Abstract
This study compared two dietary phytochemicals, grape-derived resveratrol and palm oil-derived γ-tocotrienol, either alone or in combination, on the contribution of autophagy in cardioprotection during ischaemia and reperfusion. Sprague-Dawley rats weighing between 250 and 300 g were randomly assigned to one of the following groups: vehicle, ischaemia/reperfusion (I/R), resveratrol + I/R, γ-tocotrienol + I/R, resveratrol +γ-tocotrienol + I/R. For resveratrol treatments, the rats were gavaged with resveratrol (2.5 mg/kg) for 15 days while for γ-tocotrienol experiments the rats were gavaged with γ-tocotrienol (0.3 mg/kg) for 30 days. For the combined resveratrol +γ-tocotrienol experiments, the rats were gavaged with γ-tocotrienol for 15 days, and then gavaging continued with resveratrol along with γ-tocotrienol for a further period of 15 days. After 30 days, isolated perfused hearts were subjected to 30 min. of global ischaemia followed by 2 hrs of reperfusion. Our results showed for the first time that at least in part, the cardioprotection (evidenced from the ventricular performance, myocardial infarct size and cardiomyocyte apoptosis) with resveratrol and γ-toctrienol was achieved by their abilities to induce autophagy. Most importantly, resveratrol and γ-tocotrienol acted synergistically providing greater degree of cardioprotection simultaneously generating greater amount of survival signal through the activation of Akt-Bcl-2 survival pathway. Autophagy was accompanied by the activation of Beclin and LC3-II as well as mTOR signalling, which were inhibited by either 3-methyl adenine (3-MA) or Wortmannin. The autophagy was confirmed from the results of transmission electron microscopy and light microscopy as well as with confocal microscopy. It is tempting to speculate that during ischaemia and reperfusion autophagy along with enhanced survival signals helps to recover the cells from injury.
Keywords: resveratrol, tocotrienol, autophagy
Introduction
Dietary phytochemicals are becoming popular as functional foods for the nutritional supplements. The most significant of these phytochemicals is probably resveratrol (3,4’,5-trihydroxy-trans-stilbene), a polyphenolic phytoalexin present in grapes, wines, peanuts and several other fruits and vegetables that possess numerous health benefits [1, 2], originally found to possess chemopreventive activity, resveratrol is now considered as a potent cardioprotective compound and the key element present in red wine that is responsible for so-called French Paradox [3, 4]. Extensive research has revealed the mechanisms of action for resveratrol and documented its action as a pharmacological preconditioning agent [5–7]. Resveratrol is a unique compound that can kill cancer cells on one hand and protect the heart cells on the another hand. Such dichotomy of resveratrol’s behaviour has been explained by its opposing effects on cells depending on the concentrations [8]. For example, at a lower concentrations ranging from 2.5 to 25 mg/kg, resveratrol generates a survival signal protecting cardiomyocytes from death while at a higher concentration of greater than 100 μM it generates a death signal killing the cancer cells [8]. Thus, resveratrol can function both as an anti-apoptotic compound through the activation of Akt and Bcl-2 [9] and as a pro-apoptotic compound through the inhibition of the same survival proteins [8].
Tocotrienols are a group of vitamin E isomers that are also rapidly gaining importance as dietary supplement [10]. Tocotrienols exist in four different isomers including, α, β, γ and δ, depending on the position and number of methyl groups on chromanol ring. Existing literature supports the notion that among these isomers, γ-tocotrienol possess many health benefits including cardioprotection [11]. The principle dietary sources of tocotrienols include red palm oil and rice bran oil [12]. Similar to resveratrol, γ-tocotrienol also provides cardioprotection at a relatively low concentration [12].
One of the important cellular pathways for the generation of survival signal includes autophagy. Interestingly, both resveratrol and tocotrienols have recently been implicated to induce autophagy. In a recent study, resveratrol was found to induce caspase-independent cancer cell death through autophagocytosis [13], and subsequent study showed that resveratrol-induced apoptosis depends on the formation of autophagolysosomes [14]. In another related study, tocotrienol and tocotrienol-rich factor (TRF) induced autophagy in rat pancreatic stellate cells through the mitochondrial pathway [15]. Recently, a study demonstrated that autophagy is enhanced in the survival myocytes in the ischaemic heart [16]. Although autophagy was shown to be involved in non-apoptotic form of programmed cell death, recent studies have demonstrated that autophagy can also cause cell survival, and since resveratrol and γ-tocotrienol generate a survival signal at a relatively low concentration, we hypothesized that these two popular dietary supplements might induce autophagy in cardiomyocytes and provide cardioprotection. Furthermore, we attempted to determine if resveratrol and γ-tocotrienol provide synergistic effects on cardioprotection and the role of autophagy.
Materials and methods
Chemicals
Resveratrol was of analytical grade and obtained from Sigma-Aldrich chemical company (St. Louis, MO, USA). γ-tocotrienol was obtained from Carotech (BASF Germany). All other components were of analytical grade and were obtained from Sigma-Aldrich chemical company, unless otherwise specified.
Animals
All animals used in this study received humane care in compliance with the principles of the laboratory animal care formulated by the National Society for Medical Research and Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (Publication Number NIH 85-23, revised 1996). Sprague-Dawley male rats weighing between 250 and 300 g were fed ad libitum regular rat chow (Harlan Teklad, Madison, WI, USA) with free access to water until the start of the experimental procedure. The rats were randomly assigned to one of the following groups: control I/R, resveratrol treated-I/R, tocotrienol treated-I/R, resveratrol and tocotrienol treated-I/R. A total of 2.5 mg/kg/day resveratrol was gavaged to the animals for 15 days, γ-tocotrienol was gavaged for 30 days at a dose of 0.3 mg/kg/day. In the dual treated group, during the first 15 days γ-tocotrienol was given while for the second 15 days both resveratrol and γ-tocotrienol were administrated. Previous studies from our laboratory established the appropriate dose and time periods for each compound used in this experiment [8, 12]. Wortmaninn was perfused through the aorta for 10 min. at 2*10−7M concentration. The 3-methyl adenine (3-MA) was injected two times 30 and 10 min. before the experiments at a dose of 10 mg/kg.
