Abstract
Clinical trials have shown that angiotensin II receptor blockers reduce the new onset of diabetes in hypertensives; however, the underlying mechanisms remain unknown. We investigated the effects of telmisartan on peroxisome proliferator activated receptor γ (PPAR-δ) and the adenosine monophosphate (AMP)-activated protein kinase (AMPK) pathway in cultured myotubes, as well as on the running endurance of wild-type and PPAR-δ-deficient mice. Administration of telmisartan up-regulated levels of PPAR-δ and phospho-AMPKα in cultured myotubes. However, PPAR-δ gene deficiency completely abolished the telmisartan effect on phospho-AMPKαin vitro. Chronic administration of telmisartan remarkably prevented weight gain, enhanced running endurance and post-exercise oxygen consumption, and increased slow-twitch skeletal muscle fibres in wild-type mice, but these effects were absent in PPAR-δ-deficient mice. The mechanism is involved in PPAR-δ-mediated stimulation of the AMPK pathway. Compared to the control mice, phospho-AMPKα level in skeletal muscle was up-regulated in mice treated with telmisartan. In contrast, phospho-AMPKα expression in skeletal muscle was unchanged in PPAR-δ-deficient mice treated with telmisartan. These findings highlight the ability of telmisartan to improve skeletal muscle function, and they implicate PPAR-δ as a potential therapeutic target for the prevention of type 2 diabetes.
Keywords: peroxisome proliferator activated receptor γ, adenosine monophosphate-activated protein kinase, telmisartan, skeletal muscle, running endurance
Introduction
The rising prevalence of diabetes mellitus requires new approaches to prevent the onset of diabetes among high-risk populations. Hypertension is commonly complicated by insulin resistance and type 2 diabetes. Several antihypertensive agents such as diuretics and β-adrenergic blockers may worsen insulin sensitivity and impair glucose tolerance; however, angiotensin II receptor blockers (ARBs) exert beneficial effects in hypertensives such as improving insulin sensitivity and preventing the onset of diabetes [1]. Some ARBs, such as telmisartan, can effectively activate the peroxisome proliferator activated receptor γγ (PPAR-δ), a key regulator of adipocyte differentiation and adipose insulin sensitivity [2]. However, PPAR-δ is rarely expressed in skeletal muscle. By contrast, PPAR-δ (also referred to as PPAR-β) is expressed in a wide variety of tissues, with high levels in skeletal muscle [3]. In addition to pharmacological treatments, exercise plays an important role in the prevention of hypertension and diabetes. Skeletal muscle is the main tissue that is critical not only in endurance exercise but also in controlling glucose homeostasis. Recent studies have shown that PPAR-δ and AMPK regulate the metabolic and contractile characteristics of myofibres [4]. PPAR-δ overexpressing mice have a predominance of oxidative type I muscle fibres, enhanced whole-body insulin sensitivity, and greater endurance [5]. Activation of AMPK improved mouse exercise performance by 44% over that in controls [4]. Thus, activation of the PPAR-δ/AMPK pathway may offer a promising strategy for enhancing running endurance and improving glucose homeostasis. Currently, specific PPAR-δ and AMPK agonists are not available for clinical use. Thus, it is important to determine whether an ARB (such as telmisartan) can affect PPAR-δ/AMPK and their related functions. Given the importance of skeletal muscle function in the development of type 2 diabetes, we suggested that telmisartan has direct effects on skeletal muscle function and remodelling via the PPAR-δ/AMPK pathway. Here, we present evidence that activation of the PPAR-δ/AMPK by telmisartan is a key target for enhancing the running endurance of skeletal muscle in mice.
Materials and methods
Animals and experimental procedures
Male C57BL/6J wild-type and PPAR-δ-deficient mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The animals were housed in cages at a controlled temperature (22°± 1°C) and relative humidity (55%± 15%) and kept in a 12 hrs light/12 hrs dark cycle. They were supplied with standard laboratory chow and tap water ad libitum until 8 weeks of age. Then the animals were randomly divided into telmisartan treated group (n= 10) and normal diet control group (n= 10). The control group was fed standard laboratory chow, which consists of 10% calories as fat and 68% as carbohydrate. The treated group was given telmisartan (5 mg/kg body wt) mixed in the chow. Daily food intake per mouse was recorded during the first 10 days after the start of telmisartan administration. Body masses were measured every 2 weeks throughout the experimental period. Other functional experiments were performed. Then during the 24th week, they were killed after having been made to fast for 16 hrs. Blood was obtained from the carotid arteries of mice. Serum glucose, triglyceride and insulin were measured using routine techniques. The gastrocnemius muscle was quickly removed, frozen and stored at –70°C until further processing. All the experimental procedures were performed in accordance with protocols approved by the Institutional Animal Care and Research Advisory Committee.
