Abstract
The free spindled cells of the lamina propria of the gut have been reported as showing fibroblastic, smooth-muscle and myofibroblastic differentiation. A precise understanding of the differentiation of these cells is essential for appreciating their functions, and this paper addresses this question using ultrastructural analysis. Histologically normal samples from different areas of the gastrointestinal tract were studied. Both subepithelial stromal cells, lying immediately beneath the basal lamina, and the deeper interstitial stromal cells, were studied. Subepithelial and interstitial cells had comparable features, reinforcing the idea that these formed a single reticulum of cells. Two major cell types were identified. Some were smooth-muscle cells, on the basis of abundant myofilaments with focal densities, glycogen, an irregular cell surface, focal lamina and multiple attachment plaques alternating with plasmalemmal caveolae. Some cells had a lesser expression of these markers, especially of myofilaments, and were regarded as poorly differentiated smooth-muscle cells and descriptively referred to as ‘myoid’. Other cells were fibroblastic to judge by prominent rough endoplasmic reticulum, an absence of myofilaments and lamina, but presence of focal adhesions. The fibronexus junctions of true myofibroblasts were not seen. The study emphasises that the smooth-muscle actin immunoreactivity in this anatomical site resides in smooth-muscle cells and not in myofibroblasts, a view consistent with earlier ultrastructural and immunostaining results. The recognition that these cells are showing smooth-muscle or fibroblastic but not true myofibroblastic differentiation should inform our understanding of the function of these cells.
Keywords: gastrointestinal tract, subepithelial stromal cell, smooth-muscle cell, fibroblast, electron microscopy
Introduction
The stromal or lamina propria cells of the gut lying immediately beneath the epithelial basal lamina (basement membrane) have been referred to as pericryptal, intestinal, subepithelial or lamina propria fibroblasts[1–3]. These subepithelial stromal cells are regarded as forming a reticulum with phenotypically similar cells, sometimes descriptively referred to as ‘interstitial’[4], which lack close epithelial association [5–8]. This collective stromal cell compartment is of interest for two main reasons. The subepithelial cells may have a collaborative role with epithelium in normal physiological functioning [9, 10], while both subepithelial and interstitial cells may act as precursors of myofibroblasts, cells with a major role in gastrointestinal (GI) carcinogenesis [4, 11]. Early ultrastructural studies in various species, including man, indicated a fine structure sometimes with enough rough endoplasmic reticulum (rER) to justify the name, pericryptal or intestinal fibroblast [1, 2]. Later, Richman et al. [12] described a smooth-muscle ultrastructure for these cells, and smooth-muscle actin (SMA) and H-caldesmon were shown to be positive immunohistochemically [9, 13], confirming a smooth-muscle phenotype.
Recently, a wide range of stromal cells, containing some SMA as detected immunohistochemically or modest bundles of smooth-muscle-type myofilaments seen by electron microscopy, have been described as myofibroblastic [14]. These have included pericryptal fibroblasts, and this broad definition has led to the widespread use of the term, intestinal myofibroblast[15]. It is reasonable to argue that understanding the role of these GI stromal cells in normal physiological functioning and carcinogenesis would be advanced by a precise understanding of their differentiation. However, as already noted, investigations have suggested fibroblastic, smooth-muscle and myofibroblastic phenotypes for these cells. The objective of this paper is to use electron microscopy to bring some clarification to understanding the differentiation of these cells, and to promote a more appropriate terminology than is used at present.
Materials and methods
Specimens and sampling
Histologically normal tissue samples from different areas of the GI tract were obtained from two main sources. Some were obtained as endoscopic biopsies for the investigation of enteric parasites in the clinical context of diarrhoea, mainly in male AIDS patients. They were fixed in phosphate-buffered glutaraldehyde and because no wax-embedded material was available because of the small size of the biopsies, the normality of histological structure was judged on the basis of appearances in toluidine-blue-stained epoxy resin semithin sections. These specimens showed no villous atrophy. Other specimens were from patients undergoing resection for adenocarcinoma and were sampled at some distance from the tumour. They were fixed in histological formalin, and judged to be grossly normal by their appearances in haematoxylin-and-eosin sections. Specimens from various GI sites from oesophagus to rectum were included in the study (Table 1). The samples available as small endoscopic biopsies were too small to have a part for wax embedding that would have permitted correlated immunostaining. Therefore, given that the immunophenotype of lamina propria cells was already well established from the literature, the present study was restricted to an ultrastructural analysis. All specimens were obtained under protocols conforming to local institutional ethical criteria for using human tissues in research.
