Abstract
Use of mesenchymal stem cells (MSCs) has emerged as a potential new treatment for various diseases but has generated marginally successful results. A consistent finding of most studies is massive death of transplanted cells. The present study examined the respective roles of glucose and continuous severe hypoxia on MSC viability and function with respect to bone tissue engineering. We hereby demonstrate for the first time that MSCs survive exposure to long-term (12 days), severe (pO2 < 1.5 mmHg) hypoxia, provided glucose is available. To this end, an in vitro model that mimics the hypoxic environment and cell-driven metabolic changes encountered by grafted sheep cells was established. In this model, the hallmarks of hypoxia (low pO2, hypoxia inducible factor-1α expression and anaerobic metabolism) were present. When conditions switched from hypoxic (low pO2) to ischemic (low pO2 and glucose depletion), MSCs exhibited shrinking, decreased cell viability and ATP content due to complete exhaustion of glucose at day 6; these results provided evidence that ischemia led to the observed massive cell death. Moreover, MSCs exposed to severe, continuous hypoxia, but without any glucose shortage, remained viable and maintained both their in vitro proliferative ability after simulation with blood reperfusion at day 12 and their in vivo osteogenic ability. These findings challenge the traditional view according to which severe hypoxia per se is responsible for the massive MSC death observed upon transplantation of these cells and provide evidence that MSCs are able to withstand exposure to severe, continuous hypoxia provided that a glucose supply is available.
Keywords: hypoxia, glucose, mesenchymal stem cells, marrow stromal cells, ischemia, bone
Introduction
Repair of large bone defects is still a challenge for the orthopaedic, reconstructive and maxillo-facial surgeon. Availability of pluripotent mesenchymal stem cells (MSCs) and the potential of inducing the osteogenic phenotype is motivating exploration and development of custom-tailored materials known as ‘bioengineered bone constructs’[1]. In such cases, the clinical scenario involves either expansion of stem cells in monolayers and loading them into a porous scaffold prior to surgery or direct cell expansion within the scaffold, and implanting this novel construct back into the donor patient. Although clinical studies have been initiated [2–5] and encouraging results for the repair of long bones were obtained in large animals [6–10], the therapeutic effectiveness of bone constructs has not yet met the one of autologous bone grafts (the benchmark of bone repair). The reasons for this limited osteogenic potential of bone constructs are not yet fully understood but massive death of transplanted cells after engraftment into the tissue-construct is a prime factor. Support for this implication is given by in vivo experiments in which massive cell death was observed when mesenchymal cells, loaded into scaffolds, were implanted either subcutaneously [11] or into mouse calvarial bone defects [12]. Further evidence was provided by the in vivo delivery of a single suspension of MSCs for the treatment of either cardiac ischemia (reviewed in [13]) or acute kidney injury in mice [14]. In these cases, massive MSC death was also observed upon their in vivo transplantation. Oxidative stress, hypoxia and inflammation have been involved in the early death of grafted cells upon transplantation in either the ischemic heart or kidney. In contrast, very little is known about the mechanisms involved when MSCs loaded on scaffolds are used for the repair of bone defects.
Because bone constructs are devoid of pre-existing vascularization and oxygen diffusion is effective only within 150–200 μm from a blood supply source [15–17], it is reasonable to postulate that MSCs loaded into a scaffold and transplanted into a bone defect will experience the rigors of a hypoxic microenvironment. This hypothesis is supported by the finding that in vivo oxygen tension falls to 1% O2 at bone fracture sites [18, 19] and to 12–20 mmHg in the periosteal space adjacent to an osteotomy gap between days 6 and 10 after surgery [20]. However, there are striking differences between transplanting cell suspensions and cell-loaded scaffolds. Cell suspensions are highly susceptible to anoikis (death by absence of attachment) and are exposed to abrupt changes in their physico-chemical environment upon transplantation. In contrast, cells loaded onto scaffolds are less prone to anoikis (since these cells are attached to the substrate scaffold prior to implantation), because the scaffold environment ‘protects’ the cells loaded in them; in this case, the cells tolerate changes in their physico-chemical environment because the scaffolds act as a reservoir for oxygen and nutrients. Most importantly, when loaded within three-dimensional scaffolds of significant volume, MSCs (which rely on exogenous glucose as their main fuel source) are exposed to a gradient of glucose concentration. Until vascularization of the construct is established and takes over the process of glucose supply, the amount of glucose is limited to the one present within the scaffold at the time of implantation.
The aforementioned considerations provided the impetus for establishing an in vitro model to simulate the progressive and extensive depletion of oxygen and nutrients encountered by MSCs upon transplantation. Key features of this model are the following: (i) MSCs are adherent when they are exposed to hypoxia; (ii) these cells are exposed to a severe and continuous hypoxic environment, which could be considered as anoxic, throughout the experiment and (iii) metabolic changes (i.e. depletion of glucose and lactate accumulation) are not abrupt but progressive and driven by cell metabolism. This model provides a controlled environment in which critical experimental parameters can be addressed separately and tested to access their respective contributions to the physiological and pathological aspects of the response of MSCs to ischemia. In the present study, we tested the hypothesis that glucose depletion, but not continuous severe hypoxia, impaired MSC viability and function.
Materials and methods
Chemicals
Alpha minimum essential medium (αMEM) and glucose were from Sigma-Aldrich (St. Louis, MO, USA). Antibotics, trypsin and foetal bovine serum were from PAA (Pasching, Austia). Guava reagents for flow cytometry were from Millipore (Bedford, MA, USA). The CellTiter-Glo® Luminescent cell assay was from Promega (Mannheim, Germany) and the hypoxia inducible factor-1α (HIF-1α) Activation Kit was from Thermo Scientific (Quebec, Canada).
