Abstract
Focal adhesions (FAs) are complex plasma membrane-associated macromolecular assemblies that serve to physically connect the actin cytoskeleton to integrins that engage with the surrounding extracellular matrix (ECM). FAs undergo maturation wherein they grow and change composition differentially to provide traction and to transduce the signals that drive cell migration, which is crucial to various biological processes, including development, wound healing and cancer metastasis. FA-related signalling networks dynamically modulate the strength of the linkage between integrin and actin and control the organization of the actin cytoskeleton. In this review, we have summarized a number of recent investigations exploring how FA composition is affected by the mechanical forces that transduce signalling networks to modulate cellular function and drive cell migration. Understanding the fundamental mechanisms of how force governs adhesion signalling provides insights that will allow the manipulation of cell migration and help to control migration-related human diseases.
Keywords: cell migration, focal adhesions, actin cytoskeleton
Introduction
Focal adhesions are regulated by mechanical forces
Focal adhesions-transduced signals regulate cytoskeletal mechanics
Signals targeting focal adhesions drive cell migration
Conclusion and future prospects
Introduction
Cell migration is a fundamental phenomenon that controls multiple biological processes, including embryonic development (morphogenesis), wound healing and immune responses 1. During development, dividing cells migrate to mediate various processes ranging from gastrulation to organogenesis. In addition, when there is injury to the skin or another tissue, cells migrate there to repair the damage. These include platelets, which migrate and aggregate at the injury site to stop bleeding by forming fibrin clots, macrophages and neutrophils, which migrate to kill microorganisms that cause infection, and fibroblasts and epithelial cells, which migrate to the damaged structures and provide cover for the creation of new tissue. These cells can migrate to their destinations individually over long distances or as epithelial sheets, and on arrival they perform specific functions. In both situations, the cell migration cycle is similar and is controlled by complex pathways 1, 2.
The cell migration cycle consists of the extension of the leading edge, formation of new adhesions, translocation of the cell body and detachment of the trailing edge of the cell. To achieve all the steps of the cycle, the actin cytoskeleton and adhesion organelles are reorganized spatio-temporally. When cell migration begins, dendritic actin networks are assembled by polymerizing actin filaments at the leading edge to push the membrane forward 3. This significant force involved in pushing a cell's leading edge does not involve myosin II motors acting on the actin cytoskeleton 4–7. Soon after the membrane at the leading edge protrudes, adhesion organelles are formed to attach the protrusion to the substratum. Subsequently, the actomyosin contractile force is generated by myosin II motors sliding on actin filaments, which promotes bundling of filamentous actin (stress fibres) that connect distal points of adhesions; this allows the contractile forces to propagate across the cell, and applies the force to the substratum through the adhesions; the result is that the cell body is pulled forward 8. Finally, the disassembly of adhesions at the trailing edge leads to detachment of the cell at the rear. Therefore, the dynamic response of the actin cytoskeleton and adhesion organelles is fundamental to coordinating the entire process of cell migration.
The adhesion organelles that allow cells to adhere to the substratum, which also mediate the signals that regulate cell migration, are the integrin-based FAs. FAs form when the central component, the integrin receptor, is activated by engagement with the ECM onto the substratum, which then recruits numerous FA-associated proteins to connect with the actin cytoskeleton 9–11. At the last count 12, 180 proteins had been reported to be associated with FAs to make up the integrin adhesome 12, 13; these include cytoskeletal proteins, adaptor proteins, and signalling proteins, such as kinases, phosphatases, phospholipases and regulators of small guanosine triphosphatase (GTPases). This complex molecular ensemble produces the signalling that regulates the dynamics of FAs, controls the integrity of the linkage between integrin and actin and organizes of the actin cytoskeleton; these together coordinately control cell migration 9–11, 14–18. Cell migration is central in many biological processes and disease states, and therefore an understanding of what is known about the regulation of FAs provides a resource that should help to control abnormal migration.
Focal adhesions are regulated by mechanical forces
The signalling networks in FAs are modulated by a process called FA maturation 19. During maturation, FAs grow in size and change composition after which they either stabilize or begin to disassemble. Based on their size (∼0.1–10 μm2) and localization, FAs can be classified into nascent adhesions, focal complexes and FAs (Fig. 1). Nascent adhesions assemble soon after the integrin receptors engage with the ECM at the edge of lamellipodium, and are either undergoing fast turnover during active protrusions or are evolving into focal complexes within the lamellipodial dendritic actin network. At the lamellipodium-lamellum interface, these adhesions grow and elongate into FAs that are connected by bundles of actin filaments (stress fibres), which serve to anchor the cell 4, 20, 21. All classes of FAs depend on maturation stimuli for their formation and maintenance.
Fig. 1.
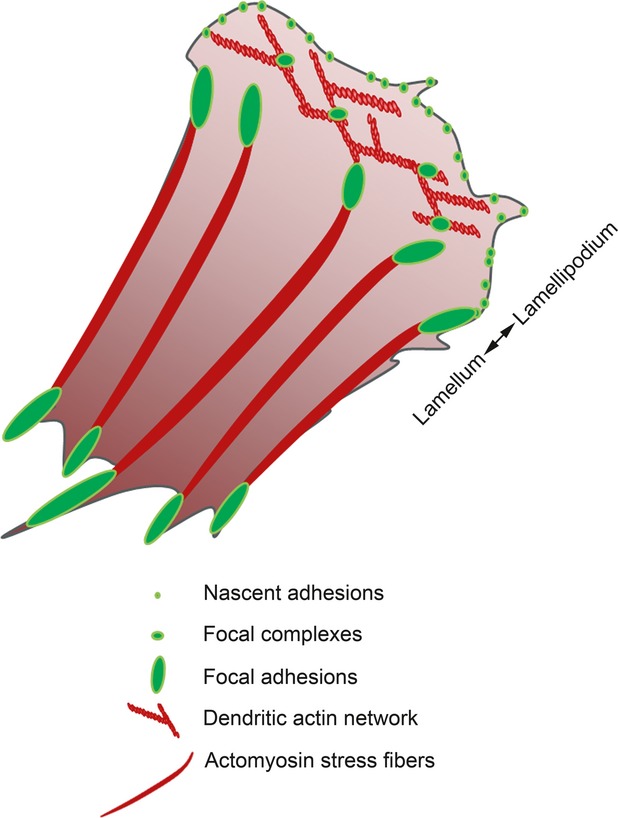
Schematic representation of the structures of the actin cytoskeleton and focal adhesions (FAs). The maturation of FAs is differentially coupled to the specific organization of actin cytoskeleton.
