Abstract
In a multicellular system, cellular communication is a must for orchestration and coordination of cellular events. Advent of the latest analytical and imaging tools has allowed us to enhance our understanding of the intercellular communication. An intercellular exchange of proteins or intact membrane patches is a ubiquitous phenomenon, and has been the subject of renewed interest, particularly in the context of immune cells. Recent evidence implicates that intercellular protein transfers, including trogocytosis is an important mechanism of the immune system to modulate immune responses and transferred proteins can also contribute to pathology. It has been demonstrated that intercellular protein transfer can be through the internalization/pathway, dissociation-associated pathway, uptake of exosomes and membrane nanotube formations. Exchange of membrane molecules/antigens between immune cells has been observed for a long time, but the mechanisms and functional consequences of these transfers remain unclear. In this review, we will discuss the important findings concerning intercellular protein transfers, possible mechanisms and highlight their physiological relevance to the immune system, with special reference to T cells such as the stimulatory or suppressive immune responses derived from T cells with acquired dendritic cell membrane molecules.
Keywords: cellular interaction, synapse, membrane protein transfer, trogocytosis, immune responses
Introduction
Cell to cell communication is essential for orchestration and coordination of cellular events in multicellular systems. A growing amount of studies is underlying the role of the synapse (establishment of close contacts between juxtaposed cells) in cellular cross-talk. The term ‘synapse’ or ‘synapsis’ was coined in 1897 by Sherrington [1, 2] to describe the functional connection between neurons. The neuronal synapse is a classical example of cellular connection that offers a structural platform for intercellular communication. In this process two cells or parts thereof, form a new functional connection for signal exchange. Neuronal synapse formation and disintegration may take minutes to few hours and retain livelong (relatively slow) structural plasticity [2]. As overall features of cellular contacts between T cells and antigen-presenting cells (APCs) were found to be similar to neuronal synaptic communication, in 1984 Norcross [3] proposed the term ‘synapse’ to describe the contacts between T cells and APCs. Later several studies gathered biophysical and molecular data and confirmed its existence between immune cells. Since then the term ‘immunological synapse’ (IS) has gained wide acceptance among immunologists [3–6]. Recently, the concept of synapse has been extended further to describe contacts between immune and non-immune cells. Interestingly, a novel type of synapse termed as ‘stromal synapse’ identified between interstitial cells of Cajal (ICC)/ICC-like cells and immunoreactive cells, apparently playing a role in tissue immune surveillance [7].
The immunological synapse is formed between an APC and a lymphocyte, when many different molecules on the APC and the lymphocyte sides (like CD28/CD80 and leucocyte function associated antigen-1 [LFA-1]/ intercellular adhesion molecule-1 [ICAM-1]) come together to form an interface and has been observed for T [5, 8], B [9] and natural killer (NK) [10] cells. The formation of IS involves several molecules in a very organized manner, leading to: (i) a central supramolecular activation cluster formed by the T-cell receptor (TCR): peptide-loaded major histocompatibility complex (pMHC) cluster; (ii) a peripheral adhesion ring junction made up of adhesion molecules that include integrins (specifically LFA-1 [αLβ2] and VLA4 [α4β1]) and adaptor proteins such as talin and (iii) a CD45 rich distal zone [11]. Better understanding of the molecular organization of immunological synapse is a prerequisite to realize its structural and functional relevance. During IS formation, critical interactions are made with the cytoskeleton. Molecular studies demonstrated that CD3 and β1 integrin (two IS-associated receptors) play important roles in Cdc42 activation and ρ guanosine triphosphatase Cdc42 regulates cytoskeletal changes at the IS which are critical to T-cell activation. It has been suggested that both IS-associated receptors probably lie on a serial molecular pathway and transduce signals through the ezrin–radixin–moesin (ERM) dependent machinery that is responsible for the remodelling and stabilization of the synapse. Members of the ERM family of proteins have been shown to play an important regulatory role during IS formation [12, 13] and T-cell activation [14] by aiding the formation of the uropod/distal pole complex, a structure essential for T-cell activation (reviewed in [14, 15]). Thus, studies are underway to enhance the knowledge of the spatiotemporal relationship between cytoskeleton, adhesion molecules, antigen receptors and costimulatory molecules during different stages of cell–cell contact. With an increasing knowledge on cell–cell interactions, it is becoming clear that the formal criteria for ‘synaptic’ signal exchange seem to comprise the following: (i) the close apposition of two membranes, which leads to (ii) uni- or bidirectional information exchange and downstream signal transduction to the nucleus for the onset of gene transcription. The IS, like T cell immunological synapse is thought to be the seat of initiation of TCR signalling events [11] leading to different lymphocytes functions such as proliferation, cytokine production to coordinate and regulate cell to cell interaction necessary to elicit immune responses. Noteworthy, there is emerging evidence supporting a role of IS to facilitate also the transfer of both extracellular receptors [16] and membrane patches between cell conjugates [17–19]. Interestingly, a new phenomenon of cellular communication through exchange of intracellular, inner membrane protein has also been demonstrated to occur between immune cells [20]. Ras proteins represent the first example of non-secreted intracellular plasma membrane (PM)-bound proteins which, upon cell-to-cell contact, are transferred from the inner surface of the PM of one cell to the interior of another cell [21].
The immune system comprised multiple cell subsets capable of performing specific functions. According to linear model of immune function, immune subsets are characterized by the functions they perform and protein molecules that are expressed on their cell surfaces play a pivotal role in their functions and form the basis of their phenotypic characterization [22]. For example, expression of CD3 indicates T cells and TCR/CD3 plus CD4 or CD8 defines CD4+ and CD8+ T cells. Similarly, cell population constitutively expressing MHC class II can be described as professional APC, and in non-human primates, NK cells could be characterized by killer Ig-like receptors (KIR) [23]. However, in the year 1973 intercellular antigen exchange was first demonstrated between lymphocytes [24]; since then, various surprising situations were observed, in which protein molecules considered specific for one cell type were seen on the surfaces of other cell types [18, 22, 25]. These include, transfer of antigenic material from macrophages to lymphocytes [24], uptake of macrophage Fc receptors and MHC molecules by T cells [26], acquisition of recipient MHC class I and II molecules on donor thymocytes in bone-marrow chimaeras [27, 28], transfer of MHC class II proteins from splenic cells to allogenic T-cell clones [29] and capture of B-cell surface immunoglobulin by T cells [30, 31]. To describe this phenomenon of intercellular transfer of membrane patches containing membrane-anchored proteins from one cell type to another following IS formation, Hudrisier and colleagues [19] coined the term ‘Trogocytosis’ from the ancient Greek word ‘trogo’ which means nibble. As of August 10, 2009, the PubMed contained >19 million references, search with keyword ‘trogocytosis’ showed 35 references, of which 30 references were identified as intercellular membrane or protein transfer related. Thus, a significant fraction (∼85%) of the worldwide trogocytosis literature is related to immune functions of the intercellular membrane transfer, reflecting a wider acceptance of this term and a biomedical importance of this phenomenon.
Intercellular membrane transfer a widespread phenomenon
After a long quiescent period, in-depth studies began to understand mechanisms of intercellular protein transfer, which was found to be a widespread event. The importance of such a cellular cross-talk is being well understood now, as new reports of intercellular membrane transfer are published [18, 22, 32–37].
Intercellular protein transfers between the cells of immune system
It was demonstrated that T cells can acquire not only MHC class I and class II proteins [38, 39], but also co-stimulatory proteins [40–42] and membrane proteins from APC [43, 44], endothelial cells [45]. Until very recent, protein transfer by trogocytosis is believed to be unidirectional in murine system [23]. However, our recent work provided the first evidence of bidirectional membrane molecule transfer between dendritic and T cells in murine system [46]. Similarly, new information on trogocytosis was acquired pertaining to NK cells. It was shown that NK cells can capture target cell-MHC class I protein both in vitro and in vivo[47–49], virus receptor (CD155) [50] and membrane fragments [51] from target cells. Both in human and mouse models it was demonstrated that NK cell receptors for MHC class I protein can be transferred to target cells [52] demonstrating bidirectional membrane transfer. B cells can capture membrane-associated antigens from target cells and amount of antigen captured correlated with the affinity of B-cell receptor (BCR) for the antigen [9, 53]. Recently, it was shown that bystander B cells could acquire antigen-specific BCR from activated B cells by membrane transfer and donated BCR was able to enhance specific antigen presentation to CD4+ T cells [54]. However, acquired BCR could not deliver normal signalling events associated with BCR-mediated B cell activation. It was found that B6.CD45.1 B cells that had acquired the MD4 BCR could not respond with Ca2+ flux on cross-linking with an IgMa-specific antibody, but strongly responded when interacted with an antibody that cross-linked with their endogenous IgMb. In addition, CD8αα intraepithelial lymphocytes were shown to snatch thymic leukaemia MHC class Iβ molecules in vitro and in vivo[55]. Whereas, γδ T cells could capture fragments of membrane from tumour cells, such as Daudi cells (a B-lymphoblastoid cell line, derived from Burkitt’s lymphoma) [56]. Similarly, dendritic cells (DCs) were shown to transfer captured allogenic MHC class I and class II proteins in vivo during transplantation [57, 58].
Using a model of viral antigen lymphocytic choriomeningitis virus (LCMV) gp33–41 recognition in P14 mice [57, 58], Riond and colleagues, clearly demonstrated the in vivo evidence of trogocytosis, i.e. the transfer of membrane fragments from APCs to lymphocytes. Authors reported that CD8+ T cells perform trogocytosis at least during, encounter with DCs in the lymph nodes, and with target cells in the periphery. Interestingly, this investigation suggested that trogocytosis may be an in vivo marker of recent interaction of CD8+ T cells with its target, as CD8+ T cells having performed trogocytosis with DCs in lymph nodes, express the CD69 activation marker [59]. Recently, Hudrisier and colleagues reported that trogocytosis on T cells are triggered by several costimulatory molecules and coreceptors, in addition to TCR/CD3 components. On the other hand, only the BCRs and MHC molecules are potentials triggers of trogocytosis on B cells. Remarkably, Aucher et al. [60] employed different inhibitors of actin polymerization or of kinases involved in intracellular signalling and demonstrated that trogocytosis by CD8+ and CD4+ T cells was inhibited partially or fully, but no effect on trogocytosis by B cells. It was further demonstrated that trogocytosis by T cells was inhibited at 4°C, whereas in B cells it was independent of temperature, indicating that unlike B cells, trogocytosis by T cells does rely on active processes. Trogocytosis thus has different requirements in different cell types. Given the varied roles proposed for trogocytosis in T-cell activation, therefore, the presence or absence of these molecules on T cells and of their ligands on APC could greatly influence the positive (i.e. activation) or negative (i.e. induction of anergy) consequences the captured material will play in subsequent T-T interactions [61, 62]. Recent studies reported that when patients with chronic lymphocytic leukaemia were treated with certain anticancer drugs/immunotherapeutic monoclonal antibodies (mAbs) (rituximab, trastuzumab, cetuximab or mAb T101) directed against CD20 on malignant B cells, induced loss of bound rituximab (RTX) and CD20 from targeted circulating malignant B cells. This loss of the RTX-CD20 immune complexes was termed as ‘shaving’, in which receptors on effector cells remove and internalize cognate ligands and cell membrane fragments from target cells by trogocytosis mechanism [63].
