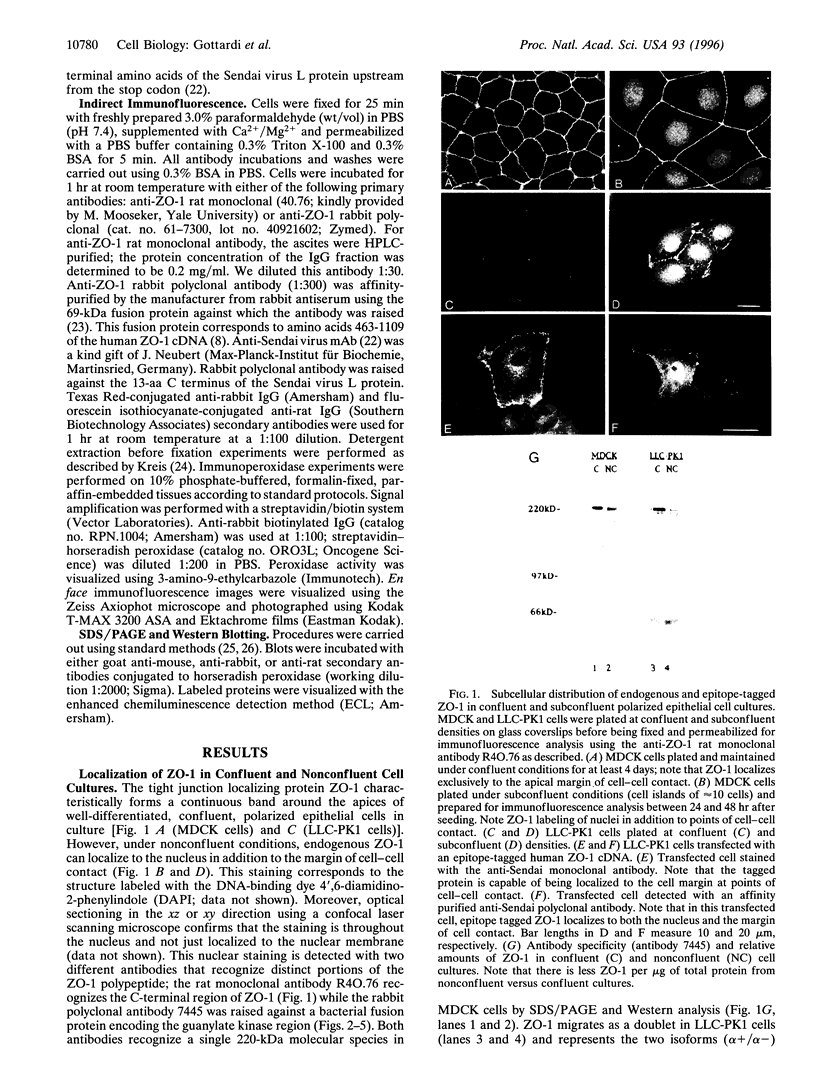
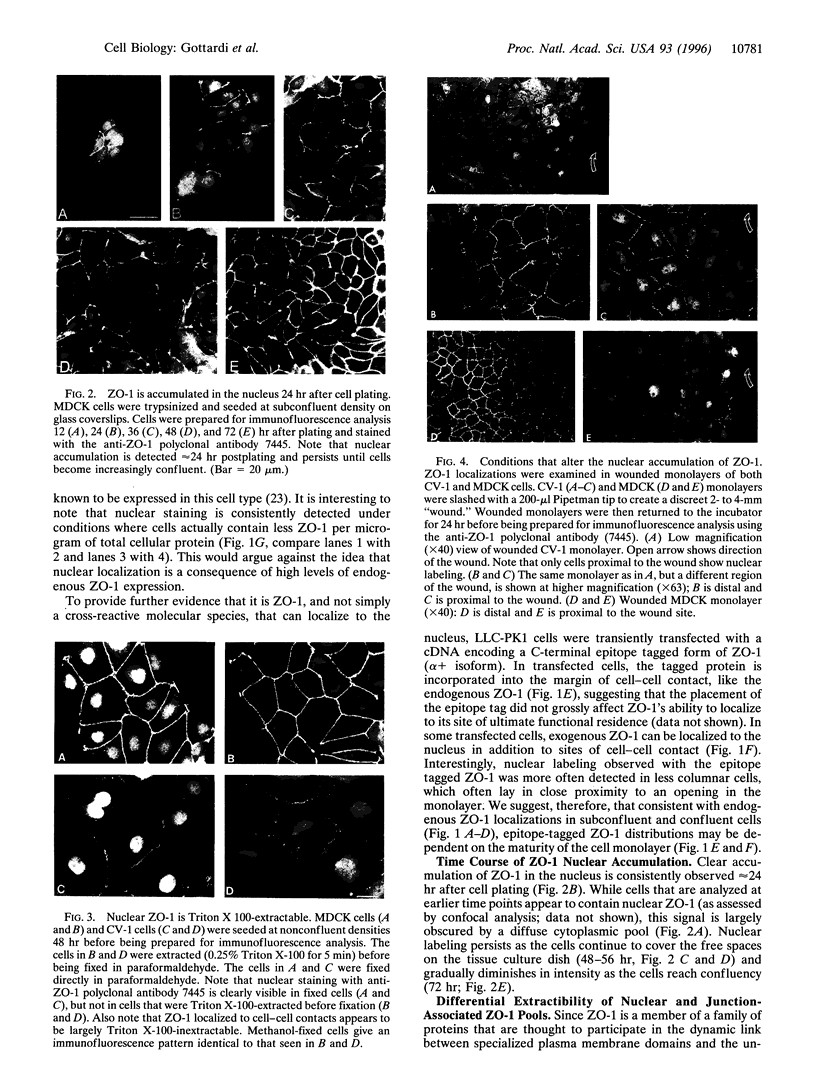
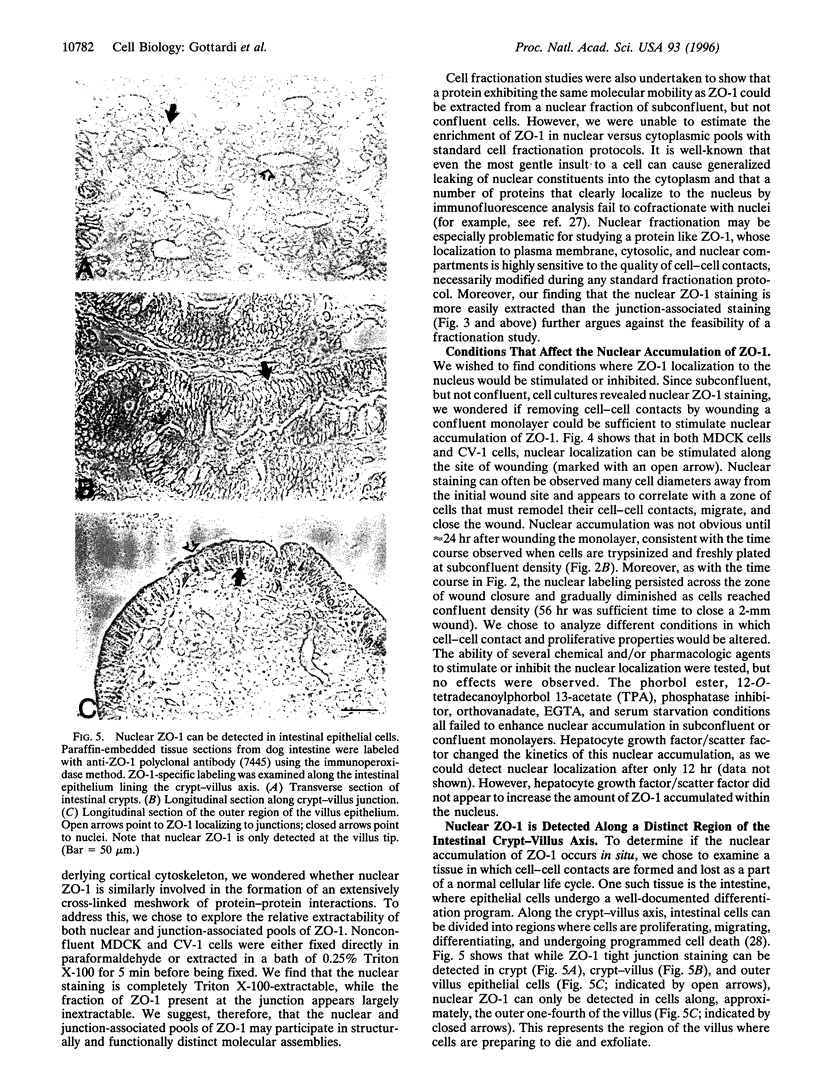
Abstract
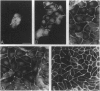
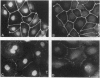
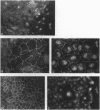
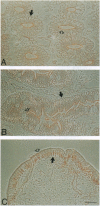
The junction-associated protein zonula occludens-1 (ZO-1) is a member of a family of membrane-associated guanylate kinase homologues thought to be important in signal transduction at sites of cell-cell contact. We present evidence that under certain conditions of cell growth, ZO-1 can be detected in the nucleus. Two different antibodies against distinct portions of the ZO-1 polypeptide reveal nuclear staining in subconfluent, but not confluent, cell cultures. An exogenously expressed, epitope-tagged ZO-1 can also be detected in the nuclei of transfected cells. Nuclear accumulation can be stimulated at sites of wounding in cultured epithelial cells, and immunoperoxidase detection of ZO-1 in tissue sections of intestinal epithelial cells reveals nuclear labeling only along the outer tip of the villus. These results suggest that the nuclear localization of ZO-1 is inversely related to the extent and/or maturity of cell contact. Since cell-cell contacts are specialized sites for signaling pathways implicated in growth and differentiation, we suggest that the nuclear accumulation of ZO-1 may be relevant for its suggested role in membrane-associated guanylate kinase homologue signal transduction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Balda M. S., Fanning A. S. The structure and regulation of tight junctions. Curr Opin Cell Biol. 1993 Oct;5(5):772–778. doi: 10.1016/0955-0674(93)90024-k. [DOI] [PubMed] [Google Scholar]
- Arpin M., Friederich E., Algrain M., Vernel F., Louvard D. Functional differences between L- and T-plastin isoforms. J Cell Biol. 1994 Dec;127(6 Pt 2):1995–2008. doi: 10.1083/jcb.127.6.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Matsuno K., Fortini M. E. Notch signaling. Science. 1995 Apr 14;268(5208):225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- Balda M. S., Anderson J. M. Two classes of tight junctions are revealed by ZO-1 isoforms. Am J Physiol. 1993 Apr;264(4 Pt 1):C918–C924. doi: 10.1152/ajpcell.1993.264.4.C918. [DOI] [PubMed] [Google Scholar]
- Balda M. S., González-Mariscal L., Contreras R. G., Macias-Silva M., Torres-Marquez M. E., García-Sáinz J. A., Cereijido M. Assembly and sealing of tight junctions: possible participation of G-proteins, phospholipase C, protein kinase C and calmodulin. J Membr Biol. 1991 Jun;122(3):193–202. doi: 10.1007/BF01871420. [DOI] [PubMed] [Google Scholar]
