscopic observations he confirmed the cell theory and established the idea of cells being the elementary units of life able to replicate themselves by division [35, 36]. In 1867, the eminent German pathologist Julius Cohnheim postulated what became known as the ‘Cohnheim hypothesis’. He suggested that all of reparative cells taking part in the regeneration of wounds come from the bloodstream (and therefore from the bone marrow) [37]. Finally, at the end of the 19th century, Barth observed that upon autologous bone transplantation in hounds the vast majority of cells die and leave a scaffolding behind to be slowly repopulated by new host cells and an adequate neovascular network [38].
In 1912, Alexis Carrel, a French scientist working at the Rockefeller Research Institute in New York, started a culture from a small slice of heart muscle taken from a chick embryo [39]. This culture continued for several decades, outliving the normal chicken’s lifespan. His belief that cells in culture were inherently immortal dominated the early 20th century until in the 1960s Leonard Hayflick and Paul Moorhead defied this dogma and proposed the ‘Hayflick limit’, according to which differentiated cells in culture are not able to replicate more than 40–60 times and are bound to display signs of senescence with successive passages [40].
However, some mutated lines possessed the capacity to replicate themselves forever, if kept undifferentiated in culture. By 1967 Leroy Stevens had developed a strain of mice, with a high incidence of spontaneous testicular teratomas. These neoplasias displayed a characteristic mixture of different tissues lined up next to each other randomly. By the end of the 1960s it was established that they originated from germ cells that were able to give rise to a plethora of different tissues. So the concept of pluripotency of germinal cells was introduced [41] and this tumour cell was named embryonal carcinoma stem cell (EC). Research with EC stem cells expanded considerably in the 1970s. In a series of experiments, chimeric mice were produced by injecting ECs into early blastocysts [42]. Because ectopic blastocyst injections were also found to generate teratomas it became soon evident that pluripotent cells could also be derived from blastocysts directly [43]. Soon the next logical step was undertaken, when Gail Martin [44] in the United States and Martin Evans [45] in England generated in 1981 a stable diploid cell line that could generate every tissue of the adult body, including germ cells. Gail Martin referred to her cells as ‘embryonic stem cells’ and gave them the nickname ‘ES cells’.
However, the human equivalent was not available at that time. Researchers performed studies on cells obtained from blastocysts of primates, including rhesus monkeys and marmosets [46] as well as human EC stem cell lines isolated from a rare tumour of the male testes, after orchiectomy procedures [47]. However, in 1998 some couples undergoing treatment for extracorporeal fertilization donated a surplus of blastocysts for experimental purposes. James Thomson isolated and cultivated a human ES line from these blastocysts [48]. A new era had dawned and the prospects of this technology seem overwhelming.
Tissue engineering
In 1981, Eugene Bell from MIT in Boston drew some attention with his work on ‘living skin equivalents’[49]. The year thereafter he received funding for preparing a cell-based vascular scaffold and in 1982 the funding companies claimed in a press conference to be ‘pursuing research and development efforts in business segments including TE and ‘smart’ computer systems’[50]. A couple of years later, just down the aisle in MIT, Joseph Vacanti of Children’s hospital approached Robert Langer with the idea of constructing custom made scaffolds for cell loading in an effort to overcome organ shortage for children in need of transplants. It was not until 1987 when during a special session of the US National Science Foundation meeting in Washington DC the term ‘tissue engineering’ was officially coined for the whole field. The first conference on the ‘engineering of living tissue’ was held in 1988 at lake Tahoe, California and the proceedings of this meeting were published a year later as a book titled ‘Tissue Engineering’[51]. In these proceedings a definition for the new field was given by Robert Nerem:
‘Tissue engineering is the application of the principles and methods of engineering and the life sciences towards the fundamental understanding of structure/function relationships in normal and pathological mammalian tissues and the development of biological substitutes to restore, maintain, or improve functions’.
This definition was cited again in brief in a 1993 publication by Vacanti and Langer in Science– maybe the most cited early review on TE [7]. Two years later the BBC broadcast of images of a mouse from Charles Vacanti’s laboratory with a tissue engineered ear ‘growing’ off its back made the round of the world. The demonstration of this rodent, given the nickname ‘auriculosaurus’, was a turning point for TE and aroused a huge public interest on new biotechnological advances [4].
