Abstract
Aortic dissection, occurring following a separation of the layers constituting the complex vascular walls, leads to the formation of a ‘false’ lumen and disrupts the regulation of aortic wall homeostasis and function. This clinical condition still represents an important health problem and is associated with high mortality. Its natural history mandates surgical intervention when exceeding 55 mm in diameter and involving the ascending portion of the aorta (Type A), on the bases of an anatomical classification dated back to 1965. An intriguing question rising is whether a dissection that overcomes that critic acute phase has still the indication to surgical intervention. Molecular analysis of chronic dissected aortic walls could help in understanding how morphology and structure are affected and whether tissue homeostasis is re-established. Thus, pursued by this consideration, we made a histological and immunohistochemical characterization of a chronic Type A dissection, reporting three major findings: endothelial cells line the aortic primitive lumen, as well as the ‘false’ one; walls of primitive and ‘false’ lumina are comparable in thickness; vascular layers in the ‘false’ lumen are made up of terminally differentiated cells. This evidence obtained in a single specimen encourages a meditation on the compulsory indication for surgical intervention.
Keywords: aortic dissection, molecular medicine, diagnostic techniques, molecular imaging
Introduction
Aortic dissection, occurring so that intima and inner media are separated from the outer media and adventitia of the aorta to create a ‘false’ lumen, represents the most common emergency condition of the aorta and is still associated with a high mortality [1, 2]. Outcome is determined by the type, the onset, the extent of dissection and also by the presence of associated complications. Initial management of aortic dissection is aimed at limiting the propagation of the ‘false’ lumen by controlling the aortic shear stress and simultaneously determining which patients will benefit from surgical intervention or endovascular repair. The extent of aortic dissection is crucial to define the outcome of this process, confirming the importance of a precise staging of the dissection as an important means to indicate the more appropriate treatment. Several systems of classification have been developed principally based on the anatomical extent (Stanford or DeBakey classification) [3], to the time from onset (acute, subacute or chronic) [4], and to the underlying pathology (European Society of Cardiologists’ system) [5].
Because the main factor to be considered in the evaluation of aortic dissection is its anatomical extention, the Stanford classification, dividing dissections in Type A, when ascending aorta is affected, and Type B, when it is not, is preferred by physicians as the standard guide to whether surgical treatment is needed. Surgical intervention is nowadays considered as the most appropriate therapeutic choice for Type A dissection, not only in acute syntomatic conditions, but also when chronic asyntomatic cases are diagnosed.
In spite of the substancial advances made in the understanding of the risk factors for ‘aortic dissection’, no further progresses have been made in therapeutic approaches. On this issue, it is noteworthy that decision about surgical intervention is still based on the Stanford/DeBakey classification, dated back to 1965. Therefore, molecular analysis, affording a deeper investigation of the characteristics of dissected aortic walls, that have overcome the acute phase, can shed new light on therapeutic strategies for chronic Type A dissections.
Prompted by these considerations, we aimed at characterizing molecular markers of the different components of vascular walls in a fragment biopsy obtained during surgery of a patient with Type A chronic aortic dissection that came to our observation. In particular, we evaluated cell proliferation, cell differentiation and the anatomic structure of all the three layers of the aortic wall.
Material and methods
Human aortic sample was obtained from a 53-year-old woman, which came to our observation after the incidental finding, obtained through a transthoracic echocardiogram, of a dilated ascending aorta. The patient was asymptomatic and reported hypertension as the only cardiovascular risk factor. A transesophageal echocardiography examination disclosed a tricuspid aortic valve with non-coronary cusp partially prolapsing, ascending aorta diameter 55 mm, moderate aortic regurgitation and the presence of false lumen in ascending aorta that extends to the origin of common carotid (Fig. 1A). A 64-slice contrast-enhanced computed tomography (MSCT; LighSpeed VCT; GE Healthcare, San Francisco, CA, USA) was performed to confirm the diagnosis (Fig. 1B and C). On physical examination, she appeared well and she did not present particular signs; electrocardiography revealed sinus rythm at a rate of 85 beats per min.; her blood pressure was 150/85 mm Hg, axillary temperature was 36.7°C, respirations were 16 breaths per min. and arterial blood gas measurements were normal. The chemistry and haematologic laboratory values were within the normal reference ranges. A chest radiography showed no abnormalities of the heart and mediastinum, and the lungs were clear. An anamnestic collection revealed a single episode of sudden chest pain occurred 11 months before her admission in our institution.
Fig 1.