The maturation stimuli can be supplied through biochemical or physical cues. Biochemical regulators of FA maturation include small G-proteins of the Rho-family, which transduce signals to regulate assembly and dynamics of FAs 20, 21. Previous studies have shown that the formation of focal complexes is signalled by the activity of small GTPase Rac1 22, while RhoA signalling promotes the formation of long-lived FAs through activating myosin II-driven contractility 23, 24. GTP-bound RhoA activates its target, Rho-associated kinase (ROCK); this increases myosin II-mediated contractility by inhibiting the myosin light chain phosphatase and directly phosphorylating myosin II regulatory light chain (MLC) 25, 26. The myosin II-generated contractile force along actin filaments provides the major cellular tension that drives FA maturation 19. Physical cues include the cellular tension generated directly from actomyosin contractility, which is also altered by ECM rigidity through feedback loops to modulate the pulling forces exerted by the cells 27–31, and forces from outside of the cell, such as variation in shear forces. Therefore, FAs are really individual mechanosensors whose maturation state is indicative of the local balance with respect to the mechanical forces generated from cellular tension or from external forces 32.
Focal adhesions in different maturation states are composed of specific protein components, which are determined by the local mechanical force 33, 34. However, it is unlikely that all proteins directly sense the mechanical force; rather the recruitment of proteins into FAs is a hierarchical cascade driven by a number of force-sensitive FA proteins 35–38. In response to mechanical force, these force-sensitive FA proteins may undergo structural rearrangement or enzymatic modification that change their binding preferences with respect to other FA-associated proteins (force-responsive FA proteins) and this then further modulates the protein association with FAs. The abundance of these proteins in FAs mainly acts to strengthen the linkage between integrin and actin filaments 39–41.
The proteins that could serve as force-sensitive or force-responsive FA proteins consist of subsets of scaffolding and regulatory proteins. The scaffolding proteins are able to physically connect the actin cytoskeleton to integrin receptors via direct or indirect interactions, while the regulatory proteins control the connection between integrin receptors and actin filaments through their abilities to modulate the activity, stability or functionality of the components in the scaffolding group. The scaffolding proteins include actin-binding proteins and adaptors. Specifically, the actin-binding proteins include proteins that are able to bind directly to the cytoplasmic domains of integrin receptors, such as talin 41–43, α-actinin 41, 44–46, and filamin A/B/C 41, 47–50, or that are able to connect with integrin receptors via other actin-binding proteins or adaptors, such as vinculin 39, 41, VASP 41, 51, 52 and zyxin 41, 52, 53. The adaptors are FA proteins containing specific domains, including src homology 2 (SH2), src homology 3 (SH3), pleckstrin homology (PH), LIM, FERM and calponin homology (CH) domains. The SH2 domain typically binds a phosphorylated tyrosine residue present on its target protein 54, 55, while the classic SH3 domain uses proline-rich peptides as its binding partners 56. PH domains can bind phosphatidylinositol lipid within biological membranes, such as phosphatidylinositol (3,4,5)-trisphosphate and phosphatidylinositol (4,5)-bisphosphate; it thus plays a role in recruiting proteins to specific membranes sites 57, 58. LIM domains have highly divergent sequences that are composed of two contiguous zinc finger motifs with a two-amino acid residue hydrophobic linker 59; these function as a protein-binding interface within many subcellular components such as FAs 60. Evidence indicates that some LIM domain-containing proteins are highly dependent on myosin II activity for FA abundance, suggesting that these proteins may undergo force-dependent unfolding to unmask the binding sites that mediate mechanotransduction 33, 34, 38. FERM domains consist of three modules (the F1, F2 and F3 subdomains) that are able to form a clover-shape structure 61; they play an important role in certain FA proteins that are able to recognize the cytoplasmic tail of β-integrin and mediate integrin activation, such as talin 42, 43 and kindlin 62–65. CH domains are mainly involved in actin binding 66. Altogether, the FA proteins in the scaffolding group may involve force-triggered unfolding or recruitment that promotes FA association of other components; these are able to produce a physical strengthening of the connection between the integrin receptors and actin filaments.
The regulatory proteins are FA components that modulate FA integrity via their enzymatic activity; they include the proteins with small GTPase activity, guanine nucleotide exchange factor (GEF) activity, GTPase-activating protein (GAP) activity, proteolytic activity and activity that regulate protein phosphorylation states. The GTPase activity of the Rho-family proteins, which includes Rac1 and RhoA, is critical for FA maturation and actin cytoskeleton organization 22, 23, 67. The activity of these GTPases is known to be regulated via a switchable cycle that involves GEFs that exchange bound GDP for GTP for activation, and GAPs that promote intrinsic GTP hydrolysis for inactivation 68, 69. Thus, the abundance of GEFs and GAPs regulates the organization of FAs and the actin cytoskeleton through a modulation of GTPase activity. The proteins with proteolytic activity function by cleaving the proteins within FAs, thereby disrupting the linkage between integrin and actin, which allows disassembly of FAs. For example, the Ca2+-dependent cysteine-type protease calpain mediates FA disassembly 70–72 via irreversibly cleaving several FA scaffolding proteins, including integrin 73, 74, paxillin 70 and talin 70, 75. In addition, the proteolytic activity of calpain also regulates the activities of protein tyrosine kinases, such as FAK (focal adhesion kinase) 70, 76, 77 and SRC 78, as well as protein tyrosine phosphatases, such as PTP-1B 78. The activities of various kinases (tyrosine kinases and serine/threonine kinases) and phosphatases (tyrosine phosphatases and serine/threonine phosphatases) trigger signalling cascades 79, 80 that control FA dynamics 81, 82.