Table 1.
Clinical data
| Case number | Site | Age and sex |
|---|---|---|
| 1 | Oesophagus | 27 years, male, AIDS, D* |
| 2 | Stomach, antrum | 28 years, female |
| 3 | Stomach, antrum | 37 years, female |
| 4 | Stomach, antrum | 60 years, male HP |
| 5 | Duodenum | 27 years, male, AIDS, D* |
| 6 | Duodenum | 36 years, male, AIDS, D |
| 7 | Duodenum | 27 years, male, AIDS, D* |
| 8 | Ileum | 61 years, male |
| 9 | Ileum | 45 years, male |
| 10 | Jejunum | 32 years, female |
| 11 | Small intestine | Not available |
| 12 | Small intestine | Not available |
| 13 | Colon | 61 years, male |
| 14 | Colon | 48 years, male |
| 15 | Colon | 24 years, male AIDS, RB |
| 16 | Colon | 56 years, female |
| 17 | Rectum | 61 years, male |
| 18 | Rectum | 60 years, female |
| 19 | Rectum | 63 years, male |
| 20 | Rectum | 70 years, male |
| 21 | Rectum | 29 years, male, AIDS, D |
Same patient, with sampling at different times. D: diarrhoea; AIDS: acquired immunodeficiency syndrome; HP: Helicobacter pylori; RB: rectal bleeding.
Electron microscopy technique
After aldehyde fixation, tissues were treated with osmium tetroxide, dehydrated in graded alcohols, then propylene oxide, and embedded in epoxy resin according to conventional procedures. Semithin sections were stained in toluidine blue for confirmation of normal histological structure and quality of preservation. Since smooth-muscle differentiation enters into the phenotypic differential of GI stromal cells, we studied cells specifically away from the GI musculature. Ultrathin sections from appropriate blocks were cut on a diamond knife and stained with uranyl acetate and lead citrate. They were examined and photographed in a Philips CM10 electron microscope (Philips; FEI, Eindhoven, The Netherlands) fitted with a Deben AMT LR44 2K × 2K digital camera (Deben, Bury St. Edmunds, UK).
Results
Subepithelial and interstitial stromal cells
The term subepithelial stromal cell is used here for the cells located close to the basal lamina separating epithelium from the underlying stroma (lamina propria). There is no precise definition for these cells, but the following two criteria were used in this paper, which were felt to be consistent with the definition of the subepithelial fibroblast, as used by Marsh and Trier [2] for the adult mouse jejunum, and the pericryptal fibroblast used by Kaye et al. [1] for rabbit and human colon. First, the term subepithelial was used for cell processes or cell bodies lying near to and in the same plane as the basal lamina, such that it was reasonable to assume that they might have a functional relationship with epithelium (Figs 1–6). The second criterion, which is less precise than the first, involves distance from the basal lamina. Kaye et al. [1] described a layer of three nucleated pericryptal fibroblasts, all of which were aligned parallel with the epithelial basal lamina, and a maximum of 6 μm away from it (Fig. 2 in Kaye et al. [1]). Marsh and Trier [2] also described a layer of 1–3 subepithelial fibroblasts, and their electron micrographic Figure 1 shows a subepithelial fibroblast, which at one point is 6.5 μm away from the basal lamina. In our paper, therefore, we have attempted to retain the essence of the Kaye, Marsh and Trier definitions with regard to orientation, but have arbitrarily set the distance of 6 μm (as measured in electron micrographs) as the limit for designating a cell as subepithelial. Elements outside this distance are here designated as interstitial stromal cells, in keeping with Adegboyega et al. [4], for example, who, in studying the colon, used the term, nonpericryptal interstitial fibroblast, the implication being that these cells are lying in the body of the interstitium, rather than being closely applied to the basal epithelial surface. At the same time, we do not wish to make too sharp a distinction between subepithelial and interstitial cells since, as mentioned above, some authors regard them as participating in a single three-dimensional network [5–8].
Fig 1.
