Abstract
Vasomotion describes oscillations of arterial vascular tone due to synchronized changes of intracellular calcium concentrations. Since increased calcium influx into vascular smooth muscle cells from spontaneously hypertensive rats (SHR) has been associated with variances of transient receptor potential canonical (TRPC) channels, in the present study we tested the hypothesis that increased vasomotion in hypertension is directly linked to increased TRPC expression. Using a small vessel myograph we observed significantly increased norepinephrine-induced vasomotion in mesenteric arterioles from SHR compared to normotensive Wistar–Kyoto (WKY) rats. Using immunoblottings we obtained significantly increased expression of TRPC1, TRPC3 and TRPC5 in mesenteric arterioles from SHR compared to WKY, whereas TRPC4 and TRPC6 showed no differences. Norepinephrine-induced vasomotion from SHR was significantly reduced in the presence of verapamil, SKF96365, 2-aminoethoxydiphenylborane (2-APB) or gadolinium. Pre-incubation of mesenteric arterioles with anti-TRPC1 and anti-TRPC3 antibodies significantly reduced norepinephrine-induced vasomotion and calcium influx. Control experiments with pre-incubation of TRPC antibodies plus their respective antigenic peptide or in the presence of anti-β-actin antibodies or random immunoglobulins not related to TRPC channels showed no inhibitory effects of norepinephrine-induced vasomotion and calcium influx. Administration of candesartan or telmisartan, but not amlodipine to SHR for 16 weeks significantly reduced either the expression of TRPC1, TRPC3 and TRPC5 as well as norepinephrine-induced vasomotion in mesenteric arterioles. In conclusion we gave experimental evidence that the increased TRPC1, TRPC3 and TRPC5 expression in mesenteric arterioles from SHR causes increased vasomotion in hypertension.
Keywords: vasomotion, transient receptor potential channels, angiotensin II 1 receptor blocker, calcium channel blocker, hypertension
Introduction
Vasomotion is the regular variation in tone of arteries. Vasomotion, e.g. oscillations of vascular tone due to synchronized oscillations of smooth muscle cell tension, occurs in arteries either spontaneously or in response to pressure, stretch or application of vasoconstrictor agonists [1–3]. Vasomotion is known to be associated with slow oscillations of smooth muscle membrane potential and of intracellular calcium concentrations. Vasomotion may also be enhanced by oscillations of transplasmamembrane calcium influx [4–6]. In several experimental models of hypertension an increased vasomotion of arteries had been described. Lefer et al. observed enhanced vasomotion of cerebral arterioles in spontaneously hypertensive rats (SHR) compared to normotensive rats [7]. An increased vasomotion was observed in segments of posterior cerebral arteries and in mesenteric arteries from spontaneously hypertensive stroke-prone rats compared to Wistar–Kyoto (WKY) rats [8, 9].
Transient receptor potential canonical (TRPC) cation channels have been described to play an important role for transplasmamembrane calcium influx [10]. Recent experimental data from our group and other groups indicated that an increased TRPC expression is associated with hypertension. Liu et al. reported increased TRPC3 expression in several tissues from SHR compared to normotensive WKY rats [11, 12]. Dietrich et al. showed that elevated TRPC3 channel expression in TRPC6 knockout mice was associated with increased vasoconstriction and increased blood pressure [13].
In the present study we therefore tested the hypothesis whether increased vasomotion in hypertension is associated with increased TRPC expression in mesenteric arterioles from SHR. Our study for the first time gives several experimental evidence that TRPC channels are responsible for increased oscillations of vascular tone in hypertension.
Materials and methods
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publications No. 85-23, revised 1996) and was approved by the local animal protection authorities.
Animals and treatment
In this study, 3-month-old SHR and WKY rats weighing 250–260 g were used. SHR were randomly divided into four groups: control group received placebo (n= 6), 5 mg/kg/day telmisartan (n= 6), 4 mg/kg/day candesartan (n= 6) and 10 mg/kg/day amlodipine (n= 6) by oral garage for 16 weeks. WKY were used as reference controls (n= 6) but were not treated with telmisartan, candisartan or amlodipine by oral garage. The rats were housed under a 12-hr/12-hr day/night cycle and given tap water and standard chow ad libitum. Systolic blood pressure was measured monthly in conscious and restrained rats by the tail-cuff method.
Preparation of mesenteric arterioles and measurements of isometric tension
At the end of treatment, rats were decapitated. The small intestine and associated mesenteric vessels were removed and placed in ice-cold oxygenated (95% O2, 5% CO2) physiological salt solution (PSS) of the following composition (in mmol/l): NaCl 119.0, KCl 4.7, KH2PO4 0.4, NaHCO3 25.0, MgSO4 1.17, CaCl2 2.5 and glucose 5.5. In Ca2+-free PSS, Ca2+ was omitted and 1 mmol/l EGTA was added. All solutions contained 2.5 mmol/l extracellular calcium if not indicated otherwise. Some experiments were performed in the absence of extracellular calcium to rule out the effects of transplasmamembrane calcium influx. Background fluorescence was determined after quenching extracellular fluorescent dye using manganese. For fluorescence measurements the vessels were mounted and stretch in the same way as in the contraction experiments. Second-order mesenteric arterioles were isolated for the study by careful removal the surrounding fat tissue; each artery was cut into cylindrical segments of 2.5 mm in length and the internal diameter of the vessels used in this study ranged between 100 and 150 μm. All the experiments were carried out on vessels with intact endothelium.
According to the method described by Huang et al. and Zhu et al.[14, 15], arterial rings were mounted in a multi myograph system (Danish Myo Technology A/S, Aarhus, Denmark) and changes of vessel tension were recorded by Power lab data recording system (AD Instrument, Castle Hill, Australia). Each arterial ring was bathed in PSS solution with 95% O2 plus 5% CO2 at 37°C, pH 7.40. The PSS solution was changed every 20 min. The arterial rings were placed under an optimal resting tension, which was the minimum level of stretch giving the largest force after administration of 60 mmol/l KCl. The rings were allowed to stabilize at optimal resting tension for 90 min. before the start of the experiments.
Vasomotion of mesenteric arterioles was induced by norepinephrine (final concentration, 10 μmol/l) in the absence and presence of TRP channel blockers, 1-[2-(4-Methoxyphenyl)-2-[3-(4-methoxyphenyl)propoxy]et hyl-1H-imidazole hydrochloride (SKF96365, final concentration, 10 μmol/l), of 2-aminoethoxydiphenylborane (2-APB, final concentration, 10 μmol/l) and gadolinium (final concentration, 100 μmol/l). According to established techniques the magnitude of vasomotion was quantified as the rhythmic oscillatory waves, expressed as the percentage of the vasoconstriction produced by 60 mmol/l KCl [7, 8]. Additional experiments were performed after calcium depletion of sarcoendoplasmic reticulum by thapsigargin (final concentration 1 μmol/l) for 20 min. in the absence of extracellular calcium.