Understanding the mechanical force-induced compositional changes in FAs provides information on the molecular complexity, diversity and signals of the integrin-mediated adhesions. The proteins that show increased force-dependent FA abundance could be either positively or negatively regulated by force; these include force-sensitive or force-responsive FA proteins (Fig. 2). To date, many studies based on microscopy and proteomics have revealed that changes in FA components occur in response to mechanical force. To understand how FA-related signalling networks modulate the strength of the linkage between integrin and actin, the force-dependent FA abundance of scaffolding and regulatory proteins is organized, as shown in Table 1. This provides a broad view of our understanding of how FAs enable cells to respond to their mechanical environment via modulation of their composition in a hierarchical cascade.
Fig. 2.
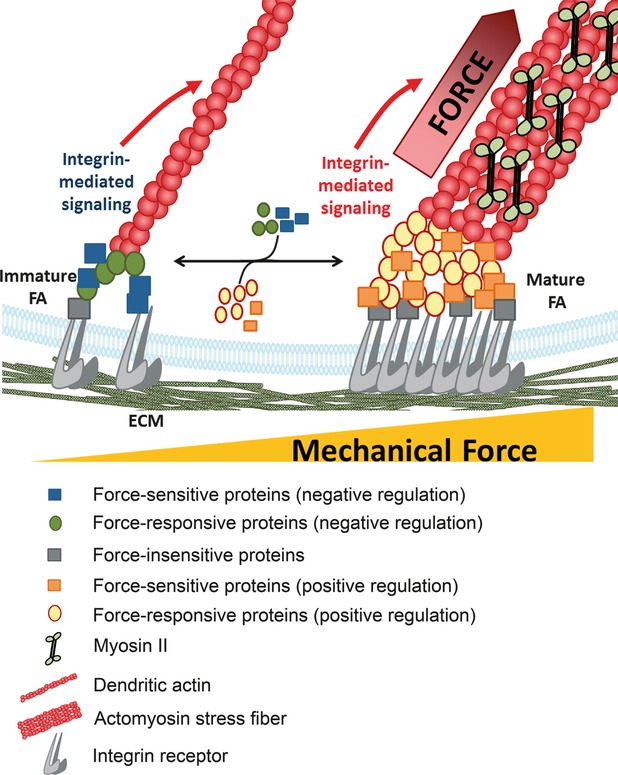
Schematic representation of how the protein composition of FAs is re-organized in response to mechanical force. Focal adhesion protein composition is altered by mechanical force. Within immature FAs, force-insensitive proteins (grey squares), force-sensitive proteins (blue shapes) and force-responsive proteins (green shapes) coordinately transmit the specific integrin-mediated signals. In response to mechanical force, focal adhesion abundance of force-sensitive proteins (blue shapes) and force-responsive proteins (green shapes) are decreased, while the abundance of force-sensitive proteins (orange shapes) and force-responsive proteins (yellow shapes) are increased. The proteins have similar levels of abundance between immature and mature FAs that are considered as force-insensitive proteins (grey squares).
Table 1.
Force-dependent focal adhesions abundance of scaffolding and regulatory proteins. The lists of scaffolding and regulatory proteins are classified into two classes: FA abundance positively regulated by force and FA abundance negatively regulated by force. The proteins in each class could contain force-sensitive or force-responsive proteins
| Scaffolding protein | |
| FA abundance positively regulated by force | ABLIM 34, ACTN1 16, 33, 34, ACTN4 33, 34, CNN1 33, 34, CNN2 33, 34, CNN3 33, 34, CORO1C 33, 34, CSRP1 33, 34, CSRP2 33, 34, FBLIM1 33, 34, FHL2 33, 34, FHL3 33, 34, FLNA 33, 34, 114, FLNB 33, 34, FLNC 33, 34, DAB2 33, 34, LIMA1 33, 34, LIMCH1 33, LIMD1 34, LMO7 33, LPP 33, 34, MYH9 33, 34, NCK1 34, PDLIM1 33, 34, PDLIM2 34, PDLIM4 33, 34, PDLIM5 33, 34, PDLIM7 33, 34, PLEC1 33, 34, 115, SH3BP4 33, SORBS3 33, 34, SPTAN1 33, TES 17, 33, 34, 116, TGFB1I1 33, 34, TLN1 33, 34, TRIP6 33, 34, VCL 33, 34, 39, ZYX 16, 17, 33, 34, 92 |
| FA abundance negatively regulated by force | ARP2/3 complex 33, 117, CAPZB 33, CRIP2 33, DBNL 33, EPB41 33, EPS8 33, 34, FHL1 33, MICALL1 33, TNS3 33 |
| Regulatory protein | |
| FA abundance positively regulated by force | ARF1 33, ARF6 33, 34, CAPN1 33, CAPN2 33, CAPN5 33, CSK 34, DDR2 33, GIT1 33, GIT2 33, 34, GNA11 33, GNA12 33, GNA13 33, GNAQ 33, GNB1 33, GNB2 33, 34, ILK 33, 34, JAK1 33, PDGFRB 33, PTK2 34, PTPN11 34, PTPN2 34, PTPN12 34, RAB1B 33, 34, RAB14 33, 34, RAB18 33, 34, RAB21 33, 34, RAB23 33, 34, RAB3B 33, RAB34 33, 34, RAB35 33, 34, RALA 33, 34, RALB 33, 34, RAP1B 33, RAP2B 33, RHOA 33, RHOB 33, ROR2 33, RRAS2 33, 34, SRC 34, YES1 33 |
| FA abundance negatively regulated by force | ARHGEF7 33, CSNK2A1 33, KRAS 33, 34, NRAS 33, PPP2CB 33, PTPRF 33, PTP4A2 33, PTPRK 33, RAB11B 33, 34, RAB13 33, RAB8A 33, RAN 33, TENC1 33 |
Focal adhesions-transduced signals regulate cytoskeletal mechanics
Focal adhesion components comprise the linkage between integrin receptors and the actin cytoskeleton and these dictate FAs dynamics (the formation, maturation and disassembly of FAs) as well as cytoskeletal organization. The initial linkage between integrin and actin is built via a FA adaptor, talin, which activates integrin receptor by binding to its cytoplasmic domain (NPXY motif) and also connects to actin filaments 42, 43. Myosin II-mediated contractile force reinforces the linkage by modulating FA composition via a hierarchical cascade. For example, force-dependent talin unfolding reinforces the linkage by binding to the actin-binding protein, vinculin 39. In addition, myosin II-dependent recruitment of the actin-binding proteins, filamin-A/B/C and the adaptor, migfilin, strengthens the linkage between integrin and actin filaments via a connection that links the integrin receptors indirectly via a FA adaptor, kindlin-2 83, 84.