Coral scaffolds
Scaffolds of natural coral (Porites) composed of spherical (630–1000 μm diameter) particles were kindly donated by Biocoral, Inc. (La Garenne Colombes, France). This material, consisting mainly (99%) of calcium carbonate in the form of aragonite and (1%) of organic material (amino acids), had open, communicating pores with a mean diameter of 250 μm and a porosity of 49 ± 2%; this substrate was previously described in detail [21]. Coral scaffold is commonly uses in the laboratory for its biocompatibility and its resorbability. Prior to the cell seeding procedure, these coral scaffolds were sterilized in an autoclave.
Oxygen measurements
Oxygen tension (pO2) was measured using the OxyLab pO2™ monitor, a fibre-optic oxygen-sensing device (Oxford Optronics, Oxford, UK) and an implantable type sensor CI/LAS-1/O/E3. Measurement of oxygen tension is based on the principle of oxygen quenching of fluorescence, and utilizes a small optical sensor featuring zero oxygen consumption at the point of measurement. The values of pO2 at 21% and 1% O2 content are 118 and 11.8 mmHg, respectively.
Culture of MSCs and experiments under normoxia and hypoxia
Bone marrow samples were harvested from the illiac crest of six sheep and expanded using established technique [21]. For experiments under normoxia, the cells were cultured under standard cell culture conditions, i.e. a sterile, 37°C, humidified, in a pre-mixed (21% O2, 5% CO2, 74% N2) gas environment in an incubator (Steri-Cult CO2 from Thermo-Scientific; Cergy Pontoise, France). For experiments under hypoxic conditions, the cells were cultured in another incubator (BINDER CO2 incubators CB-210; Binder Scientific, Nanterre, France) in a sterile, 37°C, humidified environment of a premixed gas mixture of 1% O2, 5% CO2 and 95% N2.
Passages 2–3 sheep MSCs were seeded (5 × 103 cells/cm2) into individual wells of 24-well plates and kept in a sterile, 37°C, humidified 21% O2/95% air environment overnight. At that time, the MSCs were washed twice with phosphate-buffered solution and then maintained under one of the following conditions: (1) normoxia (21% O2) under αMEM supernatant medium that contained 1 g/l glucose, and 10% foetal bovine serum (FBS) (controls); (2) hypoxia (1% O2) under αMEM supernatant medium containing 1 g/l glucose and 10% FBS; (3) high glucose/hypoxia (1% O2) under αMEM supernatant medium containing high (5 g/l) glucose and 10% FBS and (4) hypoxia (1% O2) under serum-deprived (0% FBS) αMEM medium containing 1 g/l glucose. Experiments for each one of the aforementioned conditions were performed with separate 24-well cell-culture plasticware. In order to ensure constant oxygen levels as well as cell-driven nutrient depletion, the cells were maintained undisturbed and without medium change until the end of the experiments. Evaluation of cell viability and glucose/lactate levels were performed at days 3, 6, 9 and 12 of culture unless otherwise stated. Each test was conducted in triplicate for each one of six animals.
Determination of glucose, lactate and ATP
Glucose and lactate levels were monitored using a biomedical ARCHITECT C8000 (Abbott Diagnostic, Rungis, France) robot. Intra-cellular ATP content was quantified using the CellTiter-Glo® Luminescent cell assay (Promega), according to the manufacturer’s instructions. The ATP content was expressed in fold increases over the data obtained on day 0.
The lactate yield from glucose (Ylac/glc) and the cell-specific consumption rate of glucose (qGlc; pmol/cell/day) during the first 3 days of culture were calculated using experimental concentrations of glucose and lactate and the equations made by Schop et al. [22].
Determination of cell viability and apoptosis
Viable and apoptotic cells were, respectively, identified using the Viacount assay and the annexin-V-PE/7 amino-actinomycin (7-AAD) assay, respectively, according to the manufacturer’s instructions. Briefly the Viacount assay is based on incorporation of fluorescent propidium iodide after loss of cell membrane integrity and the annexin-V-PE/7-AAD assay is based on the fact that, in early apoptosis, annexin-V binds to phosphatidylserine residues on the outer leaflet of the cell membrane, and that 7-AAD is excluded by viable cells. Acquisitions were performed with a Guava Easy-Cyte™ PCA-96 System. Analysis of data were assessed using Viacount® and Nexin® software, respectively, for viable and apoptotic cells quantification.
HIF-1α expression
MSCs in α-MEM medium containing 10% FBS and 1 g/l glucose were seeded in eight separate wells of Labteck (NUNC®) plasticware (Dominque Dutscher, Brumath, France) and exposed to either 21 or 1% O2 for 24 hrs. HIF-1α expression was assessed using the HIF-1α Activation Kit (Thermo Scientific) according to the manufacturer’s instructions. At the end of the procedure, the wells were removed from the Labteck slide and mounted using Dako Fluorescent Mounting Medium (DakoCytomation; Dako, Trappes, France). Fixed MSCs were examined using a fluorescent microscope (Nikon Eclipse TE2000-U; Nikon, Champigny sur Marne, France) equipped with a 4′,6-diamidino-2-phenylindole (DAPI) filter (λex= 377 ± 50), for nuclei visualization, and a tetramethyl rhodamine isothiocyanate (TRITC) filter (λex= 543 ± 22), for HIF-1α localization; this microscope was fitted with a digital camera (DXM1200F). The Nikon NIS element F 2.20 software was used for imaging and analysis.
In vivo assessment of the MSC osteogenic potential
MSCs were expanded either under normoxic (positive control) or serum-deprived hypoxic (hypoxic/SD) culture conditions for 12 days. The ability of the aforementioned cells to form ectopic ossicles was assessed using an in vivo animal transplantation model (8-week-old female nih/nu/xid/bg mice [Harlan Sprague-Dawley, Indianapolis, IN, USA]), which was adapted from published reports [23]. All animal procedures were performed in compliance with the guidelines published by the European Committee for Care and Use of Laboratory Animals (Directive du Conseil 24.11.1986. 86/609/CEE).