Intercellular protein transfer between the cells related – unrelated to immunity
Recently, intercellular membrane transfer has been documented between immunoreactive cells and system unrelated to immunity. In skeletal muscles, it has been demonstrated that T cells are capable of ripping membrane fragments not only from immune cells but also from human skeletal muscle derived cells through an active process that may functionally alter acquiring T cells [34]. Popescu and colleagues provided the electron microscopic evidence of a novel synapse between ICC/ICC-like cells and several types of immune cells, like lymphocytes, plasma cells, basophils, eosinophils, mast cells and macrophages in various organs: rat myometrium, gut, uterus, stomach and bladder as well as human myometrium and mammary gland. Interestingly, microvesicles were found in the synaptic cleft, authors suggested that this may correspond to an exosome-based mechanism of cellular communications [7]. However, further studies are needed to provide physiological relevance of this observation.
Intercellular protein transfer in the systems unrelated to immunity
Transfer of membrane proteins has been accounted also in the systems unrelated to immunity. Eph receptors and their membrane-associated ephrin ligands mediate cell–cell repulsion to guide migrating cells and axons. During the detachment of neuronal growth cones, bidirectional endocytosis of ephrinB–EphB complexes has been suggested to be a mechanism for the termination of adhesion, thereby allowing contact-mediated cell repulsion following intercellular interaction between two transmembrane proteins [64]. In addition, it has been reported that glycosylphosphatidylinositol (GPI) anchored proteins transfer across homotypic interactions between HeLa cells [65] and the transmembrane protein bride of sevenless is internalized from one cell by contact with another during eye development in Drosophila melanogaster[66]. The mechanisms for above mentioned protein transfers are different from the topic of this review. Interestingly, transfer of CD9 tetraspin, a membrane-organizing molecule from oocyte to sperm was reported during fertilization, in a process similar to trogocytosis. Acquisition of membrane fragments by the sperm may help membrane reorganization in sperm to facilitate fertilization [67]. Altogether, these studies conducted in various systems present strong evidence that cell surface proteins commonly transfer between cells both in vitro and in vivo[18].
The pathways responsible for the intercellular membrane transfer [18]
In general, intercellular membrane transfer between APC and T cells is considered to be an antigen-specific event that requires the formation of an IS. TCR/CD28-mediated adhesion was demonstrated to play a pivotal function in intercellular exchange of membrane between T cells and APC [68]. However, TCR independent membrane transfer between APCs and pre-activated T cells have been also reported as alternative antigen-independent pathways of trogocytosis involving engagement of CD28 and its ligands B7.1/2. Moreover, investigations demonstrated that trogocytosis can indeed be triggered by a variety of lymphocyte specific surface receptors/costimulatory molecules either individually or in combination and does not simply rely on CD28/TCR activation [62].
Trogocytosis is strictly dependent on cellular contact, as demonstrated by its complete inhibition when a semi-permeable transwell membrane separated the lymphocytes and the targets [16, 19]. Very little information is available about the nature of proteins which transfer and, which do not. Many proteins or membrane patches have been identified that are exchanged or snatched by contact dependent membrane transfer mechanism, like MHC molecules, costimulatory molecules (CD80, CD86), adhesion molecules (like ICAM), receptors (like NK cell receptor, neuropilin-1, macrophage Fc receptors, BCR, CCR5, viral receptors like Epstein-Barr virus [EBV] and CD155) and tumour antigens. Studies have reported that nanotubes can cargo various proteins and other molecules, like endosome-related organelles, lipid-anchored proteins, mitochondria, MHC molecules, Mycobacterium bovis Bacillus Calmette-Guerin, murine leukaemia virus, HIV-1 protein Gag, calcium fluxes, GPI-green fluorescent protein (GFP) [69]. However, exosomes transport diverse range of proteins and other molecules through contact independent mechanism. For example, peptide-MHC complex, tumour antigens (human EGFR2 and carcinoembryonic antigen, gp100 and tyrosinase-related protein-1), genetic material (RNA, miRNA) and Mycobacterium tuberculosis or Mycobacterium bovis derived antigens. Interestingly, exosomes secreted by DCs infected with LCMV do not bear LCMV antigens [70]. Using Western-blot technique, it was confirmed that cell-surface proteins (target cell-derived MHC class I proteins) can be transferred intact into the adopting T or NK cells [47, 52], indicating trogocytosis does not involve proteolytic cleavage. Remarkably, recent studies demonstrated that cell-surface receptors transferred from ripped off membrane patches of target cells to NK cells assumed a normal in/out transmembrane orientation in the adopting lymphocyte [20, 71]. It thus appears that when immune cells interact with their targets, several cell–cell contact dependent mechanisms of trogocytosis could operate depending on cellular milieu and cell types involved. A wide range of proteins (including NK cell receptor KIR2DL1 and Ly49A, as detected by Western blotting, was shown to transfer from NK cells to target cells during inhibitory interactions [52]. Interestingly, analysis of transferred biotinylated surface proteins demonstrated a specific selection for the proteins that transferred. Proteins remain associated with cell surfaces by hydrophobic interactions. Hence, disruption or overcome of this hydrophobic interaction is necessary to initiate the intercellular transfer of proteins [18]. Absorption, internalization and enzymatic cleavage of cell-surface proteins could also be one of the probable mechanisms for intercellular membrane transfer [31, 38, 40, 72–74]. Overall, the direct cell-to-cell contact dependent or independent intercellular transfer of proteins from APCs to T cells may be described as shown in Figure 1.
Fig 1.
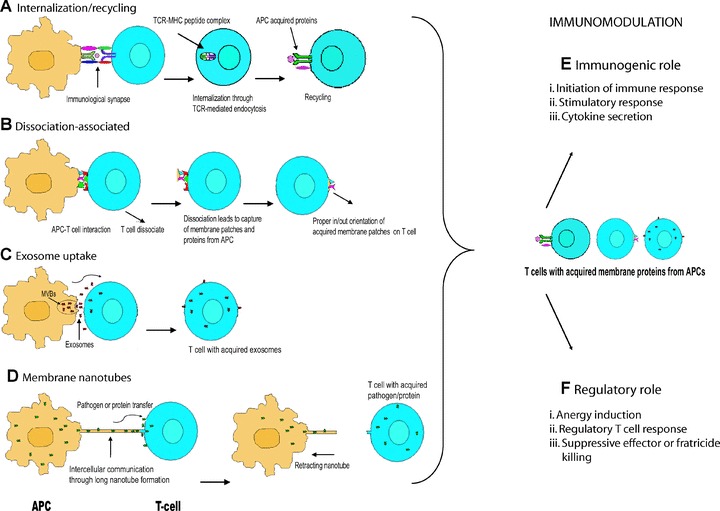
Mechanism for intercellular protein transfer between immune cells and its immunological relevance. (A) Internalization and recycling pathway; (B) dissociation-associated pathway; (C) exosome uptake; (D) membrane nanotube formation. IS or tight contact between lymphocytes and their targets enables intercellular exchange of cellular proteins. Some membrane proteins are snatched by specific receptors, and other, ‘bystander ligands’ and membrane patches can also be acquired. This intercellular exchange of proteins has an important influence on the course of T-cell-mediated immune responses. (E) In some circumstances, the intercellular transfer of cell-surface proteins from APCs to T cells can amplify immune responses or broaden cellular stimulation or activate neighbouring effector cells leading to augmenting cytokine production. (F) In some other conditions, the trogocytosis may induce anergy or tolerance, and T-cell function as regulatory T cells in subsequent immune modulation. In addition, the process of trogocytosis can dampen immune responses by fratricide killing, i.e. lysis of CTLs by neighbouring CTLs. (MVBs; multivesicular bodies).
TCR-mediated internalization and recycling
T-cell responses are initiated by TCR recognition of peptide/MHC (pMHC) on APCs [75, 76] and are the central event in developing an adaptive response. Subsequent (within minutes) to specific interactions of T cells with APCs, TCR and MHC molecules are assembled at the centre of supramolecular activation clusters at the site of T-cell contact [5, 77–79] and accessory molecules move towards the T cell/APC contact site, forming a signalling area at the interface that has been termed the IS [5]. TCR movement towards the IS reflects the intensity of antigenic stimulation [80, 81]. Using fluorescence recovery after photo-bleaching (FRAP) and fluorescence loss in photo-bleaching techniques, TCR motion on live cells has been studied [82]. TCR mobility was demonstrated on the human Jurkat T cells [83]. Krummel and colleagues also reported that TCR is mobile on murine T cells, and accelerate towards the IS during antigen recognition [84]. TCR motion towards IS requires an energy-consuming mechanism influenced by the interaction between TCR and the cortical actin cytoskeleton (CAC). Dushek and colleagues, demonstrated that increase in intracellular calcium [Ca2+]i concentration induces actin polymerization thereby, markedly reduces TCR mobility on the T-cell surface via actin cytoskeleton-dependent mechanism [85]. TCR recruitment to the centre of the IS has been associated with productive signalling [5, 86] and with TCR internalization [87, 88]. TCR-down-regulation is observed following interactions of TCR with pMHC complexes [89–91] and T cell APC interactions cause APC-derived surface molecules to adhere to the surface of T cells [92, 93]. Thereafter, these clusters are internalized through TCR-mediated endocytosis and localized in endosomes and lysosomes, followed by recycling and expression of these molecules on T-cell surfaces within 30 min. [38]. For efficient and specific acquisition of TCR-mediated pMHC complexes, a sustained TCR signalling is a prerequisite. The possibility of involvement of perforin’s cytolytic activity has been ruled out during the membrane capture process, because the kinetics of membrane capture were same both in perforin-deficient and P14-transgenic mice [43]. The peptide-MHC complexes transferred from APCs to T cells are the best studied examples of protein transfer that occurs via trogocytosis. Here, T cells can acquire MHC class I and class II proteins from APCs [39, 43, 44, 68, 94, 95]. Reports of intercellular transfer of membrane fragments from APCs to T cells [44, 68] and from target cells to NK cells [51], and even through homotypic interactions between cells like Daudi cells [96], are consistent with the membrane transfer mechanism that involves the transfer of membrane fragments derived from the intercellular contact or IS [97]. The TCR-ligand interaction could be one of the possible explanations of initiation of the membrane capture process, creating enough force ripping MHC proteins and the other APC ligand during TCR internalization. [18].
Dissociation-associated pathway
Before the advent of dissociation-associated theory, the membrane protein capture is thought to depend on TCR-mediated internalization during the direct cell-to-cell contact (as described above) or the APC-derived exosome/vesicle transfer (which will be described below). Using fibroblasts expressing a GFP-tagged I-Ek molecule with covalently attached antigenic peptide, Wetzel et al. demonstrated a third mechanism, the cellular dissociation [98]. With the help of live cell imaging, they showed that T cells, while spontaneously dissociating from APCs often capture MHC-peptide complexes directly from the IS. It was further shown that the MHC transfer is peptide specific and is enhanced by costimulation through CD28-CD80 interactions. T cells dissociated from the moth cytochrome c peptide (MCC): GFP cells were fully activated, expressing high levels of CD69. The activation phenotype is also relevant when considering the spontaneous dissociation of T cells from APCs. The acquisition of DC molecules by T cells has been previously reported. However, using 5-chloromethyl fluorescein diacetate labelled ovalbumin (OVA)-pulsed DC line DC2.4 (DC2.4(OVA)) cells with 1,1′-dioactadecyl-3,3,3′,3′-tetramethylindocarbodyanine perchlorate (Dil)-labelled OT II CD4+ T cells, we demonstrated a novel phenomenon of bidirectional membrane molecule transfer between DCs and T cells. CD4+ T cells acquired Ia(b), CD11c, CD40 and CD80 from DC2.4(OVA) cells, and conversely DC2.4(OVA) cells took up CD4, CD25, CD69 and TCR from T cells [46]. In two different studies using in vitro imaging, repeated association and dissociation of CD4+ T cells from macrophages were observed [99] and the same was the case with DCs in a three-dimensional collagen matrix [100]. To explain this phenomenon, it was shown that the cells were interacting with multiple APC partners, accumulating the activation signals until fully activated. Alternatively, abortive activation event leaving the cells partially activated was explained for the spontaneous association and dissociation of T cells. Thus, Wetzel and colleagues implicated the activation of T cells to spontaneous association and dissociation from MCC:GFP cells, as T cells formed a mature IS, expressed high levels of CD69, and displayed significant TCR-down-regulation. Removal of specific MHC-peptide ligands from APCs would limit their availability for other T cells, which may be an important event in controlling an immune response [98]. Such Ag stripping from DCs is seen in vivo, suggesting that stripping would prevent lower affinity T cells to access Ag, thereby generating a higher affinity T-cell response [101].