In 1996, the Tissue Engineering Society International (TESi) was officially founded by Joseph and Charles Vacanti, and the Asian TE societies were incorporated in TESi by 2000. Soon thereafter, Raymund E. Horch and G. Björn Stark from Freiburg encouraged the foundation of the European branch of TESi the ETES, and they hosted in 2001 the TESi meeting in Germany. Time magazine described with a cover story, tissue engineers as the ‘hottest job’ for the future: ‘With man-made skin already on the market and artificial cartilage not far behind, 25 years from now scientists expect to be pulling a pancreas out of a Petri dish’[52]. By the turn of the century, there were over 3000 people working in the sector, with funding exceeding US $580 million [53, 54].
The era of regenerative medicine
The transition from TE into RegMed was justified by dramatic developments in the financial, scientific and political landscape. From a financial point of view, the first years of the 21st century, anticipated to bring the biotechnological revolution, were characterized by a disconnect between expectations and reality. As Lysaght noted in 2004 ‘…Although aggregate development costs exceed $4.5 billion, the field has yet to produce a single profitable product’[54].
On the scientific arena new technologies had come into play, with the very term TE being unable to accommodate them: The availability of human embryonic stem cells was bringing the cellular therapy at the front line of the field. Gene technology had come to a point, where a whole mammal could be cloned or genetically manipulated (the Monsanto swine case) [55]. Drug delivery systems and nanotechnology were developed and integrated in the design of biomaterials [56]. The paramount significance of angiogenic processes [23, 24, 57] for homeostasis, biointegration and upscaling of tissue engineered constructs became clear [19, 58–60]. Experimental activities were directed to encompass integrative strategies towards generation of autonomously vascularized bioartificial tissue elements [61, 62] (Fig. 2). In addition tumour research was further advanced with the prospect of revolutionizing cancer treatment to round up progress in the biotechnological arena [8, 26].
Fig 2.
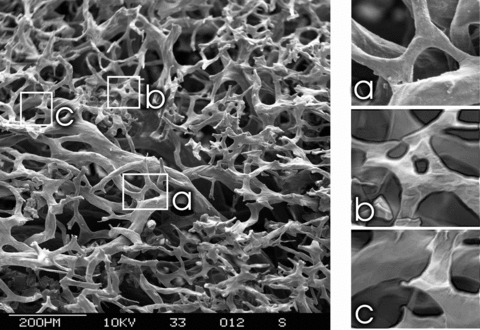
Scanning electron microscopy of a microvascular corrosion cast. This is a replica of a neovascular network created by means of an arteriovenous loop in the rat model under influence of VEGF(165). On the left side an overview demonstrating a relative nascent network (2 weeks after creation of the loop) with numerous new capillaries demonstrating different stages of arborization and remodelling. (a) Loop formation by means of intercapillary bridging. (b) Intussuceptive (non-sprouting) angiogenesis with intervascular division. This is a particularly energy saving modus of angiogenesis and remodelling. (c) Neovascular sprouts (sprouting angiogenesis) are seen all around the picture, demonstrating a vivid ongoing angiogenesis.
The course of RegMed was also shaped by a political event. The US legislature had frozen most of federal granting for stem cell research. Robert Nicholas Klein II, a lawyer and real-estate developer, whose son suffered from diabetes mellitus type I, and whose mother suffered from Alzheimer’s invested over $3 million of his own money, and organized a public effort to put pressure on the government of California to change policy on stem cell research and RegMed. On November 2, 2004, the Proposition No. 71 passed through a public ballot initiative with a 59.05% to 40.95% majority. As a response to that, the California Institute of Regenerative Medicine was established to supervise a huge funding of more than $3 billion over 10 years.
Regenerative medicine today
The recent financial meltdown knocked the air out of biotech by devastating its already floundering inflow of investment funds. From the end of 2007 until 2010 the industry had no initial public offering–introduction of shares in the public markets, and in the last 2 years we have witnessed a biotech asset yard sale with bigger pharma houses writing the cheque. The few survivors have turned their back to research altogether because like so often in science excellent scientific ideas and discoveries that provoke enthusiastic expectations turn out to be more difficult when it comes to routine clinical applications. When no immediate clinical results are visible industrial research funding becomes a problem. Investors are taking their money out of the industry thus driving stock price floors lower and by the end of 2009 as many as 79% of the biotech firms were trading under their opening price [63]. No big hope for a cyclical recovery in funding exists momentarily because, as mentioned, investors have snubbed biotech for a decade. Even at the peak of the NASDAQ in October 2007 the percentage of industry players seeing their initial public enterprise value decline (trading under their opening price) was 59%. In the aggregate, even if the capital markets recover, it is unlikely that investors will flock to biotech hedge funds like it was believed earlier. Vivid fantasies of lifesaving new cures and tantalizing financial forecasts in the 1990s have made space for tight fists and raised eyebrows among investors.