Primary culture of skeletal muscle cells
Primary cultures of skeletal muscle cells were prepared as described previously [6] with minor modifications. Briefly, muscle masses from the legs of 2–3-day-old newborn PPAR-δ-deficient and wild-type C57BL/6J mice were dissected free of skin and bones in sterile phosphate-buffered saline. Fat and connective tissues were removed. Skeletal muscle tissues were minced finely, transferred to a 0.5% trypsogen solution and incubated with gentle agitation at 37°C for 5 min. Then, undigested tissue was allowed to settle and the supernatant was collected and mixed with DMEM containing 10% foetal bovine serum (FBS). The remaining tissue was digested for two more times. Finally, cell suspensions were pooled and passed through a nylon cell strainer (80 μm mesh) to remove debris and bone fragments. After centrifugation (10 min. at 300 ×g), cells were resuspended in DMEM with 10% FBS and 1% concentrated antibiotic–antimycotic mixture. The suspension was adjusted to a final concentration of ∼5 × 105 cells/ml in 24- and 6-well plates. The plates were incubated at 37°C in an air (95%)–CO2 (5%) humidified incubator and the medium was replaced every 48 hrs. After confluence, cells were given DMEM without FBS. Telmisartan was dissolved in dimethyl sulfoxide (DMSO) and added to the media within 0.1% of volume.
Assessment of running endurance
PPAR-δ-deficient mice and wild-type mice were subjected to a treadmill running test, conducted following a protocol modified from that of Wang [5]. Briefly, prior to the exercise performance test, the mice were acclimated to the treadmill with a 5-min. run at 7 m/min. once per day for 2 days. For the test, the treadmill was started at 10 m/min. for the first 60 min., and then the speed was incrementally increased 1 m/min. every 15 min. until the mouse reached exhaustion. Exhaustion was defined as when a mouse was unable to avoid repetitive electrical shocks. Running endurance was estimated by the following two parameters: the duration of the run (in minutes) and the distance run (in metres).
Oxygen consumption analysis
Oxygen consumption was measured in PPAR-δ-deficient mice and wild-type C57BL/6J mice using the QUBIT systems respiration package (Qubit Systems, Inc., Ontario, Canada) in basal and post-exercise conditions. In the post-exercise condition the animals ran on the treadmill at 10 m/min. for 10 min. before being measured. Animals were placed individually in chambers. Results are expressed as millilitre per kilogram per minute.
Metachromatic fibre typing of skeletal muscle
Fibre typing was performed with cryostat sections of the gastrocnemius muscle using the metachromatic dye-ATPase method as described previously [7]. Muscle sections were treated in pre-incubation buffer (50 mM potassium acetate, pH 4.4, 17 mM CaCl2) for 8 min., followed with three washes in Tris rinse buffer (300 mM Tris-HCl, pH 7.8, 53 mM CaCl2). Muscle slides were then put into incubation buffer (53 mM glycine, pH 9.4, 28 mM CaCl2, 65 mM NaCl, 47.5 mM NaOH, 4 mM ATP) for 25 min. and rinsed three times in 1% (w/v) CaCl2 solution. Then, 0.1% toluidine blue was used to stain muscle fibres for 1 min. The sections were rinsed in H2O and dehydrated with ethanol. All steps were carried out at room temperature. The proportion of type I slow fibres were analysed with NIS-Elements imaging software (Nikon Corporation, Tokyo, Japan).
Semi-quantitative conventional polymerase chain reaction
mRNA levels of PPAR-δ, PPAR-δ and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control were measured in cultured primary skeletal muscle cells or skeletal muscle tissue using standard techniques as we have reported previously [8]. Total RNA was isolated with Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). RNA was used to synthesize first-strand cDNA using the Reverse Transcription System (Promega, Madison, WI, USA); 3 μg of purified mRNA was reverse-transcribed with an RT mixture consisting of oligo dT (12 to 18) and 5 U avian myeloblastosis virus (AMV) reverse transcriptase at 42°C for 60 min., followed by heating to 95°C for 5 min. The single-stranded cDNA was amplified by PCR using taq DNA polymerase (Sangon Biological Engineering, Shanghai, China). The sense and antisense primers for the coding regions of PPAR-δ, PPAR-δ or GAPDH genes were the following: PPAR-δ (Reference Sequence [Ref Seq] database accession number: NM_001473623), forward, 5′-CGCACCCTTTGTCATCCAC-3′; reverse, 5′-CGCACCTCTGCCATCTTCT-3′; expected size, 597 bp; PPAR-δ (Ref Seq NM_011146), forward, 5′-CTGACCAAAGCCAAGGCGAGGG-3′; reverse, 5′-TTCAGCAAGCCTGGGCGGTCTC-3′; expected size, 523 bp; GAPDH (Ref Seq BC095932); forward, 5′-ACCTCAACTACATGGTCTAC-3′ reverse, 5′-TTGTCATTGAGAGCAATGCC-3′; expected size, 802 bp. The reaction was initiated at 94°C for 4 min. and followed by 32 cycles of denaturation at 94°C for 30 sec., annealing at 60°C for 30 sec. and extension at 72°C for 40 sec. The reaction was achieved with a final extension at 72°C for 5 min. Each sample was run in parallel with the housekeeping gene. Control experiments in which reverse transcriptase was omitted were performed in order to exclude the possibility that the signal detected could come from the genomic DNA. A total of 14 μl of the PCR products were size fractionated on an agarose gel (1.5%) and quantified using a Gel Doc 2000 Imager (Bio-Rad, CA, USA).