- Bouvier D., Baldacci G. The N-terminus of fission yeast DNA polymerase alpha contains a basic pentapeptide that acts in vivo as a nuclear localization signal. Mol Biol Cell. 1995 Dec;6(12):1697–1705. doi: 10.1091/mbc.6.12.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer C. B., Roth M. G. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol. 1991 Aug;114(3):413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. O., Hunt C. A., Kennedy M. B. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992 Nov;9(5):929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Sharnick S. V., Laskey R. A. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell. 1982 Sep;30(2):449–458. doi: 10.1016/0092-8674(82)90242-2. [DOI] [PubMed] [Google Scholar]
- Einberger H., Mertz R., Hofschneider P. H., Neubert W. J. Purification, renaturation, and reconstituted protein kinase activity of the Sendai virus large (L) protein: L protein phosphorylates the NP and P proteins in vitro. J Virol. 1990 Sep;64(9):4274–4280. doi: 10.1128/jvi.64.9.4274-4280.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes D. J. Structure and function of the nuclear pore complex. Annu Rev Cell Biol. 1992;8:495–527. doi: 10.1146/annurev.cb.08.110192.002431. [DOI] [PubMed] [Google Scholar]
- Fortini M. E., Rebay I., Caron L. A., Artavanis-Tsakonas S. An activated Notch receptor blocks cell-fate commitment in the developing Drosophila eye. Nature. 1993 Oct 7;365(6446):555–557. doi: 10.1038/365555a0. [DOI] [PubMed] [Google Scholar]
- Funayama N., Fagotto F., McCrea P., Gumbiner B. M. Embryonic axis induction by the armadillo repeat domain of beta-catenin: evidence for intracellular signaling. J Cell Biol. 1995 Mar;128(5):959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. I., Hermiston M. L. Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr Opin Cell Biol. 1994 Dec;6(6):795–803. doi: 10.1016/0955-0674(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Stevenson B., Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988 Oct;107(4):1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth A. G., Hughes M. R., Stevenson B. R. Detection of the tight junction-associated protein ZO-1 in astrocytes and other nonepithelial cell types. Am J Physiol. 1992 Feb;262(2 Pt 1):C461–C469. doi: 10.1152/ajpcell.1992.262.2.C461. [DOI] [PubMed] [Google Scholar]