However, this is hardly a phenomenon limited to the life sciences. Since 1995 the Gartner Advisory Group has devised a methodology to describe the ‘ups and downs’ of new technologies and relate them to business considerations [64].
These common general observations from the financial world seem to be also worthwhile to be considered by life science researchers, because of their analogy to scientific developments and their relevance to potential research funding sources. According to the hype cycles theory, a new technology is bound to undergo a series of five phases. As a first event there is a ‘technology trigger’. During this step, the new technology draws considerable attention by a breakthrough or a major press release. Soon thereafter, there is a ‘peak of inflated expectations’ during which over enthusiasm and overt publicity initiates a rage of investments. During this period there is a disconnection between expectations and performance. In the ‘trough of disillusionment’ failure to meet the expectations, renders the technology unfashionable and unattractive in the public mind and for investors. During the ‘slope of enlightenment’ although public interest has grossly deteriorated, in some centres the practical application and commercial potential is reassessed. Finally, in the ‘plateau of productivity’ the field re-emerges with viable concepts and profitability. The initial ideas are translated into business applications and products (Fig. 3).
Fig 3.

The ‘Gartner Hype Cycle’ for new technological advances in regard to financial and business considerations.
Understanding innovative technologies is pivotal to developing successful investment strategies in the biotech sector and hence is influential in developing the field of RegMed. Differences around the world seem to play a role. In a European conference held in May 2010 in Brussels on innovation in healthcare with focus on small and medium enterprises (SME) relevant to the sector, there was a consensus that there are serious socio-economical barriers to be overcome, before the European landscape becomes nearly as favourable as in the United States [65]. Until then European grown talent will be constantly ‘crossing the Atlantic’ not only in respect to bioengineering expertise, but also in terms of talented fund managers. Nevertheless, within the European Framework Programme (FP7) some 15% of a total of €6 billion dedicated to health research were funnelled into SMEs. Another €2 billion has been separately invested in the Innovative Medicines Initiative, a public–private group that enhances collaboration between companies and experts on pre-competitive research projects. In autumn 2010 the European commission is about to publish the research and innovation strategy for the coming decade.
The Innovative Medicines Initiative represents an issue important to the ecosystem of health related research and development. Although state funding is key to the foundling steps of innovative health technologies, eventually these advances will translate into products and services and will be integrated into the regular system of investment and revenue. There is a grey area, however, where the biotechnological advances escape the level of basic academic research but are also a step behind from becoming a proven concept ready for clinical application. This area has been described by several authors as the ‘death valley’[66] and it is dictated not only by a ‘financial gap’ but equally by a technical one as well as a gap of information and trust. Specifically for RegMed one could add legal and ethical problems into this metaphor [67]. Small enterprises are unlikely to support a tedious effort of bringing an innovative technology to the point of applicability and profitability, whereas large-capital companies are better equipped, with a robust financial and political backbone.
Despite any financial up and downs and the driving forces of various funding sources RegMed will definitely play a major role in the future of medical therapy. Besides the ever growing knowledge of adult or embryonic stem cells and their regulation many patients might profit from new insights in the near future [68–70]. As an example, preservation of cord blood stem cells is gaining popularity after promising results on their use for brain injury [71] and type 1 diabetes mellitus [72] as well as stroke [73] and hearing loss [74].
The capacity of liver to regenerate is known by millennia, but the dogma that heart is a nonregenerating organ has been only recently challenged. Lately, niches of putative cardiac stem cells were identified in the murine and human heart [76–78]. These data engender a new direction in RegMed basic research – the necessity to discover the natural microenvironment and consequently, to engineer the artificial support essential for stem cells to act [79–81]. Finally, heart diseases might be prone to therapy by means of a new line of cells, the unique cardiac telocytes (Fig. 4) responsible for a continuous regeneration in the mammalian heart [5, 10, 75, 82] and, possible repair, eventually. Under the light of such recent achievements of research in RegMed one might come back to the saga of Prometheus to find out that it seems likely that not only the liver may carry the ability to regenerate in itself but also other vital organs like the heart might well be able to renew themselves. Further molecular and cellular research into the functions of telocytes could well reveal new tools to regenerative reprogramming of lost organ functions. It is more than tempting to speculate that telocytes could be a novel, possible target for therapeutic strategies aimed at potentiating cardiac repair and regeneration after ischemic injury.