Exosome uptake
Intercellular communication through the release of membrane vesicles or exosomes has recently become the subject of increasing interest (reviewed in [70, 102]. Exosomes (50–90 nm) i.e. enclosed membrane bodies or vesicles are of endocytic origin and represent one of the potent mechanisms of intercellular membrane transfer between various cells. Exosomes are released into the extracellular environment on fusion of multivesicular bodies with PM [103]. Many cells have the capability to release exosomes, including DCs [104], B cells [105], T cells [106], mast cells [107], reticuloendothelial cells [108], epithelial cells [109] and tumour cells [110]. Their composition may slightly differ from bulk membrane [111]. However, the protein compositions of exosomes secreted by a number of different cells have been shown to be somewhat cell-type specific [112]. Exosomes isolated from the malignant ascites of patients with cancer contain antigen specific to the tumour human epidermal growth factor receptor 2 (Her2)/ν from ovarian cancer ascites, and melanoma antigen recognized by T-cells (Mart1) from patients with melanoma [113]. Exosomes are identified by the presence of surface protein CD63-a commonly used marker of exosomes [114]. Exosomes can bind to cells through receptor-ligand interactions, similar to cell–cell communication mediating antigen presentation [105] or could fuse with the target cell membrane, delivering exosomal surface proteins and cytoplasm to recipient cell [115, 116] or internalized by the recipient cells by an endocytic mechanism [117]. Earlier, It was suggested that cells perform this phenomenon to lose potentially harmful components as in case of the recovery of human neutrophils from complement attack by shedding membrane attack complex [118]. Recently, exosome-mediated transfers of genetic materials (mRNAs and microRNAs) have been demonstrated in a mouse and a human mast cell lines. RNA loaded exosomes may represent a vehicle by which one cell communicates with another and modulating recipient-cell protein production [114]. Soluble cytokine receptors regulate inflammatory and immunological events by functioning as agonist or antagonists of cytokine signalling. Interestingly, it was reported that tumour necrosis factor receptor-1 (TNFR1) can be released from human vascular endothelial cells into the extracellular milieu as a constituent of exosome like vesicles [119] and release of exosome-like vesicles has been suggested as an alternative mechanism for generation of soluble cytokine receptors [120].
Transfer of membrane material through vesicle shedding is heavily dependent on interactions between the PM and underlying cytoskeleton. Local disruption of the cytoskeleton is known to result in membrane blebbing [25]. The generation of membrane vesicles or membrane evaginations requires cytoskeletal reorganization and membrane mobility. For example, shedding of adhesion receptors from the surface of activated platelets probably involves calpain action, with rupture of membrane-associated cytoskeleton and dissociation of membrane/cytoskeleton attachment [121]. Recent advances in biophysics like, ‘optical tweezers’ shed novel insight into the importance of membrane/cytoskeleton interactions [122]. T s technique allows a precise estimation of the force generated by small membrane tethers obtained by pulling microbeads bound to the cell surface with a force of a few pico newtons (pN). Thereby, it is possible to estimate intrinsic PM tension and energy of adhesion to cytoskeleton. The energy of adhesion to cytoskeleton accounts for 75% of the apparent membrane tension [25]. Transient disruption of cortical microfilament as a pursuant to weakening in membrane cytoskeleton interactions with a second messenger like, phosphotidylinositol 4,5 biphosphate [123] or cytosolic calcium increase [124] could facilitate a local release of the PM, thus the vesicle formation. Molecular events that stimulates cells to generate and shed exosomes are now being understood, it was reported that overexpression of TSAP6, a multi-pass transmembrane protein, could facilitate the secretion of the histamine-releasing factor via exosomes and suggested a role for TSAP6 in either transport of protein to the exosomes or in regulating exosome production [125]. Recently, Yu et al. presented the first evidence of p53 protein mediated regulation of exosome production through the ability of p53 to transcribe the TSAP6 gene whose product is sufficient to induce the secretion of exosomes [112].
Studies have shown that vesicular or exosome mediated transport of antigen/MHC class II complexes and other APC-derived molecules from the professional APCs to T cells represent an important mechanism of cellular communications in the immune system [126][29, 127]. Two mechanisms have been suggested for the release of exosomes and specific acquisition by cognate responders. First it was suggested that the exosomes (bearing class II MHC) are formed by a process that involves invagination of the limiting MHC class II endocytic compartment vesicular membrane [105, 128], resulting in a multivesicular compartment comprised smaller vesicles within a larger vesicle. Upon fusion with surface membrane, exosomes may be released into extracellular spaces and are captured by T cells. Alternatively, it has been suggested that APC surface membrane may vesiculate near the contact area between opposing APC T-cell conjugates and released to augment specific acquisition by cognate T-cell responders [95]. Recent investigation suggested that recruitment of DC derived exosomes required T-cell activation and was dependent on LFA-1 rather than on the TCR specificity [129].
Several studies have also shown that APCs capture protein molecules from leucocytes or other APCs [57, 115, 130]. Interestingly, Morelli and colleagues reported that DC derived exosomes can be internalized by immature DCs through a calcium and temperature dependent mechanism that requires participation of the DC cytoskeleton. DC derived exosomes once internalized by immature DCs, exosomal allopeptides are processed into MHC class II for presentation to CD4+ T cells. Milk fat globule (MFG)-E8/lactadherin, CD11a, CD54, phophatidyl-serine and the tetraspanins CD9 and CD81 on the exosome side as well as αv/β3 integrin, CD11a and CD54 on the DCs mediate targeting of exosomes to DCs [117]. However, our recent studies demonstrated that mature DCs pulsed with exosomes stimulate enhanced cytotoxic T-lymphocyte responses and antitumour immunity and showed that DCOVA-derived exosomes (EXOOVA) expressed pMHC I complexes, CD11c, CD40, CD80, CCR7, DEC205, toll-like receptor 4 (TLR-4), TLR-9, MyD88 and DC-SIGN molecules, but at a lower level than DCOVA. Uptake of EXOOVA by mature DCs was mediated through LFA-1/CD54 and C-type lectin/mannose (glucosamine)-rich C-type lectin receptor interactions [131]. Further, we have shown that the metastatic activity of a highly metastatic B16 melanoma cell line BL6–10 can be transferred to poorly metastatic B16 melanoma cell line F1 by uptake of highly metastatic BL6–10 tumour-released exosomes [132]. Recently, a very interesting phenomenon of exosome mediated HIV Gag secretion/shedding has been reported. It was observed that Jurkat T cells possess endosome-like domains of PM and bud exosomes from these domains and Jurkat T cells direct the key budding factor of human immunodeficiency virus-1 (HIV-1), HIV Gag to these endosome-like domains and secrete HIV Gag from cell in exosomes [133]. Interestingly, vesicle shedding within a novel type of synapse between immune and non-immune cells (i.e. ICC or ICC-like cells and immune cells in the synaptic cleft) has been reported that may correspond to an exosome-based mechanism of cellular cross-talk [7].
Membrane nanotube formation
Intercellular exchange of proteins through membrane tubes, i.e. long membrane tethers, between cells provides another probable mechanism of cell-surface protein transfer between cells. Nanotubes formation has been observed in a wide range of immune cells, including B, T and NK cells, neutrophils and monocytes, as well as glial and neuronal cells [44, 134, 135]. Rustom et al. [136] reported a unique mechanism for intercellular membrane transfer, i.e. membrane can transfer directly between cells connected by tunnelling nanotubes. Authors demonstrated that rat neuronal pheochromocytoma cell line (PC12) cells or kidney cells were connected via membrane tunnels or nanotubes. Recent published literature generally used interchangeably the terms ‘tunnelling nanotubes’ and membrane nanotubes [134, 137]. However recently, Davis [138] suggested that tunnelling and membrane nanotubes can be defined as open-ended and closed-ended membranous connections between cells, respectively. However, closed-ended membranous connections as triggered by viral proteins can be defined as viral cytonemes [139]. These nanotubes were shown to facilitate the selective transfer of membrane vesicles and organelles between cells through actin dependent mechanism. Formation of membrane nanotubes was also observed between B cells and NK cells in the event of disassembly of IS [140]. Recently, the transmission of calcium fluxes between myeloid cells has been shown to take place by nanotube formation [73, 141, 142]. Nanotube-mediated intercellular transfer of calcium fluxes induces phenotypic changes in distal DCs, which is reminiscent of response generally seen by direct antigenic stimulation. However, the molecular mechanism of calcium fluxes transmission by nanotubes is still elusive. Interestingly, the heterogeneity in the structure of membrane nanotubes connecting human macrophages is observed. Thicker nanotubes are made up of both F-actin and microtubules, whereas thinner ones contain only F-actin. It was shown that nanotubes containing microtubules transport vesicles over long distances, whereas, using a constitutive flow of nanotube surface, bacteria ‘surf’ along nanotubes that lack microtubules [135]. Surface transport along thin nanotubes was found to be dependent on adenosine triphosphate (ATP) but independent of microtubules. However, transport of vesicles, like endosomes and lysosomes were only observed inside thicker nanotubes (containing microtubules) connecting macrophages. Sowinski et al. [143] have demonstrated that the formation of T-cell nanotubes between T cells can have important consequences for allowing a rapid spread of HIV-1. Recently, HIV-1 infection of macrophages has been found to induce increased number of nanotubes formation, contributing to the pathogenesis of the AIDS by a potential route for intercellular HIV-1 trafficking [144]. Interestingly, the trogocytic transfer of CD4 molecules from target to infected cells was observed, but trogocytic transfer of membranes was not detected in the HIV transmission direction [145]. In addition, it has been shown that the mitochondria can access thick nanotubes. Rescue of aerobic respiration in cells deficient in mitochondria was demonstrated by the intercellular transfer of whole mitochondria or mitochondrial DNA from normal cells, possibly involving membrane nanotubes formation [146]. Tunnelling nanotubes are also suggested as a novel way to spread drug resistance in tumour cells [147]. At present, the best datum for functional relevance of membrane nanotubules in immune-cell biology is the demonstration that they mediate communication of antigenic signals between myeloid cells [18, 141]. Determining whether there are physiological functions for nanotubes is an intriguing new goal for cellular immunology [148].
Functional relevance to immune responses
Trogocytosis has a broader impact in immunobiology. It is well established that costimulatory or other protein molecules (extracellular and intracellular) on the cell membrane have a considerable impact on cellular function. Therefore, it is obvious that acquisition of different molecules (which is not normally transcribed) by lymphocytes or other cells through trogocytosis may directly or indirectly influence the phenotype and functions of immune subsets capturing these membrane proteins. Several studies demonstrated that trogocytosis has an important influence on the course of the immune responses (either stimulatory or suppressive immune responses) (Fig. 1) [18].