Vasomotion was also investigated in mesenteric arterioles after pre-incubation with antibodies against TRPC or against β-actin (1:200) for 40 min. at 37°C. Polyclonal anti-TRPC1, anti-TRPC3 and anti-TRPC5 antibodies generated in rabbits were purchased from Alomone Labs (Jerusalem, Israel) or Sigma-Aldrich (St. Louis, MO, USA). The selectivity and negligible cross-reactivity of these anti-TRPC antibodies for their target proteins have been previously described [15, 16]. Control experiments were conducted in mesenteric arterioles incubated in PSS with immglobulins (Sigma, 3 μg/ml) or albumin (Sigma, 3 μg/ml) or in the presence of a mixture of anti-TRPCs antibodies and respective antigen peptides incubated for 40 min. at 37°C. Incorporation of TRPC antibodies into mesenteric arteries by pinocytosis was facilitated by changing the bathing solution to a hypotonic solution (0.45% NaCl) for 20 sec. according to recent literature [17].
Immunohistochemistry
Fresh mesenteric arterioles were fixed with 10% formalin for 10 min. at room temperature, washed with phosphate-buffered saline (PBS) and then permeabilized with PBS containing 0.5% Triton X-100 for 20 min. at room temperature. Mesenteric arterioles were incubated with PBS containing with 5% bovine serum and 0.1% Triton X-100 for 1 hr at room temperature. Then arterioles were incubated with rabbit anti-TRPC1, anti-TRPC3 and anti-TRPC5 antibodies (1:200; Alomone Labs) over night at 4°C. Mesenteric arterioles were then washed and incubated with secondary antibodies conjugated with a fluorescent probe (FITC-conjugated goat anti-rabbit antibodies; Santa Cruz Biotechnology, Delaware Avenue, CA, USA; 1:200) for 30 min. at room temperature. In control experiments, the primary TRPC3 antibody was pre-incubated with antigenic peptide (1:25) for 12 hrs at 4°C. After removing the unbound secondary antibodies by washing the preparations with PBS, imaging was performed with the fluorescence microscope (Nikon, TE2000, Tokyo, Japan).
Measurement of intracellular calcium in fresh mesenteric arterioles
PBS, with the following composition (in mmol/l) 137 NaCl, 4.1 KCl, 0.66 KH2PO4, 3.4 Na2HPO4, 2.5 NaHCO3, 1.0 MgCl2 and 5 glucose, was adjusted daily to pH 7.4 at room temperature. Arterioles were loaded with the fluorescent dye fura2-acetoxymethylester (final concentration, 2 μmol/l) and 0.2% pluronic F-127 for 45 min. at 37°C in the dark. The arterioles were washed twice with PBS and 1.1 mmol/l calcium was added before measurements. We measured cytosolic calcium in fresh mesenteric arterioles as described by Fellner and Arendshorst [18]. Mesenteric arterioles were identified by morphology and centred in a glass of the optical field that was free of adventitia. Changes of cytosolic calcium after administration of norepinephrine were measured in the absence and presence of inhibitors or anti-TRPC antibodies.
Immunoblotting
Immunoblotting was performed as previously described by our group [11, 12]. Mesenteric arterioles were rapidly excised and frozen in liquid nitrogen, then were crushed in liquid nitrogen and resuspended in ice-cold lysis buffer (20 mmol/l Tris-HCl, pH 7.4, 50 mmol/l NaCl, 50 mmol/l NaF, 5 mmol/l ethylenediaminetetraacetic acid and 20 mmol/l Na4P2O7). Supernatants were collected, and protein concentrations were estimated. Lysates of VSMC) and supernatants from mesenteric arterioles were subjected to SDS-PAGE gel electrophoresis (10%). Separated proteins were then electrotransferred onto nitrocellulose. Nitrocellulose membranes were blocked in 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween-20 for 60 min. at room temperature. The membranes were incubated with primary rabbit antibodies against TRPC1, TRPC3, TRPC4, TRPC5 and TRPC6 (1:200; Alomone Labs), followed by incubation with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody. The specific bands were quantified using an image analyser (Biorad Laboratories, Philadelphia, PA, USA).
Statistics
Data are given as mean ± S.E.M. Statistical analyses of the data were performed with the two-tailed Student’s t-test for unpaired comparisons. Analysis of data ANOVA was used, because vasomotion was investigated in several unrelated vessels when comparing more than two groups. Values were considered to be significant when two-sided P was less than 0.05.
Results
Increased norepinephrine-induced vasomotion in mesenteric arterioles from SHR
After administration of 10 μmol/l norepinephrine a rapid vasoconstriction was followed by synchronized oscillations of vascular tone, i.e. vasomotion, in mesenteric arterioles from WKY (Fig. 1A) and SHR (Fig. 1B). Norepinephrine-induced vasomotion was quantified as percentages of oscillations of vascular tone in relation to the maximal contraction to KCl. (Fig. 1C). The norepinephrine-induced vasomotion was significantly higher in mesenteric arterioles from SHR compared to WKY (65.5 ± 7%versus 2.2 ± 0.5%; each n= 6, P < 0.01). The norepinephrine-induced vasoconstriction was also significantly increased in mesenteric arterioles from SHR compared to WKY (140 ± 11%versus 100 ± 3%; each n= 6, P < 0.05, Fig. 1D). Recordings for more than 1 hr confirmed increased norepinephrine-induced vasomotion in SHR compared to WKY (Fig. 1E and F).
Fig 1.
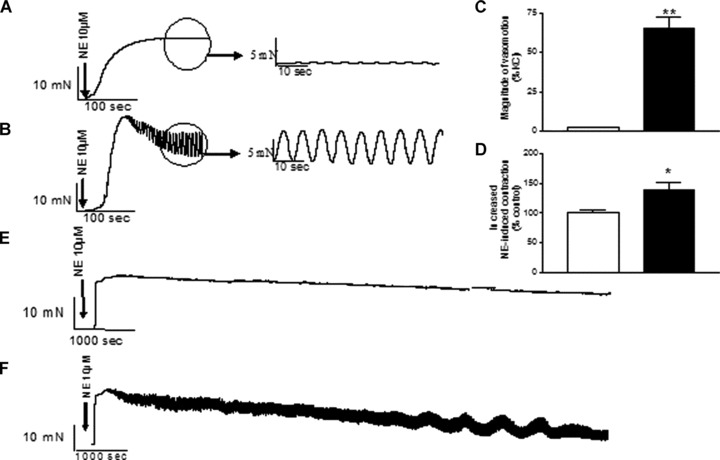
Comparison of vasomotion induced by norepinephrine (10 μmol/l) in mesenteric arterioles from WKY (A) and SHR (B). (C) Summary data for norepinephrine-induced vasomotion in mesenteric arterioles from SHR (filled bars) compared with WKY (open bars). (D) Summary data for norepinephrine-induced vasoconstriction in mesenteric arterioles from SHR (filled bars) compared with WKY (open bars). Data are given as mean ± S.E.M. **P < 0.01 or *P < 0.05 by two-tailed Student’s t-test. Representative recordings lasting for more than 1 hr showing norepinephrine-induced vasomotion in mesenteric arterioles from WKY (E) or SHR (F).