- Itoh M., Nagafuchi A., Yonemura S., Kitani-Yasuda T., Tsukita S., Tsukita S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993 May;121(3):491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis L. A., Goodenough D. A. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol. 1994 Mar;124(6):949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Niethammer M., Rothschild A., Jan Y. N., Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995 Nov 2;378(6552):85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Kim S. K. Tight junctions, membrane-associated guanylate kinases and cell signaling. Curr Opin Cell Biol. 1995 Oct;7(5):641–649. doi: 10.1016/0955-0674(95)80105-7. [DOI] [PubMed] [Google Scholar]
- Kistner U., Wenzel B. M., Veh R. W., Cases-Langhoff C., Garner A. M., Appeltauer U., Voss B., Gundelfinger E. D., Garner C. C. SAP90, a rat presynaptic protein related to the product of the Drosophila tumor suppressor gene dlg-A. J Biol Chem. 1993 Mar 5;268(7):4580–4583. [PubMed] [Google Scholar]
- Kornau H. C., Schenker L. T., Kennedy M. B., Seeburg P. H. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995 Sep 22;269(5231):1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Kreis T. E. Microtubules containing detyrosinated tubulin are less dynamic. EMBO J. 1987 Sep;6(9):2597–2606. doi: 10.1002/j.1460-2075.1987.tb02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S., Chen D. Y., Humphrey J. S., Gnarra J. R., Linehan W. M., Klausner R. D. Nuclear/cytoplasmic localization of the von Hippel-Lindau tumor suppressor gene product is determined by cell density. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):1770–1775. doi: 10.1073/pnas.93.5.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber T., Kidd S., Alcamo E., Corbin V., Young M. W. Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes Dev. 1993 Oct;7(10):1949–1965. doi: 10.1101/gad.7.10.1949. [DOI] [PubMed] [Google Scholar]
- Lorenzen J. A., Dadabay C. Y., Fischer E. H. COOH-terminal sequence motifs target the T cell protein tyrosine phosphatase to the ER and nucleus. J Cell Biol. 1995 Nov;131(3):631–643. doi: 10.1083/jcb.131.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue R. A., Marfatia S. M., Branton D., Chishti A. H. Cloning and characterization of hdlg: the human homologue of the Drosophila discs large tumor suppressor binds to protein 4.1. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9818–9822. doi: 10.1073/pnas.91.21.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J. L. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. J Membr Biol. 1990 Jun;116(2):177–184. doi: 10.1007/BF01868675. [DOI] [PubMed] [Google Scholar]