Briefly, cells were mixed with coral powder for 90 min. Each cell containing scaffold was embedded in a Tissucol® fibrin gel whose fibrinogen (18 mg/ml) and thrombin (50 UI) concentration had been optimized in pertinent previous studies in our laboratories [24]. Mice were anesthetized with ketamine and xylazine and the cell-containing constructs were transplanted subcutaneously (six constructs per animal); scaffolds without cells were used as controls. After 8 weeks, the mice were sacrificed with an overdose of pentobarbital. Explants were embedded in methacrylate, sectioned (500-μm-thick section) and stained with Stevenel Blue (nucleated cells and extracellular matrix) and van Gieson picro-fuchsin (mineralized bone), using established procedures for undecalcified histology [25]. The stained sections were examined using an inverse microscope (Leica DMRXA®; Leica, Nanterre, France). Bone formation in each of the examined histological sections of interest to the present study was quantified using Nikon NIS element F 2.20 software.
Results
Establishment of an ischemic model
To establish an in vitro model of ischemia, MSCs were cultured in a sustained 1% oxygen environment and hallmarks of ischemia (specifically, oxygen, glucose, ATP lactate levels and HIF-1α expression) were determined.
Determination of oxygen tension
The kinetics of oxygen tension was determined in the supernatant medium both in the absence and presence of cells. For this purpose, the fluorescent probe was positioned 1 mm from the bottom of a well filled with 1 ml medium. The oxygen tension dropped from 118 to 30 mmHg within 4 hrs when the samples tested were transferred from an incubator at 21% O2 to an incubator at 1% O2 (but similar other cell culture conditions) in both the absence and presence of MSCs (Fig. 1A). Whereas control conditions (no cells) led to stable oxygen tensions (i.e. 12 mmHg) at 24 hrs, the presence of MSCs, however, led to further decreases in oxygen tension which reached 2 mmHg at 6 hrs and was lower than 1.5 mmHg (i.e. 0.2% oxygen) during the culture time period from 24 hrs up to 12 days; the latter result is a direct consequence of the oxygen consumption by viable cells within the respective time frame.
Fig 1.
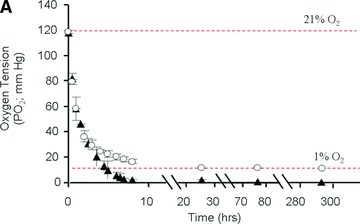
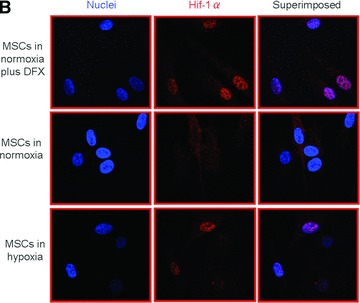
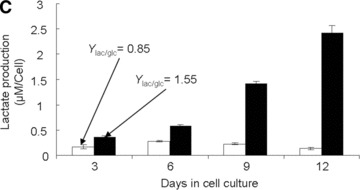
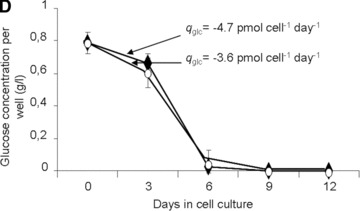
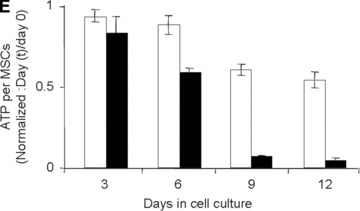
Establishment and validation of the in vitro model of hypoxia. (A) Time course of oxygen tension in the presence (black triangles) and absence (open circles) of sheep MSCs. (B) HIF-1α expression when MSCs were cultured (i) in normoxia with and without desferroxamine (DFX; positive control) and (ii) in hypoxia for 24 hrs. The cell nuclei were labelled using Hoechst stain 22232 (to visualize the cell nuclei) and dylight549 (to determine the presence of HIF-1α). (C) Time course of lactate production by sheep MSCs in normoxia (white bars) and hypoxia (black bars). (D) Time course of residual glucose in MSC cultures (per well) in normoxia (white circles) and hypoxia (black triangles). (E) Normalized (to day 0) fold increase of intracellular ATP in normoxia (white bars) and in hypoxia (black bars).
In order to ensure sustained hypoxic conditions, the following experimental conditions were strictly observed: (i) MSCs were seeded, and allowed to adhere into wells, in a 21% O2 environment overnight; (ii) these cells were then transferred to incubators with the appropriate O2 environment and were maintained undisturbed until the end of the experiments with no supernatant medium change; (iii) care was taken not to disturb the cell culture plates throughout each experiment. Under these experimental conditions, both exhaustion of nutrients and accumulation of metabolic waste were driven by cell metabolism; these conditions made the experimental setting more physiologically relevant.
Expression of HIF-1α
Expression of the transcription factor HIF-1α, a chemical essential to the response of cells to hypoxia, was assessed in MSCs Q11 cultured in either 21 or 1% O2. Whereas HIF-1α was always localize in the cytoplasm in MSCs cultured at 21% O2, it was systematically found in the nuclei of MSCs cultured in 1% O2 as early as 24 hrs after cell adhesion (Fig. 1B).
Determination of lactate level
Hypoxic conditions may exist when oxygen demand exceeds supply. Under these conditions, cells shift from aerobic to anaerobic metabolism to produce energy. The hallmark of this anaerobic shift is conversion of glucose to lactate. In order to verify that the experimental procedures used in the present study led to hypoxia, adherent MSCs were cultured in a 1% O2 environment for 12 days; the lactate concentration was determined and compared to values obtained from adherent MSCs cultured in 21% O2 for the same period of time. In the 1% O2 environment, a gradual accumulation of lactate was observed (i.e. Ylac/glc= 1.55; Fig. 1C) whereas in the 21% O2 environment, production of lactate was the lowest (i.e. Ylac/glc= 0.85) measured in the present study. These results suggest that, in the 1% O2 environment, MSCs shifted to anaerobic glycolysis.