Western blot analyses
Western blot analyses of PPAR-δ, PPAR-γ, AMPKα, phospho- AMPKα, angiotensin II type I (AT1) receptor, Troponin I-ss and fl-actin from cultured primary skeletal muscle cells or skeletal muscle tissue were performed with standard techniques as reported by our group previously [8]. Briefly, total protein of the skeletal muscle cells in primary culture or the gastrocnemius muscle was extracted and measured by the Bradford method. Equal amounts of protein samples (50 μg) were separated by SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membrane, blocked with 5% (w/v) dry milk in phosphate-buffered saline and incubated with primary antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C. After incubation with the secondary antibodies for 1 hr, the proteins were detected by enhanced chemiluminescence and quantified using a Gel Doc 2000 Imager (Bio-Rad).
Statistical analysis
Data were expressed as mean ± S.E.M. Statistical significance of differences between groups was evaluated by Student’s t-test or ANOVA with Bonferroni’s multiple comparison post hoc tests, as appropriate (SPSS Inc., Chicago, IL, USA). Two-sided P-values less than 0.05 were considered to be statistical significance.
Results
Telmisartan enhances PPAR-δ expression in cultured myotubes
First, we examined whether telmisartan affects PPAR-δ and PPAR-δ levels in cultured myotubes. After stimulation with telmisartan for 24 hrs, PPAR-δ mRNA and protein expression increased 105% and 102%, respectively, in cultured myotubes from wild-type mice. However, telmisartan did not affect the PPAR-δ mRNA or protein levels in cultured myotubes either from wild-type or PPAR-δ-deficient mice (Fig. 1A–D).
Fig 1.
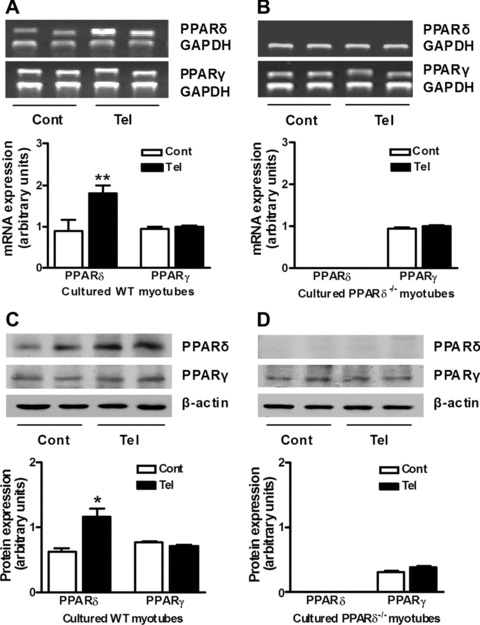
Effects of telmisartan on PPAR-δ and PPAR-δ expression in cultured myotubes. mRNA (A) and protein (C) levels of PPAR-δ and PPAR-δ were analysed in primary cultured myotubes from wild-type (WT) mice by semi-quantitative RT-PCR and Western blotting. mRNA (B) and protein (D) levels of PPAR-δ and PPAR-δ were analysed in primary cultured myotubes from PPAR-δ-deficient (PPAR-δ−/−) mice by semi-quantitative RT-PCR and Western blotting. The cells were unstimulated (Cont) or stimulated with telmisartan (Tel) in medium without FBS for 24 hrs before total RNA or protein were extracted. *P < 0.05, **P < 0.01 versus Cont (n= 4 for each group).
Telmisartan up-regulates p-AMPK levels through PPAR-δ
We found that telmisartan significantly increased phospho-AMPK α levels in cultured myotubes from wild-type mice but not in those from PPAR-δ-deficient mice (Fig. 2A). Importantly, telmisartan had no effect on AT1 receptor expression in cultured myotubes from either wild-type or PPAR-δ-deficient mice (Fig. 2B).
Fig 2.
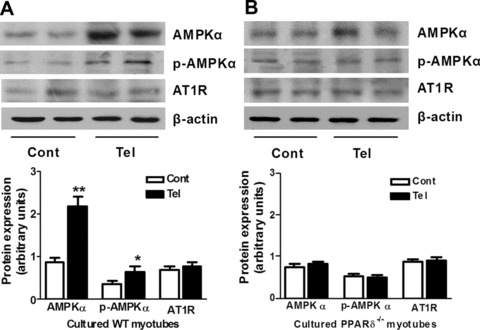
Activation of PPAR-δ by telmisartan up-regulates AMPK without affecting the AT1 receptor in cultured myotubes. Protein levels of AMPK, phosphor-AMPK (p-AMPK) and AT1 receptor (AT1R) in primary cultured myotubes from wild-type [WT, (A)] and PPAR-δ-deficient [PPAR-δ−/−, (B)] mice were analysed by Western blotting. The cells were unstimulated (Cont) or stimulated with telmisartan (Tel) in medium without FBS for 24 hrs before total protein were extracted. *P < 0.05, **P < 0.01 compared with Cont (n= 4 for each group).
Chronic treatment with telmisartan increases PPAR-δ expression in skeletal muscle
We next asked whether the effect of telmisartan on PPAR-δ expression in vitro would extend to the in vivo setting. Wild-type and PPAR-δ-deficient mice were treated with telmisartan (5 mg/kg) for 6 months. Compared with control mice, telmisartan increased PPAR-δ mRNA and protein levels (P < 0.01 and P < 0.05, respectively), but did not significantly affect PPAR-δ levels (P > 0.05) in skeletal muscle of wild-type mice (Fig. 3A and C). PPAR-δ mRNA and protein levels in skeletal muscle were unaffected in PPAR-δ-deficient mice treated with or without telmisartan (Fig. 3B and C).