Isolated working heart preparation
After completing the feeding protocol, the animals were anaesthetized with sodium pentobarbital (80 mg/kg, i.p.) (Abbott Laboratories, North Chicago, IL, USA), and intraperitonealy and heparin sodium (500 IU/kg., i.v.) (Elkins-Sinn Inc., Cherry Hill, NJ, USA) was used as an anticoagulant. After the deep anaesthesia was conformed, hearts were excised, the aorta was canulated and the hearts were perfused through the aorta in Langendorff mode at a constant (100 cm of water) perfusion pressure at 37°C with the KHB for a 5 min. washout period as described previously. The perfusion medium consisted of a modified Krebs-Henseleit bicarbonate buffer (millimolar concentration: sodium chloride 118, potassium chloride 4.7, calcium chloride 1.7, sodium bicarbonate 25, potassium dihydrogenphosphate 0.36, magnesium sulphate 1.2 and glucose 10), and after its oxygenization pH was 7.4 at 37°C. During the washout period, left atria was canulated, and the Langendorff preparation was switched to the working mode for 10 min. with a left atrial filling pressure of 17 cm H2O, aortic afterload pressure was set to 100 cm of water. At the end of 10 min., baseline cardiac function like heart rate (HR, beats/min.), aortic flow (AF, ml/min.), coronary flow (CF, ml/min.), left ventricular developed pressure (LVDP, mmHg) and first derivative of developed pressure (LVdp/dt, mmHg/sec.) were recorded. After that, 30 min. of global ischaemia was initiated by clamping the left atrial inflow and aortic outflow lines at a point close to their origins. At the end of the 30 min. of ischaemia, reperfusion was initiated for 120 min. by unclamping the atrial inflow and aortic outflow lines. The first 10 min. reperfusion was in Langendorff mode to avoid the ventricular fibrillations, after the hearts were switched to anterograde working mode [17].
Cardiac function assessment
After 10 min. of working mode perfusion baseline parameters were recorded. To monitor the recovery of the heart, the left ventricular cardiac function was recorded after 60 and 120 min. of reperfusion. A calibrated flow-meter (Gilmont Instrument Inc., Barrington, IL, USA) was used to measure the AF. CF was measured by timed collection of the coronary effluent dripping from the heart. During the entire experiment, aortic pressure was monitored using a Gould P23XL pressure transducer (Gould Instrument Systems Inc., Valley View, OH, USA) connected to a side arm of the aortic cannula, the signal was amplified using a Gould 6600 series signal conditioner. 0.8 CORDAT II real-time data acquisition and analysis system (Triton Technologies, San Diego, CA, USA) [17]. Heart Rate (HR), left ventricular developed pressure (LVDP) and the first derivative of developed pressure (LVdp/dt) were all calculated from the continuously generated pressure signal.
Infarct size estimation
Infarct size was measured according to the triphenyl tetrazolium chloride (TTC) method. After the 2 hrs of reperfusion, 40 ml of 1% (w/v) solution of TTC in phosphate buffer was infused into aortic cannula, and the heart samples were stored at −70°C for subsequent analysis. Sections of frozen heart were fixed in 2% paraformaldehyde, placed between two cover slips and digitally imaged using a Microtek ScanMaker 600z. To quantitate the areas of infarct in pixels, standard NIH image program was used. The infarct size was quantified and expressed in pixels [17].
Assessment of apoptotic cell death
Immunohistochemical detection of apoptotic cells was carried out using the TUNEL method (Promega, Madison, WI, USA) [18]. Briefly, after the isolated heart experiments the heart tissues were immediately put in 10% formalin and fixed in an automatic tissue fixing machine. The tissues were carefully embedded in the molten paraffin in metallic blocks. Prior to analysis of tissues for apoptosis, the samples were sectioned and placed on glass slide. The tissue sections were deparaffinized with xylene, washed and rehydrated by sequential washing with different concentrations of ethanol (absolute, 95%, 85%, 70%, 50%). Then the TUNEL staining was performed according to the manufacturer’s instructions. The fluorescence staining was viewed with a fluorescence microscope (AXIOPLAN2 IMAGING, Carl Zeiss Microimaging Inc., NY, USA) at 520 ± 20 nm for green fluorescence of fluorescein and at 620 nm for red fluorescence of propidium iodide. The number of apoptotic cells was counted and expressed as a per cent of total myocyte population.
Western blot analysis
Left ventricles from the hearts were homogenized in 1 ml of buffer (25 mM Tris-HCl, 25 mM NaCl, 1 mM orthovanadate, 10 mM NaF, 10 mM pyrophosphate, 10 mM okadaic acid, 0.5 mM EDTA, 1 mM PMSF and 1× protease inhibitor cocktail). The homogenates were centrifuged at 2000 rpm at 4°C for 10 min. The supernatant was centrifuged at 10,000 rpm at 4°C for 20 min. The resultant supernatant was the cytosolic fraction. The cytosolic extracts were aliquoted, snap frozen and stored at −80°C until use. Total protein concentrations in cytosolic extracts were determined using a BCA Protein Assay Kit (Pierce, Rockford, IL, USA) [18].
Proteins were separated in SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked in 5% non-fat dry milk and probed with primary antibody 1:1000 dilution overnight. The following primary antibodies were obtained from Cell Signaling Technology (Boston, MA, USA): Bcl-2, mTOR, p-mTOR and caspase 3. The following primary antibodies: p-Akt, Akt, Beclin-1, LC3 and glyceraldehydes-6-phosphate dehydrogenase (GAPDH) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The protein bands were detected using horseradish peroxidase conjugated secondary antibody (1:2000 dilution) and Western blot luminol reagent (Santa Cruz Biotechnology). GAPDH was used for the cytosolic loading control. The bands were digitized, subjected to densitometric scanning using a standard NIH image program and normalized against the loading control.
Transmission electron microscopy
Small sample of myocardium from control, IR resveratrol, resveratrol + 3-methyl adenine, γ-tocotrienol, γ-tocotrienol + 3-methyl adenine, γ-tocotrienol + resveratrol, γ-tocotrienol + resveratrol + 3-methyl adenine treated hearts were fixed in 4% glutaraldehyde. For the correct identification of autophagosomes by transmission electron microscopy (TEM), we have used the helpful notes detailed in two articles [19, 20]. Membranes contrast was enhanced using an osmium-ferrocyanide mixture in 0.1M cacodylate buffer in the post-fixation step. Subsequently, the samples were dehydrated, infiltrated and embedded in Epon 812 at 60°C for 48 hrs. Light microscopy was performed on 1 μm semithin section stained with 1% toluidine blue and digital images were recorded using a CCD Axiocam HRc Zeiss camera with AxioVision software (Carl Zeiss Imaging solution GmbH, Germany) on Nikon Eclipse E600 microscope (Nikon Instruments, Inc.). Routine 60 nm ultrathin sections were cut with a diamond knife, mounted on formwar-coated grids and stained with 1% uranyl acetate and Reynolds’s lead citrate. Ultrathin sections were examined using a Morgagni 286 TEM (FEI Company, Eindhoven, The Netherlands) at 60 kV. Digital electron micrographs were recorded with a MegaView III CCD using iTEM-SIS software (Olympus, Soft Imaging System GmbH, Germany).