Increased expression of TRPC1, TRPC3 and TRPC5 channels in mesenteric arterioles from SHR
Using immunoblotting we demonstrated the expression of TRPC1, TRPC3, TRPC4, TRPC5 and TRPC6 channels in mesenteric arterioles (Fig. 2A). Immunoblottings showed that the expressions of TRPC1, TRPC3 and TRPC5 channels were significantly higher in mesenteric arteries from SHR compared to WKY (TRPC1 expression for WKY 1.00 ± 0.13 versus 1.60 ± 0.10 for SHR; P < 0.01; TRPC3 expression for WKY 1.00 ± 0.18 versus 1.70 ± 0.26 for SHR; P < 0.05; TRPC5 expression for WKY 1.00 ± 0.13 versus 1.41 ± 0.10 for SHR; P < 0.05; each n= 6; Fig. 2B). On the other hand, the expressions of TRPC4 and TRPC6 channels were not significantly different between the strains (TRPC4 expression for WKY 1.00 ± 0.09 versus 1.16 ± 0.08 for SHR; TRPC6 expression for WKY 1.00 ± 0.18 versus 1.09 ± 0.06 for SHR; each n= 6; P > 0.05). After pre-incubation of TRPC3 antibodies with their respective antigen no bands could be observed any longer. Immunohistochemistry confirmed the expression of TRPC1, TRPC3 and TRPC5 were significantly increased in mesenteric arterioles from SHR compared to WKY (Fig. 2C SHR and Fig. 2D WKY).
Fig 2.
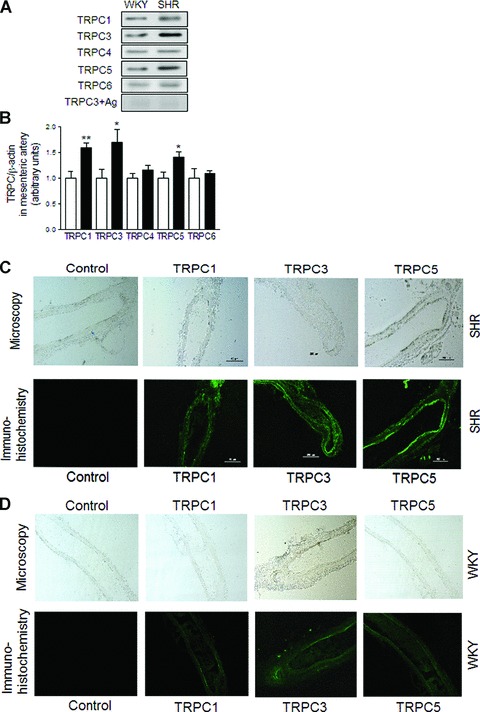
Expression of TRPC1, TRPC3 and TRPC5 channels in mesenteric arterioles from SHR. Representative immunoblottings (A) and summary data (B) of TRPC1, TRPC3, TRPC4, TRPC5,TRPC6 channels and TRPC3 antibody with its respective antigenic peptide the protein expression in mesenteric arterioles from WKY (open bars) and SHR (filled bars). Data are mean ± S.E.M. of n= 4 independent experiments. *P < 0.05 or **P < 0.01 by two-tailed Student’s t-test. (C) Light microscopy of mesenteric arterioles from SHR (upper panels) and immunohistochemistry (lower panels) of TRPC1, TRPC3 and TRPC5 protein expression in mesenteric arterioles from SHR. (D) Light microscopy of mesenteric arterioles from WKY (upper panels) and immunohistochemistry (lower panels) of TRPC1, TRPC3 and TRPC5 protein expression in mesenteric arterioles from WKY. Immunohistochemistry using specific antibodies labelled with green shows TRPC1, TRPC3 and TRPC5 protein expression in mesenteric arterioles. Control indicates immunohistochemistry after pre-incubation of the primary TRPC3 antibody with antigenic peptide for 12 hrs at 4°C. Bar denotes 200 μm. (43.0 ± 15%; P= n.s.; n= 6, Fig. 4D). In the presence of anti-TRPC5 antibodies the norepinephrine-induced vasomotion was not significantly affected (45.0 ± 11%; P= n.s. Fig. 4E). In the presence of anti-TRPC1 plus anti-TRPC3 antibodies the norepinephrine-induced vasomotion was significantly reduced to 29.0 ± 8% (P < 0.05; n= 6, Fig. 4F). Furthermore, pre-incubation of mesenteric arterioles with anti-β-actin antibodies did not significantly affect norepinephrine-induced vasomotion when compared to control conditions (59.8 ± 15%, P= n.s.; n= 6, Fig. 4G). Incorporation of TRPC antibodies into mesenteric arteries by pinocytosis was facilitated by changing the bathing solution to a hypotonic solution according to recent literature [17].
Inhibition of TRPC significantly attenuates norepinephrine-induced vasomotion in SHR
After calcium-depletion of sarcoendoplasmic reticulum calcium stores by 1 μmol/l thapsigargin in the absence of extracellular calcium, the administration of extracellular calcium and norepinephrine repeatedly induced vasomotion in mesenteric arterioles from SHR. In the absence of calcium the thapsigarin-induced calcium increase was 76 ± 13 nmol/l. After return of cytosolic calcium to baseline values the subsequent administration of norepinephrine did not significantly change cytosolic calcium from 65 ± 12 nmol/l to 70 ± 12 nmol/l; n= 10; P > 0.05). These data indicate that intracellular stores are empty after administration of thapsigargin. As shown in Fig. 3, compared to control conditions (57.0 ± 6%, n= 6, Fig. 3A), the norepinephrine-induced vasomotion was significantly reduced in the presence of several chemically unrelated TRPC blockers, i.e. in the presence of gadolinium reduced to 48.5 ± 6% (Fig. 3B), in the presence of 2-APB reduced to 30.3 ± 3% (Fig. 3C) and in the presence of SKF96365 reduced to 23.5 ± 9% (Fig. 3D) (each n= 6; *P < 0.05 or **P < 0.01 by ANOVA, Fig. 3I). On the other hand, vasomotion may be evoked and affected by transplasmamembrance calcium influx through several different types of calcium channels. To distinguish the effects of different channel types in the present study we used blocker for TRP channels as well as verapamil. We evaluated effects of verapamil on norepinephrine-induced vasomotion. The experiments indicated that verapamil also reduces norepinephrine-induced vasomotion. However, the inhibitory effect of verapamil plus gadolinium, verapamil plus 2-APB, or verapamil plus SKF96365 was more pronounced. As shown in Fig. 3(E)–(H), in the presence of verapamil (final concentration 10−7 mol/l), the norepinephrine-induced vasomotion was significantly reduced to 30.0 ± 5% of the maximal contraction to KCl (Fig. 3E) compared to control conditions. It should be noted that in the presence of verapamil plus 10−4 mol/l Gd3+ (Fig. 3F), verapamil plus 10−5 mol/l 2-APB (Fig. 3G) or verapamil plus 10−5 mol/l SKF96365 (Fig. 3H), the norepinephrine-induced vasomotion was significantly reduced to 18.2 ± 5%, 13.7 ± 4%, or 2.9 ± 0.5% of the maximal contraction to KCl, respectively (each n= 6, *P < 0.05 or **P < 0.01 compared to control conditions).