Fig 4.

Digitally coloured transmission electron micrographs of mouse heart show telocytes (TC) in blue. (A) Cardiac structural unit composed by cardiomyocyte (CM), blood vessel and TC. Telocyte extends its characteristic, very long and thin, process (Tp-telopode) between arteriole and cardiomyocyte. (B) Regenerative cardiac unit composed by cardiomyocyte progenitor (CMP), capillary (cap), nerve and TC.
Conclusion
Many research projects in the field of RegMed are dependent on state und industrial funding because public money is not available in sufficient amounts. The recent financial collapse has served as the straw that broke the camel’s back in a decade-long erosion in the business fundamentals of the biotech industry. Big pharmaceutical corporations have been acquiring some of the most significant biotech independents, possibly marking a new era where RegMed leaves the academic labs of universities and the small boundaries of SMEs for gainful applications in the marketplace. However, RegMed definitely represents much more than dividends to shareholders. The quest for regeneration is one of the primordial dreams of mankind, like flying, remote communication and setting foot on the moon. It is the destiny of mankind to reach for its visions, and therefore RegMed is bound to succeed sooner or later.
Acknowledgments
We thank the Hanns Georg and Xue Hong Geis Foundation for financial support of research in Regenerative Medicine and Tissue Engineering.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Santayana G. Reason in common sense. The life of reason. 2. New York: Charles Scribner’s Sons; 1905. p. 284. [Google Scholar]
- 2.Daar AS, Greenwood HL. A proposed definition of regenerative medicine. J Tissue Eng Regen Med. 2007;1:179–84. doi: 10.1002/term.20. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Lau SF, Heng BF, et al. Generation of easily accessible human kidney tubules on two-dimensional surfaces in vitro. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01113.x. Doi: 10.1111/j.1582-4934.2010.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spadaccio C, Rainer A, Trombetta M, et al. A G-CSF functionalized scaffold for stem cells seeding: a differentiating device for cardiac purposes. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01100.x. Doi: 10.1111/j.1582-4934.2010.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaetani R, Rizzitelli G, Chimenti I, et al. Cardiospheres and tissue engineering for myocardial regeneration: potential for clinical application. J Cell Mol Med. 2010;14:1071–7. doi: 10.1111/j.1582-4934.2010.01078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi YS, Dusting GJ, Stubbs S, et al. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J Cell Mol Med. 2010;14:878–89. doi: 10.1111/j.1582-4934.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan IM, Scott T, Tandle AT, et al. Radiosensitization of glioma cells by modulation of Met signaling with the hepatocyte growth factor neutralizing antibody, AMG102. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01122.x. Doi: 10.1111/j.1582-4934.2010.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pneumaticos SG, Triantafyllopoulos GK, Basdra EK, et al. Segmental bone defects: from cellular and molecular pathways to the development of novel biological treatments. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01062.x. Doi: 10.1111/j.1582-4934.2010.01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Zhou J, Liu Z, et al. Injectable cardiac tissue engineering for the treatment of myocardial infarction. J Cell Mol Med. 2010;14:1044–55. doi: 10.1111/j.1582-4934.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mollmann H, Nef HM, Voss S, et al. Stem cell-mediated natural tissue engineering. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00972.x. Doi: 10.1111/j.1582-4934.2009.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballyns JJ, Bonassar LJ. Image-guided tissue engineering. J Cell Mol Med. 2009;13:1428–36. doi: 10.1111/j.1582-4934.2009.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nesselmann C, Ma N, Bieback K, et al. Mesenchymal stem cells and cardiac repair. J Cell Mol Med. 2008;12:1795–810. doi: 10.1111/j.1582-4934.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiegel HC, Pryymachuk G, Rath S, et al. Foetal hepatocyte transplantation in a vascularized AV-Loop transplantation model in the rat. J Cell Mol Med. 2010;14:267–74. doi: 10.1111/j.1582-4934.2008.