Fig 3.
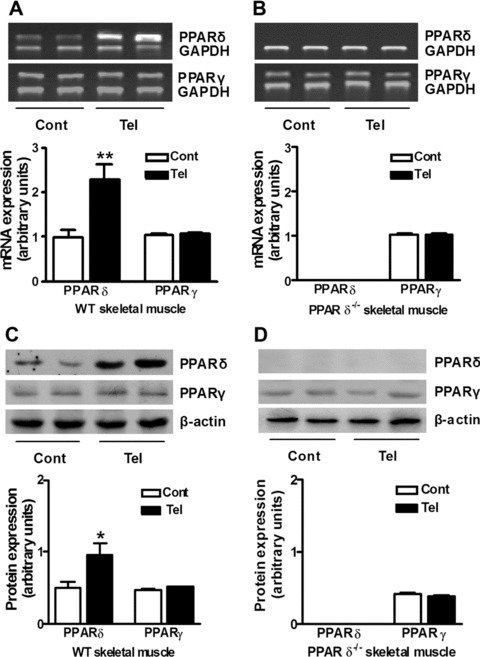
Long-term telmisartan intervention increases PPAR-δ, but not PPAR-γ expression in mice skeletal muscle. mRNA (A) and protein (C) levels of PPAR-δ and PPAR-γ in skeletal muscle tissue from wild-type mice (WT) by Western blotting or semi-quantitative RT-PCR. mRNA (B) and protein (D) levels of PPAR-δ and PPAR-γ in skeletal muscle tissue from PPAR-δ-deficient mice (PPAR-δ−/−) were analysed by Western blotting or semi-quantitative RT-PCR. Mice were fed a regular diet (Cont) or a telmisartan-containing diet (Tel) for 24 weeks before being killed. *P < 0.05, **P < 0.01 versus Cont (n= 4 for each group).
Effect of telmisartan on metabolic profile in vivo
To evaluate changes of phenotype after telmisartan treatment, body weight, food intake and blood metabolic parameters were examined. Our study revealed that long-term telmisartan reduced weight gain in wild-type mice (P < 0.05) but not in PPAR-δ-deficient mice (Fig. 4A and B). Furthermore, telmisartan significantly reduced the weight of visceral and subcutaneous fat in wild-type mice, but this effect was absent in PPAR-δ-deficient mice (Fig. 4G and H). Food intake was not affected in either group (Fig. 4C). Also serum insulin, triglyceride and glucose levels did not differ between mouse strains with or without telmisartan treatment (Fig. 4D–F).
Fig 4.
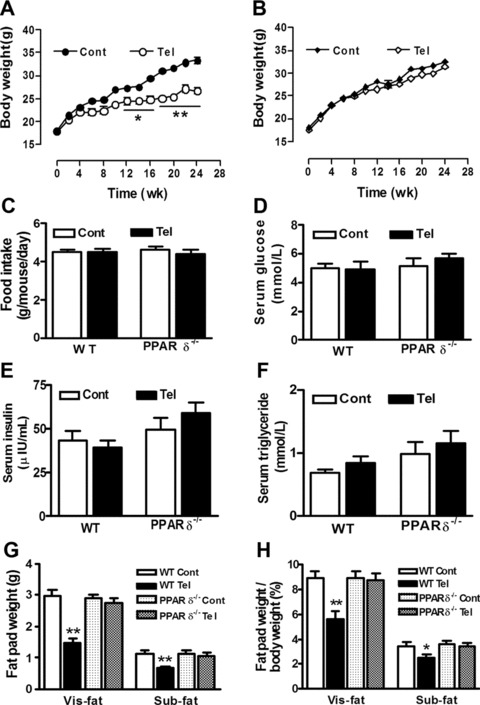
Effects of chronic treatment with telmisartan on weight gain, food intake, serum parameters and fat pad weight in wild-type (WT) and PPAR-δ-deficient (PPAR-δ−/−) mice. Mice were fed a regular diet (Cont) or regular diet plus telmisartan (Tel). (A), (B) Body weights were measured every 2 weeks throughout the experimental period. (C), Daily food intake per mouse was recorded during the first 10 days after the start of telmisartan administration. Fasting serum levels of glucose (D), insulin (E) and triglyceride (F) were examined using routine methods after 24 weeks’ administration. (G) Subcutaneous fat (Sub-fat) and visceral fat (Vis-fat) were isolated and weighed when mice were killed after 24 weeks’ administration. H The ratio of fat pad weight to whole body weight. *P < 0.05; **P < 0.01 compared with Cont (n= 10 for each group).
Telmisartan enhances AMPK phosphorylation through PPAR-δ in skeletal muscle
Results in vitro showed that up-regulation of phospho-AMPK α levels by telmisartan occurred in a PPAR-δ-dependent manner. Long-term administration of telmisartan increased phospho-AMPK α protein levels in skeletal muscle of wild-type mice (P < 0.05), but not in that of PPAR-δ-deficient mice (Fig. 5A and B). Interestingly, telmisartan did not influence AT1 receptor expression in skeletal muscle from either wild-type or PPAR-δ-deficient mice (Fig. 5A and B).