Fig 3.
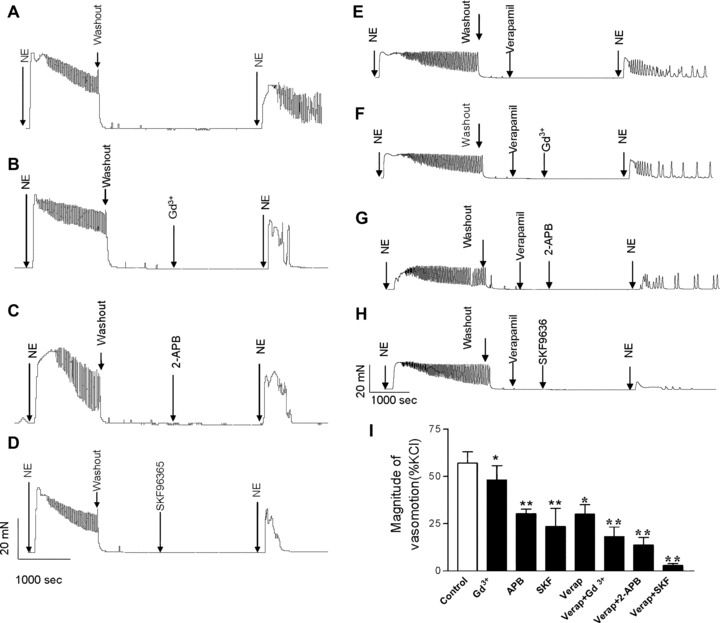
Inhibition of TRPC significantly attenuates norepinephrine-induced vasomotion in SHR. Vasomotion was induced by norepinephrine after depletion of sarcoendoplasmic reticulum calcium stores by 1 μmol/l thapsigargin (TG) in mesenteric arterioles from SHR under control conditions (A), in the presence of gadolinium (Gd3+) (B), 2-APB (C) or SKF96365 (D), in the presence of verapamil (E), or in the presence of verapamil plus Gd3+ (F), verapamil plus 2-APB (G) or verapamil plus SKF96365 (H). (I) Summary data are shown as mean ± S.E.M. of n= 6 independent experiments. *P < 0.05 or **P < 0.01 by ANOVA.
As previously indicated by Andresen and Yang [19] and Hajduczok [20] we occasionally noted that Gd3+ at a concentration of 1 mmol/l turned the buffer solution slightly cloudy probably due to gadolinium precipitation. Additional experiments comparing the gadolinium effects using cells suspended in HEPES-buffered solution in the absence and presence of 0.78 mmol/l phosphate were performed. The effect of gadolinium on vasomotion was not significantly different in the absence or presence of 0.78 mmol/l phosphate (48.5 ± 6%versus 49.3 ± 7%.; n= 6; P > 0.05).
Inhibition of norepinephrine-induced vasomotion in mesenteric arterioles from SHR by specific TRPC antibodies
We also examined the effects of specific anti-TRPC antibodies on norepinephrine-induced vasomotion in mesenteric arterioles from SHR. Under control conditions, i.e. in the absence of TRPC antibodies, the norepinephrine-induced vasomotion was 57.0 ± 6% of the maximal contraction to KCl (Fig. 4A). Compared to control conditions, the norepinephrine-induced vasomotion was significantly reduced in the presence of anti-TRPC1 antibodies to 32.0 ± 7% (P < 0.05; n= 6, Fig. 4B) and in the presence of anti-TRPC3 antibodies to 30.0 ± 7% (P < 0.05; n= 6, Fig. 4C). On the other hand, to support the specific effects of anti-TRPC antibodies on norepinephrine-induced vasomotion additional control experiments were performed with the respective antigenic peptides. Pre-incubation with respective antigenic peptide significantly abolished the inhibitory effect of TRPC antibodies on norepinephrine-induced vasomotion. In the presence of TRPC3 antigenic peptide and anti-TRPC3 antibody the norepinephrine-induced vasomotion was not significantly different compared to control conditions The magnitude of vasomotion of the maximal contraction to KCl was 57.6 ± 5% under control conditions with isotonic NaCl, it was 54.0 ± 7% under control conditions after short-term exposure to hypotonic 0.45% NaCl without antibody, and it was 23.4 ± 4% after short-term exposure to hypotonic 0.45% NaCl plus TRPC3 antibody (P < 0.01.; n= 6, Fig. 4H–J).
Fig 4.
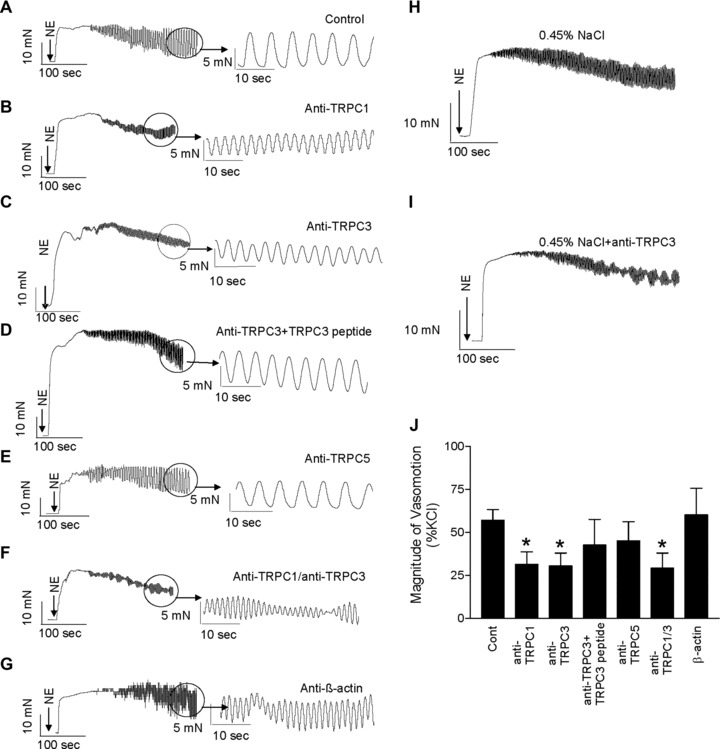
Inhibition of norepinephrine-induced vasomotion in mesenteric arterioles from SHR by specific anti-TRPC antibodies Representative tracings of norepinephrine-induced vasomotion in mesenteric arterioles from SHR under control conditions (A), in the presence of anti-TRPC1 antibodies (B), anti-TRPC3 antibodies (C), anti-TRPC3 antibodies with antigenic peptide (D), anti-TRPC5 antibodies (E), anti-TRPC1 plus anti-TRPC3 antibodies (F) or in the presence of anti-β-actin antibodies (G). Representative tracings of norepinephrine-induced vasomotion in mesenteric arterioles from SHR under control conditions after short-term exposure to hypotonic 0.45% NaCl without antibody (H), or after short-term exposure to hypotonic 0.45% NaCl plus TRPC3 antibody (I). Summary data (J) show that inhibition of TRPC channels by specific anti-TRPC antibodies significantly attenuates norepinephrine-induced vasomotion in SHR. Data are mean ± S.E.M. of n= 6 independent experiments. *P < 0.05 by ANOVA.