Determination of glucose and ATP
Under the oxygen conditions tested, the kinetics of glucose consumption led to complete depletion of glucose between days 6 and 9 of culture (Fig. 1D). Such glucose exhaustion was paralleled by a drastic decrease in ATP content at days 9 and 12 (Fig. 1E) when adherent MSCs were cultured in a 1%, but not in a 21%, O2 environment. During that time, the qGlc increased from 3.6 to 4.7 pmol (P < 0.001) per cell per day at 21% and 1% oxygen, respectively (Fig. 1D).
Taken together these data indicate that, under the chosen experimental conditions, MSCs are exposed to hypoxia (i.e. low oxygen tension) from day 0 to day 6 and to ischemia (low oxygen tension and glucose exhaustion) from day 6 to day 12 of culture.
The fate of MSCs in the hypoxia/ischemia model
In a 21% O2 environment, MSCs retained their typical stellate morphology up to 6 days of culture but, when they reached confluence, they exhibited a cobblestone appearance for the remainder of the study, i.e. 12 days (Fig. 2A, Normoxia). In contrast, in the 1% O2 environment, MSCs exhibited their typical stellate morphology up to day 6 but shrank progressively thereafter (Fig. 2A, Hypoxia). Such reduction in cell size was documented by measuring the cell surface area (Fig. 2B, b). At day 12, the average cell surface area was 360 ± 10 and 160 ± 60 pixel/μm2 (P < 0.001) when adherent MSCs were cultured in a 21% and 1% O2 environment, respectively. Morphological changes were also attested by flow cytometry (FACS) analysis which also revealed size reduction of the MSCs exposed to 1% O2 (Fig. 2B, a).
Fig 2.
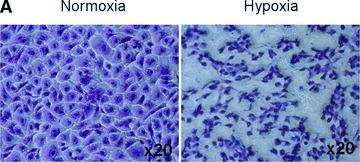
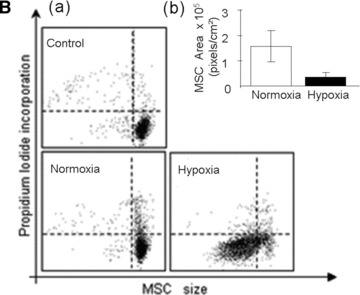
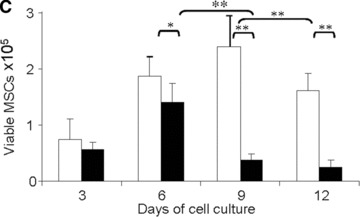
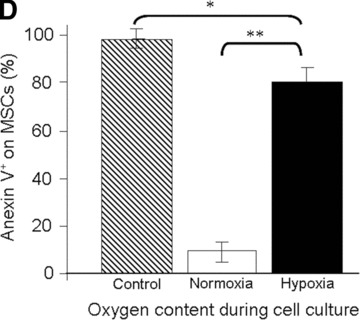
Viability of MSCs: in vitro model. (A) Morphology of MSCs either maintained in normoxia or exposed to hypoxia for 12 days. Stain: haematoxylin and eosin. Light microscopy magnification: ×20. (B) FACS analysis of cell size and area of MSCs either maintained in normoxia or exposed to hypoxia for 12 days. (C) Time course of cell viability when MSCs were either maintained in normoxia (white bars) or were exposed to hypoxia (black bars). (D) FACS analysis of apoptotic MSCs stained with annexin-V following either maintenance in normoxia (white bar); hypoxia (black bar) for 12 days or positive control in normoxia (striped bar). *P < 0.05 and **P < 0.001.
Under both oxygen-related conditions tested, an increase in viable cell number was observed at days 3 and 6 (Fig. 2C). However, in the 1% O2 environment, a significant (P < 0.001) decrease in the viable cell number was observed between days 6 and 9. At day 12, however, the number of viable MSCs was drastically reduced (specifically, 2.5 ± 1.2 × 104versus 16 ± 3 × 104 cells; P < 0.001) when the MSCs were cultured in the 1%versus the 21% O2 environment. In addition, FACS analysis revealed that 80% of residual cells cultured in the 1% O2 environment were positive in annexin-V, a marker of early apoptotic cells (Fig. 2D). Taken together, these data establish that, under ischemic conditions (i.e. hypoxia combined with absence of glucose), the MSCs did not survive.
Respective role of continuous hypoxia and glucose supply in MSC survival
The death of MSCs cultured in a 1% O2 environment might be due to either a shortage of glucose or to the continuous exposure of MSCs to hypoxia. The effect of hypoxia per se on MSC survival was, therefore, first assessed in an experimental setting that prevented exposure of MSCs to glucose shortage (i.e. the MSCs were exposed to continuous hypoxia in serum-free conditions but to a medium that contained 1% glucose). Under such conditions, no shortage of glucose was observed during the 12 days of culture (Fig. 3B, b). Under these conditions, the MSCs retained their typical stellate morphology (Fig. 3A, Hypoxia/SD); during that time the MSCs produced lactate suggesting that they had shifted to anaerobic metabolism (data not shown). Interestingly, these MSCs remained quiescent for up to 6 days of culture but proliferated thereafter (Fig. 3B, a). At day 12, the percentage of apoptotic cells was similar to that observed when MSCs were cultured in a 21% O2 environment (specifically, 10.2 ± 3.5%versus 9.93 ± 4.14%). These results suggest that exposure to continuous hypoxia did not affect the viability of MSCs.
Fig 3.

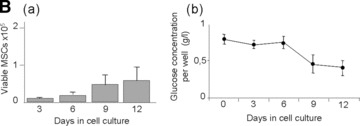
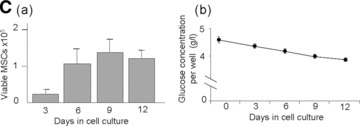
Effect of glucose supply on MSCs viability and function. (A) Morphology of MSCs either maintained in normoxia (Normoxia), in serum-deprived hypoxia (Hypoxia/SD) or in high glucose hypoxia (Hypoxia/HG); Stain: haematoxylin and eosin; Light microscopy magnification: ×20. (B, a) Time course of cell viability and (B, b) residual glucose concentration in the supernatant (per well) when MSCs were cultured in serum-deprived hypoxia for 12 days. (C, a) Time course of cell viability and (C, b) residual glucose concentration in the supernatant (per well) when MSCs were cultured in high glucose hypoxia for 12 days. *P < 0.05 and **P < 0.001.