Fig 5.
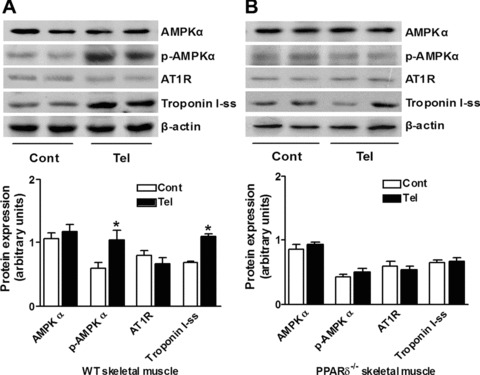
Telmisartan-mediated PPAR-δ activation increases AMPK phosphorylation and troponin I-ss expression without involvement of the AT1 receptor in skeletal muscle. Protein levels of AMPK, phosphor-AMPK (p-AMPK), AT1 receptor (AT1R) and troponin I-ss, a marker of type I fibres, in skeletal muscle tissue from wild-type mice [WT, (A)] and PPAR-δ-deficient mice [PPAR-δ−/−, (B)] was analysed by Western blotting. Mice were fed a regular diet (Cont) or a telmisartan-containing diet (Tel) for 24 weeks before being killed. *P < 0.01 versus Cont (n= 4 for each group).
Chronic telmisartan treatment enhances running endurance, reduces fat mass and remodels myofibres
Skeletal muscle fibres are generally classified as type I (oxidative/slow) or type II (glycolytic/fast) fibres. Recent studies showed that PPAR-δ/AMPK activation induces a transformation from fast glycolytic to slow oxidative fibres [4, 5]. To determine whether telmisartan has a similar effect, we assessed running time, running distance, basal and post-exercise oxygen consumption. Compared with control mice, wild-type mice treated with telmisartan had greater running endurance and more post-exercise oxygen consumption (Fig. 6A–D). However, PPAR-δ-deficient mice treated with telmisartan displayed no phenotypic change relative to untreated PPAR-δ-deficient mice. The skeletal muscle mass was not different between mice with and without telmisartan treatment (Fig. 6E), but the ratio of skeletal muscle weight to whole body weight was significantly higher in mice treated with telmisartan than untreated mice (Fig 6F). We further analysed the myofibre composition of gastrocnemius muscle and found that the proportion of type I slow fibres was significantly higher in telmisartan-treated wild-type mice than in control wild-type mice, whereas there were no difference between two groups of PPAR-δ-deficient mice (Fig. 6G and H). In accordance with the fibre composition, the biomarker for slow skeletal muscle fibre (troposin I-ss) was significantly up-regulated in wild-type mice receiving telmisartan but not in telmisartan-treated PPAR-δ-deficient mice (Fig. 5A and B).
Fig 6.
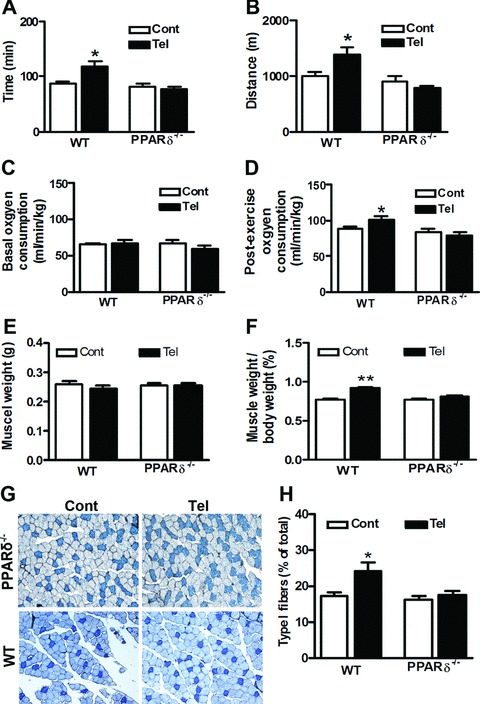
Chronic telmisartan treatment enhances running endurance and remodels myofibres. Mice were fed a normal diet (Cont) or a normal diet plus telmisartan (Tel). During weeks 23–24 of the experimental period, running endurance and oxygen consumption of the wild-type mice (WT) and PPAR-δ-deficient mice (PPAR-δ−/−) were analysed (n= 5 per group). Running endurance was estimated by the duration (A) and the distance (B) of the run. Oxygen consumption included basal (C) and post-exercise (D) conditions. After 24 weeks’ treatment, mice were killed and gastrocnemius muscle was isolated immediately. (E) Weight of gastrocnemius muscle. (F) The ratio of gastrocnemius muscle weight to whole body weight. Skeletal muscle fibre typing was performed with cryostat sections of the gastrocnemius muscle using the metachromatic dye-ATPase method. (G) Representative figures of fibre typing from four independent experiments were shown. Type I fibres were stained dark blue. (H) The percentage of type I fibres was calculated. *P < 0.01 versus Cont (n= 4 per group).
Discussion
Previous studies have highlighted a potential role for ARBs in activation of PPAR-δ in adipose tissue and the vasculature [9–11].