Control experiments were performed to underline the significance of using TRPC antibodies for inhibition of norepinephrine-induced vasomotion. First, destruction of the antibody by heating abolished its effects on vasomotion. After heating of the anti-TRPC3 antibody to 95°C for 5 min. no effect on norepinephrine-induced vasomotion could be observed (58.0 ± 7%versus 56.3 ± 5%, each n= 6; P > 0.05). Second, we compared TRPC antibodies supplied from different sources. Additional experiments using anti-TRPC3 antibodies from a different supplier also showed the reduction of norepinephrine-induced vasomotion in mesenteric arteries to 30.4 ± 9% of the maximal contraction to KCl (n= 6; P < 0.05 compared to control). Third, we used random immunoglobulins not related to TRPC channels. Administration of random immunoglobulins did not affect norepinephrine-induced vasomotion (53.0 ± 7%; n= 4, P > 0.05 compared to control), thus excluding non-specific effects of immunoglobulins. Furthermore, exposure of a high albumin concentration did not affect the norepinephrine-induced vasomotion in mesenteric arteries from SHR (55.4 ± 7%; n= 4; P > 0.05 compared to control). These data underline that specific interactions with TRPC channels can modify norepinephrine-induced vasomotion.
Inhibition of norepinephrine-induced calcium increase in mesenteric arterioles from SHR by specific TRPC antibodies
We further evaluated the effects of specific anti-TRPC antibodies on norepinephrine-induced calcium increase in mesenteric arterioles. As shown in Fig. 5, under control conditions, the norepinephrine-induced calcium increase in mesenteric arterioles was 299 ± 20 nmol/l (n= 8). In the presence of anti-TRPC1 antibodies, the norepinephrine-induced calcium increase was significantly reduced to 150 ± 31 nmol/l (P < 0.01; n= 8, Fig. 5A). In the presence of anti-TRPC3 antibodies, the norepinephrine-induced calcium increase was significantly reduced to 188 ± 37 nmol/l (P < 0.05; n= 8, Fig. 5B). In the presence of anti-TRPC5 antibodies, the norepinephrine-induced calcium increase was significantly reduced to 185 ± 48 nmol/l (P < 0.05; n= 8, Fig. 5C). In the presence of anti-TRPC1 plus anti-TRPC3 and anti-TRPC5 antibodies the norepinephrine-induced calcium increase was significantly reduced to 100 ± 18 nmol/l (P < 0.01; n= 8, Fig. 5D). However, in the presence of anti-TRPC3 antibodies plus TRPC3 antigenic peptide, the norepinephrine-induced calcium increase was not significantly different compared to control conditions (298 ± 14 nmol/l, P > 0.05; n= 8, Fig. 5E). Furthermore, anti-β-actin antibodies did not significantly affect norepinephrine-induced calcium increase when compared to control conditions (278 ± 13 nmol/l, P > 0.05; n= 8, Fig. 5F).
Fig 5.
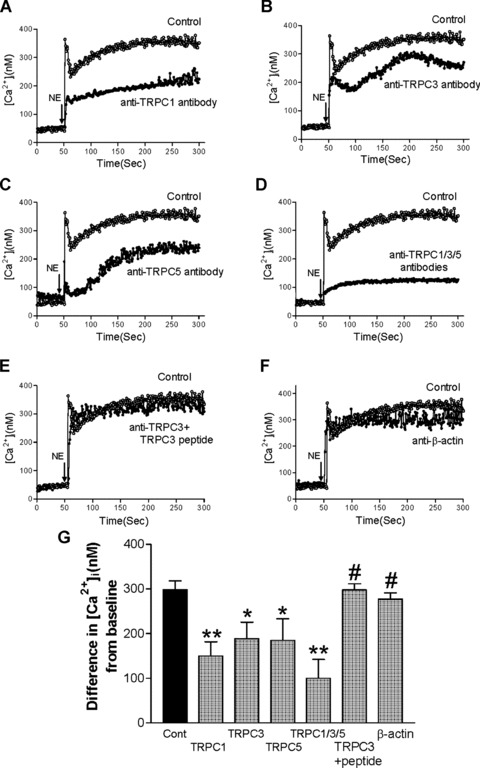
Inhibition of norepinephrine-induced calcium increase in mesenteric arterioles from SHR by specific anti-TRPC antibodies. Representative tracings of norepinephrine-induced calcium increase in mesenteric arterioles from SHR under control conditions and in the presence of anti-TRPC1 antibodies (A), anti-TRPC3 antibodies (B), anti-TRPC5 antibodies (C), anti-TRPC1 plus anti-TRPC3 plus anti-TRPC5 antibodies (D), anti-TRPC3 antibodies plus TRPC3 antigenic peptide (E) or anti-β-actin antibodies (F). Summary data (G) shows the effects of specific anti-TRPC antibodies on norepinephrine-induced calcium influx in mesenteric arterioles from SHR. Data are mean ± S.E.M. of n= 8 independent experiments. *P < 0.05, **P < 0.01 or #P > 0.05 compared to control conditions by ANOVA.
Effect of candesartan or telmisartan but not amlodipine on the rhythmic oscillatory vasomotion of mesenteric arterioles
After oral administration of candesartan (4 mg/kg/day), telmisartan (5 mg/kg/day) or amlodipine (10 mg/kg/day) for 16 weeks to hypertensive rats, systolic blood pressure was significantly lower compared to placebo-treated SHR (placebo, 222 ± 7 mmHg; candesarten, 123 ± 7 mmHg; telmisartan 115 ± 6 mmHg; amlodipine, 122 ± 5 mmHg; P < 0.01; each n= 6; Fig. 6A). After treatment with candesartan or telmisartan for 16 weeks, norepinephrine-induced vasomotion was significantly reduced in mesenteric arterioles compared to placebo-treated SHR. Vasomotion was 66 ± 7% in SHR under placebo control conditions, 14 ± 3% in SHR treated with candesartan and 8 ± 2% in SHR treated by telmisartan (P < 0.01; each n= 6; Fig. 6B). It should be noted that vasomotion was not significantly affected in amlodipine-treated SHR. Representative tracings are shown in Fig. 6(C)–(F).