To further investigate the essential role of glucose in the survival of MSCs, these cells were cultured in a 1% O2 environment in the presence of 10% serum and excess of glucose (5 g/l). Under such conditions, no shortage of glucose was observed for 12 days of culture (Fig. 3C, b). During that time, the MSCs retained their typical stellate morphology (Fig. 3A, Hypoxia/HG) and proliferated for up to 6 days of culture; at that time cell proliferation reached a plateau (Fig. 3C, a). At day 12, the percentage of apoptotic cells in the 1% O2 environment was similar to that obtained with MSCs cultured in a 21% O2 environment (specifically, 9.5 ± 4%versus 9.93 ± 4.14%). To confirm these results, MSCs were cultured in the absence of glucose in a 1% O2 environment with or without serum. Under such conditions, no viable cells were observed at day 3 of culture.
In vitro assessment of MSC function after ischemia
To assess the function of MSCs after a 12 day exposure to continuous hypoxia, we simulated the conditions of blood reperfusion. For this purpose, MSCs were first cultured under continuous hypoxic conditions in serum-free medium for 12 days and were then transferred to standard (21% O2) culture conditions for up to six more days. In this case, the MSC numbers were assessed at days 15 and 18 of culture (Fig. 4A) and provided evidence that MSCs proliferated. To further assess the function of MSCs cultured under continuous hypoxic conditions, these cells were detached and re-seeded (50,000 cells per well) at day 12. The proliferation of these cells was compared to that of MSCs cultured under standard (21% O2) culture conditions; the respective doubling time was 25 ± 2 and 30 ± 2 hrs (P < 0.001). This result provided evidence of enhanced proliferation upon reperfusion of the MSCs which were pre-exposed to continuous hypoxia.
Fig 4.
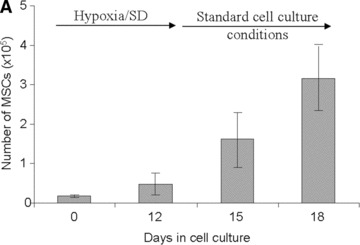
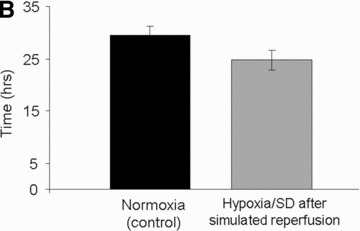

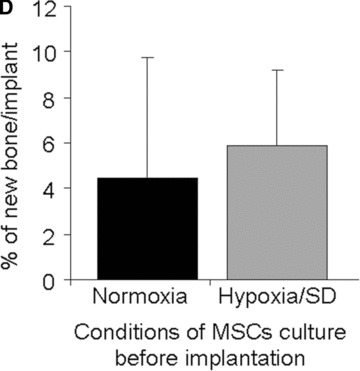
Assessment of MSC function. (A) Number of viable MSCs before and after simulated reperfusion in hypoxia/SD in vitro. (B) Doubling time of MSCs exposed to hypoxia/SD after simulated reperfusion (grey bar) compared to that of MSCs maintained under standard culture conditions (black bar) in vitro. (C) Representative histology results of MSC-containing constructs after 2 months of subcutaneous implantation in mice: (a) Transplanted implant without MSCs, (b) transplanted implant with MSCs cultured in normoxia and (c) transplanted implant with MSCs cultured in hypoxia/SD (including magnification of new formed bone also delineated by doted black lines for quantification), before implantation. Stain: Stevenel Blue and von Gieson picro-fuchsin (bone); (magnification: ×2). (d) Magnification (×20) of new formed bone with osteocytes and lacunae (arrows). (D) New bone quantification in MSC-containing constructs after 2 months of subcutaneous implantation in mice (normalized with respect to control scaffolds without MSCs).
In vivo assessment of MSC function after ischemia
Since the long-term objective of the present study is to use MSC to enhance bone repair, the bone-forming capability of MSCs (after an ischemic episode) was assessed in vivo. For this purpose, MSCs (cultured under continuous hypoxia in serum-free conditions for 12 days) were loaded onto coral particles, and were entrapped in fibrin gel. The osteogenic capability of these MSCs was compared to that of MSCs cultured under standard culture conditions (i.e. 21% O2; 10% serum; medium changes every 3 days) after subcutaneous implantation in mice. Scaffolds without cells were implanted in the same animal as a negative control. Whereas scaffolds without cells did not induce any new bone formation, all scaffolds loaded with MSCs either pre-exposed to an either ischemic episode or maintained under standard cell culture conditions exhibited active osteogenesis translated by woven bone with active osteoblasts (Fig. 4C). Histomorphometric analysis revealed that the ostegenic capabilities of MSCs either pre-exposed to an ischemic episode or maintained under standard cell culture conditions were similar (Fig. 4D).
Discussion
The primary objective of the present study was to establish an in vitro model that mimics the hypoxic environment and cell-driven changes experienced by grafted cells prior to vascularization of tissue constructs is completed in vivo.