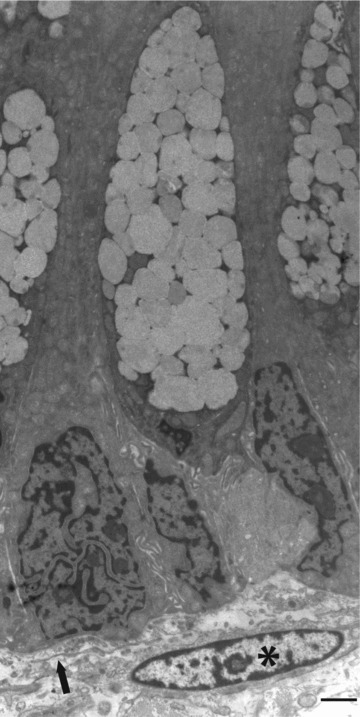
Classical arrangement of a subepithelial stromal cell (*) in relation to basal region of epithelium containing goblet cells. Note that there is a slender cell process (arrow) nearer the epithelium, which also belongs to a subepithelial stromal cell. Normal colon, case 15. Bar, 2 μm.
Fig 2.
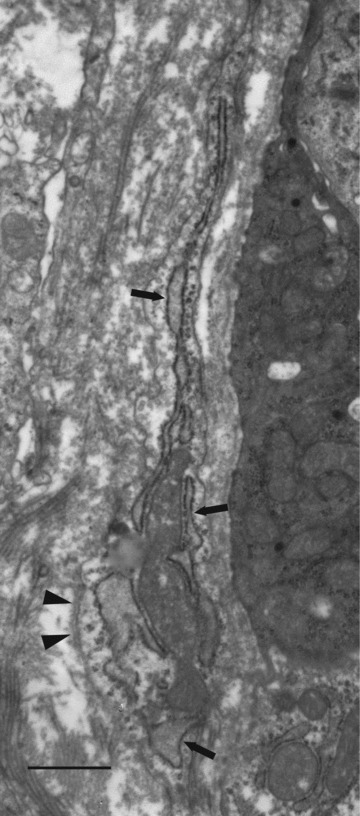
Subepithelial stromal cell process containing prominent rER (arrows) and showing a short stretch of external lamina (arrowheads). Normal small intestine, case 11. Bar, 1 μm.
Using the above criteria, subepithelial and interstitial stromal cells were seen in all parts of the digestive system studied – oesophagus, stomach, duodenum, jejunum, ileum, colon and rectum – with the following ultrastructural features. Figures 1–6 show subepithelial stromal cells, while Figures 6A and 7 show interstitial stromal cells. Often, slender cell processes were found in both subepithelial and interstitial locations, which had too small a cytoplasmic volume or cell surface area to show clearly their cellular differentiation (Figs 1 and 6A). However, slightly coarser processes showed varying combinations of rER (Fig. 2), limited stretches of ‘external’ lamina (i.e. incomplete lamina, or ‘foci’ of lamina) (Figs 2 and 4) or bundles of myofilaments under the plasmalemma (Fig. 3). Nuclei varied from showing smooth contours (Figs 1, 6A and 7) to exhibiting the many irregularities (concertina-nuclei) (Fig. 3) typical of smooth muscle cells (Table 2). Other subepithelial cells showed more definite evidence of smooth-muscle differentiation – more abundant myofilaments with focal densities, glycogen, a more irregular cell surface, focal lamina and multiple attachment plaques alternating with plasmalemmal caveolae (Figs 5 and 6). Other subepithelial stromal cells had a cytoplasm dominated by rER cisternae, and lacking cytoplasmic myofilaments and cell-associated lamina, but had focal adhesions, thereby suggesting a fibroblastic phenotype.
Fig 6.