- Rajasekaran A. K., Hojo M., Huima T., Rodriguez-Boulan E. Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol. 1996 Feb;132(3):451–463. doi: 10.1083/jcb.132.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff P., Speicher D. W., Husain-Chishti A. Molecular identification of a major palmitoylated erythrocyte membrane protein containing the src homology 3 motif. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6595–6599. doi: 10.1073/pnas.88.15.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel K. L., Beckerle M. C. The LIM domain is a modular protein-binding interface. Cell. 1994 Oct 21;79(2):211–219. doi: 10.1016/0092-8674(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Siegfried E., Perrimon N. Drosophila wingless: a paradigm for the function and mechanism of Wnt signaling. Bioessays. 1994 Jun;16(6):395–404. doi: 10.1002/bies.950160607. [DOI] [PubMed] [Google Scholar]
- Siliciano J. D., Goodenough D. A. Localization of the tight junction protein, ZO-1, is modulated by extracellular calcium and cell-cell contact in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1988 Dec;107(6 Pt 1):2389–2399. doi: 10.1083/jcb.107.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B. R., Siliciano J. D., Mooseker M. S., Goodenough D. A. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986 Sep;103(3):755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G., Fitzgerald K., Greenwald I. Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell. 1993 Jul 30;74(2):331–345. doi: 10.1016/0092-8674(93)90424-o. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Itoh M., Nagafuchi A., Yonemura S., Tsukita S. Submembranous junctional plaque proteins include potential tumor suppressor molecules. J Cell Biol. 1993 Dec;123(5):1049–1053. doi: 10.1083/jcb.123.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott E., Balda M. S., Fanning A. S., Jameson B., Van Itallie C., Anderson J. M. The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7834–7838. doi: 10.1073/pnas.90.16.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott E., Balda M. S., Heintzelman M., Jameson B., Anderson J. M. Localization and differential expression of two isoforms of the tight junction protein ZO-1. Am J Physiol. 1992 May;262(5 Pt 1):C1119–C1124. doi: 10.1152/ajpcell.1992.262.5.C1119. [DOI] [PubMed] [Google Scholar]
- Woods D. F., Bryant P. J. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991 Aug 9;66(3):451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Zhao L. J., Padmanabhan R. Nuclear transport of adenovirus DNA polymerase is facilitated by interaction with preterminal protein. Cell. 1988 Dec 23;55(6):1005–1015. doi: 10.1016/0092-8674(88)90245-0. [DOI] [PubMed] [Google Scholar]