Mechanical force modulates the integrin-mediated signals transduced from the force-sensitive and force-responsive FA proteins. In response to myosin II activity, the abundance of RhoA enhancers, such as TRIP6 (thyroid hormone receptor interactor 6) 85, testin 86 and GEF-H1 87, is increased in FAs. In addition, FA abundance of actin-bundling proteins, such as α-actinin 88, synaptopodin-2 89 and supervillin 90, 91 as well as several cytoskeletal LIM domain-containing adaptors 33, 34, 38, such as zyxin 92–94, PDLIM1 95, PDLIM2 95, PDLIM4 95, PDLIM5 95, PDLIM7 95 and FHL2 96, is enhanced. This suggests that mechanical force could promote the level of cellular tension in a positive feedback loop through promoting the association of specific FA components that allows the maturation of FAs and creates bundles of filamentous actin (stress fibres) 33.
Cellular tension also contributes to FA turnover 97, as mature FAs disassembly is blocked by myosin II inhibition 98. Previous experiments have revealed that the Ca2+-activated protease calpain mediates proteolysis of FA proteins 71, 72, 75 and endocytosis-mediated pathways are able to recycle FA components; these serve as important mediators in regulating the disassembly of FAs 99, 100. Some disassembly factors are recruited to mature FAs 33, which may explain how actomyosin contractility mediates FA turnover at the retracting edge of the cells.
Myosin II-mediated contractile force also influences the protein association of immature FAs that transduce signals to promote lamellipodial protrusion 33, 101. In the lamellipodium, actin is arranged as a dendritic network by continuous actin polymerization 3. This cytoskeletal structure is mainly regulated by the Rho GTPase Rac1, but is also induced by myosin II inhibition 5. Inhibition of actomyosin contractility enhances the abundance into immature FAs of Rac1 activators, such as RacGEF β-PIX (PAK-interacting exchange factor-β) 102, RacGEF modulator EPS8 (epidermal growth factor receptor pathway substrate 8) 103, MIF (macrophage migration inhibitory factor) 104 and PKA (protein kinase A) 105, of Rac1 downstream effectors, such as IRSp53 (insulin receptor tyrosine kinase substrate p53) 106 and N-WASP (neuronal Wiskott–Aldrich Syndrome protein) 106, 107, and of Rac1 downstream targets, such as Arp2/3 complex 108, cofilin 109 and the actin monomer binding protein Cap1 110. Previous studies have shown that the Arp2/3 complex serves as the primary mediator of actin polymerization during lamellipodial protrusion, and Rac1 is sufficient to induce Arp2/3-dependent lamellipodium extension via the Rac1 downstream effectors, IRSP53 and N-WASP. FA association of the actin depolymerization factor cofilin promotes actin polymerization at the lamellipodia through the generation of new barbed ends for binding and this affects the Arp2/3 complex. Therefore, FA association of the Rac1 regulatory modules within the immature FAs explains the negative feedback mechanism of actomyosin contractility on the propagation of continuous membrane protrusions 33. Taken together, the biochemical signals associated with FAs are adjusted by the local balance of mechanical forces; this dictates FA dynamics, cytoskeletal organization and the nature of cellular tension.
Signals targeting focal adhesions drive cell migration
Cell migration, a highly dynamic and well regulated process, consists of well-defined steps that include the following: extension of the leading edge and the formation of immature FAs; FA maturation and cell body translocation; the FA disassembly and rear retraction. Integrin-mediated signals from the FAs steps (assembly, maturation and disassembly), which are adjusted by the local balance of cellular tension and the mechanical properties of the environment, regulate actin polymerization and organization. During the migrating cycle, FA dynamics and cytoskeletal organization conjoin to drive this coordinated process 111.
The initial step of the migration cycle is the extension of the leading edge and formation of nascent adhesions (immature FAs) beneath the lamellipodium. These nascent adhesions not only stabilize the protrusion, but also transduce specific signals that continuously promote membrane protrusion. The protein components of nascent adhesions include the Rac1 regulatory module (Rac1 activators, Rac1 downstream effectors and Rac1 downstream targets), which promotes dendritic/branched actin polymerization for continuous protrusion extension, and positively enhances the assembly of immature FAs (nascent adhesions and focal complexes) 33, 101. Soon after, the immature FAs connect with bundles of actin filaments at the lamellipodia-lamella interface and they undergo a compositional reorganization and enlarge into mature FAs. This compositional reorganization includes force-sensitive and force-responsive FA proteins; these coordinate to reinforce the linkage between integrin and actin, help to form mature FAs and aid bundling of filamentous actin (stress fibres) 33, 34, 38.