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroehne V, Heschel I, Schugner F, et al. Use of a novel collagen matrix with oriented pore structure for muscle cell differentiation in cell culture and in grafts. J Cell Mol Med. 2008;12:1640–8. doi: 10.1111/j.1582-4934.2008.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eyden B. The myofibroblast: phenotypic characterization as a prerequisite to understanding its functions in translational medicine. J Cell Mol Med. 2008;12:22–37. doi: 10.1111/j.1582-4934.2007.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horch RE. Future perspectives in tissue engineering. J Cell Mol Med. 2006;10:4–6. doi: 10.1111/j.1582-4934.2006.tb00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horch RE, Kopp J, Kneser U, et al. Tissue engineering of cultured skin substitutes. J Cell Mol Med. 2005;9:592–608. doi: 10.1111/j.1582-4934.2005.tb00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boos AM, Loew JS, Deschler G, et al. Directly auto-transplanted mesenchymal stem cells induce bone formation in a ceramic bone substitute in an ectopic sheep model. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01131.x. Doi: 10.1111/j.1582-4934.2010.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong LM, Liao CG, Chen L, et al. Promoter hypomethylation up-regulates CD147 expression through increasing Sp1 binding and associates with poor prognosis in human hepatocellular carcinoma. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01124.x. Doi: 10.1111/j.1582-4934.2010.01124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu S, Li Y, Liu S, et al. Engineered heart tissue graft derived from somatic-cell-nuclear-transferred embryonic stem cells improve myocardial performance in infarcted rat heart. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01112.x. Doi: 10.1111/j.1582-4934.2010.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satija NK, Singh VK, Verma YK, et al. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med. 2009;13:4385–402. doi: 10.1111/j.1582-4934.2009.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang F, Zhang G, Hashimoto I, et al. Neovascularization in an arterio-venous loop-containing tissue engineering chamber: role of NADPH oxidase. J Cell Mol Med. 2008;12:2062–72. doi: 10.1111/j.1582-4934.2008.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steffens L, Wenger A, Stark GB, et al. In vivo engineering of a human vasculature for bone tissue engineering applications. J Cell Mol Med. 2009;13:3380–6. doi: 10.1111/j.1582-4934.2008.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vacanti CA. The history of tissue engineering. J Cell Mol Med. 2006;10:569–76. doi: 10.1111/j.1582-4934.2006.tb00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutmacher DW, Horch RE, Loessner D, et al. Translating tissue engineering technology platforms into cancer research. J Cell Mol Med. 2009;13:1417–27. doi: 10.1111/j.1582-4934.2009.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mason C. The time has come to engineer tissues and not just tissue engineer. Regen Med. 2006;1:303–6. doi: 10.2217/17460751.1.3.303. [DOI] [PubMed] [Google Scholar]
- 28.Aeschylus . Prometheus bound. Athens; Patakis; 415 B.C. [Google Scholar]
- 29.Rosenthal N. Prometheus’s vulture and the stem-cell promise. N Engl J Med. 2003;349:267–74. doi: 10.1056/NEJMra020849. [DOI] [PubMed] [Google Scholar]
- 30.Aristotle . The complete works: the revised Oxford edition. Princeton, NJ: Princeton University Press; 1984. [Google Scholar]
- 31.Genesis . I:1. Wheaton: Crossway Publications; pp. 21–2. [Google Scholar]
- 32.Matthews LG. SS. Cosmas and Damian – patron saints of medicine and pharmacy their cult in England. Med Hist. 1968;12:281–8. [Google Scholar]
- 33.Asenjo A. Neurosurgical techniques. Springfield: Charles C Thomas; 1963. [Google Scholar]
- 34.Dinsmore E. A history of regeneration research: milestones in the evolution of a science. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- 35.Coleman W. Biology in the nineteenth century: problems of form, function and transformation. 2nd ed. Cambridge: Cambridge University Press; 1978. [Google Scholar]
- 36.Stocum D. Regenerative biology and medicine. 1st ed. Oxford: Academic Press; 2006. An overview of regenerative biology and medicine; pp. 1–20. [Google Scholar]
- 37.Wohlrab F, Henoch U. The life and work of Carl Weigert (1845–1904) in Leipzig 1878–1885. Zentralbl Allg Pathol. 1988;134:743–51. [PubMed] [Google Scholar]
- 38.Barth A. Ueber histologische Befunde nach Knochenimplantationen. Arch Klin Chir. 1893;46:409–17. [Google Scholar]