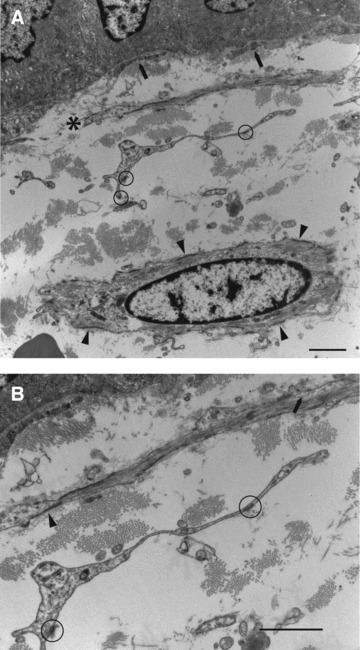
(A) (top): Cell processes and a nucleated cell profile. Closest to the epithelium (∼80 nm) are slender subepithelial stromal cell processes of indeterminate nature (arrows). Next, in terms of distance from the epithelium, is a slender cell process (*) showing smooth-muscle features (many myofilaments and multiple attachment plaques). This is ∼1.3 μm from the basal lamina at its nearest and so by definition is subepithelial. Then, there is a cell process ∼5 μm away from the basal lamina, and is also therefore, by definition, subepithelial: it is of fibroblastic appearance – no lamina or filaments, but focal adhesions (circles). Finally, at the bottom of the figure is an interstitial stromal cell, ∼10 μm distant from the basal lamina, with unambiguous smooth-muscle features (prominent myofilaments and attachment plaques, arrowheads). (B) (bottom): Details of Figure 6A showing lamina (arrowhead), myofilaments and plasmalemmal caveolae (arrow) of the smooth-muscle type subepithelial stromal cell and the focal adhesions (circles) of the fibroblastic subepithelial stromal cell process. Normal colon, case 15. Bar, 1 μm.
Fig 4.

Subepithelial stromal cell profile showing a stretch of external lamina, faithfully following the cell surface contour (arrowheads) and containing modestly developed myofilaments with focal densities (arrow). E: epithelium. Normal duodenum, case 5. Bar 500 nm.
Fig 3.

A subepithelial stromal cell showing the concertina-nucleus typical of a smooth-muscle cell. The cytoplasm contains rER (*) and some peripheral myofilaments with focal densities (arrows). Normal duodenum, case 5. Bar, 1 μm.
Fig 7.

An interstitial stromal cell with the appearance of a fibroblast – rER (arrows) and focal adhesions (circle). Normal colon, case 15. Bar, 1 μm.
Table 2.
Comparative ultrastructural features of fibroblasts, smooth-muscle cells and myofibroblasts*
| Fibroblast | Smooth-muscle cell | Myofibroblast | |
|---|---|---|---|
| Rough endoplasmic reticulum | Abundant | Scarce | Abundant |
| Collagen secretion granules | Present | Absent | Present |
| Smooth-muscle myofilaments† | Absent | Abundant | Slender, peripheral bundles |
| External lamina | Absent | Present | Absent |
| Focal adhesion‡ | Present | Absent | Absent |
| Fibronexus | Absent | Absent§ | Conspicuous |
| Nuclear irregularity | Smooth | Irregular | Mostly smooth |
| Cell-surface irregularity | Smooth | Small-scale irregularities¶ | Smooth |
Fig 5.
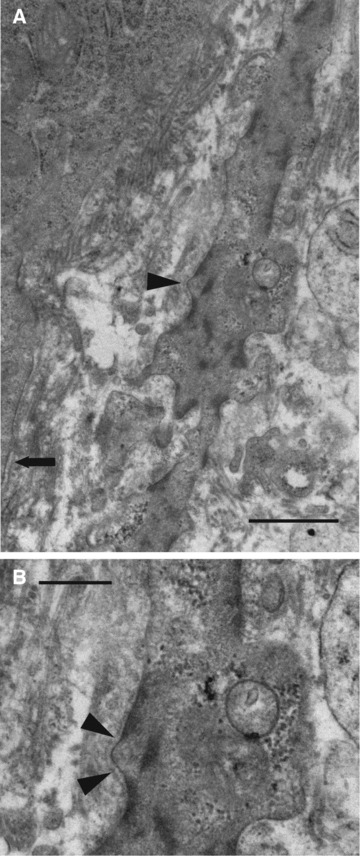
(A) (top): A subepithelial stromal cell process 300 nm away from the epithelial basal lamina (arrow) at its closest point, and showing unambiguous smooth-muscle features – prominent smooth-muscle myofilaments with focal densities, glycogen, attachment plaques, an irregular cell surface and foci of lamina (arrowhead). (B) (bottom): Details of lamina (arrowheads) and glycogen. Normal rectum, case 21. Bar, 1 μm (A) and 500 nm (B).
Interstitial stromal cells had the same ultrastructural features as subepithelial cells, showing either the myofilaments, attachments plaques and lamina of smooth-muscle cells (Fig. 6A) or the rER and focal adhesions of fibroblasts (Fig. 7). All the subepithelial and interstitial cells lacked an observable association with vessels, and were therefore not countenanced as pericytes. Further, all of them were solitary cells, in the sense of not being part of a smooth-muscle bundle or layer. Finally, no fibronectin fibrils or fibronexus junctions, regarded as important ultrastructural markers of myofibroblastic differentiation [16, 17], were identified.