The local pO2 to which MSCs in culture are exposed is the result of the balance between the rate of oxygen consumption by the cells and the delivery of oxygen transferred across the thickness of the supernatant aqueous medium layer. Because it is affected by cell type and density, specific rate of O2 utilization, architectural features of the culture plate (such as, geometry, size of the cell culture containers) as well as the depth of the supernatant medium [26–29], determination of the local pO2 using an equation such as the one proposed by Metzen [27] is challenging. We, therefore, decided to use an empirical method in order to gain insight of the time course of oxygen transport in the model used in the present study. The results provided evidence that, in the absence of cells, it took 10 hrs for the pO2 inside the 24-well plate to equilibrate with the oxygen content in the gas environment (i.e. 1% O2). In contrast, in the presence of cells, it took a shorter period of time (i.e. 5 hrs) to reach the pO2 of the gas environment as previously observed [28] and 10 hrs to reach 0.2% oxygen. Because the MSCs were exposed to hypoxia for 12 consecutive days, they experienced a continuous and severe depletion of oxygen (i.e. pO2≤ 0.2%) in the present study. This condition is critical because brief duration exposures to hypoxia may protect cells from lethal injury [28]. Last, but not least, the present model is physiologically relevant because it exposed cells not only to continuous hypoxia but also to oxygen tension levels which are similar to the ones observed in vivo (i.e. 1 to 3% O2) [18–20].
Hypoxia is a pathological condition in which oxygen demand by cells exceeds supply [30]. As was mentioned earlier in this discussion section, cell type, function and metabolism affect oxygen consumption, the pO2 at which cells will face hypoxia will differ from cell type to cell type and will be influenced by the experimental conditions. A physical measurement of pO2per se is not, therefore, sufficient to establish that cells are exposed to hypoxia during experiments. For this reason, response of the MSCs to hypoxia was confirmed by demonstrating that: (i) MSCs relied mainly on anaerobic glycolysis to produce energy (i.e.γlac/glc= 1.55 in hypoxia and γlac/glc= 0.85 in normoxia) and (ii) MSCs expressed HIF-1α in their nuclei (an hallmark of hypoxia). The fact that under 0.2% oxygen conditions, the MSCs proliferated with no morphological changes for up to 6 days of culture provided the first evidence that MSCs have the ability to withstand severe continuous hypoxia. Nevertheless, exposure to the severe continuous hypoxia conditions tested in the present study led to decreased cell numbers suggesting a requirement for a possible acclimatization period [31]. In the present experimental setting, day 6 of culture was a critical point as MSCs switched from hypoxia to ischemia (i.e. severe continuous hypoxia and glucose depletion from days 6 to 12 of culture). In the interval between days 9 and 12 of culture, the cell size (Fig. 2A and B), number of viable cells (Fig. 2C) and ATP content per cell (Fig. 1E) decreased drastically. At day 12, more than 80% of residual MSCs were apoptotic (i.e. annexin-V+; Fig. 2D). The observed apoptotic cell death is consistent with studies reported in the literature which established apoptosis as the primary mode of cell death in vitro in the short-term (less than 3 days) ischemic model [32–34]. Taken together, these data suggest that MSCs survived exposure to severe, continuous hypoxia but failed to survive exposure to severe continuous hypoxia in the absence of glucose.
The second major objective of the present study was to test the hypothesis that glucose depletion (but not exposure to continuous severe hypoxia alone) is responsible for the impairment of MSC survival and function. To this aim, MSCs were exposed to continuous hypoxia in serum-free conditions (but in a medium which contained 1 g/l glucose) for 12 days; these conditions prevented a glucose shortage. In this case, the MSCs remained quiescent for up to 6 days of culture but proliferated thereafter (Fig. 3B, a) suggesting that exposure to severe, continuous hypoxia under these conditions did not affect the viability of MSCs. To confirm these results, MSCs were also exposed to severe, continuous hypoxia in the presence of an excess of glucose (i.e. 5 g/l) and 10% serum. Under these conditions, the MSCs retained their typical stellate morphology and proliferated for up to day 6 of culture; at that time, cell proliferation reached a plateau (Fig. 3C, a). At day 12, 90% of cells were viable confirming that exposure to long-term hypoxia per se was not detrimental to MSCs. These results confirmed, but also extended, the time course of the studies by Mylotte [35], which reported that rat MSCs rely on anaerobic glycolysis rather than mitochondrial respiration to tolerate a short-term (72 hrs) exposure to hypoxia (0.5% O2) [35]. All together, the results of the present study demonstrate for the first time that MSCs survive a long-term episode of severe hypoxia (i.e. 0.2% O2) provided that an appropriate supply of glucose is available. Most importantly, the findings of the present study challenge the traditional hypothesis that severe, continuous hypoxia per se is responsible for the massive cell death observed upon transplantation.
Because the ultimate efficacy of a tissue-engineered bone suitable for repairing large bone defects relies on the presence of functional cells within the bone construct [36], we assessed the function of MSCs after a long-term episode of continuous severe hypoxia. We first demonstrated in vitro that exposure of MSCs to such treatment did not affect their in vitro proliferative ability upon reperfusion. In fact, the population doubling time of MSCs maintained under hypoxic conditions was lower compared to that of MSCs maintained under standard culture conditions. We then investigated the in vivo osteogenic ability of MSCs, pre-exposed in vitro to long-term severe hypoxia and demonstrated for the first time that MSCs, exposed to such in vitro treatment in the presence of sufficient glucose, maintained their in vivo osteogenic capability when transplanted in a subcutaneous mice model. Taken together, these data strongly suggest that a balance between cell number (initially loaded into a scaffold) and glucose content of the scaffold are required in order to prevent glucose depletion inside the scaffold before vascularization of the tissue-engineered construct is achieved in vivo.
Because in the past most studies investigating the role of hypoxia on MSC function used different cell isolation methods, experimental parameters, oxygen tension and evaluation techniques but failed to monitor the local pO2, it has been extremely difficult to reach a consensus on the exact effect of oxygen on MSC function (for review [37]). For these reasons, it is our belief that detailed description of experimental settings, systematic monitoring of pO2 and standardization of models and procedures, which are reproducible in all laboratories and at all times, are needed.