Immunofluorescence techniques and image analysis
Heart tissue samples collected at the end of experiments were fixed in 4% buffered paraformaldehyde (pH 7.4), embedded in paraffin and sectioned. After deparaffinizing the sections, the antigen retrieval treatment was performed using 10 mM sodium citrate containing 0.05% Tween 20 at 90–95°C for 30 min. After washing with PBS, the slides were blocked with Powerblock (BioGenex, San Ramon, CA, USA) for 10 min. Slides were washed with PBS and incubated with primary antibodies (rabbit LC3II; 1:50 dilution) in PBS containing 1% BSA for 2 hrs. After washing, the slides were incubated with fluorescein-conjugated secondary antibodies (anti-rabbit Alexa Fluor 488, green, and anti-goat Alexa Fluor 594, red, both at 1:1000 dilutions) in the dark for 45 min. For nuclear staining, To-Pro 3 iodide (1:1000 dilution) was used for 45 min. in the dark. The slides were washed and covered with mounting medium. Confocal microscopic images were obtained using a Zeiss LSM 510 (Thornwood, NY, USA) confocal laser scanning microscope with 40 × 1.3 oil immersion objective by simultaneous recording in the 488 λ, 560 λ, and/or 615 λ channels as appropriate [21]. Quantification of LC3-II staining was performed from five random frames of confocal microscopic images obtained at 400× magnification from each group. Particles were counted using Adobe Photoshop and Scion Image.
Statistical analysis
The values for myocardial functional parameters, infarct size and apoptosis were expressed as the mean ± standard error of mean (S.E.M.). A one-way analysis of variance was first carried out to test for any differences in mean values between groups. If differences were established, the values of the drug-treated groups were compared with those of the drug-free group by modified t-test. The results were considered significant if P < 0.05.
Results
Synergistic effect of resveratrol and γ-tocotrienol on post-ischaemic ventricular recovery
Table 1 shows the recovery of post-ischaemic ventricular function such as CF, AF, LVDP and LVdp/dt of isolated hearts subjected to 30 min. of global ischaemia followed by 120 min. of reperfusion. As expected, both resveratrol and γ-tocotrienol treatment protected the hearts against ischaemia/reperfusion injury as evidenced by improved post-ischaemic AF, LVDP and LVdp/dt in comparison with the vehicle treated control group (Table 1). However, there were no significant differences between the resveratrol or γ-tocotrienol groups.
Table 1.
Effects of Resveratrol and γ-Tocotrienol on myocardial ischaemia and reperfusion
| Pre-ischaemic values | After 60 min. of reperfusion | After 120 min. of reperfusion | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CF | AF | LVDP | LVdp/dt | CF | AF | LVDP | LVdp/dt | CF | AF | LVDP | LVdp/dt | |
| Control | 24.00 ± 0.53 | 50.7 ± 1.3 | 115.0 ± 2 | 3133 ± 59 | 20.83 ± 0.42 | 21.8 ± 1.9 | 81.7 ± 3.7 | 1892 ± 39 | 19.5 ± 0.48 | 7.8 ± 1.7 | 55.5 ± 4.3 | 1118 ± 47 |
| Resveratrol | 24.75 ± 0.73 | 52.6 ± 1.5 | 114.8 ± 1.7 | 3292 ± 66 | 22.45 ± 0.54 | 34.3 ± 1.4* | 101 ± 4* | 2358 ± 42* | 21.58 ± 0.37 | 22.2 ± 1*,† | 86.7 ± 3.2* | 1702 ± 60*,† |
| γ-Tocotrienol | 25.42 ± 0.55 | 50.4 ± 1 | 116.1 ± 1.8 | 3227 ± 81 | 22.58 ± 0.71 | 30.6 ± 0.8*,† | 99 ± 2.8* | 2221 ± 87*,† | 21.5 ± 0.67 | 21.2 ± 0.9*,† | 84.8 ± 2.7* | 1652 ± 58*,† |
| Resveratrol +γ-Tocotrienol | 25.57 ± 0.44 | 54.7 ± 1.2 | 118.6 ± 1.6 | 3471 ± 65 | 23.87 ± 0.46 | 36.7 ± 0.8* | 105.3 ± 3.1* | 2576 ± 58* | 21.75 ± 0.51* | 27.1 ± 0.7* | 95.5 ± 3.6* | 1954 ± 69* |
Isolated hearts obtained from vehicle (control), Resveratrol, γ-Tocotrienol and Resveratrol and γ-Tocotrienol treated animals were subjected to 30 min. ischaemia followed by 120 min. of reperfusion. Cardiac functions were measures prior to ischaemia and 60 and 120 min. of reperfusion. Results are expressed as means ± S.E.M. n= 6. CF = coronary flow, AF = aortic flow, LVDP = left ventricle developed pressure, LVdp/dt = maximum first derivative of LVDP. *P < 0.05 versus control; †P < 0.05 versus Resveratrol +γ-Tocotrienol.
The hearts obtained from the resveratrol +γ-tocotrienol treated group exhibited enhanced cardiac function after 2 hrs of reperfusion compared to either resveratrol or γ-tocotrienol alone, suggesting an additive effect between resveratrol and γ-tocotrienol on improving cardiac function. In dual treated group, besides the other functional parameters, CF was significantly higher compared to the control group.
As shown in Table 2, Wortmannin treatment abolished the protective effect of both the mono therapies and the dual treatment (Table 2). Similar to Wortmannin, 3-MA also suppressed the post-ischaemic functional parameters; however it should be noted that the effect of 3-MA on the suppression of cardiac function was to a lesser extent than that of Wortmannin.
Table 2.