Discussion
Early ideas on the nature of stromal or lamina propria cells lying close to the GI basal lamina, particularly studied in colon and variously called pericryptal or subepithelial fibroblasts [1, 2], were based on ultrastructural studies. These revealed a varying appearance, sometimes nondescript but sometimes characterized by prominent rER, the hallmark in free spindled stromal cells of the fibroblast. Later, the demonstration of SMA and H-caldesmon [9, 13] and further ultrastructural observations [12] suggested a smooth-muscle phenotype, with H-caldesmon in particular being regarded as a highly specific smooth-muscle cell marker not found in granulation tissue or tumour stromal myofibroblasts [18–20]. These conflicting interpretations appeared to be resolved by the introduction of the term, subepithelial (intestinal) myofibroblast, in a study of the rat [7] and taken up and promoted by Powell and colleagues [14, 15]. The main point of the present study and our argument is that cells with the ultrastructural features of myofibroblasts are absent from normal GI stroma.
True myofibroblasts (those of granulation tissue and tumour stroma) were originally defined by electron microscopy [21–23] in studies that predated those of investigators who expanded the term myofibroblast to other somewhat different cells: the term ‘myofibroblast’, therefore, has precedence for granulation tissue and tumour stroma. Building on these early studies of Gabbiani and colleagues, there is now a comprehensive histological, immunohistochemical and ultrastructural definition for the myofibroblast [24–28], which allows distinction from several other mesenchymally derived cells with which the myofibroblast has some overlapping features (Table 2). True myofibroblasts have been argued as having inter alia SMA and extra-long domain A (EDA) fibronectin, but not desmin: the latter, despite often being cited as a myofibroblast marker in the literature, is only expressed in rare cells in granulation tissue and tumour stromal myofibroblasts [29], such that it cannot justifiably count as a good myofibroblast marker [27]. True myofibroblasts also have prominent rER, peripheral smooth-muscle myofilaments and the cell-to-matrix adhesional specialization known as the fibronexus [16, 17, 24–28] (Table 2). Morphological electron microscopy observations and immuno-ultrastructural studies for fibronectin and collagen type IV [17] suggest that lamina is not a component of the true myofibroblast surface (discussed in [26]).
This comprehensive definition contrasts with a broader definition using slightly different criteria that is widely used in the literature, and based very largely on a flattened or spindle-cell morphology in combination with SMA immunostaining or the presence of modest numbers of peripheral actin filaments [14, 25]. This broad definition applies to a wide range of cells of diverse anatomical site, function and especially ultrastructure – true granulation tissue myofibroblasts, pericytes, interstitial contractile cells of the lung, interstitial cells of Cajal, astrocytes, mesenchymal cells of placental villi, thecal cells of ovary, Leydig cells and, importantly for the present discussion, pericryptal (subepithelial) fibroblasts [14, 25]. It is undoubtedly convenient to have a term ‘myofibroblast’ for all these cells, but a definition encompassing such anatomical and functional diversity cannot, in our opinion, deliver the precision required for the detailed investigation of the mechanisms of normal physiological cell function and tumour formation in which these cells probably participate [30]. The value of ultrastructure is that it can indeed make a distinction between many of these cells (Table 2).
In the present context, electron microscopy shows that the GI subepithelial and interstitial stromal cells exhibit a somewhat complex differentiation, including fibroblastic and smooth-muscle but not myofibroblastic phenotypes. Some appear to be fibroblastic on the basis of presence of rER, absence of lamina and presence of focal adhesions. However, one cannot exclude the possibility that these are very poorly differentiated smooth-muscle cells which, although lacking ultrastructural features of smooth-muscle, may harbour submicroscopic levels of smooth-muscle proteins: a precedent is in the lamina propria cells of the human uterine cervix which have a fibroblastic appearance but nevertheless stain for the muscle marker, desmin [31]. Nonetheless, on the basis of current information, it is reasonable to designate these cells as ‘fibroblasts’. The postulate of fibroblastic cells in this anatomical locality is consistent with Adegboyega’s description of SMA-negative fibroblasts in the normal colonic interstitium [4].