Stimulatory effect on immune responses
Various immunological cell capture protein molecules/membrane patches from their targets. The intercellular transfer of membrane molecules can provide signals for immune responses with varying outcomes. For example, membrane-tethered antigens are internalized by B cells for processing and subsequent presenting them to T cells [9, 53]. Usually, APCs such as DCs can acquire antigens and subsequently present the processed peptide-MHC class I and II complexes to T cells [57]. Acquisition of APC cell-surface MHC and associated molecules by T cells endows T cells with novel functions. We have recently demonstrated that during intercellular membrane transfer, CD4+ T cells derived from the wild-type OVA-specific TCR transgenic OT II mice can not only acquire the synapse comprised MHC class II and costimulatory molecules (CD54 and CD80), but also the bystander pMHC I complexes from OVA-pulsed DCs (DCOVA) [149]. This phenomenon is seen because the bystander pMHC I and the pMHC II complexes localize in the same IS formed between DCs and CD4+ T cells [150]. These CD4+ T cells are type 1 helper T (Th) cells since they secrete IFN-γ, TNF-α and IL-2, but no IL-4. These CD4+ T cells (CD4+ Th1-APCs) carrying acquired Ag-presenting machinery from DCOVA can act as APCs in stimulation of OVA-specific CD8+ cytotoxic T-lymphocyte (CTL) responses [42, 149, 151]. In addition, CD4+ Th1-APCs also induce OVA-specific antitumour immunity in C57BL/6 mice against the OVA expressing murine melanoma line BL6-10OVA cells. Interestingly, the stimulatory effect of CD4+ Th1-APCs is mediated through its endogenous CD40L and acquired CD80 costimulation and IL-2 secretion [152]. Importantly, the acquired pMHC I complexes on CD4+ Th1-APCs play an important role in targeting the stimulatory effect of CD4+ Th-APCs to naïve CD8+ T cells in vivo[152–154]. We have also demonstrated the role of exosome acquired T cells in breaking CD4+25+ regulatory T (Tr) cell-mediated immune suppression and stimulating efficient antigen-specific CD8+ CTL response [155, 156]. We found that uptake of exosomes with, but not without pMHC I complexes endowed CD4+ T cells with the ability to stimulate OVA-specific CD8+ CTL responses. These data clearly elucidate an important role of acquired pMHC I complex on CD4+ T cells in targeting the stimulatory effect of CD4+ T cells to CD8+ T cells in vivo. In comparison to effector memory CD8+CTL responses stimulated by DC, CD4+ Th-APCs induced central memory CD8+ T-cell responses [152].
Naïve CD8+ cytotoxic T (Tc) cells also acquire pMHC I and costimulatory CD54 and CD80 molecules through DCOVA stimulation, and act as Tc-APCs. These Tc-APCs can play both negative and positive modulations in antitumour immune responses by eliminating DCOVA and neighbouring Tc-APCs, and by stimulating OVA-specific CD8+ central memory T responses and antitumour immunity via targeting role of acquired pMHC I complexes [157]. In a human melanoma in vivo model, Machlenkin and colleagues demonstrated that adoptive transfer of membrane capturing, peptide-specific T cells, but not non-capturing or bulk CD8+ T cells, inhibits tumour progression [158]. In addition, it has also been demonstrated that MHC class II and CD80 which had been acquired from APCs by CD4+ T cells remain functional and could sustain T-cell activation in the absence of APCs [159]. Sustained activity of transcriptional factors such as nuclear factor-κB and activator protein-1 (AP1) was seen in T cells with acquired CD80 molecules. T cells, upon CD80 acquisition could up-regulate the signal transducer and activator of transcription-5 (Stat5) in the absence of APCs or exogenous signal 1 [159]. Furthermore, Brandes et al. [160] have demonstrated that human γδ T cells expressing MHC II and costimulatory molecules can also act as APCs and stimulate proliferation and differentiation of naïve γδ T cells. Recent study demonstrated that tumour-experienced T cells can regulate NK cell-mediated antitumour response. It was observed that T-cells (CD4+, CD8+ and resting T cells) on contact with tumour cells actively capture NKG2DLs and NKp46Ls from tumour cells (trogocytosis), and promote degranulation and IFN-γ secretion by NK cells, thus antitumour immunity [161].
Suppressive effect on immune responses
CD4+ T cells that have captured agonist pMHC II complexes can subsequently present them to adjacent CD4+ T cells, and these T cells can proliferate in response to T-cell mediated presentation [151], but as the number of activated cells increases, this T-T cell interaction can result in apoptosis or the induction of anergy or tolerance or regulatory T cells [162–164]. Recently, a novel negative feedback regulatory mechanism of CD4+ T cell immune response has been documented. It was shown that CD4+ T cells that acquire MHC/peptide complex from APCs could present the same to Ag-experienced CD4+ T cells too, thereby inhibiting their recruitment into the response while allowing recruitment of naïve T cells to generate repertoire variety [165]. These mechanisms may serve to limit the clonol expansion [162]. The adoptive antigen-specific CD4+ regulatory T cells including type 1 regulatory T (Tr1) cells and Th3 play an important role in immune suppression of autoimmune diseases and antitumour immunity [166]. However, the molecular mechanisms for antigen-specificity acquisition of adoptive CD4+ Tr cells are elusive. We have recently demonstrated that the tolerogenic OVA-pulsed DCOVA expressing the immune suppressive cytokine IL-10 could in vitro and in vivo induce responses of Tr1 cells secreting IL-10 and IFN-γ[167]. These CD4+ Tr1 cells acquired pMHC I by tolerogenic DCOVA activation and efficiently inhibited immunogenic DCOVA-mediated CD8+ CTL responses and antitumour immunity. Importantly, the acquired pMHC I complexes on CD4+ Tr1 cells lead to an enhanced suppression by 7-fold relative to analogous CD4+ Tr1 cells without acquired pMHC I, indicating that the antigen-specificity acquisition of adoptive CD4+ regulatory T cells are via acquired pMHC I complexes. Interestingly, the nonspecific CD4+25+ Tr cells can also become antigen specific and more immunosuppressive in inhibition of antigen-specific CD8+ CTL responses after uptake of antigen-specific DC released exosomal pMHC I complexes. These data indicate that the antigen-specificity acquisition of CD4+ Tr cells via acquiring DC’s pMHC I may be an important means in augmenting CD4+ Tr cell’s suppression. In addition, CD4+ Th-APC expressing OVA-specific TCR, FasL and perforin could kill DCOVA and neighbouring Th-APC expressing endogenous and acquired pMHC II. Taken together, we show that CD4+ Th-APC can modulate immune responses by stimulating CD4+ Th1 and central memory CD8+ T-cell responses and eliminating DCOVA and neighbouring Th-APC. Recently, Mostbock and colleagues [168] demonstrated that acquisition of antigen presentasome (APS), an MHC/costimulatory (CD80 molecules) complex, was an important factor for memory T-cell homeostasis. They suggested that acquisition of APS by memory T cells could lead to negative regulatory consequences, as it activated BAX/BAK and perforin pathways leading to cell death of CD4/CD80 acquired T cells. In another recent study, it was reported that acquisition of the bystander MHC class I-peptide complexes by CD4+ Th cells made them become targets for specific CTL killing [169]. This study suggested that the mechanism of Ag-specific CD4+ T-cell regulation may have important roles during the immunopathology of viral infection such as HIV-1. It was shown that CD8+ T cells which had acquired cognate pMHC I complexes became susceptible to antigen-specific lysis or fratricide killings, thereby contributed to effector clearance [38, 43, 170].
Intercellular transfer of proteins from T cells to APCs might also balance the immune responses, as anergic or regulatory T-cell-derived vesicles have been shown to induce a tolerogenic phenotype in APCs [171]. Interestingly, Busch et al. [172] demonstrated the transfer of human and murine T-cell surface receptors to APCs after cognate interaction that could play an important immunomodulatory role. Intercellular protein transfer occurred in two phases. First group of molecules transferred (e.g. CD2) from T cells to DC was a rapid event (after 2h) that might facilitate the disengagement of CD4+ T cell from the DC. Transfer of the second group of molecules occurred later after 10–16 hrs, involving CD3/TCR complex, CD27, and OX40. DCs that acquired TCR molecules from CD4+ T cell showed reduced ability to stimulate naive CD4+ T cells without losing the capacity to stimulate cognate CD8+ T cells, suggesting a role of the transferred CD4+ T-cell molecules in the regulation of specific CD4+ T immune response. Regulatory T cells (Tregs) play a vital role in the development and controlling of various immunopathologies, like autoimmune diseases. Recent data demonstrated that αβ-TCR+CD3+CD4−CD8−NK1.1− double negative Tregs acquire foreign peptide in TCR-specific manner and express them on their cell surfaces and specifically suppress syngenic CD8+ T cells that carry the same TCR specificity [173]. Intercellular transfer of proteins can also regulate NK cell functions. Acquisition of MHC class I molecules by NK cells from tumour cells resulted into a reduced NK cytotoxic function in mice [48]. In addition, contact between NK cells and target cells, which express NKG2D and MIC, respectively, led to intercellular exchange of NKG2D and MIC that correlated with the reduction in NKG2Ddependent NK cell cytotoxicity in subsequent interactions [73, 174]. Recently, studies demonstrated that alternatively activated monocytes (alt-monocytes), obtained by stimulation with IL-4 or IL-13, undertake an intensive synaptic transfer (trogocytosis) with IL-2-activated NK cells. Trogocytosis between NK cells and alt-monocytes correlated with an efficient killing of alt-monocytes, mediated by natural cytotoxicity receptors, thereby, trogocytosis between NK cells and autologous-activated monocytes modulate inflammatory responses [175].
Intercellular membrane transfer and its consequences discussed above are important for a quantitative standpoint, providing either positive or negative modulation. For example, CD4+ T cells that have acquired pMHC 1 containing membrane protein from DCs stimulate enhanced CD8+ CTL responses [149, 152]. Whereas, CD4+ T cells that have captured agonist pMHC II complexes induce anergy [151]. Similarly, NK cytotoxic function is reduced when NK cells acquire MHC class I molecules from tumour cells [48]. However, if trogocytosis involves unusual (i.e. rarely expressed) and/or functionally atypical molecule, then it may induce qualitative changes in the phenotype and functional characteristics of a particular cell. An example of this specialized regulatory role is the expression or the acquisition of human leucocyte antigen (HLA)-G. HLA-G is a non-classical HLA class I molecule characterized by a strong immunosuppressive function. It is expressed in some types of cancers, transplantations, autoimmune diseases, inflammatory conditions and viral infections. HLA-G was found to inhibit functions of NK cells and CTLs [176], induce regulatory cells [177–179], to inhibit allogenic responses [177, 178] and DC maturation [179], and up-regulate inhibitory receptor expression [180]. HLA-G has been shown to transfer from APCs to T cells resulting in functional consequences. LeMoult et al. [37] suggested that the HLA-G-associated trogocytosis could have a major impact on immune responses, with which highly efficient regulatory T cells could be generated by reversing the function of effector immune cells. Besides, by transferring HLA-G1 onto activated NK cells, HLA-G1 expressing tumour cells might protect themselves from cytolytic destruction, thereby, trogocytosis of HLA-G can constitute an efficient immune escape mechanism of tumour [32]. They have emphasized the need for monitoring HLA-G expression in pathologic context and incorporation of HLA-G blocking strategies into immunotherapies.