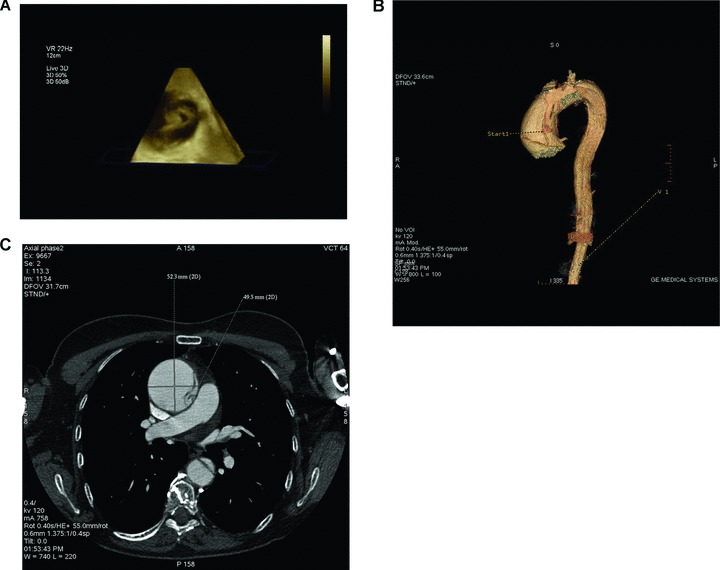
In vivo imaging showing Type A aortic dissection. (A) Real-time 3D TEE indicates the primary tear located 7 mm above the sinotubular junction dissection flap, true and false lumina. (B) 3D 64-slice volume rendering contrast-enhanced computed tomography (CT) recontruction shows the primary tear on the left side of the ascending aortic wall. (C) 64-slice CT scan axial view shows the dissection flap, the true (T) and false (F) lumina on both the ascending and discending aortic walls.
After anatomical examination, the fragment biopsy obtained during surgery was rinsed with ice-cold phosphate buffered saline (PBS) and then a portion of the tissue (including the point of dissection) was immersion-fixed with 4% paraformaldehyde, flesh-frozen and sectioned (10 μm) on a cryostat for histological examination and immunohistochemistry thereafter.
Histological examination was carried out with haematoxylin and eosin staining. For immunostaining, sections were pre-treated with 3% H2O2 in methanol for 10 min. at room temperature to exhaust endogenous peroxidase activities and then blocked in PBS containing 1% normal serum and 1% Tween-20 (T-PBS) for 30 min. The following primary antibodies were used: mouse anti-αSMA antibody (1:400; Sigma-Aldrich, St. Louis, MO, USA); goat anti-PECAM1 (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Then sections were incubated with horseradish peroxidase-conjugated secondary antibodies (Vector Laboratories, Burlingame, CA, USA) diluted 1:200. Finally, the colour was developed with 0.1% 3,3%-diaminobenzidine. For immunofluorescence, sections were incubated with the following primary antibodies after blocking: mouse anti-CD34 (1:200; AbD Serotec) and goat anti-Ki67 (1:200; SantaCruz). Secondary antibodies were Cy3-conjugated anti-mouse IgG (1:200; Jackson ImmunoResearch, West Grove, PA, USA); biotinylated anti-goat IgG (1:200; Vector Laboratories, Burlingame, CA, USA) followed by streptavidin Alexa Fluor 488 (1:200; Molecular Probes, The Netherlands). All images were acquired using a DMI3000B Leica fluorescence microscope provided of a Leica DFC340FX camera (Leica Microsystems, Wetzlar, Germany).
Results
First of all, we examined the fragment biopsy of the aorta that was consistent with dissection of the ascending portion of the aorta, with morphological features suggestive of a chronic dissection (Fig. 2A). Then the tissue was processed for histological and immunohistochemical analyses. Haematoxylin and eosin staining allowed to reveal the comparable morphological characteristics of both primitive and ‘false’ lumina (Fig. 2B). To better characterize the structural components of the aortic walls, we performed an immunoistochemistry for PECAM-1, an antigen expressed by a completely differentiated endothelial cells [6]. As shown in Figure 2C, PECAM-1 was expressed by both primitive and ‘false’ lumina (indicated in figure by numbers 3 and 4, respectively), thus revealing a reendothelization of the dissected aortic walls. Moreover, staining with α-SMA, an antigen expressed by vascular smooth muscle cells, indicated that the thickness of muscular wall of the ‘false’ lumen was comparable to the one of the primitive lumen, as shown in Figure 2C. This result was fully supported by the virtual imaging reconstruction of in vivo analysis [3D 64-slice computed tomography (CT)] showing a comparable thickness of true (T) and false lumen (F) walls (Fig. 2D). Finally, because immunofluorescence for typical markers of cell proliferation, CD34 (specific for endothelial progenitor cells) and Ki67 (non-specific proliferation marker) showed no positivity, we reasoned that the vascular layers in the ‘false’ lumen should be completely differentiated (Fig. 2E). Overall our observations highlight three findings: (1) PECAM-1 positive staining show that endothelial cells line the aortic primitive lumen, as well as the ‘false’ one; (2) both in vivo imaging and immunohistochemical analysis demonstrate that walls of primitive and ‘false’ lumina are of comparable thickness and (3) finally, the absence of proliferation antigens and the positivity for markers of terminally differentiated cells in the vascular layers of the ‘false’ lumen are suggestive of a completed reparative process following the dissection.