Effects of Wortmannin and 3 methyl-adenine on Resveratrol and/or γ-Tocotrienol treatment
| Pre-ischaemic values | 60 min. of repefusion | 120 min. of Reperfusion | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CF | AF | LVDP | LVdp/dt | CF | AF | LVDP | LVdp/dt | CF | AF | LVDP | LVdp/dt | |
| Resveratrol | 24.75 ± 0.73 | 52.6 ± 1.5 | 114.8 ± 1.7 | 3292 ± 66 | 22.45 ± 0.54 | 34.3 ± 1.4* | 101 ± 4* | 2358 ± 42 | 21.58 ± 0.37 | 22.2 ± 1 | 86.7 ± 3.2 | 1702 ± 60 |
| Resveratrol + Wortmannin | 24 ± 0.63 | 52.0 ± 1.2 | 122.5 ± 2.1 | 3325 ± 69 | 19.08 ± 0.42*,† | 17.3 ± 1.1* | 81.5 ± 2.1*,† | 1877 ± 50* | 17.83 ± 0.4*,† | 6.7 ± 0.5*,† | 63.3 ± 2.6*,† | 1317 ± 37*,† |
| Resveratrol + 3MA | 25.08 ± 0.49 | 51.5 ± 0.9 | 115.7 ± 2.3 | 3347 ± 111 | 22.25 ± 0.51 | 24.2 ± 3.1* | 94.8 ± 2.8 | 2167 ± 117 | 20.5 ± 0.6 | 10.7 ± 1.2* | 75.2 ± 3.2* | 1539 ± 51 |
| γ-Tocotrienol | 25.42 ± 0.55 | 50.4 ± 1 | 116.1 ± 1.8 | 3227 ± 81 | 22.58 ± 0.71 | 30.6 ± 0.8 | 99 ± 2.8 | 2221 ± 87 | 21.5 ± 0.67 | 21.2 ± 0.9 | 84.8 ± 2.7 | 1652 ± 58 |
| γ-Tocotrienol + Wortmanin | 24.08 ± 0.44 | 50.1 ± 1.2 | 111.5 ± 2.1 | 3354 ± 39 | 18.83 ± 0.44* | 15.2 ± 1* | 83.2 ± 2.2* | 1596 ± 58*,† | 17.17 ± 0.46* | 6.5 ± 0.4* | 60.7 ± 2.7* | 1161 ± 48*,† |
| γ-Tocotrienol + 3MA | 24.5 ± 0.44 | 49.8 ± 0.6 | 116 ± 3.1 | 3249 ± 80 | 22.17 ± 1.01 | 18.8 ± 1.4* | 90.7 ± 4.1 | 1999 ± 114 | 20.5 ± 1.38 | 9.3 ± 1.3* | 67.7 ± 3.2* | 1376 ± 62* |
| Resveratrol +γ-Tocotrienol | 25.57 ± 0.44 | 54.7 ± 1.2 | 118.6 ± 1.6 | 3471 ± 65 | 23.87 ± 0.46 | 36.7 ± 0.8 | 105.3 ± 3.1 | 2576 ± 58 | 21.75 ± 0.51 | 27.1 ± 0.7 | 95.5 ± 3.6 | 1954 ± 69 |
| Resveratrol +γ-Tocotrienol+Wortmannin | 24.42 ± 0.43 | 52.5 ± 1.8 | 113.5 ± 1.8 | 3274 ± 87 | 18.67 ± 0.56*,† | 20.1 ± 1*,† | 88.7 ± 2.3 | 1992 ± 52* | 17.58 ± 0.59*,† | 9 ± 0.9*,† | 67 ± 3.1 | 1344 ± 53*,† |
| Resveratrol +γ-Tocotrienol + 3MA | 24.67 ± 0.36 | 51.5 ± 0.8 | 115.2 ± 2 | 3400 ± 92 | 21.83 ± 0.54* | 24.9 ± 1.3* | 94 ± 2.2 | 2198 ± 88* | 20.08 ± 0.58 | 14.5 ± 1.1* | 76 ± 3.2 | 1577 ± 47* |
Isolated hearts obtained from Resveratrol, γ-Tocotrienol and Resveratrol and γ-Tocotrienol treated animals were treated with Wortmannin or 3 methyl-adenine (3MA) and subjected to 30 min. ischaemia followed by 120 min. of reperfusion. Cardiac functions were measured prior to ischaemia and 60 and 120 min. of reperfusion. Results are expressed as means ± S.E.M. n= 6. CF = coronary flow, AF = aortic flow, LVDP = left ventricle developed pressure, LVdp/dt = maximum first derivative of LVDP. The effects of the blockers were reflected to the own treated groups.
Reduction of ischaemia/reperfusion-mediated infarct size and apoptosis by resveratrol and/or γ-tocotrienol
Both resveratrol and γ-tocotrienol treatments significantly reduced the I/R-induced infarct size compared to the drug-free control, and further reduction in infarct size was observed in the hearts with dual therapy, although it was not statistically significant compared to the resveratrol or γ-tocotrienol treated group alone (Fig. 1). As the infarct size is contributed from both necrosis and apoptosis, we estimated the apoptosis employing TUNEL assay. In consonance with the infarct size, both of the mono therapies significantly decreased the percentage of apoptotic cells, and a more pronounced decline in case of dual treatment was observed, although it was not statistically significant compared to mono therapies (Fig. 2a). Similar to infarct size, Wortmannin abolished the protective effect of resveratrol or tocotrienol on apoptosis induced by ischaemia/reperfusion. As shown in Figure 2B, consistent with our TUNEL assay results, we have detected higher level of procaspase 3 in the mono treated groups as well as in the dual treated group, which were decreased after Wortmannin treatment, indicating the cleavage and activation of caspase 3.
Fig 1.
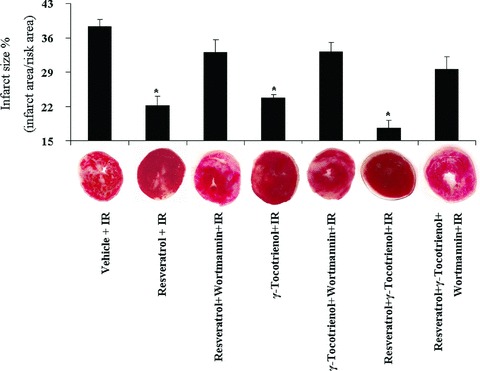
The effect of resveratrol and/or γ-tocotrienol on infarct size. After 30 min. ischaemia and 120 min. reperfusion, the hearts obtained from resveratrol and/or γ-tocotrienol treated animals were perfused with TTC solution and infarct size were calculated as a ratio of infarct area/risk area. Values are expressed as mean ± S.E.M., n= 3 in each group. *P < 0.05.
Fig 2.
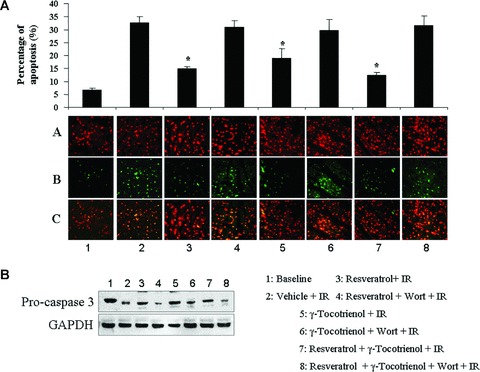
(A) Cardiomyocyte apoptosis was measured by TUNEL assay. Panel a shows the total no. of cells, panel b shows the apoptotic cells and panel c shows the merged pictures of panel a and panel b. Data were expressed as a ratio of apoptotic cardiomyocytes/total cardiomyocytes population. Values are expressed as mean ± S.E.M., n= 3 in each group. *P < 0.05. (B) Western blot analysis of caspase 3, GAPDH was used as loading control. Figures are representative images of three different groups, and each experiment was repeated at least three times.
Synergistic effect of resveratrol and/or γ-tocotrienol on the induction of survival signals in the ischaemic reperfused heart
In order to examine the effect of the mono and dual therapies on survival signal, we examined the level of Bcl-2 and the activation of Akt, which was studied by measuring the ratio of phosphorylation of Akt at 473 to total Akt by Western blot. Both resveratrol and γ-tocotrienol induced extensive activation of Akt (Fig. 3). However, dual treated hearts showed more pronounced activation of Akt and dramatically increased Bcl-2 level compared to the monotherapies, further suggesting a synergic effect on the induction of survival by these two compounds. It appears from our results that resveratrol has more pronounced effect on activation of the Akt where as γ-tocotrienol enhances the level of Bcl-2 (Fig. 3). Furthermore, perfusing the hearts with the PI3 kinase inhibitor Wortmannin diminished the activation of Akt as well as decreased the level of Bcl-2 induced by mono and dual treatments.
Fig 3.