Unusual phenotypes and negative consequences
Intercellular transfer of proteins not normally transcribed by the cells might endow the cells with properties not normally associated with that of particular cell type. It has been shown that the intercellular transfer of GPI-anchored prion proteins might be important in the pathogenesis of the prion proteins [181]. Development of multidrug resistance in tumours has been demonstrated to be due to the intercellular transfer of P-glycoproteins that can pump many chemotherapeutic agents out of tumour cells [182]. There is evidence that cell-to-cell contact allows the transfer onto NK cells of the B-cell marker and receptor for EBV, CD21 and contributes to the infection of NK cells in vitro, and could be the basis for EBV associated infection of NK cells in nasopharyngeal NK-cell lymphoma [183]. Similarly, the intercellular transfer of the chemokine receptor and HIV co-receptor CC-chemokine receptor 5 (CCR5) can render cells susceptible to HIV infection in vitro[184]. Thus, there is considerable evidence showing that the intercellular protein transfer can contribute to several pathologies.
Technological consequences
So far, analyses of antigen or pathogen specific T cells are hindered by the requirement of prior knowledge of specific epitopes and specialized reagents, for example, peptide-MHC tetramer, intracellular cytokine staining, enzyme-linked immunospot. Remarkably, to overcome these limitations, a new ‘Trogocytosis Analysis Protocol (TRAP)’ assay has been developed [185–187]. In TRAP assay, the knowledge that lymphocytes acquire surface proteins from APCs, has been exploited to detect antigen-specific T cells. This method is based on biotinylation and streptavidin-fluorchrome labelling of APCs and detection of T cell acquired labelled APC proteins using flow cytometry. This method was found to be a versatile and reliable method of detection and quantification of virus specific T cells [186, 187] and tumour reactive CTL in melanoma patients [158]. Recently, TRAP assay along with other standard assays has been utilized to assess the immune responses following vaccination with recombinant adenylate cyclase of Bordetella pertusis carrying antigen. The TRAP assay was found to be useful in studying vaccine responses as well as phenotypic characterization of antigen-specific lymphocytes [188]. As knowledge on the mechanisms and consequences of intercellular transfer of membrane proteins is growing, the phenomenon of trogocytosis is now entering in its translational phase as an immunodiagnostic.
Perspectives
Recent research developments have well established that quality and quantity of immune responses are unequivocally influenced by cellular protein repertoires (intracellular, extracellular and transmembrane proteins alone or in combination) expressed on immune cells. It is well known that the actions of individual immune cells are independent, but the immune response is the net outcome of consequential interactions between various immune cells and their environments. Obviously, anticipation of a possible impact that, a new partner protein(s) which is not normally expressed on cells (trogocytosis) may have on ensuing immune responses is unquestionable. Intercellular protein transfer is now a well documented ubiquitous mechanism of cellular cross-talk between interacting cells. However, it is still elusive how cells generate the forces needed to overcome the strong hydrophobic interaction at an IS to allow cells to break up. However, it has been suggested that transfer of MHC class I or class II protein might coincide with MHC protein or other APC ligands being pulled during T-cell-receptor internalization [18], thereby might break up high-avidity protein-protein interactions to allow cells to move apart as has been shown in the case of neuronal growth cones [49]. To date, even when in vitro evidence grows, the question remains of the relevance of this type of micro-environment interference. Existence of fratricide killing mechanism after target cell HLA class I acquisition by CTL has been questioned under in vivo condition due to high density antigen requirements [38]. Competence of APC-like T cells [42, 149, 151]vis-à-vis professional APC has also been debated [23]. However, our recent work clearly demonstrated that in comparison to DC, Th-APC endowed enhanced central memory CTL responses [152]. Besides, physiological relevance of acquired MHC class II and CD80 from APC in CD4+ T cells have also been demonstrated, as signalling events observed in the absence of APCs [152, 159]. It has been also shown that acquired intact proteins or receptors from NK cells remain functional in the acquiring target cells, as signalling proteins were able to transduce signal in the acquiring cells [52]. Clearly, a number of evidence demonstrated that the cell-surface proteins can transfer between diverse cells both in vitro and in vivo, and this widespread intercellular transfer of cell-surface proteins has an important role in modulation of immune responses [37, 38, 42, 43, 149, 151].
Now the most challenging question in this fascinating area of immunology is to establish the functional consequences of intercellular membrane transfer in vivo. However, a major obstacle, towards finding answer to the above question, is the lack of precise methods to detect occurrence of this interesting phenomenon in a physiological condition. Therefore, we urgently need to devise the methods and ways for improvement of automated detection and intravital imaging of the process of intercellular membrane transfer. Currently, concerted efforts are being made to address these issues [189], as have been demonstrated for imaging immunological nanotubes [143, 190] and live ISs by a novel imaging strategy combining optical tweezers and confocal microscopy [191]. Improved live imaging will certainly lead to precisely delineate and understand the process of trogocytosis and its consequences in vivo. Considering the significant influence of intercellular membrane transfer in diverse immunological and pathological circumstances, a better understanding of intercellular membrane transfer will eventually lead to translate this knowledge into therapeutic interventions, and as a diagnostic tool.
References
- 1.Sherrington CS. The central nervous system. In: Foster M, editor. A text-book of physiology. 7th ed. London: Macmillan; 1897. pp. 916–1252. [Google Scholar]
- 2.Friedl P, Storim J. Diversity in immune-cell interactions: states and functions of the immunological synapse. Trends Cell Biol. 2004;14:557–67. doi: 10.1016/j.tcb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Norcross MA. A synaptic basis for T-lymphocyte activation. Ann Immunol. 1984;135D:113–34. doi: 10.1016/s0769-2625(84)81105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bromley SK, Burack WR, Johnson KG, et al. The immunological synapse. Annu Rev Immunol. 2001;19:375–96. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 5.Grakoui A, Bromley SK, Sumen C, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 6.Makrogianneli K, Carlin LM, Keppler MD, et al. Integrating receptor signal inputs that influence small Rho GTPase activation dynamics at the immunological synapse. Mol Cell Biol. 2009;29:2997–06. doi: 10.1128/MCB.01008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popescu LM, Gherghiceanu M, Cretoiu D, et al. The connective connection: interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells. Electron microscope study in situ. J Cell Mol Med. 2005;9:714–30. doi: 10.1111/j.1582-4934.2005.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monks CR, Freiberg BA, Kupfer H, et al. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 9.Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489–94. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 10.Vyas YM, Mehta KM, Morgan M, et al. Spatial organization of signal transduction molecules in the NK cell immune synapses during MHC class I-regulated noncytolytic and cytolytic interactions. J Immunol. 2001;167:4358–67. doi: 10.4049/jimmunol.167.8.4358. [DOI] [PubMed] [Google Scholar]
- 11.Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat Immunol. 2000;1:23–9. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- 12.Faure S, Salazar-Fontana LI, Semichon M, et al. ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nat Immunol. 2004;5:272–9. doi: 10.1038/ni1039. [DOI] [PubMed] [Google Scholar]
- 13.Roumier A, Olivo-Marin JC, Arpin M, et al. The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity. 2001;15:715–28. doi: 10.1016/s1074-7613(01)00225-4. [DOI] [PubMed] [Google Scholar]
- 14.Shaffer MH, Dupree RS, Zhu P, et al. Ezrin and moesin function together to promote T cell activation. J Immunol. 2009;182:1021–32. doi: 10.4049/jimmunol.182.2.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dustin ML. Stop and go traffic to tune T cell responses. Immunity. 2004;21:305–14. doi: 10.1016/j.immuni.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Sprent J. Swapping molecules during cell–cell interactions. Sci STKE. 2005;2005 doi: 10.1126/stke.2732005pe8. :pe8. [DOI] [PubMed] [Google Scholar]
- 17.Bossi G, Trambas C, Booth S, et al. The secretory synapse: the secrets of a serial killer. Immunol Rev. 2002;189:152–60. doi: 10.1034/j.1600-065x.2002.18913.x. [DOI] [PubMed] [Google Scholar]
- 18.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol. 2007;7:238–43. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 19.Joly E, Hudrisier D. What is trogocytosis and what is its purpose. Nat Immunol. 2003;4:815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 20.Rechavi O, Goldstein I, Vernitsky H, et al. Intercellular transfer of oncogenic H-Ras at the immunological synapse. PLoS ONE. 2007;2:e1204. doi: 10.1371/journal.pone.0001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rechavi O, Goldstein I, Kloog Y. Intercellular exchange of proteins: the immune cell habit of sharing. FEBS Lett. 2009;583:1792–9. doi: 10.1016/j.febslet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 22.LeMaoult J, Caumartin J, Carosella ED. Exchanges of membrane patches (trogocytosis) split theoretical and actual functions of immune cells. Hum Immunol. 2007;68:240–3. doi: 10.1016/j.humimm.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Caumartin J, Lemaoult J, Carosella ED. Intercellular exchanges of membrane patches (trogocytosis) highlight the next level of immune plasticity. Transpl Immunol. 2006;17:20–2. doi: 10.1016/j.trim.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Bona C, Robineaux R, Anteunis A, et al. Transfer of antigen from macrophages to lymphocytes. II. Immunological significance of the transfer of lipopolysaccharide. Immunology. 1973;24:831–40. [PMC free article] [PubMed] [Google Scholar]
- 25.Hudrisier D, Bongrand P. Intercellular transfer of antigen-presenting cell determinants onto T cells: molecular mechanisms and biological significance. FASEB J. 2002;16:477–86. doi: 10.1096/fj.01-0933rev. [DOI] [PubMed] [Google Scholar]
- 26.Lee ST, Paraskevas F. Macrophage–T cell interactions. I. The uptake by T cells of Fc receptors released from macrophages. Cell Immunol. 1978;40:141–53. doi: 10.1016/0008-8749(78)90322-2. [DOI] [PubMed] [Google Scholar]
- 27.Sharrow SO, Ozato K, Sachs DH. Phenotypic expression of I-A and I-E/C subregion determinants on murine thymocytes. J Immunol. 1980;125:2263–8. [PubMed] [Google Scholar]