The RhoA regulatory module associated with mature FAs activates myosin II through the action of downstream effector, ROCK, on up-regulating of MLC phosphorylation 24. Myosin II activation sustains the myosin II-mediated contractile force and this further enhances the magnitude of the cellular tension. This enhanced cellular tension transmits the pulling force along the actin bundles to the adhesion sites, thereby translocating the cell body forward. The last step of the migration cycle is disassembly of mature FAs at the cell rear, which is also contractile force-dependent 98. Actomyosin contractility promotes FA association with the disassembly factors, including proteases 71, 73, 75 and the components of endocytosis pathways 99, 100. This disrupts the linkage between integrin and actin by cleaving and recycling the structural proteins that form the mature FAs 33. Following the action of the disassembly factors, the pulling force supplied by the actomyosin contractility retracts the trailing edge of the cell, completing the migration cycle. Altogether, FAs not only serve as mechanosensors that re-organize their composition in response to mechanical forces, but also function as mechanotransducers that mediate specific cellular signalling pathways that regulate FA turnover and cytoskeletal organization, thereby controlling cell behaviour and driving cell migration.
Conclusion and future prospects
In response to mechanical force, FAs reorganize their protein composition in a hierarchical cascade to assemble FAs in different maturation states. The proposed model is shown in Figure 2. Within immature and mature FAs, some FA-associated proteins have similar levels of abundance, indicating that they serve as force-insensitive proteins. Some FA-associated proteins (force-sensitive proteins) show negative or positive regulation in response to mechanical force, which may alter their FA abundance, conformation, or enzymatic activity, thereby changing the association of FAs with other FA proteins (force-responsive proteins) to assemble FAs in different maturation states. In immature FAs, force-insensitive proteins, force-sensitive proteins and force-responsive proteins coordinately transmit specific integrin-mediated signals to promote dendritic actin polymerization and the formation of immature FAs for membrane protrusion. In response to mechanical force, force-sensitive proteins in immature FAs are negatively regulated and decrease their FA abundance, thereby driving the dissociation of force-responsive proteins from FAs. By contrast, subjecting force-sensitive proteins in mature FAs to mechanical force enhances their FA abundance and triggers the association of force-responsive proteins to assemble mature FAs. FAs serve as force transmission pathways to sense the local balance of mechanical forces.
Focal adhesions enable cells to respond to their various environments, which contain diverse mechanical properties. They do this by manipulating their protein compositions, which allows the transmission of specific biochemical signals that mediate cellular behaviour. Within a range of tissue microenvironments, cells feel and sense the proper matrix elasticity, thus displaying their specific biological function in specific tissues. However, the mechanical properties of the matrix in some disordered tissues can mislead the cells and cause disease progression. For example, matrix remodelling and stiffening promote breast tumorigenesis and malignancy 112. In liver fibrosis, fibril-forming collagens facilitate further progression of chronic liver disease 113. Therefore, understanding the molecular details of how FAs respond to mechanical force will provide a resource that will aid the discovery of new therapeutic strategies. Although the details of the control of cellular phenomena in vivo are complicated, systems analysis using proteomics-related techniques, protein microarrays, or phospho-kinase antibody arrays is able to globally explore signalling modules and networks of FAs in the specific cells cultured under conditions of tissue-level matrix stiffness. For a particular signalling network, tracking a FA protein tagged with a fluorescent protein using microscopy-based technologies, such as live-cell imaging techniques, enables observation and quantification at high spatial and temporal resolution. Further illustration of the integrin-mediated signalling pathways in different cell types or under different physiological conditions will provide a possible foundation for designing therapeutic strategies for some human diseases.
Acknowledgments
We apologize to those authors whose papers could not be cited because of space constraints. This study was supported by research grants from the National Science Council of Taiwan (NSC 101-2320-B-010-034 and NSC 101-2628-B-010-003-MY3), the UST-UCSD International Center of Excellence in Advanced Bio-engineering sponsored by the Taiwan National Science Council I-RICE Program (NSC-100-2911-I-009-101), and a grant from Ministry of Education, Aim for the Top University Plan.
Conflicts of interest
The author confirms that there are no conflicts of interest.