The present study provides novel evidence that the effect of telmisartan on skeletal muscle function is PPAR-δ dependent. In vitro, telmisartan up-regulated levels of PPAR-δ and phospho-AMPKα in cultured myotubes, but this effect was absent in cultured myotubes from PPAR-δ-deficient mice. In vivo, phospho-AMPKα levels in skeletal muscle were up-regulated in wild-type mice treated with telmisartan. In contrast, AMPKα expression in skeletal muscle was unchanged in PPAR-δ-deficient mice treated with telmisartan. Chronic administration of telmisartan remarkably prevented weight gain, enhanced running endurance and post-exercise oxygen consumption and promoted the production of slow-twitch skeletal muscle fibres in wild-type mice but not in PPAR-δ-deficient mice. The present findings suggest that activation of the PPAR-δ / AMPK pathway could be considered as a novel therapeutic target for the prevention of diabetes.
Several large-scale clinical trials showed a 14–34% reduction in the incidence of type 2 diabetes in hypertensive patients treated with either ACE inhibitors or ARBs for 3–6 years compared with those treated with thiazide diuretic, β-adrenergic blocker and/or the calcium channel antagonist [12–14]. From a clinical perspective, these studies form the basis of a new strategy to reduce the ongoing epidemic and burden of type 2 diabetes. However, the mechanisms whereby inhibition of the rennin-angiotensin system improves glucose homeostasis are complex. They may include improvement of blood flow through the microcirculation of skeletal muscles, protection of the pancreatic islets from glucotoxicity and restoration of β-cell mass, and inhibition of oxidative stress [15–18]. In addition, ARBs are known to activate PPAR-δ, which is responsible for their insulin-sensitizing effects [19]. PPAR-δ is abundant in adipose tissues, functioning as the key transcription factor for adipocyte differentiation and lipid storage [10]. However, PPAR-δ is the predominant PPAR isoform in skeletal muscle [3]. Activation of PPAR-δ causes several changes in metabolism, including enhanced fatty acid oxidation, improved lipid profiles and reduced adiposity [20, 21]. It also regulates CB1 expression in adipose tissue [22], increases glucose metabolism and promotes gene regulatory responses in cultured human skeletal muscle [6, 23]. Muscle-specific PPAR-δ overexpression results in a profound change in fibre composition due to hyperplasia and/or a shift to more oxidative fibres, resulting in increased enzymatic activity and running endurance [5].
Although telmisartan is regarded as a partial PPAR-δ agonist [2], little is known about whether telmisartan has an effect on PPAR-δ. Our study shows for the first time that telmisartan can up-regulate PPAR-δ expression in skeletal muscle. First, we demonstrated that telmisartan can up-regulate PPAR-δ without affecting PPAR-δ in cultured myotubes. Second, long-term administration of telmisartan markedly increased PPAR-δ levels but not PPAR-δ levels in skeletal muscle of wild-type mice. Third, no telmisartan stimulatory effect was observed in skeletal muscle from PPAR-δ-deficient mice. This evidence strongly supports the notion that telmisartan directly acts on PPAR-δ in skeletal muscle.
In mammals, AMPK is known to contribute to glucose homeostasis, appetite and exercise physiology [4, 24, 25]. Activation of AMPK in skeletal muscle increases glucose uptake, fatty acid oxidation and mitochondrial biogenesis by increasing gene expression in these pathways [26]. Administration of PPAR-δ agonist or AMPK agonist increased oxidative fibre content and running endurance [4, 5]. In this study, we showed that telmisartan promoted AMPK phospherylation through PPAR-δ activation. A recent study showed that PPAR-δ agonist AICAR can increase AMPK phospherylation and expression [27]. Our results in vitro indicated that telmisartan increases phospho-AMPK expression in cultured myotubes from wild-type mice but not in myotubes from PPAR-δ-deficient mice. Similar findings were demonstrated in vivo. Chronic telmisartan treatment increased phospho-AMPK in the skeletal muscle of wild-type mice but not in PPAR-δ-deficient mice. The lack of an effect of telmisartan on the AT1 receptor in cultured myotubes and skeletal muscle is noteworthy. These results indicate that telmisartan stimulates the PPAR-δ/AMPK pathway in skeletal muscle by a mechanism independent of AT1 receptor blockade.
An important aspect of the present study was the finding that telmisartan’s effect on the PPAR-δ/AMPK pathway is linked with the skeletal muscle composition and function in vivo. In wild-type mice, chronic administration of telmisartan enhanced running endurance, increased post-exercise oxygen consumption, reduced body fat and increased the proportion of type I fibres in skeletal muscle. In contrast, telmisartan had no effect on body fat accumulation and skeletal muscle function or remodelling in PPAR-δ-deficient mice. Post-exercise oxygen consumption is accompanied by an elevated consumption of fuel [28]. In response to exercise, fat stores are broken down and free fatty acids are released into the blood. In recovery, the direct oxidation of free fatty acids as fuel and the energy consuming reconversion of free fatty acids back into fat stores both take place [29].