Fig 6.
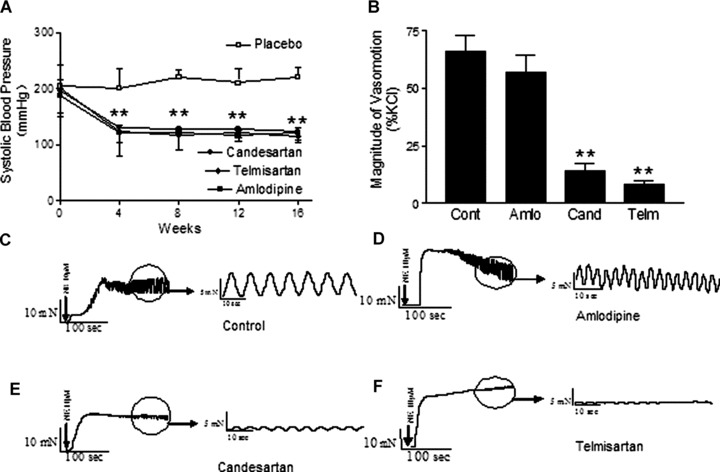
Effect of candesartan or telmisartan but not amlodipine on norepinephrine-induced vasomotion in mesenteric arterioles. Long-term administration of angiotensin AT1 receptor antagonist telmisartan or candesartan or calcium channel blocker amlodipine reduces blood pressure in vivo. The angiotensin AT1 receptor antagonist telmisartan (5 mg/kg per day) or candisartan (4 mg/kg per day), calcium channel blocker amlodipine (10 mg/kg per day) or placebo were administered to SHR by gavage for 16 weeks. (A) Summary data for systolic blood pressure from SHR. (B) Summary data for the rhythmic oscillatory vasomotion in mesenteric arterioles from SHR after treatment with candesartan, telmisartan, or amlodipine for 16 weeks. Representative tracings of norepinephrine-induced vasomotion in mesenteric arterioles from SHR under control conditions (C), administration of amlodipine (D), candesartan (E) or telmisartan (F). **P < 0.01 compared with placebo by ANOVA.
Effect of candesartan or telmisartan but not amlodipine on TRPC expression in mesenteric arterioles
As shown in Fig. 7 after treatment with candesartan or telmisartan for 16 weeks, TRPC1, TRPC3 and TRPC5 channel expressions were significantly reduced in mesenteric arterioles from candesartan or telmisartan-treated SHR compared to placebo-treated SHR as control group (each P < 0.05; n= 5). It should be noted that TRPC1, TRPC3 and TRPC5 channel expression were not significantly affected in amlodipine-treated SHR compared to placebo-treated SHR (each P= n.s.; n= 5).
Fig 7.
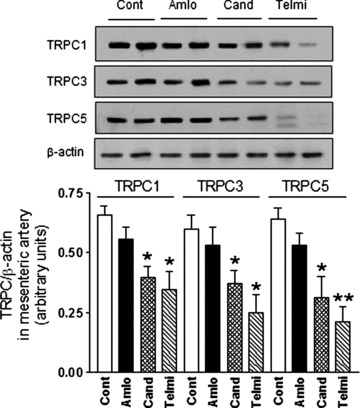
Effect of candesartan or telmisartan but not amlodipine on TRPC expression in mesenteric arterioles. Long-term administration of angiotensin AT1 receptor antagonist telmisartan or candesartan, but not of calcium channel blocker amlodipine reduces TRPC1, TRPC3 and TRPC5 channel protein expression in vivo. The angiotensin AT1 receptor antagonist telmisartan (5 mg/kg per day) or candesartan (4 mg/kg per day), calcium channel blocker amlodipine (10 mg/kg per day), or placebo were administered to SHR by gavage for 16 weeks. Representative immunoblottings of TRPC channel protein expressions in mesenteric arterioles from treated SHR (Fig. 7A) and summary data are shown (Fig. 7B). *P < 0.05; **P < 0.01 compared with placebo by ANOVA.
Discussion
The major finding of the present study was that increased oscillations of arterial vascular tone due to synchronized changes of intracellular calcium concentrations are linked to increased TRPC expression in hypertension. We gave several experimental evidence: First, significantly increased expression of TRPC1, TRPC3 and TRPC5 channels in mesenteric arterioles from SHR compared to WKY; second, increased vasomotion in mesenteric arterioles from SHR compared to WKY; third, reduced vasomotion in the presence of TRPC blockers and anti-TRPC antibodies and fourth, reduction of TRPC expression and vasomotion in SHR after long-term administration of candesartan or telmisartan, but not amlodipine.
Vasomotion which occurs in small resistance vessels of the microcirculation, as well as in larger arteries, is expected to increase flow to assist in tissue perfusion especially during periods of altered metabolism or perfusion pressure [1]. On the other hand, increased vasomotion may be responsible for development and maintenance of pathophysiological states including hypertension [2]. The present study confirmed previous reports showing increased vasomotion in hypertension [7–9]. Earlier genetic studies based on crossbreeding showed a close association between hypertension and increased oscillations of arterial vascular tone [21, 22]. That close association in genetic studies suggests that the underlying molecular mechanisms causing hypertension and vasomotion may be linked to each other.
Which are the underlying mechanisms of norepinephrine-induced vasomotion in mesenteric arterioles? The application of norepinephrine activates G-protein coupled receptors, and subsequent release of G proteins stimulates phospholipase Cβ1 to produce diacylglycerol and inositoltrisphosphate. Diacylglycerol directly activates so-called second-messenger-operated TRPC channels [22–24]. TRPC1, TRPC3 and TRPC5 channels have been reported to form second-messenger-operated calcium entry channels [22–24]. Furthermore, TRPC1, TRPC3 and TRPC5 channels show also characteristics of store-operated TRP channels [25–28]. Subsequent calcium influx or oscillatory release of calcium from intracellular stores then activates calcium-dependent chloride channels which intermittently depolarize the membrane potential [1, 2]. Depolarization and calcium influx through voltage-dependent calcium channels has been shown to be an important step in coordinating the individual oscillators for example in rat mesenteric arteries [29]. Hence, the increased expression of TRPC channels in rat mesenteric arteries may be the initial trigger for increased oscillations of arterial vascular tone in hypertensive vasculature. It cannot be ruled out that part of the inhibitory effects observed on norepinephrine-induced vasomotion by several inhibitors or TRPC antibodies may be mediated by their action on endothelium [2, 3]. Although antibodies against TRPC3 or TRPC5 reduced cytosolic calcium to a similar extent, their effects on vasomotion remained different, indicating specific interaction of the antibody with their respective proteins. In the present study whole cytosolic calcium was measured in mesenteric arterioles. On the other hand, oscillations of intracellular calcium can only be observed in single-cell measurements using confocal images of cytosolic calcium over time as the changes in the mean intensity of fluorescence within some regions of interest in smooth muscle cells [5].