Fig 2.
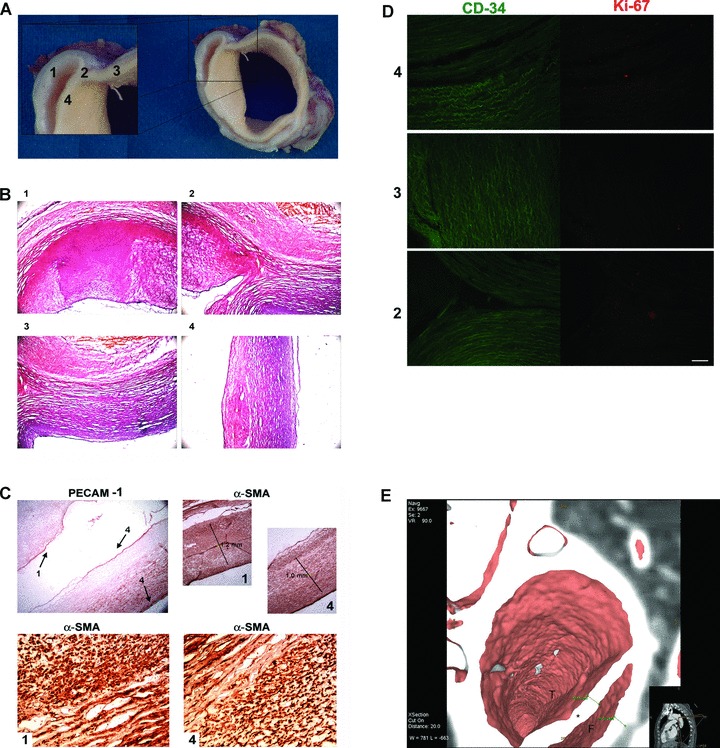
(A) Fragment biopsy examination. Photograph in A shows anatomical examination consistent with dissection of the ascending portion of the aorta. Numbers indicate walls of true lumen (3 and 4), the point of dissection (2) and wall of ‘false’ lumen (1) and will be used also in the following figures for identification of vessel segments. (B) Histological examination with haematoxylin and eosin staining showing comparable morphological characteristics of both true and ‘false’ lumina. Numbers indicate aortic segments shown in A. (C) Endothelial and smooth muscle stainings of aorta dissection. Pecam-1 staining (upper left) indicates the presence of an endothelial layer both in true lumen (4, black arrow) and in ‘false’ lumen (1 and 4, black arrows). α-SMA staining (upper right) shows muscle layers of approximative equal thickness in both vessels walls (‘false’ lumen in 1, true lumen in 4). In lower panels, a particular of α-SMA staining at greater magnification showing a comparable distribution of smooth muscle layers in the two vascular walls. (D) Assessment of proliferation markers: double staining for CD34/Ki67 shows no immunoreactivity, indicating no cell proliferation and differentiated aortic walls. (E) In vivo imaging: 3D 64-slice CT virtual dissection shows true lumen (T), false lumen (F) and dissection flap (*).
Discussion
The classification systems that also nowadays are adopted as guidelines for clinical practice in the treatment of aortic dissection, were formulated several years ago and were mainly based on anatomical and epidemiological data. This issue arouses important questions on the medical need to investigate at the molecular level the features of such a clinical problem. This sounds particularly important when considering cases of those asyntomatic patients that have passed the critical window of acute phase of aortic dissection, associated with high mortality and, in spite of all, they have to be surgically treated.