Although telmisartan does not influence serum glucose directly, it affects several pathways related to glucose haemostasis. Besides improvement of skeletal muscle function, telmisartan also improves blood flow through the microcirculation of skeletal muscles, protects the pancreatic islets from glucotoxicity and restores β-cell mass, inhibits oxidative stress, prevents weight gain and activates PPAR-δ. These factors are critical in the development of insulin resistance and type 2 diabetes [15–19, 30].
Skeletal muscle is the largest organ in the human body. The mass and composition of skeletal muscle are critical for its functions and the whole body energy metabolism, such as exercise, energy expenditure and glucose metabolism [31–33]. In human beings, three major muscle fibre types are distinguished, including type I, type IIA and type IIB. However, human type IIB gene is very homologous to rat type IIX gene [34].
Type I fibres are mitochondria-rich and mainly use oxidative metabolism for energy production, which provides a stable supply of ATP, and thus are fatigue resistant. In mice, type II fibres comprise three subtypes, IIA, IIX and IIB. Type IIB fibres have the lowest levels of mitochondrial and oxidative enzymes, rely on glycolytic metabolism and are susceptible to fatigue, whereas the oxidative and contraction functions of type IIA and IIX lie between type I and IIB [5]. However, we did not successfully verify IIA, IIX, IIB fibre subtypes of skeletal muscle in mice because of the methodological problem. This weakness limits us to further evaluate the skeletal muscle function. Mass, fibre size and fibre composition are regulated in adult skeletal muscle in response to changes in physical activity, environment or pathological conditions [35]. Skeletal muscle in obese and diabetic patients had a lower oxidative capacity, increased glycolytic capacity and a decreased percentage of type I fibres [36, 37]. Therefore, PPAR-δ activation induced by telmisartan not only increases oxidative fibres and running endurance, but also substantially contributes to enhanced mitochondria function and lipid oxidation.
In summary, these findings support the notion of a previously unrecognized role of telmisartan in skeletal muscle metabolism. We provide evidence for the first time that telmisartan increases running endurance through activation of the PPAR-δ/AMPK pathway, and this may have the additional benefit of preventing diabetes.
From a clinical point of view, it is tempting to speculate that our data provide the molecular basis for the prevention of diabetes in hypertensives using ARBs. A randomized clinical trial is needed to verify whether ARBs show additional benefits in improving skeletal muscle function in hypertensive patients.
Acknowledgments
We thank Drs. William J. Arendshorst and Wing Tak Wong for expert advice and discussions. This study was funded by Natural Science Foundation of China (Grant No. 30890042) and National Basic Research Program (973 Program) grants of China (Grant Nos. 2006CB503905 and 2006CB503804).
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Andraws R, Brown DL. Effect of inhibition of the renin-angiotensin system on development of type 2 diabetes mellitus (meta-analysis of randomized trials) Am J Cardiol. 2007;99:1006–12. doi: 10.1016/j.amjcard.2006.10.068. [DOI] [PubMed] [Google Scholar]
- 2.Benson SC, Pershadsingh HA, Ho CI, et al. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- 3.Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144:2201–7. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- 4.Narkar VA, Downes M, Yu RT, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–15. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang YX, Zhang CL, Yu RT, et al. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2:1532–9. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer DK, Al-Khalili L, Perrini S, et al. Direct activation of glucose transport in primary human myotubes after activation of peroxisome proliferator-activated receptor delta. Diabetes. 2005;54:1157–63. doi: 10.2337/diabetes.54.4.1157. [DOI] [PubMed] [Google Scholar]
- 7.Ogilvie RW, Feeback DL. A metachromatic dye-ATPase method for the simultaneous identification of skeletal muscle fiber types I, IIA, IIB and IIC. Stain Technol. 1990;65:231–41. doi: 10.3109/10520299009105613. [DOI] [PubMed] [Google Scholar]
- 8.Zhang LL, Yan Liu D, Ma LQ, et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007;100:1063–70. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]
- 9.Zorad S, Dou JT, Benicky J, et al. Long-term angiotensin II AT1 receptor inhibition produces adipose tissue hypotrophy accompanied by increased expression of adiponectin and PPARgamma. Eur J Pharmacol. 2006;552:112–22. doi: 10.1016/j.ejphar.2006.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janke J, Schupp M, Engeli S, et al. Angiotensin type 1 receptor antagonists induce human in-vitro adipogenesis through peroxisome proliferator-activated receptor-gamma activation. J Hypertens. 2006;24:1809–16. doi: 10.1097/01.hjh.0000242405.68461.84. [DOI] [PubMed] [Google Scholar]
- 11.Imayama I, Ichiki T, Inanaga K, et al. Telmisartan downregulates angiotensin II type 1 receptor through activation of peroxisome proliferator-activated receptor gamma. Cardiovasc Res. 2006;72:184–90. doi: 10.1016/j.cardiores.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Sleight P, Yusuf S, Pogue J, et al. Blood-pressure reduction and cardiovascular risk in HOPE study. Lancet. 2001;358:2130–1. doi: 10.1016/S0140-6736(01)07186-0. [DOI] [PubMed] [Google Scholar]