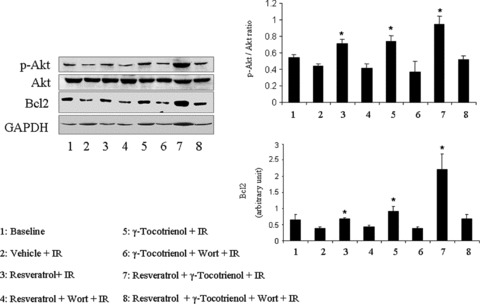
Western blot analysis of p-Akt, Akt and Bcl-2. GAPDH was used as loading control. Figures are representative images of three different groups, and each experiment was repeated at least three times.
Induction of autophagy by resveratrol and γ-tocotrienol
To determinate whether autophagy contributes to the cardioprotective effect of resveratrol and/or γ-tocotrienol, we determine the level of Beclin-1 and the ratio of LC3II/LC3I (Fig. 4). As expected, ischaemia/reperfusion slightly enhanced the ratio of LC3II/LC3I and the level of Beclin-1. As shown in Figure 4, significant increment in the ratio of LC3II/LC3I as well as in the level of Beclin-1 was found in the hearts treated with resveratrol or γ-tocotrienol alone. Similar to survival signalling molecules, Wortmannin treatment abolished the effect of resveratrol or γ-tocotrienol on the level of Beclin-1 and ratio of LC3II/LC3I. Significant and more extensive increase in the level of Beclin-1 and LC3II/LC3I ratio were found in the dual treated hearts, which further supports the existence of the synergic effects between resveratrol and γ-tocotrienol.
Fig 4.

Western blot analysis of LC3I/LC3II and Beclin-1. GAPDH was used as loading control. Figures are representative images of three different groups, and each experiment was repeated at least three times.
Consistent with our Western blot data, our fluorescent microscopy revealed that normal myocardium contained a few LC3 stained cells, which were enhanced after ischaemia/reperfusion. The hearts obtained either from resveratrol or γ-tocotrienol treated animals exhibited more LC3 positive cells, and further increment was found in the dual treated animals’ hearts after ischaemia and reperfusion. Wortmannin treatment reduced the number of LC3 stained positive cells (Fig. 5). The light microscopy on semithin section of samples treated with resveratrol, γ- tocotrienol and resveratrol +γ-tocotrienol showed almost normal morphology (Fig. 6c, e and g) with very few degenerative changes on isolated cardiomyocytes. However, the myocardium in hearts treated with 3-MA clearly showed oncotic changes with myofibrillar contraction bands and vacuolar degeneration (Fig. 6d, f and h).
Fig 5.
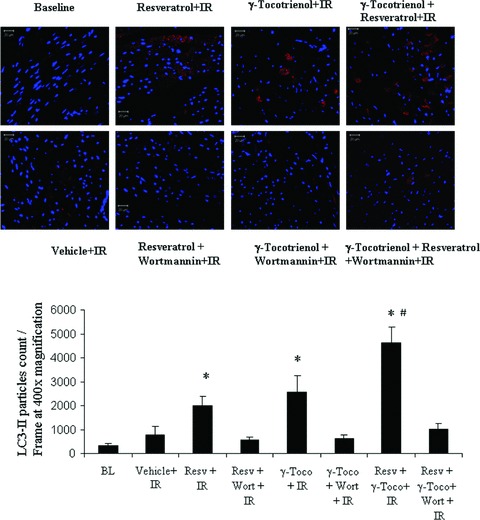
Confocal microscopic pictures showing the LC3II staining in red and the staining of nucleus in blue.
Fig 6.
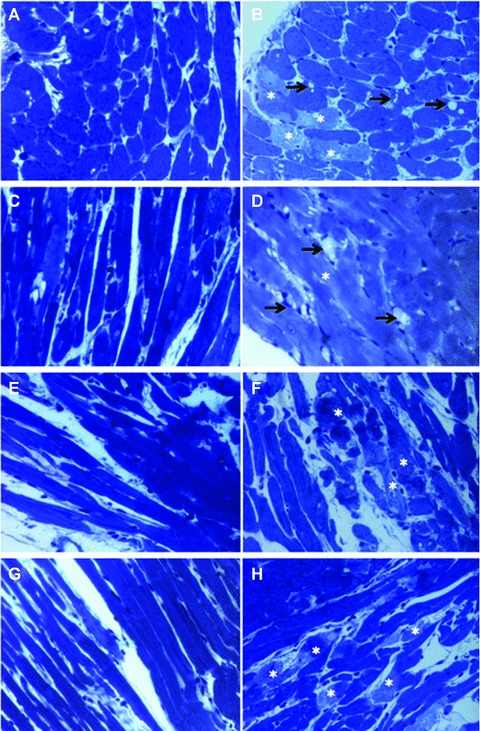
Light microscopy of toluidine blue stained semi-fine sections of Epon-embedded tissue. a – control, b – IR, c – Resveratrol, d – Resveratrol + 3 MA, e –γ-Tocotrienol, f –γ-Tocotrienol + 3 MA, g – Resveratrol +γ-Tocotrienol, h – Resveratrol +γ-Tocotrienol + 3 MA. Cardiac tissue proves to have almost normal structure on light microscopy in control (a), Resveratrol (c), γ-Tocotrienol (e) and Resveratrol +γ-Tocotrienol (g) treated samples. Oncotic changes of cardiomyocytes characterized by contraction band necrosis (*) and vacuolar degeneration (arrows) are visible in IR (b) and 3MA supplementary treatment (d, f, h).
To visualize the autophagosomes, transmission electron microscopy was employed. As shown in TEM images (Fig. 7), autophagosomes have been identified as intracellular structures with double limiting membrane (smooth ribosome free double membrane) that contain morphologically intact cytoplasmic material. Our results revealed that control myocardium showed a normal morphology (Fig. 6a) without ultrastructural changes (Fig. 7a). Ischaemia-reperfusion induced oncotic changes in the myocardium (Fig. 6b) with myofibrils disorganization, mitochondrial swelling and cellular lyses (Fig. 7b). Few small autophagosomes were seen in both cases.
Fig 7.

Transmission electron microscopy images showing the ultrastructural findings in a – control, b – IR, c – Resveratrol, d – Resveratrol + 3 MA, e –γ-Tocotrienol, f –γ-Tocotrienol + 3 MA, g – Resveratrol +γ-Tocotrienol, h – Resveratrol +γ-Tocotrienol + 3 MA. (A) Cardiomyocyte from control shows normal ultrastructure and a small autophagosome (arrow). (B) Cardiomyocytes in IR show ischaemic lesions with myofibrillar disorganization of (*) and mitochondria with swelling and dense structures (arrowheads). (C) Two early autophagosomes contain mitochondria (m) and small amount of cytoplasm (c) enclosed in a double membrane (arrows). (D) Cardiomyocytes with swelled mitochondria, segmental loss of myofibrils (*) and a small autophagosome (arrow). (E) Early autophagosome containing five mitochondria (m) limited by the double membrane (arrow). (F) Oncotic cardiomyocyte shows condensed sarcomeres (*), mitochondrial swelling, a lysosome (ly) and an autophagosome (arrow). (G) An autophagosome that contains two mitochondria (m) and cytoplasmic material (c). Arrow points to the characteristic double membrane of the autophagosome. (H) Cardiomyocyte with normal ultrastructure (left side) contains an autophagosome (arrow) next to a cardiomyocyte with swelled mitochondria (m) and disrupted myofibrils (*). P – pericyte, cap – capillary.