- 39.Leff D. The cell: interand intra-relationships. New Jersey: Avery Publishing Group; 1983. New biological assembly line. [Google Scholar]
- 40.Hayflick L. Entropy explains aging, genetic determinism explains longevity, and undefined terminology explains misunderstanding both. PLoS Genet. 2007;3:e220. doi: 10.1371/journal.pgen.0030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleinsmith LJ, Pierce GB., Jr Multipotentiality of single embryonal carcinoma cells. Cancer Res. 1964;24:1544–51. [PubMed] [Google Scholar]
- 42.Papaioannou VE, McBurney MW, Gardner RL, et al. Fate of teratocarcinoma cells injected into early mouse embryos. Nature. 1975;258:70–3. doi: 10.1038/258070a0. [DOI] [PubMed] [Google Scholar]
- 43.Damjanov I. Teratocarcinoma: neoplastic lessons about normal embryogenesis. Int J Dev Biol. 1993;37:39–46. [PubMed] [Google Scholar]
- 44.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 46.Thomson JA, Kalishman J, Golos TG, et al. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol Reprod. 1996;55:254–9. doi: 10.1095/biolreprod55.2.254. [DOI] [PubMed] [Google Scholar]
- 47.Andrews PW. Human teratocarcinomas. Biochim Biophys Acta. 1988;948:17–36. doi: 10.1016/0304-419x(88)90003-0. [DOI] [PubMed] [Google Scholar]
- 48.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 49.Bell E, Ehrlich HP, Buttle DJ, et al. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science. 1981;211:1052–4. doi: 10.1126/science.7008197. [DOI] [PubMed] [Google Scholar]
- 50.Lysaght MJ, Crager J. Origins. Tissue Eng A. 2009;15:1449–50. doi: 10.1089/ten.tea.2007.0412. [DOI] [PubMed] [Google Scholar]
- 51.Skalak R, Fox C. Proceedings of a workshop held at Granlibakken, Lake Tahoe, California. New York: Liss; 1989. Tissue engineering. [Google Scholar]
- 52.Time Magazine. What will be the 10 hottest jobs. http://www.time.com/time/reports/v21/work/mag_ten_hottest_jobs.html. . Accessed September 20, 2009.
- 53.Kemp P. History of regenerative medicine: looking backwards to move forwards. Regen Med. 2006;1:653–69. doi: 10.2217/17460751.1.5.653. [DOI] [PubMed] [Google Scholar]
- 54.Lysaght MJ, Hazlehurst AL. Tissue engineering: the end of the beginning. Tissue Eng. 2004;10:309–20. doi: 10.1089/107632704322791943. [DOI] [PubMed] [Google Scholar]
- 55.Wilmut I, Schnieke AE, McWhir J, et al. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–3. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 56.Beier JP, Klumpp D, Rudisile M, et al. Collagen matrices from sponge to nano: new perspectives for tissue engineering of skeletal muscle. BMC Biotechnol. 2009;9:34. doi: 10.1186/1472-6750-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manetti M, Guiducci S, Ibba-Manneschi L, et al. Mechanisms in the loss of capillaries in systemic sclerosis: angiogenesis versus vasculogenesis. J Cell Mol Med. 2010;14:1241–54. doi: 10.1111/j.1582-4934.2010.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vacanti JP, Langer R, Upton J, et al. Transplantation of cells in matrices for tissue regeneration. Adv Drug Deliv Rev. 1998;33:165–82. doi: 10.1016/s0169-409x(98)00025-8. [DOI] [PubMed] [Google Scholar]
- 59.Mooney DJ, Mikos AG. Growing new organs. Sci Am. 1999;280:60–5. doi: 10.1038/scientificamerican0499-60. [DOI] [PubMed] [Google Scholar]
- 60.Polykandriotis E, Euler S, Arkudas A, et al. Regression and persistence: remodelling in a tissue engineered axial vascular assembly. J Cell Mol Med. 2009;13:4166–75. doi: 10.1111/j.1582-4934.2009.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Polykandriotis E, Arkudas A, Horch RE, et al. Autonomously vascularized cellular constructs in tissue engineering: opening a new perspective for biomedical science. J Cell Mol Med. 2007;11:6–20. doi: 10.1111/j.1582-4934.2007.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Polykandriotis E, Tjiawi J, Euler S, et al. The venous graft as an effector of early angiogenesis in a fibrin matrix. Microvasc Res. 2008;75:25–33. doi: 10.1016/j.mvr.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Booth BL. Beyond the biotech IPO: a brave new world. Nat Biotechnol. 2009;27:705–9. doi: 10.1038/nbt0809-705. [DOI] [PubMed] [Google Scholar]
- 64.Fenn J, Raskino M. Mastering the hype cycle: how to choose the right innovation at the right time. Boston: Mcgraw-Hill Professional; 2008. [Google Scholar]
- 65.EurActiv.com PLC. Biotech start-ups turn to US for finance http://www.euractiv.com/en/enterprise-jobs/biotech-start-ups-turn-to-us-for-finance-news-494455 Accessed June 28, 2010.