However, we also show strong evidence for true smooth-muscle differentiation, as indicated by prominent smooth-muscle myofilaments with focal densities, glycogen, attachment plaques alternating with plasmalemmal caveolae and lamina – all classical markers of smooth-muscle cell ultrastructure [26, 32, 33]. It is true that some cells (Fig. 3) had fewer myofilaments than expected in a fully differentiated smooth-muscle cell as found in the muscularis mucosae, for example, but we believe that there are almost certainly different populations of cells in different anatomical locations, which are essentially smooth-muscle in nature, but showing varying levels of smooth-muscle differentiation. Some of the placental villus stromal cells, for example, stain for desmin and have a well-formed lamina, suggesting smooth-muscle differentiation: however, they have few myofilaments indicating a less than complete level of differentiation [34]. Some authors will prefer the term ‘myoid’ for the GI cells showing an incomplete smooth-muscle phenotype [35] and we also have found this terminology acceptable.
Perhaps the most important point that our results emphasise is that in no cells in the 21 samples from different regions of the gut from oesophagus to rectum were the fibronectin fibrils or fibronexus junctions of true myofibroblasts [16, 17, 26–28] observed. In addition, these myofibroblastic features have never been seen in the literature. In one of the few recent in vivo studies [36], the irregular surface and the faint lamina of smooth-muscle are seen. We therefore emphasise that the SMA immunoreactivity in the free stromal cells of the GI tract resides in smooth-muscle cells and not true myofibroblasts. This is consistent with earlier more limited ultrastructural observations [12] and the published immunostaining results of non-myofibroblastic H-caldesmon and desmin [13, 37], which suggest true smooth-muscle rather than myofibroblastic differentiation. We therefore advocate abandoning such terms as ‘intestinal myofibroblast’, in favour of general terms such as GI stromal or lamina propria cell, or more specific terms such as fibroblast, smooth-muscle or myoid cell. These more precise terms will surely help our understanding of cell function.
In the context of understanding cell function in the gut, it is, finally, of considerable interest to speculate on the nature of the fibroblastic cells described here in relation to the concept of telocytes [38], formerly referred to as Interstitial Cajal-Like Cells[39]. These cells have been distinguished from fibroblasts on the basis of a number of features [39], but notably their exceptionally long slender processes (‘telopodes’) [38], an example of which is arguably present in our Figure 6B. However, telocytes share some features of fibroblasts –e.g. rER [38, 39]– and the relationship of fibroblastic stromal cells to telocytes in the gut is clearly a subject for further research.
Acknowledgments
We thank the staff of the Medical Illustration Department (The Christie) and Mrs. Collette Curry for digital preparation of the images.
Conflicts of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Kaye GI, Lane N, Pascal RR. Colonic pericryptal fibroblast sheath: replication, migration, and cytodifferentiation of a mesenchymal cell system in adult tissue. II. Fine structural aspects of normal rabbit and human colon. Gastroenterology. 1968;54:852–65. [PubMed] [Google Scholar]
- 2.Marsh MN, Trier JS. Morphology and cell proliferation of subepithelial fibroblasts in adult mouse jejunum. I. Structural features. Gastroenterology. 1974;67:622–35. [PubMed] [Google Scholar]
- 3.Brenmoehl J, Falk W, Göke M, et al. Inflammation modulates fibronectin isoform expression in colonic lamina propria fibroblasts (CLPF) Int J Colorectal Dis. 2008;23:947–55. doi: 10.1007/s00384-008-0523-z. [DOI] [PubMed] [Google Scholar]
- 4.Adegboyega PA, Mifflin RC, DiMari JF, et al. Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps, and adenomatous colorectal polyps. Arch Pathol Lab Med. 2002;126:829–36. doi: 10.5858/2002-126-0829-ISOMIN. [DOI] [PubMed] [Google Scholar]