Electron microscopy examination of resveratrol, γ-tocotrienol and resveratrol +γ-tocotrienol treated samples showed an almost normal ultrastructure and the presence of numerous autophagosomes in different stages of maturation. Early autophagic vacuoles contain still identifiable organelles. The autophagosomes enclose single or grouped mitochondria, lamellar structures and cytoplasmic content (Fig. 7c, e and g). Few small autophagosomes have been seen in samples with 3-methyl adenine but ultrastructural ischaemic changes (mitochondrial swelling, dilated cisternae of endoplasmic reticulum, myofibrils contraction or disruption) were more prominent (Fig. 7d, f and h).
Differential effects of resveratrol and γ-tocotrienol on activation of mTor
To examine the mechanisms of initiation of the autophagy, we studied the level and the phosphorylation status of mTOR. We found extensive phosphorylation of mTOR at baseline condition, which was slightly lowered after ischaemia/reperfusion (Fig. 8). In case of resveratrol treatment, the phosphorylation of mTOR was almost identical to the vehicle treated I/R control, and enhanced after Wortmannin treatment. In contrast, γ-tocotrienol treated hearts displayed lower level of p-mTOR as well as mTOR. Similar to the γ-tocotrienol treated hearts, the dual treated hearts also showed lower level of p-mTOR (Fig. 8).
Fig 8.
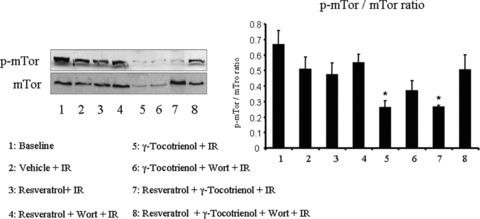
Western blot analysis of p-mTOR and mTOR. Figures are representative images of three different groups, and each experiment was repeated at least three times.
Discussion
The results of the present study have demonstrated for the first time that, the cardioprotection with resveratrol and γ-tocotrienol is attributed at least in part by their abilities to induce autophagy. Most importantly, resveratrol and γ-tocotrienol can act synergistically providing greater degree of cardioprotection through the induction of Akt-Bcl-2 survival pathway and enhancement of autophagy. At relatively low concentrations, resveratrol (2.5 mg/kg) and γ-tocotrienol (0.3 mg/kg) induced autophagy as evidenced by their abilities to induce autophagic marker proteins LC3-II and Beclin-1. For γ-tocotrienol, induction of autophagy was more dependent on mTOR while resveratrol-induced autophagy was more independent on mTOR pathway. Inhibition of autophagy with an inhibitor of autophagic/lysosomal protein degradation, Wortmannin and 3-methyl adenine (3-MA) also induced cardiac death. Such death signal was evidenced by the induction of apoptotic cell death, and the reduced level of procaspase 3, which indicates the activation of caspase 3.
Although autophagy was initially believed to be involved in non-apoptotic form of programmed cell death, recent studies have changed this concept by demonstrating that autophagy can also cause cell survival. For example, up-regulation of autophagy was observed in chronically ischaemic swine myocardium, and autophagy was more pronounced in the surviving area [16].
Another related study demonstrated the protective role of macroautophagy in cardiac HL-1 cells challenged by ischaemic/reperfusion injury [22]. Recently, Yitzhaki et al. showed that autophagy is necessary to achieve the cardioprotection induced by CCPA, a selective agonist of adenosine A1 receptor [23]. In our own study, we found that myocardial ischaemic preconditioning induces autophagy through the induction of Bag-1 survival protein, where inhibition of Bag-1 attenuated the autophagy and simultaneously reversing the cell survival process [21]. However, the role of autophagy in mediating cell death or survival remains controversial and the underlying signalling mechanisms are unclear. The word ‘autophagy’ denotes a process of self-cannibalization through a lysosomal degradation pathway that involves sequestration of intracellular organelles into autophagosomes [24]. In this respect, autophagy is a self-clearing process to remove the dying or unwanted cells, an alternate mechanism for proteasomal degradation, which can generate a survival signal, as in the case of myocardial ischaemia [25].
Our results show that at a low concentration, resveratrol and γ-tocotrienol induced autophagy and generated a survival signal. These results are consistent with previous reports, which shows that resveratrol [2.5–25 μM] and γ-tocotrienol [0.3 mg/kg] at low concentrations could protect the myocardium from ischaemia reperfusion injury [8, 12]. At these concentrations, both resveratrol and γ-tocotrienol generate the survival signal through the activation of Akt and induction of Bcl-2 protein leading to the inhibition of cardiomyocyte apoptosis and necrosis thereby reducing myocardial infarct size. It is well known that resveratrol-mediated survival signal is realized by its ability to induce pharmacological preconditioning, a state-of-the-art technique of myocardial preservation [26]. The present study further shows that these two phytochemicals lead to cell survival and cardioprotection as the inhibition of survival signals and autophagy by Wortmannin abolished resveratrol- and/or γ-tocotrienol-mediated cardioprotection.
Previously, we have shown the time and dose-dependent effect of cardioprotection of γ-tocotrienol and resveratrol [12]. We have found that 0.03 mg/kg bw of γ-tocotrienol treatment for a month did not show any significant cardioprotective effect. But 0.3 and 3 mg/kg bw γ- tocotrienol treatment showed a similar cardioprotective effect. Since the treatment with 3 mg/kg γ-tocotrienol did not give any additional effect, we have chosen the 0.3 mg/kg dose for the current study. In case of resveratrol, we have studied the effect of different doses of resveratrol on cardioprotection. We have demonstrated the dose response of resveratrol on cardioprotection, where 2 weeks of treatment with 2.5 and 5 mg/kg bw of resveratrol showed a similar degree of cardioprotection [8].
Recently, Xi and colleagues have shown that resveratrol could protect the heart also when it was administrated at the onset of reperfusion. The authors found that resveratrol could protect the heart from reperfusion injury by modulating the mPTP opening in a GSK-3β-dependent manner [27]. In the current study, interventions were done prior to the induction of ischaemia/reperfusion event. It is not known whether acute treatment during ischaemia or reperfusion could induce autophagy as observed after chronic treatment in ischaemic-reperfused myocardium.
It should be noted that infarct size and apoptotic cell death lowering ability of resveratrol and tocotrienol were additive, because when the hearts were simultaneously treated with both the compounds, the myocardial infarct size and cardiomyocyte apoptosis were further lowered. There is very limited information regarding induction of autophagy by resveratrol, while almost no information is available on tocotrienols’ ability to cause autophagy. The synergistic effects of resveratrol and tocotrienol suggest that the signalling pathways leading to the autophagy are different. Indeed, different pathways were indicated when the autophagy was inhibited by blocking class III PI3-kinase with Wortmannin.