References
- 1.Alberts B, Johnson A, Lewis J, et al. Molecular biology of the cell. 5th ed. New York: Garland Science, Taylor and Francis Group, LCC; 2008. [Google Scholar]
- 2.Ananthakrishnan R, Ehrlicher A. The forces behind cell movement. Int J Biol Sci. 2007;3:303–17. doi: 10.7150/ijbs.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnette DT, Manley S, Sengupta P, et al. A role for actin arcs in the leading-edge advance of migrating cells. Nat Cell Biol. 2011;13:371–81. doi: 10.1038/ncb2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi CK, Vicente-Manzanares M, Zareno J, et al. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008;10:1039–50. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Even-Ram S, Doyle AD, Conti MA, et al. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol. 2007;9:299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- 6.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 7.Small JV, Stradal T, Vignal E, et al. The lamellipodium: where motility begins. Trends Cell Biol. 2002;12:112–20. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- 8.Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 9.Jockusch BM, Bubeck P, Giehl K, et al. The molecular architecture of focal adhesions. Annu Rev Cell Dev Biol. 1995;11:379–416. doi: 10.1146/annurev.cb.11.110195.002115. [DOI] [PubMed] [Google Scholar]
- 10.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 11.Burridge K, Fath K, Kelly T, et al. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- 12.Zaidel-Bar R, Geiger B. The switchable integrin adhesome. J Cell Sci. 2010;123:1385–8. doi: 10.1242/jcs.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaidel-Bar R, Itzkovitz S, Ma'ayan A, et al. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–67. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–74. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 15.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 16.Zamir E, Geiger B, Kam Z. Quantitative multicolor compositional imaging resolves molecular domains in cell-matrix adhesions. PLoS ONE. 2008;3:e1901. doi: 10.1371/journal.pone.0001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaidel-Bar R, Cohen M, Addadi L, et al. Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans. 2004;32:416–20. doi: 10.1042/BST0320416. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helfman DM, Levy ET, Berthier C, et al. Caldesmon inhibits nonmuscle cell contractility and interferes with the formation of focal adhesions. Mol Biol Cell. 1999;10:3097–112. doi: 10.1091/mbc.10.10.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaverina I, Krylyshkina O, Small JV. Regulation of substrate adhesion dynamics during cell motility. Int J Biochem Cell Biol. 2002;34:746–61. doi: 10.1016/s1357-2725(01)00171-6. [DOI] [PubMed] [Google Scholar]
- 21.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633–43. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 23.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–15. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishizaki T, Naito M, Fujisawa K, et al. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–24. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- 25.Leung T, Chen XQ, Manser E, et al. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–27. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maekawa M, Ishizaki T, Boku S, et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–8. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 27.Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 28.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–7. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 30.Wolfenson H, Bershadsky A, Henis YI, et al. Actomyosin-generated tension controls the molecular kinetics of focal adhesions. J Cell Sci. 2011;124:1425–32. doi: 10.1242/jcs.077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang HB, Dembo M, Hanks SK, et al. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci USA. 2001;98:11295–300. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–95. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- 33.Kuo JC, Han X, Hsiao CT, et al. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol. 2011;13:383–93. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiller HB, Friedel CC, Boulegue C, et al. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 2011;12:259–66. doi: 10.1038/embor.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang G, Giannone G, Critchley DR, et al. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature. 2003;424:334–7. doi: 10.1038/nature01805. [DOI] [PubMed] [Google Scholar]
- 36.Sawada Y, Tamada M, Dubin-Thaler BJ, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–26. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grashoff C, Hoffman BD, Brenner MD, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–6. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallegos L, Ng MR, Brugge JS. The myosin-II-responsive focal adhesion proteome: a tour de force? Nat Cell Biol. 2011;13:344–6. doi: 10.1038/ncb0411-344. [DOI] [PubMed] [Google Scholar]
- 39.del RA, Perez-Jimenez R, Liu R, et al. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–41. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stossel TP, Condeelis J, Cooley L, et al. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2:138–45. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- 41.Calderwood DA, Shattil SJ, Ginsberg MH. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J Biol Chem. 2000;275:22607–10. doi: 10.1074/jbc.R900037199. [DOI] [PubMed] [Google Scholar]
- 42.Moser M, Legate KR, Zent R, et al. The tail of integrins, talin, and kindlins. Science. 2009;324:895–9. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- 43.Tadokoro S, Shattil SJ, Eto K, et al. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–6. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 44.Mimura N, Asano A. Further characterization of a conserved actin-binding 27-kD fragment of actinogelin and alpha-actinins and mapping of their binding sites on the actin molecule by chemical cross-linking. J Biol Chem. 1987;262:4717–23. [PubMed] [Google Scholar]
- 45.Mimura N, Asano A. Isolation and characterization of a conserved actin-binding domain from rat hepatic actinogelin, rat skeletal muscle, and chicken gizzard alpha-actinins. J Biol Chem. 1986;261:10680–7. [PubMed] [Google Scholar]
- 46.Otey CA, Pavalko FM, Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990;111:721–9. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardel ML, Nakamura F, Hartwig JH, et al. F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc Natl Acad Sci USA. 2006;103:1762–7. doi: 10.1073/pnas.0504777103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loo DT, Kanner SB, Aruffo A. Filamin binds to the cytoplasmic domain of the beta1-integrin. Identification of amino acids responsible for this interaction. J Biol Chem. 1998;273:23304–12. doi: 10.1074/jbc.273.36.23304. [DOI] [PubMed] [Google Scholar]