- 28.Sharrow SO, Mathieson BJ, Singer A. Cell surface appearance of unexpected host MHC determinants on thymocytes from radiation bone marrow chimeras. J Immunol. 1981;126:1327–35. [PubMed] [Google Scholar]
- 29.Lorber MI, Loken MR, Stall AM, et al. I-A antigens on cloned alloreactive murine T lymphocytes are acquired passively. J Immunol. 1982;128:2798–803. [PubMed] [Google Scholar]
- 30.Hudson L, Sprent J, Miller JF, et al. B cell-derived immunoglobulin on activated mouse T lymphocytes. Nature. 1974;251:60–2. doi: 10.1038/251060a0. [DOI] [PubMed] [Google Scholar]
- 31.Hudson L, Sprent J. Specific adsorption of IgM antibody onto H-2-activated mouse T lymphocytes. J Exp Med. 1976;143:444–9. doi: 10.1084/jem.143.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caumartin J, Favier B, Daouya M, et al. Trogocytosis-based generation of suppressive NK cells. EMBO J. 2007;26:1423–33. doi: 10.1038/sj.emboj.7601570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rafii A, Mirshahi P, Poupot M, et al. Oncologic trogocytosis of an original stromal cells induces chemoresistance of ovarian tumours. PLoS ONE. 2008;3:e3894. doi: 10.1371/journal.pone.0003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waschbisch A, Meuth SG, Herrmann AM, et al. Intercellular exchanges of membrane fragments (trogocytosis) between human muscle cells and immune cells: a potential mechanism for the modulation of muscular immune responses. J Neuroimmunol. 2009;209:131–8. doi: 10.1016/j.jneuroim.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Zhang QJ, Li XL, Wang D, et al. Trogocytosis of MHC-I/peptide complexes derived from tumors and infected cells enhances dendritic cell cross-priming and promotes adaptive T cell responses. PLoS ONE. 2008;3:e3097. doi: 10.1371/journal.pone.0003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed KA, Munegowda MA, Xie Y, et al. Intercellular trogocytosis plays an important role in modulation of immune responses. Cell Mol Immunol. 2008;5:261–9. doi: 10.1038/cmi.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeMaoult J, Caumartin J, Daouya M, et al. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood. 2007;109:2040–8. doi: 10.1182/blood-2006-05-024547. [DOI] [PubMed] [Google Scholar]
- 38.Huang JF, Yang Y, Sepulveda H, et al. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286:952–4. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 39.Arnold PY, Davidian DK, Mannie MD. Antigen presentation by T cells: T cell receptor ligation promotes antigen acquisition from professional antigen-presenting cells. Eur J Immunol. 1997;27:3198–205. doi: 10.1002/eji.1830271217. [DOI] [PubMed] [Google Scholar]
- 40.Hwang I, Huang JF, Kishimoto H, et al. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med. 2000;191:1137–48. doi: 10.1084/jem.191.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baba E, Takahashi Y, Lichtenfeld J, et al. Functional CD4 T cells after intercellular molecular transfer of 0×40 ligand. J Immunol. 2001;167:875–83. doi: 10.4049/jimmunol.167.2.875. [DOI] [PubMed] [Google Scholar]
- 42.Tatari-Calderone Z, Semnani RT, Nutman TB, et al. Acquisition of CD80 by human T cells at early stages of activation: functional involvement of CD80 acquisition in T cell to T cell interaction. J Immunol. 2002;169:6162–9. doi: 10.4049/jimmunol.169.11.6162. [DOI] [PubMed] [Google Scholar]
- 43.Hudrisier D, Riond J, Mazarguil H, et al. Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J Immunol. 2001;166:3645–9. doi: 10.4049/jimmunol.166.6.3645. [DOI] [PubMed] [Google Scholar]
- 44.Stinchcombe JC, Bossi G, Booth S, et al. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 200:751–61. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 45.Brezinschek RI, Oppenheimer-Marks N, Lipsky PE. Activated T cells acquire endothelial cell surface determinants during transendothelial migration. J Immunol. 1999;162:1677–84. [PubMed] [Google Scholar]
- 46.He T, Tang C, Liu Y, et al. Bidirectional membrane molecule transfer between dendritic and T cells. Biochem Biophys Res Commun. 2007;359:202–8. doi: 10.1016/j.bbrc.2007.05.099. [DOI] [PubMed] [Google Scholar]
- 47.Carlin LM, Eleme K, McCann FE, et al. Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J Exp Med. 2001;194:1507–17. doi: 10.1084/jem.194.10.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sjostrom A, Eriksson M, Cerboni C, et al. Acquisition of external major histocompatibility complex class I molecules by natural killer cells expressing inhibitory Ly49 receptors. J Exp Med. 2001;194:1519–30. doi: 10.1084/jem.194.10.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmer J, Ioannidis V, Held W. H-2D ligand expression by Ly49A+ natural killer (NK) cells precludes ligand uptake from environmental cells: implications for NK cell function. J Exp Med. 2001;194:1531–9. doi: 10.1084/jem.194.10.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuchs A, Cella M, Giurisato E, et al. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155) J Immunol. 2004;172:3994–8. doi: 10.4049/jimmunol.172.7.3994. [DOI] [PubMed] [Google Scholar]
- 51.Tabiasco J, Espinosa E, Hudrisier D, et al. Active trans-synaptic capture of membrane fragments by natural killer cells. Eur J Immunol. 2002;32:1502–8. doi: 10.1002/1521-4141(200205)32:5<1502::AID-IMMU1502>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 52.Vanherberghen B, Andersson K, Carlin LM, et al. Human and murine inhibitory natural killer cell receptors transfer from natural killer cells to target cells. Proc Natl Acad Sci USA. 2004;101:16873–8. doi: 10.1073/pnas.0406240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleire SJ, Goldman JP, Carrasco YR, et al. B cell ligand discrimination through a spreading and contraction response. Science. 2006;312:738–41. doi: 10.1126/science.1123940. [DOI] [PubMed] [Google Scholar]
- 54.Quah BJ, Barlow VP, McPhun V, et al. Bystander B cells rapidly acquire antigen receptors from activated B cells by membrane transfer. Proc Natl Acad Sci USA. 2008;105:4259–64. doi: 10.1073/pnas.0800259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pardigon N, Takeda K, Saunier B, et al. CD8 alpha alpha-mediated intraepithelial lymphocyte snatching of thymic leukemia MHC class Ib molecules in vitro and in vivo. J Immunol. 2006;177:1590–8. doi: 10.4049/jimmunol.177.3.1590. [DOI] [PubMed] [Google Scholar]
- 56.Espinosa E, Tabiasco J, Hudrisier D, et al. Synaptic transfer by human gamma delta T cells stimulated with soluble or cellular antigens. J Immunol. 2002;168:6336–43. doi: 10.4049/jimmunol.168.12.6336. [DOI] [PubMed] [Google Scholar]
- 57.Russo V, Zhou D, Sartirana C, et al. Acquisition of intact allogeneic human leukocyte antigen molecules by human dendritic cells. Blood. 2000;95:3473–7. [PubMed] [Google Scholar]
- 58.Herrera OB, Golshayan D, Tibbott R, et al. A novel pathway of alloantigen presentation by dendritic cells. J Immunol. 2004;173:4828–37. doi: 10.4049/jimmunol.173.8.4828. [DOI] [PubMed] [Google Scholar]
- 59.Riond J, Elhmouzi J, Hudrisier D, et al. Capture of membrane components via trogocytosis occurs in vivo during both dendritic cells and target cells encounter by CD8(+) T cells. Scand J Immunol. 2007;66:441–50. doi: 10.1111/j.1365-3083.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- 60.Aucher A, Magdeleine E, Joly E, et al. Capture of plasma membrane fragments from target cells by trogocytosis requires signaling in T cells but not in B cells. Blood. 2008;111:5621–8. doi: 10.1182/blood-2008-01-134155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wetzel SA, Parker DC. MHC transfer from APC to T cells following antigen recognition. Crit Rev Immunol. 2006;26:1–21. doi: 10.1615/critrevimmunol.v26.i1.10. [DOI] [PubMed] [Google Scholar]
- 62.Hudrisier D, Aucher A, Puaux AL, et al. Capture of target cell membrane components via trogocytosis is triggered by a selected set of surface molecules on T or B cells. J Immunol. 2007;178:3637–47. doi: 10.4049/jimmunol.178.6.3637. [DOI] [PubMed] [Google Scholar]
- 63.Beum PV, Mack DA, Pawluczkowycz AW, et al. Binding of rituximab, trastuzumab, cetuximab, or mAb T101 to cancer cells promotes trogocytosis mediated by THP-1 cells and monocytes. J Immunol. 2008;181:8120–32. doi: 10.4049/jimmunol.181.11.8120. [DOI] [PubMed] [Google Scholar]
- 64.Zimmer M, Palmer A, Kohler J, et al. EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol. 2003;5:869–78. doi: 10.1038/ncb1045. [DOI] [PubMed] [Google Scholar]
- 65.Anderson SM, Yu G, Giattina M, et al. Intercellular transfer of a glycosylphosphatidylinositol (GPI)-linked protein: release and uptake of CD4-GPI from recombinant adeno-associated virus-transduced HeLa cells. Proc Natl Acad Sci USA. 1996;93:5894–8. doi: 10.1073/pnas.93.12.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cagan RL, Kramer H, Hart AC, et al. The bride of sevenless and sevenless interaction: internalization of a transmembrane ligand. Cell. 1992;69:393–9. doi: 10.1016/0092-8674(92)90442-f. [DOI] [PubMed] [Google Scholar]
- 67.Barraud-Lange V, Naud-Barriant N, Bomsel M, et al. Transfer of oocyte membrane fragments to fertilizing spermatozoa. FASEB J. 2007;21:3446–9. doi: 10.1096/fj.06-8035hyp. [DOI] [PubMed] [Google Scholar]
- 68.Patel DM, Mannie MD. Intercellular exchange of class II major histocompatibility complex/peptide complexes is a conserved process that requires activation of T cells but is constitutive in other types of antigen presenting cell. Cell Immunol. 2001;214:165–72. doi: 10.1006/cimm.2001.1897. [DOI] [PubMed] [Google Scholar]
- 69.Gurke S, Barroso JF, Gerdes HH. The art of cellular communication: tunneling nanotubes bridge the divide. Histochem Cell Biol. 2008;129:539–50. doi: 10.1007/s00418-008-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 71.McCann FE, Eissmann P, Onfelt B, et al. The activating NKG2D ligand MHC class I-related chain A transfers from target cells to NK cells in a manner that allows functional consequences. J Immunol. 2007;178:3418–26. doi: 10.4049/jimmunol.178.6.3418. [DOI] [PubMed] [Google Scholar]
- 72.Groh V, Wu J, Yee C, et al. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 73.Roda-Navarro P, Vales-Gomez M, Chisholm SE, et al. Transfer of NKG2D and MICB at the cytotoxic NK cell immune synapse correlates with a reduction in NK cell cytotoxic function. Proc Natl Acad Sci USA. 2006;103:11258–63. doi: 10.1073/pnas.0600721103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hwang I, Sprent J. Role of the actin cytoskeleton in T cell absorption and internalization of ligands from APC. J Immunol. 2001;166:5099–107. doi: 10.4049/jimmunol.166.8.5099. [DOI] [PubMed] [Google Scholar]
- 75.Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–99. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 76.Davis MM, Boniface JJ, Reich Z, et al. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–44. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 77.Monks CR, Kupfer H, Tamir I, et al. Selective modulation of protein kinase C-theta during T-cell activation. Nature. 1997;385:83–6. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 78.Dustin ML, Olszowy MW, Holdorf AD, et al. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 1998;94:667–77. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- 79.Viola A, Schroeder S, Sakakibara Y, et al. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–2. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 80.Purtic B, Pitcher LA, van Oers NS, et al. T cell receptor (TCR) clustering in the immunological synapse integrates TCR and costimulatory signaling in selected T cells. Proc Natl Acad Sci USA. 2005;102:2904–9. doi: 10.1073/pnas.0406867102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Depoil D, Zaru R, Guiraud M, et al. Immunological synapses are versatile structures enabling selective T cell polarization. Immunity. 2005;22:185–94. doi: 10.1016/j.immuni.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 82.Sloan-Lancaster J, Presley J, Ellenberg J, et al. ZAP-70 association with T cell receptor zeta (TCRzeta): fluorescence imaging of dynamic changes upon cellular stimulation. J Cell Biol. 1998;143:613–24. doi: 10.1083/jcb.143.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Favier B, Burroughs NJ, Wedderburn L, et al. TCR dynamics on the surface of living T cells. Int Immunol. 2001;13:1525–32. doi: 10.1093/intimm/13.12.1525. [DOI] [PubMed] [Google Scholar]