The present findings are particularly relevant in developing tissue-engineered constructs as they suggest that transplanting MSCs into glucose-enriched scaffolds enhances their survival after transplantation. Nevertheless, these findings must be confirmed by further relevant in vivo studies. Most importantly, the required glucose concentration must be optimized because this chemical compound markedly affects gene regulation, proliferation and differentiation of human MSCs [38]. Last, but not least, it should be taken into consideration that expression of HIF-1α (a transcription factor that regulates the expression of many hypoxia-responsive genes including vascular endothelial growth factor, erythropoietin, glucose transporters, etc.) is regulated in vitro by the concentration of glucose [39].
In vivo, under ischemic conditions, MSCs experienced not only hypoxia but also reduced supply of critical metabolic nutrients and inadequate metabolic waste removal (for review, see [40]). Although the present study focused on the role of glucose, accumulation of metabolic waste is another significant aspect of the model. In the present experimental setting, the maximal lactate concentration in glucose-enriched hypoxic conditions was 20 mM. Such concentration is lower than the cytotoxic lactate concentration (i.e. 40 mM), determined for MSCs in another study in our lab [41] and could not account for the limited cell proliferation observed between days 6 and 12 of culture under glucose-enriched hypoxic conditions. Because in the present model, metabolic waste accumulation is cell driven and allows a gradual metabolic adaptation of cells to environmental changes (such as, pO2, pH, nutrient depletion and waste accumulation), the present model mimics closely the in vivo conditions and, thus, improves on previous studies which evaluated the effect of abrupt environmental changes driven by exogenous addition of a metabolic waste entity into the supernatant cell culture medium. The present study established a two-dimensional model that simulated major insults experienced by MSCs under conditions of ischemia. Although the present study focused on bone tissue engineering, it is tempting to speculate that the knowledge gained can be extended to other tissues where therapy involving MSCs is pertinent.
Conclusions
The present study was motivated by the need to elucidate the effect of ischemia on MSC survival and function after implantation. Using an in vitro model of hypoxia/ischemia validated by physical and biological measurements, we showed that long-term exposure to severe hypoxia for 12 days did not affect MSC viability when an appropriate glucose supply was present. In addition, we demonstrated that MSCs maintained their function (specifically, morphology, doubling time and in vivo osteogenic ability) after exposure to long-term, severe hypoxia. The present findings are particularly relevant in developing tissue-engineered constructs because they suggest that MSCs loaded into glucose-enriched scaffolds can overcome an exposure of long-term, severe hypoxia and remain functional.
Acknowledgments
We thank Biocoral, Inc., for donating the coral implants, Professor R. Bizios for her valuable comments on the manuscript and Ms. Y. Calando for immunochemistry advice regarding HIF-1α. We also acknowledge the financial support of Fonds d’amorçage Biothérapie BTH06003, the ANR 07-RIB-011-01 MYOCELLOS and ANR 08-TECS-004 GLASSBONE and contrat d’interface AP-HP/INSERM. Apart from the donation of implants, no financial support was received from Biocoral, Inc.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Logeart-Avramoglou D, Anagnostou F, Bizios R, et al. Engineering bone: challenges and obstacles. J Cell Mol Med. 2005;9:72–84. doi: 10.1111/j.1582-4934.2005.tb00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–6. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 3.Ohgushi H, Kotobuki N, Funaoka H, et al. Tissue engineered ceramic artificial joint–ex vivo osteogenic differentiation of patient mesenchymal cells on total ankle joints for treatment of osteoarthritis. Biomaterials. 2005;26:4654–61. doi: 10.1016/j.biomaterials.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 4.Kitoh H, Kitakoji T, Tsuchiya H, et al. Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis–a preliminary result of three cases. Bone. 2004;35:892–8. doi: 10.1016/j.bone.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Vacanti CA, Bonassar LJ, Vacanti MP, et al. Replacement of an avulsed phalanx with tissue-engineered bone. N Engl J Med. 2001;344:1511–4. doi: 10.1056/NEJM200105173442004. [DOI] [PubMed] [Google Scholar]
- 6.Viateau V, Guillemin G, Bousson V, et al. Long-bone critical-size defects treated with tissue-engineered grafts: a study on sheep. J Orthop Res. 2007;25:741–9. doi: 10.1002/jor.20352. [DOI] [PubMed] [Google Scholar]
- 7.Petite H, Viateau V, Bensaid W, et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18:959–63. doi: 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- 8.Mastrogiacomo M, Corsi A, Francioso E, et al. Reconstruction of extensive long bone defects in sheep using resorbable bioceramics based on silicon stabilized tricalcium phosphate. Tissue Eng. 2006;12:1261–73. doi: 10.1089/ten.2006.12.1261. [DOI] [PubMed] [Google Scholar]
- 9.Bruder SP, Kraus KH, Goldberg VM, et al. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998;80:985–96. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Bensaid W, Oudina K, Viateau V, et al. De novo reconstruction of functional bone by tissue engineering in the metatarsal sheep model. Tissue Eng. 2005;11:814–24. doi: 10.1089/ten.2005.11.814. [DOI] [PubMed] [Google Scholar]
- 11.Logeart-Avramoglou D, Oudina K, Bourguignon M, et al. In vitro and in vivo bioluminescent quantification of viable stem cells in engineered constructs. Tissue Eng C Methods. 2009 doi: 10.1089/ten.TEC.2009.0004. [DOI] [PubMed] [Google Scholar]