We used several chemically unrelated TRPC blockers, including gadolinium, 2-APB, or SKF96365, as recently described by Rose et al.[30]. Several investigators reported that 2-APB or SKF-96365 are able to reduce cation influx through TRP channels [25, 31, 32]. Higher gadolinium concentrations in the micromolar range are well-known blockers of TRPC channels [33]. However, in the present study all three chemical distinct substances which are known to affect TRPC channels also affected vasomotion. In the presence of gadolinium, SKF-96365 or 2-APB the norepinephrine-induced vasomotion was reduced to 48%, 30%, or 24%, respectively. Furthermore, we showed that verapamil blocked norepinephrine-induced vasomotion, but the inhibitory effect of verapamil plus gadolinium, or plus 2-APB, or plus SKF96365 was more pronounced, indicating that verapamil and TRP blockers showed additive effects, whereas administration of several TRP blockers did not show additive effects. Although several investigators used TRPC channel blockers including 2-APB and SKF96365 [23, 29, 30], limitations of the specificity of inhibitory agents of TRPC channels including gadolinium, 2-APB or SKF96365 have been shown. However, all three chemical distinct substances which are known to affect TRPC channels also affected vasomotion in the present study.
Furthermore we confirmed the cooperation of TRPC channels for norepinephrine-induced vasomotion using anti-TRPC antibodies. Using these commercially available anti-TRPC antibodies Peppiatt-Wildman et al. and Saleh et al. reported a high degree of selectivity for TRPC channels [15, 16]. Furthermore, they found that anti-TRPC1 antibodies produced marked inhibition of store-operated calcium activity in mesenteric arteries [15, 16]. Now we showed that in the presence of anti-TRPC1 antibodies or anti-TRPC3 antibodies the norepinephrine-induced vasomotion was reduced to 32% or 30%, respectively. Recent study confirmed that incorporation of macromolecules including antibodies into mesenteric arteries is facilitated by changing the bathing solution to hypotonic solutions [17]. Our experiments using facilitated loading of TRPC antibodies into mesenteric arteries underscored the importance of TRPC channels for vasomotion. These experiments extended our findings that TRPC channels are associated with vasomotion.
On the other hand, control experiments in the presence of anti-β-actin antibodies showed no effect. Using random immunoglobulins which were not related to TRPC channels or using a high albumin concentration did not affect norepinephrine-induced vasomotion. Furthermore, control experiments in the presence of anti-β-actin antibodies, in the presence of random immunoglobulins or high albumin concentrations and finally pre-incubation of anti-TRPC antibodies with their respective antigenic peptides excluded the possibility of non-specific effects on vasomotion. We showed that TRPC antibodies may reduce vasomotion. However, the physicochemical properties of the antibodies may affect their inhibitory function. It may be suggested that antibodies may cross the membrane to exert the inhibitory effects, whereas the antibody–antigen complexes cannot. These results finally indicate that activation of increased TRPC expression in rat mesenteric arteries triggers increased vasomotion in hypertensive vasculature.
In the present study we used several drugs for chronic treatment of rats in order to evaluate whether lowering of blood pressure per se or the specific interaction with the angiotensin receptor was necessary to show any effect on TRPC expression and vasomotion. To answer that question we used different types of drugs. Our study showed an association between reduced TRPC expression and reduced vasomotion after long-term administration of angiotensin AT1 receptor blockers, candesartan or telmisartan, but not after administration of the calcium channel blocker, amlodipine. These findings were in accordance with earlier reports, indicating that vasomotion seemed to be less prevalent in hypertensive rats treated with angiotensin converting enzyme inhibitors [34, 35]. However, these earlier studies did not investigate TRPC channel expression. Previous results and our present findings may endorse a role for angiotensin II for the regulation of TRPC expression in vasculature and vasomotion. Furthermore, since vasomotion was not affected in the amlodipine group, even though blood pressure values were similarly reduced in the candesartan, telmisartan and amlodipine groups, changes of molecular structures including TRPC expression observed in hypertension rather than blood pressure level per se may be important for the observed coincidence of hypertension and increased vasomotion. Our results indicated that lowering blood pressure alone was not sufficient to affect TRPC expression and vasomotion. But blocking of angiotensin receptors reduced blood pressure, TRPC expression and vasomotion.
In conclusion we gave experimental evidence that the increased TRPC1, TRPC3 and TRPC5 expression in mesenteric arterioles from SHR causes increased vasomotion in hypertension.
Acknowledgments
This study was supported by grants 2006CB503905 and 2006CB503804 from 973 program (Z.Z.) and grant 81070208 from the National Natural Science Foundation of China (D.L.).