- 13.Kjeldsen SE, Julius S. Hypertension mega-trials with cardiovascular end points: effect of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Am Heart J. 2004;148:747–54. doi: 10.1016/j.ahj.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 14.Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–31. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 15.Velloso LA, Folli F, Sun XJ, et al. Cross-talk between the insulin and angiotensin signaling systems. Proc Natl Acad Sci USA. 1996;93:12490–5. doi: 10.1073/pnas.93.22.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiuchi T, Iwai M, Li HS, et al. Angiotensin II type-1 receptor blocker valsartan enhances insulin sensitivity in skeletal muscles of diabetic mice. Hypertension. 2004;43:1003–10. doi: 10.1161/01.HYP.0000125142.41703.64. [DOI] [PubMed] [Google Scholar]
- 17.Shao J, Iwashita N, Ikeda F, et al. Beneficial effects of candesartan, an angiotensin II type 1 receptor blocker, on beta-cell function and morphology in db/db mice. Biochem Biophys Res Commun. 2006;344:1224–33. doi: 10.1016/j.bbrc.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Iwase M, Imoto H, Oku M, et al. Acarbose feeding increases pancreatic islet blood flow in obese glucose-intolerant Otsuka Long-Evans Tokushima fatty rats. Pancreas. 2008;37:228–30. doi: 10.1097/MPA.0b013e318164c41e. [DOI] [PubMed] [Google Scholar]
- 19.Schupp M, Janke J, Clasen R, et al. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109:2054–7. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka T, Yamamoto J, Iwasaki S, et al. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci USA. 2003;100:15924–9. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang YX, Lee CH, Tiep S, et al. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113:159–70. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 22.Yan ZC, Liu DY, Zhang LL, et al. Exercise reduces adipose tissue via cannabinoid receptor type 1 which is regulated by peroxisome proliferator-activated receptor-delta. Biochem Biophys Res Commun. 2007;354:427–33. doi: 10.1016/j.bbrc.2006.12.213. [DOI] [PubMed] [Google Scholar]
- 23.Lee CH, Olson P, Hevener A, et al. PPARdelta regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci USA. 2006;103:3444–9. doi: 10.1073/pnas.0511253103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li HB, Ge YK, Zheng XX, et al. Salidroside stimulated glucose uptake in skeletal muscle cells by activating AMP-activated protein kinase. Eur J Pharmacol. 2008;588:165–9. doi: 10.1016/j.ejphar.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 25.Kubota N, Yano W, Kubota T, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Osler ME, Zierath JR. Adenosine 5’-monophosphate-activated protein kinase regulation of fatty acid oxidation in skeletal muscle. Endocrinology. 2008;149:935–41. doi: 10.1210/en.2007-1441. [DOI] [PubMed] [Google Scholar]
- 27.Kramer DK, Al-Khalili L, Guigas B, et al. Role of AMP kinase and PPARdelta in the regulation of lipid and glucose metabolism in human skeletal muscle. J Biol Chem. 2007;282:19313–20. doi: 10.1074/jbc.M702329200. [DOI] [PubMed] [Google Scholar]
- 28.Bahr R. Excess postexercise oxygen consumption–magnitude, mechanisms and practical implications. Acta Physiol Scand Suppl. 1992;605:1–70. [PubMed] [Google Scholar]
- 29.Bahr R, Hostmark AT, Newsholme EA, et al. Effect of exercise on recovery changes in plasma levels of FFA, glycerol, glucose and catecholamines. Acta Physiol Scand. 1991;143:105–15. doi: 10.1111/j.1748-1716.1991.tb09205.x. [DOI] [PubMed] [Google Scholar]
- 30.He H, Yang D, Ma L, et al. Telmisartan prevents weight gain and obesity through activation of peroxisome proliferator-activated receptor-delta-dependent pathways. Hypertension. 2010;55:869–79. doi: 10.1161/HYPERTENSIONAHA.109.143958. [DOI] [PubMed] [Google Scholar]
- 31.Zurlo F, Larson K, Bogardus C, et al. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. 1990;86:1423–7. doi: 10.1172/JCI114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Virkamaki A, Korsheninnikova E, Seppala-Lindroos A, et al. Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes. 2001;50:2337–43. doi: 10.2337/diabetes.50.10.2337. [DOI] [PubMed] [Google Scholar]
- 33.Mensink M, Blaak EE, Vidal H, et al. Lifestyle changes and lipid metabolism gene expression and protein content in skeletal muscle of subjects with impaired glucose tolerance. Diabetologia. 2003;46:1082–9. doi: 10.1007/s00125-003-1152-2. [DOI] [PubMed] [Google Scholar]
- 34.Bottinelli R, Reggiani C. Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol. 2000;73:195–262. doi: 10.1016/s0079-6107(00)00006-7. [DOI] [PubMed] [Google Scholar]
- 35.Fluck M, Hoppeler H. Molecular basis of skeletal muscle plasticity–from gene to form and function. Rev Physiol Biochem Pharmacol. 2003;146:159–216. doi: 10.1007/s10254-002-0004-7. [DOI] [PubMed] [Google Scholar]
- 36.Tanner CJ, Barakat HA, Dohm GL, et al. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab. 2002;282:E1191–6. doi: 10.1152/ajpendo.00416.2001. [DOI] [PubMed] [Google Scholar]
- 37.Mogensen M, Sahlin K, Fernstrom M, et al. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes. 2007;56:1592–9. doi: 10.2337/db06-0981. [DOI] [PubMed] [Google Scholar]