- 12.Degano IR, Vilalta M, Bago JR, et al. Bioluminescence imaging of calvarial bone repair using bone marrow and adipose tissue-derived mesenchymal stem cells. Biomaterials. 2008;29:427–37. doi: 10.1016/j.biomaterials.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Haider H, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45:554–66. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Togel F, Yang Y, Zhang P, et al. Bioluminescence imaging to monitor the in vivo distribution of administered mesenchymal stem cells in acute kidney injury. Am J Physiol Renal Physiol. 2008;295:F315–21. doi: 10.1152/ajprenal.00098.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colton CK. Implantable biohybrid artificial organs. Cell Transplant. 1995;4:415–36. doi: 10.1177/096368979500400413. [DOI] [PubMed] [Google Scholar]
- 16.Folkman J, Hochberg M. Self-regulation of growth in three dimensions. J Exp Med. 1973;138:745–53. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutherland RM, Sordat B, Bamat J, et al. Oxygenation and differentiation in multicellular spheroids of human colon carcinoma. Cancer Res. 1986;46:5320–9. [PubMed] [Google Scholar]
- 18.Brighton CT, Krebs AG. Oxygen tension of healing fractures in the rabbit. J Bone Joint Surg Am. 1972;54:323–32. [PubMed] [Google Scholar]
- 19.Heppenstall RB, Grislis G, Hunt TK. Tissue gas tensions and oxygen consumption in healing bone defects. Clin Orthop Relat Res. 1975:357–65. doi: 10.1097/00003086-197501000-00048. [DOI] [PubMed] [Google Scholar]
- 20.Epari DR, Lienau J, Schell H, et al. Pressure, oxygen tension and temperature in the periosteal callus during bone healing–an in vivo study in sheep. Bone. 2008;43:734–9. doi: 10.1016/j.bone.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Guillemin G, Patat JL, Fournie J, et al. The use of coral as a bone graft substitute. J Biomed Mater Res. 1987;21:557–67. doi: 10.1002/jbm.820210503. [DOI] [PubMed] [Google Scholar]
- 22.Schop D, Janssen FW, van Rijn LD, et al. Growth, metabolism, and growth inhibitors of mesenchymal stem cells. Tissue Eng A. 2009;15:1877–86. doi: 10.1089/ten.tea.2008.0345. [DOI] [PubMed] [Google Scholar]
- 23.Piersanti S, Sacchetti B, Funari A, et al. Lentiviral transduction of human postnatal skeletal (stromal, mesenchymal) stem cells: in vivo transplantation and gene silencing. Calcif Tissue Int. 2006;78:372–84. doi: 10.1007/s00223-006-0001-y. [DOI] [PubMed] [Google Scholar]
- 24.Bensaid W, Triffitt JT, Blanchat C, et al. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24:2497–502. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari A, Hannouche D, Oudina K, et al. In vivo tracking of bone marrow fibroblasts with fluorescent carbocyanine dye. J Biomed Mater Res. 2001;56:361–7. doi: 10.1002/1097-4636(20010905)56:3<361::aid-jbm1104>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Pettersen EO, Larsen LH, Ramsing NB, et al. Pericellular oxygen depletion during ordinary tissue culturing, measured with oxygen microsensors. Cell Prolif. 2005;38:257–67. doi: 10.1111/j.1365-2184.2005.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metzen E, Wolff M, Fandrey J, et al. Pericellular PO2 and O2 consumption in monolayer cell cultures. Respir Physiol. 1995;100:101–6. doi: 10.1016/0034-5687(94)00125-j. [DOI] [PubMed] [Google Scholar]
- 28.Allen CB, Schneider BK, White CW. Limitations to oxygen diffusion and equilibration in in vitro cell exposure systems in hyperoxia and hypoxia. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1021–7. doi: 10.1152/ajplung.2001.281.4.L1021. [DOI] [PubMed] [Google Scholar]
- 29.Chapman JD, Sturrock J, Boag JW, et al. Factors affecting the oxygen tension around cells growing in plastic Petri dishes. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17:305–28. doi: 10.1080/09553007014550381. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberger C, Griethe W, Gruber G, et al. Cellular responses to hypoxia after renal segmental infarction. Kidney Int. 2003;64:874–86. doi: 10.1046/j.1523-1755.2003.00159.x. [DOI] [PubMed] [Google Scholar]
- 31.Grayson WL, Zhao F, Izadpanah R, et al. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol. 2006;207:331–9. doi: 10.1002/jcp.20571. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Baydoun AR, Xu R, et al. Lysophosphatidic acid protects mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis. Stem Cells. 2008;26:135–45. doi: 10.1634/stemcells.2007-0098. [DOI] [PubMed] [Google Scholar]
- 33.Gui C, Wang JA, He AN, et al. Heregulin protects mesenchymal stem cells from serum deprivation and hypoxia-induced apoptosis. Mol Cell Biochem. 2007;305:171–8. doi: 10.1007/s11010-007-9541-3. [DOI] [PubMed] [Google Scholar]
- 34.Zhu W, Chen J, Cong X, et al. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells. 2006;24:416–25. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]
- 35.Mylotte LA, Duffy AM, Murphy M, et al. Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment. Stem Cells. 2008;26:1325–36. doi: 10.1634/stemcells.2007-1072. [DOI] [PubMed] [Google Scholar]
- 36.Kruyt MC, de Bruijn JD, Wilson CE, et al. Viable osteogenic cells are obligatory for tissue-engineered ectopic bone formation in goats. Tissue Eng. 2003;9:327–36. doi: 10.1089/107632703764664792. [DOI] [PubMed] [Google Scholar]
- 37.Ma T, Grayson WL, Frohlich M, et al. Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol Prog. 2009;25:32–42. doi: 10.1002/btpr.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li YM, Schilling T, Benisch P, et al. Effects of high glucose on mesenchymal stem cell proliferation and differentiation. Biochem Biophys Res Commun. 2007;363:209–15. doi: 10.1016/j.bbrc.2007.08.161. [DOI] [PubMed] [Google Scholar]
- 39.Vordermark D, Kraft P, Katzer A, et al. Glucose requirement for hypoxic accumulation of hypoxia-inducible factor-1alpha (HIF-1alpha) Cancer Lett. 2005;230:122–33. doi: 10.1016/j.canlet.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 40.Russ AL, Haberstroh KM, Rundell AE. Experimental strategies to improve in vitro models of renal ischemia. Exp Mol Pathol. 2007;83:143–59. doi: 10.1016/j.yexmp.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Deschepper M, Bensidhoum M, Logeart-Avramoglou D, et al. Glucose supply, but not long-term exposure to continuous hypoxia, affect mesenchymal stem cells (MSCs) function. Poster presentation at TERMIS World Congress Seoul, 2009.