- 84.Moss WC, Irvine DJ, Davis MM, et al. Quantifying signaling-induced reorientation of T cell receptors during immunological synapse formation. Proc Natl Acad Sci USA. 2002;99:15024–9. doi: 10.1073/pnas.192573999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dushek O, Mueller S, Soubies S, et al. Effects of intracellular calcium and actin cytoskeleton on TCR mobility measured by fluorescence recovery. PLoS ONE. 2008;3:734–8. doi: 10.1371/journal.pone.0003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cemerski S, Das J, Locasale J, et al. The stimulatory potency of T cell antigens is influenced by the formation of the immunological synapse. Immunity. 2007;26:345–55. doi: 10.1016/j.immuni.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Varma R, Campi G, Yokosuka T, et al. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–27. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee KH, Dinner AR, Tu C, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–22. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 89.Valitutti S, Muller S, Cella M, et al. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–51. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 90.Cai Z, Kishimoto H, Brunmark A, et al. Requirements for peptide-induced T cell receptor downregulation on naive CD8+ T cells. J Exp Med. 1997;185:641–51. doi: 10.1084/jem.185.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Preckel T, Grimm R, Martin S, et al. Altered hapten ligands antagonize trinitrophenyl-specific cytotoxic T cells and block internalization of hapten-specific receptors. J Exp Med. 1997;185:1803–13. doi: 10.1084/jem.185.10.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Antigen Presentation Functions of the MHC. Keystone Symposia on Molecular and Cellular Biology. March 5–11, 1992. Abstracts. J Cell Biochem Suppl. 1992;16D:1–84. [PubMed] [Google Scholar]
- 93.Nepom JT, Benacerraf B, Germain RN. Acquisition of syngeneic I-A determinants by T cells proliferating in response to poly (Glu60Ala30Tyr10) J Immunol. 1981;127:888–92. [PubMed] [Google Scholar]
- 94.Puaux AL, Campanaud J, Salles A, et al. A very rapid and simple assay based on trogocytosis to detect and measure specific T and B cell reactivity by flow cytometry. Eur J Immunol. 2006;36:779–88. doi: 10.1002/eji.200535407. [DOI] [PubMed] [Google Scholar]
- 95.Patel DM, Arnold PY, White GA, et al. Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition. J Immunol. 1999;163:5201–10. [PubMed] [Google Scholar]
- 96.Poupot M, Fournie JJ. Spontaneous membrane transfer through homotypic synapses between lymphoma cells. J Immunol. 2003;171:2517–23. doi: 10.4049/jimmunol.171.5.2517. [DOI] [PubMed] [Google Scholar]
- 97.Davis DM. Intrigue at the immune synapse. Sci Am. 2006;294:48–55. doi: 10.1038/scientificamerican0206-48. [DOI] [PubMed] [Google Scholar]
- 98.Wetzel SA, McKeithan TW, Parker DC. Peptide-specific intercellular transfer of MHC class II to CD4+ T cells directly from the immunological synapse upon cellular dissociation. J Immunol. 2005;174:80–9. doi: 10.4049/jimmunol.174.1.80. [DOI] [PubMed] [Google Scholar]
- 99.Underhill DM, Bassetti M, Rudensky A, et al. Dynamic interactions of macrophages with T cells during antigen presentation. J Exp Med. 1999;190:1909–14. doi: 10.1084/jem.190.12.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gunzer M, Schafer A, Borgmann S, et al. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13:323–32. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- 101.Kedl RM, Schaefer BC, Kappler JW, et al. T cells down-modulate peptide-MHC complexes on APCs in vivo. Nat Immunol. 2002;3:27–32. doi: 10.1038/ni742. [DOI] [PubMed] [Google Scholar]
- 102.Simons M, Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–81. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 103.van Niel G, Porto-Carreiro I, Simoes S, et al. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 103.Thery C, Regnault A, Garin J, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Blanchard N, Lankar D, Faure F, et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–41. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 106.Raposo G, Tenza D, Mecheri S, et al. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell. 1997;8:2631–45. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–78. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 108.van Niel G, Raposo G, Candalh C, et al. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2000;121:337–49. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 109.Mears R, Craven RA, Hanrahan S, et al. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics. 2004;4:4019–31. doi: 10.1002/pmic.200400876. [DOI] [PubMed] [Google Scholar]
- 110.Van Blitterswijk WJ, De Veer G, Krol JH, et al. Comparative lipid analysis of purified plasma membranes and shed extracellular membrane vesicles from normal murine thymocytes and leukemic GRSL cells. Biochim Biophys Acta. 1982;688:495–504. doi: 10.1016/0005-2736(82)90361-3. [DOI] [PubMed] [Google Scholar]
- 111.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 112.Andre F, Schartz NE, Movassagh M, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 113.Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 114.Denzer K, van Eijk M, Kleijmeer MJ, et al. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000;165:1259–65. doi: 10.4049/jimmunol.165.3.1259. [DOI] [PubMed] [Google Scholar]
- 115.Clayton A, Turkes A, Dewitt S, et al. Adhesion and signaling by B cell-derived exosomes: the role of integrins. FASEB J. 2004;18:977–9. doi: 10.1096/fj.03-1094fje. [DOI] [PubMed] [Google Scholar]
- 116.Morelli AE, Larregina AT, Shufesky WJ, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–66. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 117.Morgan BP, Dankert JR, Esser AF. Recovery of human neutrophils from complement attack: removal of the membrane attack complex by endocytosis and exocytosis. J Immunol. 1987;138:246–53. [PubMed] [Google Scholar]
- 118.Hawari FI, Rouhani FN, Cui X, et al. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci USA. 2004;101:1297–302. doi: 10.1073/pnas.0307981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levine SJ. Mechanisms of soluble cytokine receptor generation. J Immunol. 2004;173:5343–8. doi: 10.4049/jimmunol.173.9.5343. [DOI] [PubMed] [Google Scholar]
- 120.Fox JE. Shedding of adhesion receptors from the surface of activated platelets. Blood Coagul Fibrinolysis. 1994;5:291–304. doi: 10.1097/00001721-199404000-00020. [DOI] [PubMed] [Google Scholar]
- 121.Sheetz MP. Cell control by membrane-cytoskeleton adhesion. Nat Rev Mol Cell Biol. 2001;2:392–6. doi: 10.1038/35073095. [DOI] [PubMed] [Google Scholar]
- 122.Raucher D, Stauffer T, Chen W, et al. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell. 2000;100:221–8. doi: 10.1016/s0092-8674(00)81560-3. [DOI] [PubMed] [Google Scholar]
- 123.Richelme F, Benoliel AM, Bongrand P. Dynamic study of cell mechanical and structural responses to rapid changes of calcium level. Cell Motil Cytoskeleton. 2000;45:93–105. doi: 10.1002/(SICI)1097-0169(200002)45:2<93::AID-CM2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 124.Amzallag N, Passer BJ, Allanic D, et al. TSAP6 facilitates the secretion of translationally controlled tumor protein/histamine-releasing factor via a nonclassical pathway. J Biol Chem. 2004;279:46104–12. doi: 10.1074/jbc.M404850200. [DOI] [PubMed] [Google Scholar]
- 125.Arnold PY, Mannie MD. Vesicles bearing MHC class II molecules mediate transfer of antigen from antigen-presenting cells to CD4+ T cells. Eur J Immunol. 1999;29:1363–73. doi: 10.1002/(SICI)1521-4141(199904)29:04<1363::AID-IMMU1363>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 126.Yu DT, McCune JM, Fu SM, et al. Two types of Ia-positive T cells. Synthesis and exchange of Ia antigens. J Exp Med. 1980;152:89s–98s. [PubMed] [Google Scholar]
- 127.Geuze HJ. The role of endosomes and lysosomes in MHC class II functioning. Immunol Today. 1998;19:282–7. doi: 10.1016/s0167-5699(98)01269-9. [DOI] [PubMed] [Google Scholar]
- 128.Nolte-’t Hoen EN, Buschow SI, Anderton SM, et al. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113:1977–81. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 129.Russo V, Tanzarella S, Dalerba P, et al. Dendritic cells acquire the MAGE-3 human tumor antigen from apoptotic cells and induce a class I-restricted T cell response. Proc Natl Acad Sci USA. 2000;97:2185–90. doi: 10.1073/pnas.040540197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hao S, Bai O, Li F, et al. Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity. Immunology. 2007;120:90–102. doi: 10.1111/j.1365-2567.2006.02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hao S, Ye Z, Li F, et al. Epigenetic transfer of metastatic activity by uptake of highly metastatic B16 melanoma cell-released exosomes. Exp Oncol. 2006;28:126–31. [PubMed] [Google Scholar]
- 132.Booth AM, Fang Y, Fallon JK, et al. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172:923–35. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gerdes HH, Bukoreshtliev NV, Barroso JF. Tunneling nanotubes: a new route for the exchange of components between animal cells. FEBS Lett. 2007;581:2194–201. doi: 10.1016/j.febslet.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 134.Onfelt B, Nedvetzki S, Benninger RK, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476–83. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- 135.Rustom A, Saffrich R, Markovic I, et al. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–10. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 137.Davis DM, Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat Rev Mol Cell Biol. 2008;9:431–6. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]
- 138.Davis DM. Mechanisms and functions for the duration of intercellular contacts made by lymphocytes. Nat Rev Immunol. 2009;9:543–55. doi: 10.1038/nri2602. [DOI] [PubMed] [Google Scholar]
- 139.Sherer NM, Lehmann MJ, Jimenez-Soto LF, et al. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat Cell Biol. 2007;9:310–5. doi: 10.1038/ncb1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Onfelt B, Nedvetzki S, Yanagi K, et al. Cutting edge: membrane nanotubes connect immune cells. J Immunol. 2004;173:1511–3. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]