References
- 1.Haddock RE, Hill CE. Rhythmicity in arterial smooth muscle. J Physiol. 2005;566:645–56. doi: 10.1113/jphysiol.2005.086405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nilsson H, Aalkjaer C. Vasomotion: mechanisms and physiological importance. Mol Interv. 2003;3:79–89. doi: 10.1124/mi.3.2.79. [DOI] [PubMed] [Google Scholar]
- 3.Seppey D, Sauser R, Koenigsberger M, et al. Does the endothelium abolish or promote arterial vasomotion in rat mesenteric arteries? Explanations for the seemingly contradictory effects. J Vasc Res. 2008;45:416–26. doi: 10.1159/000124283. [DOI] [PubMed] [Google Scholar]
- 4.Oishi H, Schuster A, Lamboley M, et al. Role of membrane potential in vasomotion of isolated pressurized rat arteries. Life Sci. 2002;71:2239–48. doi: 10.1016/s0024-3205(02)02014-3. [DOI] [PubMed] [Google Scholar]
- 5.Peng H, Matchkov V, Ivarsen A, et al. Hypothesis for the initiation of vasomotion. Circ Res. 2001;88:810–15. doi: 10.1161/hh0801.089603. [DOI] [PubMed] [Google Scholar]
- 6.Rahman A, Matchkov V, Nilsson H, et al. Effects of cGMP on coordination of vascular smooth muscle cells of rat mesenteric small arteries. J Vasc Res. 2005;42:301–11. doi: 10.1159/000086002. [DOI] [PubMed] [Google Scholar]
- 7.Lefer DJ, Lynch CD, Lapinski KC, et al. Enhanced vasomotion of cerebral arterioles in spontaneously hypertensive rats. Microvasc Res. 1990;39:129–39. doi: 10.1016/0026-2862(90)90065-y. [DOI] [PubMed] [Google Scholar]
- 8.Osol G, Halpern W. Spontaneous vasomotion in pressurized cerebral arteries from genetically hypertensive rats. Am J Physiol. 1988;254:H28–33. doi: 10.1152/ajpheart.1988.254.1.H28. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Fu S, Liu S, et al. The therapeutic effect of Ginkgo biloba extract in SHR rats and its possible mechanisms based on cerebral microvascular flow and vasomotion. Clin Hemorheol Microcirc. 2000;23:133–8. [PubMed] [Google Scholar]
- 10.Nilius B, Owsianik G, Voets T, et al. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 11.Liu DY, Scholze A, Kreutz R, et al. Monocytes from spontaneously hypertensive rats show increased store-operated and second messenger-operated calcium influx mediated by transient receptor potential canonical type 3 channels. Am J Hypertens. 2007;20:1111–8. doi: 10.1016/j.amjhyper.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Liu D, Yang D, He H, et al. Increased transient receptor potential canonical type 3 channels in vasculature from hypertensive rats. Hypertension. 2009;53:70–6. doi: 10.1161/HYPERTENSIONAHA.108.116947. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich A, Mederos Y, Schnitzler M, et al. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25:6980–9. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Chan FL, Lau CW, et al. Roles of cyclic AMP and Ca2+-activated K+ channels in endothelium-independent relaxation by urocortin in the rat coronary artery. Cardiovasc Res. 2003;57:824–33. doi: 10.1016/s0008-6363(02)00773-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Z, Tepel M, Neusser M, et al. Effect of captopril on vasoconstriction and Ca2+ fluxes in aortic smooth muscle. Hypertension. 1993;22:806–11. doi: 10.1161/01.hyp.22.6.806. [DOI] [PubMed] [Google Scholar]
- 16.Peppiatt-Wildman CM, Albert AP, Saleh SN, et al. Endothelin-1 activates a Ca2+-permeable cation channel with TRPC3 and TRPC7 properties in rabbit coronary artery myocytes. J Physiol. 2007;580:755–64. doi: 10.1113/jphysiol.2006.126656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saleh SN, Albert AP, Peppiatt-Wildman CM, et al. Diverse properties of store-operated TRPC channels activated by protein kinase C in vascular myocytes. J Physiol. 2008;586:2463–76. doi: 10.1113/jphysiol.2008.152157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mather S, Dora KA, Sandow SL, et al. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- 19.Fellner SK, Arendshorst WJ. Store-operated Ca2+ entry is exaggerated in fresh preglomerular vascular smooth muscle cells of SHR. Kidney Int. 2002;61:2132–41. doi: 10.1046/j.1523-1755.2002.00383.x. [DOI] [PubMed] [Google Scholar]
- 20.Andresen MC, Yang M. Gadolinium and mechanotransduction of rat aortic baroreceptors. Am J Physiol. 1992;262:1415–21. doi: 10.1152/ajpheart.1992.262.5.H1415. [DOI] [PubMed] [Google Scholar]
- 21.Hajduczok G, Chapleau MW, Ferlic RJ, et al. Gadolinium inhibits mechanoelectrical transduction in rabbit carotid baroreceptors. Implication of stretch-activated channels. J Clin Invest. 1994;94:2392–6. doi: 10.1172/JCI117605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulvany MJ. Resistance vessel structure and function in the etiology of hypertension studied in F2-generation hypertensive-normotensive rats. J Hypertens. 1988;6:655–63. doi: 10.1097/00004872-198808000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann T, Obukhov AG, Schaefer M, et al. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–63. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 24.Lee YM, Kim BJ, Kim HJ, et al. TRPC5 as a candidate for the nonselective cation channel activated by muscarinic stimulation in murine stomach. Am J Physiol Gastrointest Liver Physiol. 2003;284:G604–16. doi: 10.1152/ajpgi.00069.2002. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Bandyopadhyay BC, Singh BB, et al. Molecular analysis of a store-operated and 2-acetyl-sn-glycerol-sensitive non-selective cation channel. Heteromeric assembly of TRPC1-TRPC3. J Biol Chem. 2005;280:21600–6. doi: 10.1074/jbc.C400492200. [DOI] [PubMed] [Google Scholar]
- 26.Kiselyov K, Xu X, Mozhayeva G, et al. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–82. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 27.Trebak M, Bird GS, McKay RR, et al. Comparison of human TRPC3 channels in receptor-activated and store-operated modes. Differential sensitivity to channel blockers suggests fundamental differences in channel composition. J Biol Chem. 2002;277:21617–23. doi: 10.1074/jbc.M202549200. [DOI] [PubMed] [Google Scholar]
- 28.Zagranichnaya TK, Wu X, Villereal ML. Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem. 2005;280:29559–69. doi: 10.1074/jbc.M505842200. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida J, Ishibashi T, Imaizumi N, et al. Capacitative Ca2+ entries and mRNA expression for TRPC1 and TRPC5 channels in human epidermoid carcinoma A431 cells. Eur J Pharmacol. 2005;510:217–22. doi: 10.1016/j.ejphar.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Rahman A, Hughes A, Matchkov V, et al. Antiphase oscillations of endothelium and smooth muscle [Ca2+]i in vasomotion of rat mesenteric small arteries. Cell Calcium. 2007;42:536–47. doi: 10.1016/j.ceca.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Rose RA, Hatano N, Ohya S, et al. C-type natriuretic peptide activates a non-selective cation current in acutely isolated rat cardiac fibroblasts via natriuretic peptide C receptor-mediated signalling. J Physiol. 2007;580:255–74. doi: 10.1113/jphysiol.2006.120832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baba A, Yasui T, Fujisawa S, et al. Activity-evoked capacitative Ca2+ entry: implications in synaptic plasticity. J Neurosci. 2003;23:7737–41. doi: 10.1523/JNEUROSCI.23-21-07737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu X, Jiang M, Birnbaumer L. Receptor-activated Ca2+ influx via human Trp3 stably expressed in human embryonic kidney (HEK)293 cells. Evidence for a non-capacitative Ca2+ entry. J Biol Chem. 1998;273:133–42. doi: 10.1074/jbc.273.1.133. [DOI] [PubMed] [Google Scholar]
- 34.Hajduczok G, Chapeau MW, Ferlic RJ, et al. Gadolinium inhibits mechanoelectrical transduction in rabbit carotid baroreceptors. J Clin Invest. 1994;94:2392–6. doi: 10.1172/JCI117605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sada T, Koike H, Ikeda M, et al. Cytosolic free calcium of aorta in hypertensive rats. Chronic inhibition of angiotensin converting enzyme. Hypertension. 1990;16:245–51. doi: 10.1161/01.hyp.16.3.245. [DOI] [PubMed] [Google Scholar]