- 5.Güldner F-H, Wolff JR, Graf Keyserlingk D. Fibroblasts as a part of the contractile system in duodenal villi of rat. Z Zellforsch. 1972;135:349–60. doi: 10.1007/BF00307181. [DOI] [PubMed] [Google Scholar]
- 6.Desaki J, Fujiwara T, Komuro T. A cellular reticulum of fibroblast-like cells in the rat intestine: scanning and transmission electron microscopy. Arch Histol Jpn. 1984;47:179–86. doi: 10.1679/aohc.47.179. [DOI] [PubMed] [Google Scholar]
- 7.Joyce NC, Haire MF, Palade GE. Morphologic and biochemical evidence for a contractile cell network within the rat intestinal mucosa. Gasteroenterology. 1987;92:68–81. doi: 10.1016/0016-5085(87)90841-9. [DOI] [PubMed] [Google Scholar]
- 8.Komuro T, Hashimoto Y. Three-dimensional structure of the rat intestinal wall (mucosa and submucosa) Arch Histol Cytol. 1990;53:1–21. doi: 10.1679/aohc.53.1. [DOI] [PubMed] [Google Scholar]
- 9.Sappino A-P, Dietrich P-Y, Skalli O, et al. Colonic pericryptal fibroblasts. Differentiation pattern in embryogenesis and phenotypic modulation in epithelial proliferative lesions. Virchows Arch. 1989;415:551–7. doi: 10.1007/BF00718649. [DOI] [PubMed] [Google Scholar]
- 10.Simon-Assmann P, Kedinger M, De Arcangelis A, et al. Extracellular matrix components in intestinal development. Experientia. 1995;51:883–900. doi: 10.1007/BF01921739. [DOI] [PubMed] [Google Scholar]
- 11.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–38. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 12.Richman PI, Tilly R, Jass JR, et al. Colonic pericrypt sheath cells: characterization of cell type with new monoclonal antibody. J Clin Pathol. 1987;40:593–600. doi: 10.1136/jcp.40.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakayama H, Miyazaki E, Enzan H. Differential expression of high molecular weight caldesmon in colorectal pericryptal fibroblasts and tumour stroma. J Clin Path. 1999;52:785–6. doi: 10.1136/jcp.52.10.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell DW, Mifflin RC, Valentich JD, et al. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol Cell Physiol. 1999;277:C1–19. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 15.Powell DW, Adegboyega PA, DiMari JF, et al. Epithelial cells and their neighbors. I. Role of intestinal myofibroblasts in development, repair, and cancer. Am J Physiol Gastrointest Liver Physiol. 2005;289:G2–7. doi: 10.1152/ajpgi.00075.2005. [DOI] [PubMed] [Google Scholar]
- 16.Eyden BP. The myofibroblast: an assessment of controversial issues and a definition useful in diagnosis and research. Ultrastruct Pathol. 2001;25:39–50. doi: 10.1080/019131201300004672. [DOI] [PubMed] [Google Scholar]
- 17.Eyden BP. The fibronexus in reactive and tumoral myofibroblasts: further characterization by electron microscopy. Histol Histopathol. 2001;16:57–70. doi: 10.14670/HH-16.57. [DOI] [PubMed] [Google Scholar]
- 18.Ueki N, Sobue K, Kanda K, et al. Expression of high and low molecular weight caldesmons during phenotypic modulation of smooth muscle cells. Proc Natl Acad Sci USA. 1987;84:9049–53. doi: 10.1073/pnas.84.24.9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazard D, Sastre X, Frid MG, et al. Expression of smooth muscle-specific proteins in myoepithelium and stromal myofibroblasts of normal and malignant human breast tissue. Proc Natl Acad Sci USA. 1993;90:993–1003. doi: 10.1073/pnas.90.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe K, Kusakabe T, Hoshi N, et al. h-Caldesmon in leiomyosarcoma and tumors with smooth muscle-like differentiation: its specific expression in the smooth muscle cell tumor. Hum Pathol. 1999;30:392–6. doi: 10.1016/s0046-8177(99)90113-2. [DOI] [PubMed] [Google Scholar]
- 21.Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–50. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 22.Majno G, Gabbiani G, Hirschel BJ, et al. Contraction of granulation tissue in vitro: similarity to smooth muscle. Science. 1971;173:548–50. doi: 10.1126/science.173.3996.548. [DOI] [PubMed] [Google Scholar]