- 141.Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–18. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 142.Baluska F, Hlavacka A, Volkmann D, et al. Getting connected: actin-based cell-to-cell channels in plants and animals. Trends Cell Biol. 2004;14:404–8. doi: 10.1016/j.tcb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 143.Sowinski S, Jolly C, Berninghausen O, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–9. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- 144.Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: a potential mechanism for intercellular HIV trafficking. Cell Immunol. 2009;254:142–8. doi: 10.1016/j.cellimm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Massanella M, Puigdomenech I, Cabrera C, et al. Antigp41 antibodies fail to block early events of virological synapses but inhibit HIV spread between T cells. AIDS. 2009;23:183–8. doi: 10.1097/QAD.0b013e32831ef1a3. [DOI] [PubMed] [Google Scholar]
- 146.Spees JL, Olson SD, Whitney MJ, et al. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA. 2006;103:1283–8. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ambudkar SV, Sauna ZE, Gottesman MM, et al. A novel way to spread drug resistance in tumor cells: functional intercellular transfer of P-glycoprotein (ABCB1) Trends Pharmacol Sci. 2005;26:385–7. doi: 10.1016/j.tips.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goncharova LB, Tarakanov AO. Nanotubes at neural and immune synapses. Curr Med Chem. 2008;15:210–8. doi: 10.2174/092986708783497265. [DOI] [PubMed] [Google Scholar]
- 149.Xiang J, Huang H, Liu Y. A new dynamic model of CD8+ T effector cell responses via CD4+ T helper-antigen-presenting cells. J Immunol. 2005;174:7497–505. doi: 10.4049/jimmunol.174.12.7497. [DOI] [PubMed] [Google Scholar]
- 150.He T, Zong S, Wu X, et al. CD4+ T cell acquisition of the bystander pMHC I colocalizing in the same immunological synapse comprising pMHC II and costimulatory CD40, CD54, CD80, OX40L, and 41BBL. Biochem Biophys Res Commun. 2007;362:822–8. doi: 10.1016/j.bbrc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 151.Game DS, Rogers NJ, Lechler RI. Acquisition of HLA-DR and costimulatory molecules by T cells from allogeneic antigen presenting cells. Am J Transplant. 2005;5:1614–25. doi: 10.1111/j.1600-6143.2005.00916.x. [DOI] [PubMed] [Google Scholar]
- 152.Umeshappa CS, Huang H, Xie Y, et al. CD4+ Th-APC with acquired peptide/MHC class I and II complexes stimulate type 1 helper CD4+ and central memory CD8+ T cell responses. J Immunol. 2009;182:193–206. doi: 10.4049/jimmunol.182.1.193. [DOI] [PubMed] [Google Scholar]
- 153.Ahmed KA, Xie Y, Zhang X, et al. Acquired pMHC I complexes greatly enhance CD4(+) Th cell’s stimulatory effect on CD8(+) T cell-mediated diabetes in transgenic RIP-mOVA mice. Cell Mol Immunol. 2008;5:407–15. doi: 10.1038/cmi.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ye Z, Ahmed KA, Hao S, et al. Active CD4+ helper T cells directly stimulate CD8+ cytotoxic T lymphocyte responses in wild-type and MHC II gene knockout C57BL/6 mice and transgenic RIP-mOVA mice expressing islet beta-cell ovalbumin antigen leading to diabetes. Autoimmunity. 2008;41:501–11. doi: 10.1080/08916930802069256. [DOI] [PubMed] [Google Scholar]
- 155.Hao S, Liu Y, Yuan J, et al. Novel exosome-targeted CD4+ T cell vaccine counteracting CD4+25+ regulatory T cell-mediated immune suppression and stimulating efficient central memory CD8+ CTL responses. J Immunol. 2007;179:2731–40. doi: 10.4049/jimmunol.179.5.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hao S, Yuan J, Xiang J. Nonspecific CD4(+) T cells with uptake of antigen-specific dendritic cell-released exosomes stimulate antigen-specific CD8(+) CTL responses and long-term T cell memory. J Leukoc Biol. 2007;82:829–38. doi: 10.1189/jlb.0407249. [DOI] [PubMed] [Google Scholar]
- 157.Xia D, Hao S, Xiang J. CD8+ cytotoxic T-APC stimulate central memory CD8+ T cell responses via acquired peptide-MHC class I complexes and CD80 costimulation, and IL-2 secretion. J Immunol. 2006;177:2976–84. doi: 10.4049/jimmunol.177.5.2976. [DOI] [PubMed] [Google Scholar]
- 158.Machlenkin A, Uzana R, Frankenburg S, et al. Capture of tumor cell membranes by trogocytosis facilitates detection and isolation of tumor-specific functional CTLs. Cancer Res. 2008;68:2006–13. doi: 10.1158/0008-5472.CAN-07-3119. [DOI] [PubMed] [Google Scholar]
- 159.Zhou J, Tagaya Y, Tolouei-Semnani R, et al. Physiological relevance of antigen presentasome (APS), an acquired MHC/costimulatory complex, in the sustained activation of CD4+ T cells in the absence of APCs. Blood. 2005;105:3238–46. doi: 10.1182/blood-2004-08-3236. [DOI] [PubMed] [Google Scholar]
- 160.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–8. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 161.Domaica CI, Fuertes MB, Rossi LE, et al. Tumour-experienced T cells promote NK cell activity through trogocytosis of NKG2D and NKp46 ligands. EMBO Rep. 2009;10:908–15. doi: 10.1038/embor.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tsang JY, Chai JG, Lechler R. Antigen presentation by mouse CD4+ T cells involving acquired MHC class II: peptide complexes: another mechanism to limit clonal expansion. Blood. 2003;101:2704–10. doi: 10.1182/blood-2002-04-1230. [DOI] [PubMed] [Google Scholar]
- 163.Carlin LM, Yanagi K, Verhoef A, et al. Secretion of IFN-gamma and not IL-2 by anergic human T cells correlates with assembly of an immature immune synapse. Blood. 2005;106:3874–9. doi: 10.1182/blood-2005-03-0996. [DOI] [PubMed] [Google Scholar]
- 164.Lombardi G, Sidhu S, Batchelor R, et al. Anergic T cells as suppressor cells in vitro. Science. 1994;264:1587–9. doi: 10.1126/science.8202711. [DOI] [PubMed] [Google Scholar]
- 165.Helft J, Jacquet A, Joncker NT, et al. Antigen-specific T-T interactions regulate CD4 T-cell expansion. Blood. 2008;112:1249–58. doi: 10.1182/blood-2007-09-114389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 167.Hao S, Yuan J, Xu S, et al. Antigen specificity acquisition of adoptive CD4+ regulatory T cells via acquired peptide-MHC class I complexes. J Immunol. 2008;181:2428–37. doi: 10.4049/jimmunol.181.4.2428. [DOI] [PubMed] [Google Scholar]
- 168.Mostbock S, Catalfamo M, Tagaya Y, et al. Acquisition of antigen presentasome (APS), an MHC/costimulatory complex, is a checkpoint of memory T-cell homeostasis. Blood. 2007;109:2488–95. doi: 10.1182/blood-2006-09-047290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cox JH, McMichael AJ, Screaton GR, et al. CTLs target Th cells that acquire bystander MHC class I-peptide complex from APCs. J Immunol. 2007;179:830–6. doi: 10.4049/jimmunol.179.2.830. [DOI] [PubMed] [Google Scholar]
- 170.Hudrisier D, Riond J, Garidou L, et al. T cell activation correlates with an increased proportion of antigen among the materials acquired from target cells. Eur J Immunol. 2005;35:2284–94. doi: 10.1002/eji.200526266. [DOI] [PubMed] [Google Scholar]
- 171.Nolte-’t Hoen EN, Wagenaar-Hilbers JP, Peters PJ, et al. Uptake of membrane molecules from T cells endows antigen-presenting cells with novel functional properties. Eur J Immunol. 2004;34:3115–25. doi: 10.1002/eji.200324711. [DOI] [PubMed] [Google Scholar]
- 172.Busch A, Quast T, Keller S, et al. Transfer of T cell surface molecules to dendritic cells upon CD4+ T cell priming involves two distinct mechanisms. J Immunol. 2008;181:3965–73. doi: 10.4049/jimmunol.181.6.3965. [DOI] [PubMed] [Google Scholar]
- 173.Ford McIntyre MS, Young KJ, Gao J, et al. Cutting edge: in vivo trogocytosis as a mechanism of double negative regulatory T cell-mediated antigen-specific suppression. J Immunol. 2008;181:2271–5. doi: 10.4049/jimmunol.181.4.2271. [DOI] [PubMed] [Google Scholar]
- 174.Roda-Navarro P, Reyburn HT. Intercellular protein transfer at the NK cell immune synapse: mechanisms and physiological significance. FASEB J. 2007;21:1636–46. doi: 10.1096/fj.06-7488rev. [DOI] [PubMed] [Google Scholar]
- 175.Poupot M, Fournie JJ, Poupot R. Trogocytosis and killing of IL-4-polarized monocytes by autologous NK cells. J Leukoc Biol. 2008;84:1298–305. doi: 10.1189/jlb.0508278. [DOI] [PubMed] [Google Scholar]
- 176.Carosella ED, Moreau P, Le Maoult J, et al. HLA-G molecules: from maternal-fetal tolerance to tissue acceptance. Adv Immunol. 2003;81:199–252. doi: 10.1016/s0065-2776(03)81006-4. [DOI] [PubMed] [Google Scholar]
- 177.LeMaoult J, Krawice-Radanne I, Dausset J, et al. HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc Natl Acad Sci USA. 2004;101:7064–9. doi: 10.1073/pnas.0401922101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Le Rond S, Azema C, Krawice-Radanne I, et al. Evidence to support the role of HLA-G5 in allograft acceptance through induction of immunosuppressive/regulatory T cells. J Immunol. 2006;176:3266–76. doi: 10.4049/jimmunol.176.5.3266. [DOI] [PubMed] [Google Scholar]
- 179.Ristich V, Liang S, Zhang W, et al. Tolerization of dendritic cells by HLA-G. Eur J Immunol. 2005;35:1133–42. doi: 10.1002/eji.200425741. [DOI] [PubMed] [Google Scholar]
- 180.LeMaoult J, Zafaranloo K, Le Danff C, et al. HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. FASEB J. 2005;19:662–4. doi: 10.1096/fj.04-1617fje. [DOI] [PubMed] [Google Scholar]
- 181.Liu T, Li R, Pan T, et al. Intercellular transfer of the cellular prion protein. J Biol Chem. 2002;277:47671–8. doi: 10.1074/jbc.M207458200. [DOI] [PubMed] [Google Scholar]
- 182.Levchenko A, Mehta BM, Niu X, et al. Intercellular transfer of P-glycoprotein mediates acquired multidrug resistance in tumor cells. Proc Natl Acad Sci USA. 2005;102:1933–8. doi: 10.1073/pnas.0401851102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tabiasco J, Vercellone A, Meggetto F, et al. Acquisition of viral receptor by NK cells through immunological synapse. J Immunol. 2003;170:5993–8. doi: 10.4049/jimmunol.170.12.5993. [DOI] [PubMed] [Google Scholar]
- 184.Mack M, Kleinschmidt A, Bruhl H, et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000;6:769–75. doi: 10.1038/77498. [DOI] [PubMed] [Google Scholar]
- 185.Daubeuf S, Puaux AL, Joly E, et al. A simple trogocytosis-based method to detect, quantify, characterize and purify antigen-specific live lymphocytes by flow cytometry, via their capture of membrane fragments from antigen-presenting cells. Nat Protoc. 2006;1:2536–42. doi: 10.1038/nprot.2006.400. [DOI] [PubMed] [Google Scholar]
- 186.Tomaru U, Yamano Y, Nagai M, et al. Detection of virus-specific T cells and CD8+ T-cell epitopes by acquisition of peptide-HLA-GFP complexes: analysis of T-cell phenotype and function in chronic viral infections. Nat Med. 2003;9:469–76. doi: 10.1038/nm845. [DOI] [PubMed] [Google Scholar]
- 187.Beadling C, Slifka MK. Quantifying viable virus-specific T cells without a priori knowledge of fine epitope specificity. Nat Med. 2006;12:1208–12. doi: 10.1038/nm1413. [DOI] [PubMed] [Google Scholar]
- 188.Daubeuf S, Preville X, Momot M, et al. Improving administration regimens of CyaA-based vaccines using TRAP assays to detect antigen-specific CD8(+) T cells directly ex vivo. Vaccine. 2009;27:5565–73. doi: 10.1016/j.vaccine.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 189.Daubeuf S, Bordier C, Hudrisier D, et al. Suitability of various membrane lipophilic probes for the detection of trogocytosis by flow cytometry. Cytometry A. 2008;75:380–9. doi: 10.1002/cyto.a.20679. [DOI] [PubMed] [Google Scholar]
- 190.Hodneland E, Lundervold A, Gurke S, et al. Automated detection of tunneling nanotubes in 3D images. Cytometry A. 2006;69:961–72. doi: 10.1002/cyto.a.20302. [DOI] [PubMed] [Google Scholar]
- 191.Oddos S, Dunsby C, Purbhoo MA, et al. High-speed high-resolution imaging of intercellular immune synapses using optical tweezers. Biophys J. 2008;95:L66–8. doi: 10.1529/biophysj.108.143198. [DOI] [PMC free article] [PubMed] [Google Scholar]