- 66.Auerswald P, Branscomb L. Valleys of death and Darwinian seas: financing the invention to innovation transition in the United States. J Technol Transfer. 2003;28:227–39. [Google Scholar]
- 67.Brindley D, Davie N. Regenerative medicine through a crisis: social perception and the financial reality. Rejuvenation Res. 2009;12:455–61. doi: 10.1089/rej.2009.0981. [DOI] [PubMed] [Google Scholar]
- 68.Pirraco RP, Marques AP, Reis RL. Cell interactions in bone tissue engineering. J Cell Mol Med. 2010;14:93–102. doi: 10.1111/j.1582-4934.2009.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swetha G, Chandra V, Phadnis S, et al. Glomerular parietal epithelial cells of adult murine kidney undergo EMT to generate cells with traits of renal progenitors. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00937.x. Doi: 10.1111/j.1582-4934.2009.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y, Xiang LX, Shao JZ, et al. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J Cell Mol Med. 2010;14:1494–508. doi: 10.1111/j.1582-4934.2009.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cotten M, Fisher K. Autologous cord blood cells for hypoxic ischemic encephalopathy study 1. Phase I study of feasibility and safety. North Carolina: Duke University; 2008. [Google Scholar]
- 72.Haller MJ, Wasserfall CH, McGrail KM, et al. Autologous umbilical cord blood transfusion in very young children with type 1 diabetes. Diabetes Care. 2009;32:2041–6. doi: 10.2337/dc09-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vendrame M, Gemma C, De Mesquita D, et al. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 2005;14:595–604. doi: 10.1089/scd.2005.14.595. [DOI] [PubMed] [Google Scholar]
- 74.Revoltella RP, Papini S, Rosellini A, et al. Cochlear repair by transplantation of human cord blood CD133+ cells to nod-scid mice made deaf with kanamycin and noise. Cell Transplant. 2008;17:665–78. doi: 10.3727/096368908786092685. [DOI] [PubMed] [Google Scholar]
- 75.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kostin S. Myocardial telocytes: a specific new cellular entity. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01111.x. Doi: 10.1111/j.1582-4934.2010.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gherghiceanu M, Popescu LM. Cardiomyocyte precursors and telocytes in epicardial stem cell niche: electron microscope images. J Cell Mol Med. 2010;14:871–7. doi: 10.1111/j.1582-4934.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Popescu LM, Faussone-Pellegrini MS. TELOCYTES – a case of serendipity: the winding way from interstitial cells of Cajal (ICC), via interstitial Cajal-like cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kupcsik L, Meurya T, Flury M, et al. Statin-induced calcification in human mesenchymal stem cells is cell death related. J Cell Mol Med. 2009;13:4465–73. doi: 10.1111/j.1582-4934.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eyckmans J, Roberts SJ, Schrooten J, et al. A clinically relevant model of osteoinduction: a process requiring calcium phosphate and BMP/Wnt signalling. J Cell Mol Med. 2010;14:1845–56. doi: 10.1111/j.1582-4934.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Osch GJ, Brittberg M, Dennis JE, et al. Cartilage repair: past and future – lessons for regenerative medicine. J Cell Mol Med. 2009;13:792–810. doi: 10.1111/j.1582-4934.2009.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bani D, Formigli L, Gherghiceanu M, et al. Telocytes as supporting cells for myocardial tissue organization in developing and adult heart. J Cell Mol Med. 2010;14:2531–9. doi: 10.1111/j.1582-4934.2010.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