We have used Beclin-1 and LC3-II proteins as the markers for autophagy. Beclin-1 is the mammalian homologue of the yeast Atg6, which remains bound to the anti-apoptotic protein Bcl-2 [28, 29]. Ischaemia/reperfusion-mediated autophagy occurs via up-regulation of Beclin-1 [30], and the activation of Beclin-1 at the site of injury denotes the induction of autophagy. Beclin-1-independent autophagy may also occur in certain cases [31, 32]. Our results show that both resveratrol and γ-tocotrienol enhance the level of Beclin-1, where resveratrol enhances Beclin-1 relatively more. The treatment with both phytochemicals markedly increased the level of Beclin-1 in comparison to single treatment. Similar to Beclin-1, LC3-II, a mammalian homologue of yeast Apg8p, is also a credible marker for autophagy [33]. LC3-I, a cytosolic form of LC3, is usually present in the cytosol, and becomes converted into LC3-II when autophagy is induced [34, 35]. The results of the present study show a significantly higher amount of LC3-II formation with resveratrol. When resveratrol and tocotrienol were used simultaneously, significant and more pronounced induction of LC3-II formation occurred compared to either resveratrol or γ-tocotrienol alone indicating a synergistic effects of these compounds for autophagy.
In concert, both resveratrol and γ-tocotrienol caused the activation of the survival proteins Akt and Bcl-2. A large number of studies exist in the literature demonstrating that resveratrol and γ-tocotrienol can protect the ischaemic myocardium through the activation of Akt and Bcl-2 [8]. Our present results are also consistent with these previous reports. We found that both of the agents alone enhanced the level of these two survival proteins and when we treated the animals with both of them the level of Bcl-2 and the ratio of p-Akt/Akt were further increased suggesting the additive effect of these two compounds in induction of survival pathways.
It appears from our results that ischaemia/reperfusion itself decreases the p-mTOR/mTOR ratio indicating slight activation of autophagy. Resveratrol further decreased the ratio of mTOR, and the treatment with Wortmannin brings back the ratio closer to the normal value. We found lower ratio of p-mTOR/mTOR in case of tocotrienol treated hearts suggesting that autophagy induced by tocotrienol occurs through mTOR pathway. Further increment was noticed in dual treated heart which was lowered by Wortmannin.
Inhibition of resveratrol-mediated autophagy with Wortmannin abolished the cardioprotective abilities of resveratrol further confirming the generation of a survival signal by autophagy. Both 3-MA and Wortmannin inhibit the survival signalling by inhibiting class III PI-3-kinase [36, 37]. A previous study also indicated that 3-MA inhibited autophagy in hepatocytes [38] and resulted in impaired turnover of lysosomes due to the inhibition of autophagy [39]. Although we used both Wortmannin and 3-MA to block class III PI-3-kinase (3-MA and Wortmannin inhibit the same signalling pathway), we have furnished for survival signal data only for Wortmannin. It is to be noted that though Wortmannin and 3-methyladenine are not specific inhibitors of autophagy, they both have been commonly used in many studies including our previous one [21, 40] to inhibit the autophagic process due to the unavailability of specific autophagic inhibitor. The results showed that Wortmannin inhibited resveratrol- and γ-tocotrienol- mediated activation of Akt, Bcl-2 and lowered the LC3II/LC3I ratio, suggesting that inhibition of class III PI-3-kinase could block resveratrol and γ-tocotrienol-mediated activation of survival signalling. It should be noted, however, that tocotrienol, but not resveratrol, abolished the activation [phosphorylation] of mTOR suggesting that resveratrol function more independently from mTOR, whereas tocotrienol activates autophagy via mTOR-dependent pathway.
Consistent with these results, light microscopic data showed the presence of oncotic myocytes characterized by contraction band necrosis and vacuolar degeneration in the tissues obtained from ischaemia/reperfusion and those treated with 3-MA indicating that resveratrol- and γ-tocotrienol-mediated cardioprotection was inhibited by 3-MA. Transmission electron microscopy echoed these results, which clearly demonstrate that the normal structure was disrupted after ischaemia/reperfusion resulting in disorganization of myofibrils and mitochondria with swelling and dense structures. Both resveratrol and tocotrienol resulted in autophagy as indicated by the presence of autophagosomes containing mitochondria enclosed in a double membrane.
In summary, for the first time we have demonstrated that resveratrol and γ-tocotrienol to some extent possess synergic effect against ischaemia reperfusion injury. The effect is mediated in part via the Akt-Bcl-2 survival pathway and enhanced autophagy leading to cardioprotection against I/R injury. However the precise mechanisms by which these natural products induce autophagy remains to be elucidated. It appears from our results that γ-tocotrienol-induced autophagy at least in part is being mediated through mTOR pathway, and resveratrol-mediated autophagy is less dependent on mTOR. It is tempting to speculate that autophagy along with an enhanced survival signal helps to maintain the basic cell function, and later when the danger is over helps to recover the cell.
Acknowledgments
This study was supported by National Heart, Lung, and Blood Institute Grants NIH HL-22559, HL-33889, HL-34360, OTKA 72315, OTKA 78223, and TAMOP-4.2.2-08/01-2008-0007.