- 49.Pfaff M, Liu S, Erle DJ, et al. Ginsberg, Integrin beta cytoplasmic domains differentially bind to cytoskeletal proteins. J Biol Chem. 1998;273:6104–9. doi: 10.1074/jbc.273.11.6104. [DOI] [PubMed] [Google Scholar]
- 50.Calderwood DA, Huttenlocher A, Kiosses WB, et al. Increased filamin binding to beta-integrin cytoplasmic domains inhibits cell migration. Nat Cell Biol. 2001;3:1060–8. doi: 10.1038/ncb1201-1060. [DOI] [PubMed] [Google Scholar]
- 51.Bachmann C, Fischer L, Walter U, et al. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J Biol Chem. 1999;274:23549–57. doi: 10.1074/jbc.274.33.23549. [DOI] [PubMed] [Google Scholar]
- 52.Bubeck P, Pistor S, Wehland J, et al. Ligand recruitment by vinculin domains in transfected cells. J Cell Sci. 1997;110:1361–71. doi: 10.1242/jcs.110.12.1361. [DOI] [PubMed] [Google Scholar]
- 53.Reinhard M, Zumbrunn J, Jaquemar D, et al. An alpha-actinin binding site of zyxin is essential for subcellular zyxin localization and alpha-actinin recruitment. J Biol Chem. 1999;274:13410–8. doi: 10.1074/jbc.274.19.13410. [DOI] [PubMed] [Google Scholar]
- 54.Pawson T, Gish GD, Nash P. SH2 domains, interaction modules and cellular wiring. Trends Cell Biol. 2001;11:504–11. doi: 10.1016/s0962-8924(01)02154-7. [DOI] [PubMed] [Google Scholar]
- 55.Huang H, Li L, Wu C, et al. Defining the specificity space of the human SRC homology 2 domain. Mol Cell Proteomics. 2008;7:768–84. doi: 10.1074/mcp.M700312-MCP200. [DOI] [PubMed] [Google Scholar]
- 56.Alexandropoulos K, Cheng G, Baltimore D. Proline-rich sequences that bind to Src homology 3 domains with individual specificities. Proc Natl Acad Sci USA. 1995;92:3110–4. doi: 10.1073/pnas.92.8.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang DS, Miller R, Shaw R, et al. The pleckstrin homology domain of human beta I sigma II spectrin is targeted to the plasma membrane in vivo. Biochem Biophys Res Commun. 1996;225:420–6. doi: 10.1006/bbrc.1996.1189. [DOI] [PubMed] [Google Scholar]
- 58.Wang DS, Shaw G. The association of the C-terminal region of beta I sigma II spectrin to brain membranes is mediated by a PH domain, does not require membrane proteins, and coincides with a inositol-1,4,5 triphosphate binding site. Biochem Biophys Res Commun. 1995;217:608–15. doi: 10.1006/bbrc.1995.2818. [DOI] [PubMed] [Google Scholar]
- 59.Kadrmas JL, Beckerle MC. The LIM domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cell Biol. 2004;5:920–31. doi: 10.1038/nrm1499. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Gilmore TD. Zyxin and paxillin proteins: focal adhesion plaque LIM domain proteins go nuclear. Biochim Biophys Acta. 2003;1593:115–20. doi: 10.1016/s0167-4889(02)00349-x. [DOI] [PubMed] [Google Scholar]
- 61.Pearson MA, Reczek D, Bretscher A, et al. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell. 2000;101:259–70. doi: 10.1016/s0092-8674(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 62.Kloeker S, Major MB, Calderwood DA, et al. The Kindler syndrome protein is regulated by transforming growth factor-beta and involved in integrin-mediated adhesion. J Biol Chem. 2004;279:6824–33. doi: 10.1074/jbc.M307978200. [DOI] [PubMed] [Google Scholar]
- 63.Shi X, Ma YQ, Tu Y, et al. The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J Biol Chem. 2007;282:20455–66. doi: 10.1074/jbc.M611680200. [DOI] [PubMed] [Google Scholar]
- 64.Montanez E, Ussar S, Schifferer M, et al. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008;22:1325–30. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moser M, Nieswandt B, Ussar S, et al. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14:325–30. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 66.Castresana J, Saraste M. Does Vav bind to F-actin through a CH domain? FEBS Lett. 1995;374:149–51. doi: 10.1016/0014-5793(95)01098-y. [DOI] [PubMed] [Google Scholar]
- 67.Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–8. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- 68.Ellenbroek SI, Collard JG. Rho GTPases: functions and association with cancer. Clin Exp Metastasis. 2007;24:657–72. doi: 10.1007/s10585-007-9119-1. [DOI] [PubMed] [Google Scholar]
- 69.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–5. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 70.Carragher NO, Levkau B, Ross R, et al. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp 125(FAK), paxillin, and talin. J Cell Biol. 1999;147:619–30. doi: 10.1083/jcb.147.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Franco SJ, Huttenlocher A. Regulating cell migration: calpains make the cut. J Cell Sci. 2005;118:3829–38. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- 72.Bhatt A, Kaverina I, Otey C, et al. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. J Cell Sci. 2002;115:3415–25. doi: 10.1242/jcs.115.17.3415. [DOI] [PubMed] [Google Scholar]
- 73.Du X, Saido TC, Tsubuki S, et al. Calpain cleavage of the cytoplasmic domain of the integrin beta 3 subunit. J Biol Chem. 1995;270:26146–51. doi: 10.1074/jbc.270.44.26146. [DOI] [PubMed] [Google Scholar]
- 74.Pfaff M, Du X, Ginsberg MH. Calpain cleavage of integrin beta cytoplasmic domains. FEBS Lett. 1999;460:17–22. doi: 10.1016/s0014-5793(99)01250-8. [DOI] [PubMed] [Google Scholar]
- 75.Franco SJ, Rodgers MA, Perrin BJ, et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6:977–83. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 76.Carragher NO, Westhoff MA, Fincham VJ, et al. A novel role for FAK as a protease-targeting adaptor protein: regulation by p42 ERK and Src. Curr Biol. 2003;13:1442–50. doi: 10.1016/s0960-9822(03)00544-x. [DOI] [PubMed] [Google Scholar]
- 77.Chan KT, Bennin DA, Huttenlocher A. Regulation of adhesion dynamics by calpain-mediated proteolysis of focal adhesion kinase (FAK) J Biol Chem. 2010;285:11418–26. doi: 10.1074/jbc.M109.090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sato K, Kawashima S. Calpain function in the modulation of signal transduction molecules. Biol Chem. 2001;382:743–51. doi: 10.1515/BC.2001.090. [DOI] [PubMed] [Google Scholar]
- 79.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–9. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 80.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 81.Nayal A, Webb DJ, Brown CM, et al. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J Cell Biol. 2006;173:587–9. doi: 10.1083/jcb.200509075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zaidel-Bar R, Milo R, Kam Z, et al. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci. 2007;120:137–48. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