Our observation made on a single case of chronic Type A dissection indicates that a full aortic wall, complete of terminally differentiated endothelium and smooth muscle layers, was completely regenerated also in the ‘false’ lumen. In particular, when we analysed the expression of the endothelial antigen PECAM-1, we found that both primitive and ‘false’ lumina were endothelized. Moreover, analysis of α-SMA, an antigen expressed by the vascular smooth muscle cells, revealed that aortic walls around both lumina had comparable thickness. Furthermore, absence of positivity for the proliferation markers CD-34 and Ki67, suggests that the ‘regenerative’ process that could have allowed the reconstruction of well-structured aortic walls even in the ‘false’ lumen, should have been completed.
Although our results have been obtained on a single specimen and thus need to be confirmed on a wider sample, they prompt us to suggest a meditation concerning the ‘mandatory indication’ for surgical intervention in chronic Type A aortic dissection with a maximal ascending aortic diameter superior than 55 mm, opening the possibility to monitor over time such a clinical condition in patients free from signs and symptoms, without a connective tissue disorder. Our report is not intended to change/modify the conventional and well-established guidelines concerning surgical treatment of chronic Type A aortic dissection. However, our observation based on the assessment of molecular markers, which better define the architecture of such an artery dissection, suggests that patients surviving to an initial acute episode of Type A aortic dissection may have passed the ‘critical window’ and could be possibly at lower risk of death if correctly managed with medical therapy [7]. On this issue, it is noteworthy to quote that a molecular characterization of acute aortic dissections resulted useful in stratifying patients with suspected acute aortic dissection. To date, although there are no biochemical tests that can be reliably used to identify acute aortic dissection, biomarkers are available to rule out an appropriate diagnosis on algorithm of imaging investigations, and subsequently the surgical intervention [8–10].
Further analysis on a significant number of cases should be afforded to reinforce and sustain our hypothesis, which would be also a stimulus to search for novel approaches of in vivo molecular imaging (possibly exploiting the molecular markers described earlier). A better characterization of the clinical condition of ascending aortic dissection based on molecular markers, will be an unvaluable stepforward to identify patients that might benefit from a conservative approach as an alternative to immediate surgical therapy. Indeed, our findings indicate a completed repair process of the dissected aortic walls, as shown by the presence of molecular markers expressed by differentiated cells, at the time of the surgery that, probably, could have been not mandatory.
Acknowledgments
The authors thank Antonino Marullo, Mariangela Peruzzi, David Rose, Massimo Ricci and Giada Mascio for technical assistance.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Erbel R, Alfonso F, Boileau C, et al. Task Force on Aortic Dissection, European Society of Cardiology. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22:1642–81. doi: 10.1053/euhj.2001.2782. [DOI] [PubMed] [Google Scholar]
- 2.Golledge J, Eagle KA. Acute aortic dissection. Lancet. 2008;372:55–6. doi: 10.1016/S0140-6736(08)60994-0. [DOI] [PubMed] [Google Scholar]
- 3.Debakey ME, Henly WS, Cooley DA, et al. Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg. 1965;49:130–49. [PubMed] [Google Scholar]
- 4.Hiratzka LF, Bakris GL, Beckman JA, et al. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiolo-gists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55:e27–129. doi: 10.1016/j.jacc.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Scholl FG, Coady MA, Davies R, et al. Interval or permanent nonoperative management of acute type A aortic dissection. Arch Surg. 1999;134:402–5. doi: 10.1001/archsurg.134.4.402. [DOI] [PubMed] [Google Scholar]
- 6.Krenning G, van der Strate BW, Schipper M, et al. CD34+ cells augment endothelial cell differentiation of CD14+ endothelial progenitor cells in vitro. J Cell Mol Med. 2009;13:2521–33. doi: 10.1111/j.1582-4934.2008.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuda Y, Yamada Z, Morooka N, et al. Prognosis of patients with medically treated aortic dissections. Circulation. 1991;84:III7–3. [PubMed] [Google Scholar]
- 8.Suzukia T, Distanteb A, Eagle K. Biomarker-assisted diagnosis of acute aortic dissection: how far we have come and what to expect. Curr Opin Cardiol. 2010;25:541–5. doi: 10.1097/HCO.0b013e32833e6e13. [DOI] [PubMed] [Google Scholar]
- 9.Botta DM., Jr Biomarkers for diagnosis in thoracic aortic disease: PRO. Cardiol Clin. 2010;28:207–11. doi: 10.1016/j.ccl.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Suzukia T, Distante A, Zizza A, et al. Diagnosis of acute aortic dissection by D-dimer: the International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation. 2009;119:2702–7. doi: 10.1161/CIRCULATIONAHA.108.833004. [DOI] [PubMed] [Google Scholar]