References
- 1.Das DK, Maulik N. Resveratrol in cardioprotection: a therapeutic promise of alternative medicine. Mol Interv. 2006;6:36–47. doi: 10.1124/mi.6.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Goswami SK, Das DK. Resveratrol and chemoprevention. Cancer Lett. 2009;18:1–6. doi: 10.1016/j.canlet.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Das DK, Sato M, Ray PS, et al. Cardioprotection of red wine: role of polyphenolic antioxidants. Drugs Exp Clin Res. 1999;25:115–20. [PubMed] [Google Scholar]
- 4.Giovannini L, Migliori M, Longoni BM, et al. Resveratrol, a polyphenol found in wine, reduces ischemia reperfusion injury in rat kidneys. J Cardiovasc Pharmacol. 2001;37:262–70. doi: 10.1097/00005344-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Das S, Tosaki A, Bagchi D, et al. Potentiation of a survival signal in the ischemic heart by resveratrol through p38 mitogen-activated protein kinase/mitogen- and stress-activated protein kinase 1/cAMP response element-binding protein signaling. J Pharmacol Exp Ther. 2006;317:980–8. doi: 10.1124/jpet.105.095133. [DOI] [PubMed] [Google Scholar]
- 6.Raval AP, Lin HW, Dave KR, et al. Resveratrol and ischemic preconditioning in the brain. Curr Med Chem. 2008;15:1545–51. doi: 10.2174/092986708784638861. [DOI] [PubMed] [Google Scholar]
- 7.Bezstarosti K, Das S, Lamers JM, et al. Differential proteomic profiling to study the mechanism of cardiac pharmacological preconditioning by resveratrol. J Cell Mol Med. 2006;10:896–907. doi: 10.1111/j.1582-4934.2006.tb00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Dudley J, Das S, Mukherjee S, et al. Resveratrol, a unique phytoalexin present in red wine, delivers either survival signal or death signal to the ischemic myocardium depending on dose. J Nutr Biochem. 2009;20:443–52. doi: 10.1016/j.jnutbio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Dudley JI, Lekli I, Mukherjee S, et al. Does white wine qualify for French paradox? Comparison of the cardioprotective effects of red and white wines and their constituents: resveratrol, tyrosol, and hydroxytyrosol. J Agric Food Chem. 2008;22:9362–73. doi: 10.1021/jf801791d. [DOI] [PubMed] [Google Scholar]
- 10.Sen CK, Khanna S, Roy S. Tocotrienol: the natural vitamin E to defend the nervous system? Ann N Y Acad Sci. 2004;1031:127–42. doi: 10.1196/annals.1331.013. [DOI] [PubMed] [Google Scholar]
- 11.Das M, Das S, Wang P, et al. Caveolin and proteasome in tocotrienol mediated myocardial protection. Cell Physiol Biochem. 2008;22:287–94. doi: 10.1159/000149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das S, Lekli I, Das M, et al. Cardioprotection with palm oil tocotrienols: comparision of different isomers. Am J Physiol Heart Circ Physiol. 2008;294:H970–8. doi: 10.1152/ajpheart.01200.2007. [DOI] [PubMed] [Google Scholar]
- 13.Opipari AW, Jr, Tan L, Boitano AE, et al. Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res. 2004;64:696–703. doi: 10.1158/0008-5472.can-03-2404. [DOI] [PubMed] [Google Scholar]
- 14.Trincheri NF, Follo C, Nicotra G, et al. Resveratrol-induced apoptosis depends on the lipid kinase activity of Vps34 and on the formation of autophagolysosomes. Carcinogenesis. 2008;29:381–9. doi: 10.1093/carcin/bgm271. [DOI] [PubMed] [Google Scholar]
- 15.Rickmann M, Vaquero EC, Malagelada JR, et al. Tocotrienols induce apoptosis and autophagy in rat pancreatic stellate cells through the mitochondrial death pathway. Gastroenterology. 2007;132:2518–32. doi: 10.1053/j.gastro.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 16.Yan L, Vatner DE, Kim SJ, et al. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci USA. 2005;102:13807–12. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lekli I, Szabo G, Juhasz B, et al. Protective mechanisms of resveratrol against ischemia-reperfusion-induced damage in hearts obtained from Zucker obese rats: the role of GLUT-4 and endothelin. Am J Physiol Heart Circ Physiol. 2008;294:H859–66. doi: 10.1152/ajpheart.01048.2007. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee S, Gangopadhyay H, Das DK. Broccoli: a unique vegetable that protects mammalian hearts through the redox cycling of the thioredoxin superfamily. J Agric Food Chem. 2008;56:609–17. doi: 10.1021/jf0728146. [DOI] [PubMed] [Google Scholar]
- 19.Eskelinen EL. To be or not to be? Examples of incorrect identification of autophagic compartments in conventional transmission electron microscopy of mammalian cells. Autophagy. 2008;4:257–60. doi: 10.4161/auto.5179. [DOI] [PubMed] [Google Scholar]
- 20.Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–75. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurusamy N, Lekli I, Gorbunov NV, et al. Cardioprotection by adaptation to ischaemia augments autophagy in association with BAG-1 protein. J Cell Mol Med. 2009;13:373–87. doi: 10.1111/j.1582-4934.2008.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–87. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 23.Yitzhaki S, Huang C, Liu W, et al. Autophagy is required for preconditioning by the adenosine A1 receptor-selective agonist CCPA. Basic Res Cardiol. 2009;104:157–67. doi: 10.1007/s00395-009-0006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurusamy N, Das DK. Autophagy, redox signaling and ventricular remodeling. Antioxid Redox Signal. 2009;11:1975–88. doi: 10.1089/ars.2009.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottlieb RA, Finley KD, Mentzer RM., Jr Cardioprotection requires taking out the trash. Basic Res Cardiol. 2009;104:169–80. doi: 10.1007/s00395-009-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das S, Das DK. Resveratrol: a therapeutic promise for cardiovascular diseases. Recent Pat Cardiovasc Drug Discov. 2007;2:133–8. doi: 10.2174/157489007780832560. [DOI] [PubMed] [Google Scholar]
- 27.Xi J, Wang H, Mueller RA, et al. Mechanism for resveratrol-induced cardioprotection against reperfusion injury involves glycogen synthase kinase 3beta and mitochondrial permeability transition pore. Eur J Pharmacol. 2009;604:111–6. doi: 10.1016/j.ejphar.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boya P, Kroemer G. Beclin 1: a BH3-only protein that fails to induce apoptosis. Oncogene. 2009;28:2125–7. doi: 10.1038/onc.2009.83. [DOI] [PubMed] [Google Scholar]
- 29.Zalckvar E, Berissi H, Eisenstein M, et al. Phosphorylation of Beclin 1 by DAP-kinase promotes autophagy by weakening its interactions with Bcl-2 and Bcl-X(L) Autophagy. 2009;5:720–2. doi: 10.4161/auto.5.5.8625. [DOI] [PubMed] [Google Scholar]
- 30.Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–22. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 31.Pimkina J, Humbey O, Zilfou JT, et al. ARF induces autophagy by virtue of interaction with Bcl-xl. J Biol Chem. 2009;284:2803–10. doi: 10.1074/jbc.M804705200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scarlatti F, Maffei R, Beau I, et al. Non-canonical autophagy: an exception or an underestimated form of autophagy? Autophagy. 2008;4:1083–5. doi: 10.4161/auto.7068. [DOI] [PubMed] [Google Scholar]
- 33.Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadowaki M, Karim MR. Cytosolic LC3 ratio as a quantitative index of macroautophagy. Methods Enzymol. 2009;452:199–213. doi: 10.1016/S0076-6879(08)03613-6. [DOI] [PubMed] [Google Scholar]
- 35.Karim MR, Kanazawa T, Daigaku Y, et al. Cytosolic LC3 ratio as a sensitive index of macroautophagy in isolated rat hepatocytes and H4-II-E cells. Autophagy. 2007;3:553–60. doi: 10.4161/auto.4615. [DOI] [PubMed] [Google Scholar]
- 36.Ito S, Koshikawa N, Mochizuki S, et al. 3-Methyladenine suppresses cell migration and invasion of HT1080 fibrosarcoma cells through inhibiting phosphoinositide 3-kinases independently of autophagy inhibition. Int J Oncol. 2007;31:261–8. [PubMed] [Google Scholar]
- 37.Itakura E, Kishi C, Inoue K, et al. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell. 2008;19:5360–72. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA. 1982;79:1889–92. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stroikin Y, Dalen H, Loof S, et al. Inhibition of autophagy with 3-methyladenine results in impaired turnover of lysosomes and accumulation of lipofuscin-like material. Eur J Cell Biol. 2004;83:583–90. doi: 10.1078/0171-9335-00433. [DOI] [PubMed] [Google Scholar]
- 40.Gutierrez MG, Master SS, Singh SB, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–66. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]