- 83.Meves A, Stremmel C, Gottschalk K, et al. The Kindlin protein family: new members to the club of focal adhesion proteins. Trends Cell Biol. 2009;19:504–13. doi: 10.1016/j.tcb.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 84.Tu Y, Wu S, Shi X, et al. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell. 2003;113:37–47. doi: 10.1016/s0092-8674(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 85.Bai CY, Ohsugi M, Abe Y, et al. ZRP-1 controls Rho GTPase-mediated actin reorganization by localizing at cell-matrix and cell-cell adhesions. J Cell Sci. 2007;120:2828–37. doi: 10.1242/jcs.03477. [DOI] [PubMed] [Google Scholar]
- 86.Griffith E, Coutts AS, Black DM. RNAi knockdown of the focal adhesion protein TES reveals its role in actin stress fibre organisation. Cell Motil Cytoskeleton. 2005;60:140–52. doi: 10.1002/cm.20052. [DOI] [PubMed] [Google Scholar]
- 87.Ren Y, Li R, Zheng Y, et al. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J Biol Chem. 1998;273:34954–60. doi: 10.1074/jbc.273.52.34954. [DOI] [PubMed] [Google Scholar]
- 88.Otey CA, Carpen O. Alpha-actinin revisited: a fresh look at an old player. Cell Motil Cytoskeleton. 2004;58:104–11. doi: 10.1002/cm.20007. [DOI] [PubMed] [Google Scholar]
- 89.Schroeter MM, Beall B, Heid HW, et al. In vitro characterization of native mammalian smooth-muscle protein synaptopodin 2. Biosci Rep. 2008;28:195–203. doi: 10.1042/BSR20080079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y, Takizawa N, Crowley JL, et al. F-actin and myosin II binding domains in supervillin. J Biol Chem. 2003;278:46094–106. doi: 10.1074/jbc.M305311200. [DOI] [PubMed] [Google Scholar]
- 91.Wulfkuhle JD, Donina IE, Stark NH, et al. Domain analysis of supervillin, an F-actin bundling plasma membrane protein with functional nuclear localization signals. J Cell Sci. 1999;112:2125–36. doi: 10.1242/jcs.112.13.2125. [DOI] [PubMed] [Google Scholar]
- 92.Zaidel-Bar R, Ballestrem C, Kam Z, et al. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116:4605–13. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- 93.Hirata H, Tatsumi H, Sokabe M. Zyxin emerges as a key player in the mechanotransduction at cell adhesive structures. Commun Integr Biol. 2008;1:192–5. doi: 10.4161/cib.1.2.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hirata H, Tatsumi H, Sokabe M. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J Cell Sci. 2008;121:2795–804. doi: 10.1242/jcs.030320. [DOI] [PubMed] [Google Scholar]
- 95.Harris BZ, Lim WA. Mechanism and role of PDZ domains in signaling complex assembly. J Cell Sci. 2001;114:3219–31. doi: 10.1242/jcs.114.18.3219. [DOI] [PubMed] [Google Scholar]
- 96.Schuh M, Ellenberg J. A new model for asymmetric spindle positioning in mouse oocytes. Curr Biol. 2008;18:1986–92. doi: 10.1016/j.cub.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 97.Broussard JA, Webb DJ, Kaverina I. Asymmetric focal adhesion disassembly in motile cells. Curr Opin Cell Biol. 2008;20:85–90. doi: 10.1016/j.ceb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 98.Crowley E, Horwitz AF. Tyrosine phosphorylation and cytoskeletal tension regulate the release of fibroblast adhesions. J Cell Biol. 1995;131:525–37. doi: 10.1083/jcb.131.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10:843–53. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 100.Ezratty EJ, Bertaux C, Marcantonio EE, et al. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733–47. doi: 10.1083/jcb.200904054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Legg K. Cell migration: keeping young and mobile with beta-PIX. Nat Rev Mol Cell Biol. 2011;12:278. doi: 10.1038/nrm3104. [DOI] [PubMed] [Google Scholar]
- 102.ten Klooster JP, Jaffer ZM, Chernoff J, et al. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J Cell Biol. 2006;172:759–69. doi: 10.1083/jcb.200509096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Innocenti M, Tenca P, Frittoli E, et al. Mechanisms through which Sos-1 coordinates the activation of Ras and Rac. J Cell Biol. 2002;156:125–36. doi: 10.1083/jcb.200108035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rendon BE, Roger T, Teneng I, et al. Regulation of human lung adenocarcinoma cell migration and invasion by macrophage migration inhibitory factor. J Biol Chem. 2007;282:29910–8. doi: 10.1074/jbc.M704898200. [DOI] [PubMed] [Google Scholar]
- 105.O'Connor KL, Mercurio AM. Protein kinase A regulates Rac and is required for the growth factor-stimulated migration of carcinoma cells. J Biol Chem. 2001;276:47895–900. doi: 10.1074/jbc.M107235200. [DOI] [PubMed] [Google Scholar]
- 106.Miki H, Yamaguchi H, Suetsugu S, et al. IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature. 2000;408:732–5. doi: 10.1038/35047107. [DOI] [PubMed] [Google Scholar]
- 107.Eden S, Rohatgi R, Podtelejnikov AV, et al. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418:790–3. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- 108.Weed SA, Karginov AV, Schafer DA, et al. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J Cell Biol. 2000;151:29–40. doi: 10.1083/jcb.151.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oser M, Condeelis J. The cofilin activity cycle in lamellipodia and invadopodia. J Cell Biochem. 2009;108:1252–62. doi: 10.1002/jcb.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bertling E, Hotulainen P, Mattila PK, et al. Cyclase-associated protein 1 (CAP1) promotes cofilin-induced actin dynamics in mammalian nonmuscle cells. Mol Biol Cell. 2004;15:2324–34. doi: 10.1091/mbc.E04-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells – over and over and over again. Nat Cell Biol. 2002;4:E97–100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- 112.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang C, Zeisberg M, Mosterman B, et al. Liver fibrosis: insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology. 2003;124:147–59. doi: 10.1053/gast.2003.50012. [DOI] [PubMed] [Google Scholar]
- 114.Ehrlicher AJ, Nakamura F, Hartwig JH, et al. Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature. 2011;478:260–3. doi: 10.1038/nature10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gad A, Lach S, Crimaldi L, et al. Plectin deposition at podosome rings requires myosin contractility. Cell Motil Cytoskeleton. 2008;65:614–25. doi: 10.1002/cm.20287. [DOI] [PubMed] [Google Scholar]
- 116.Zamir E, Katz M, Posen Y, et al. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat Cell Biol. 2000;2:191–6. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]
- 117.DeMali KA, Barlow CA, Burridge K. Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J Cell Biol. 2002;159:881–91. doi: 10.1083/jcb.200206043. [DOI] [PMC free article] [PubMed] [Google Scholar]