- 23.Ryan GB, Cliff WJ, Gabbiani G, et al. Myofibroblasts in human granulation tissue. Hum Pathol. 1974;5:55–67. doi: 10.1016/s0046-8177(74)80100-0. [DOI] [PubMed] [Google Scholar]
- 24.Schürch W, Seemayer TA, Gabbiani G. The myofibroblast. A quarter century after its discovery. Am J Surg Pathol. 1998;22:141–7. doi: 10.1097/00000478-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Schürch W, Seemayer TA, Hinz B. Myofibroblast. In: Mills SE, et al., editors. Histology for pathologists. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins; 2007. pp. 123–64. [Google Scholar]
- 26.Eyden B. The myofibroblast. A study of normal, reactive and neoplastic tissues with an emphasis on ultrastructure. J Submicrosc Cytol Pathol. 2007:7–166. [PubMed] [Google Scholar]
- 27.Eyden B. The myofibroblast: phenotypic characterization as a prerequisite to understanding its functions in translational medicine. J Cell Mol Med. 2008;12:22–37. doi: 10.1111/j.1582-4934.2007.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eyden B, Banerjee SS, Shenjere P, et al. The myofibroblast and its tumours. J Clin Pathol. 2009;62:236–49. doi: 10.1136/jcp.2008.061630. [DOI] [PubMed] [Google Scholar]
- 29.Truong LD, Rangdaeng S, Cagle P, et al. The diagnostic utility of desmin. A study of 584 cases and review of the literature. Am J Clin Pathol. 1990;93:305–14. doi: 10.1093/ajcp/93.3.305. [DOI] [PubMed] [Google Scholar]
- 30.Eyden B. The myofibroblast, electron microscopy and cancer research. Int J Cancer. 2009;125:1743–5. doi: 10.1002/ijc.24550. [DOI] [PubMed] [Google Scholar]
- 31.Montes GS, Zugaib M, Joazeiro PP, et al. Phenotypic modulation of fibroblastic cells in the mucous layer of the human uterine cervix at term. Reproduction. 2001;124:783–90. doi: 10.1530/rep.0.1240783. [DOI] [PubMed] [Google Scholar]
- 32.Rhodin JAG. An atlas of histology. New York: Oxford University Press; 1975. [Google Scholar]
- 33.Gabella G. General aspects of the fine structure of smooth muscles. In: Motta PM, editor. Ultrastructure of smooth muscle. Boston: Kluwer Academic Publishers; 1990. pp. 1–22. [Google Scholar]
- 34.Kohnen G, Kertschanska S, Demir R, et al. Placental villous stroma as a model system for myofibroblast differentiation. Histochem Cell Biol. 1996;105:415–29. doi: 10.1007/BF01457655. [DOI] [PubMed] [Google Scholar]
- 35.Faussone-Pellegrini M-S. Relationships between neurokinin receptor-expressing interstitial cells of Cajal and tachykinergic nerves in the gut. J Cell Mol Med. 2006;10:20–32. doi: 10.1111/j.1582-4934.2006.tb00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahida YR, Beltinger J, Makh S, et al. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol. 1997;273:G1341–8. doi: 10.1152/ajpgi.1997.273.6.G1341. [DOI] [PubMed] [Google Scholar]
- 37.Ban S, Mitsuhashi T, Shimizu M. Immunohistochemical study of myofibroblasts in colorectal epithelial lesions. Arch Pathol Lab Med. 2003;127:1551–3. doi: 10.5858/2003-127-924-AROAFL. [DOI] [PubMed] [Google Scholar]
- 38.Popescu LM, Faussone-Pellegrini M-S. TELOCYTES – a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pieri L, Vannucchi MG, Faussone-Pellegrini M-S. Histochemical and ultrastructural characteristics of an interstitial cell type different from ICC and resident in the muscle coat of human gut. J Cell Mol Med. 2008;12:1944–55. doi: 10.1111/j.1582-4934.2008.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirra SS, Miles ML. Subplasmalemmal linear density: a mesodermal feature and a diagnostic aid. Hum Pathol. 1982;13:365–80. doi: 10.1016/s0046-8177(82)80226-8. [DOI] [PubMed] [Google Scholar]
- 41.Dingemans KP, Teeling P, Lagenduijk JH, et al. Extracellular matrix of the human aortic media: an ultrastructural histochemical and immunohistochemical study of the adult aortic media. Anat Rec. 2000;258:1–14. doi: 10.1002/(SICI)1097-0185(20000101)258:1<1::AID-AR1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 42.Dixon JS, Gosling JA. Ultrastructure of smooth muscle cells in the urinary system. In: Motta PM, editor. The ultrastructure of smooth muscle. Boston: Kluwer Academic Publishers; 1990. pp. 153–69. [Google Scholar]


