Abstract
Cardiovascular diseases remain an important cause of morbi-mortality. Atherosclerosis, which predisposes to cardiovascular disorders such as myocardial infarction and stroke, develops silently over several decades. Identification of circulating biomarkers to evaluate cardiovascular event risk and pathology prognosis is of particular importance. Microparticles (MPs) are small vesicles released from cells upon apoptosis or activation. Microparticles are present in blood of healthy individuals. Studies showing a modification of their concentrations in patients with cardiovascular risk factors and after cardiovascular events identify MPs as potential biomarkers of disease. Moreover, the pathophysiological properties of MPs may contribute to atherosclerosis development. In addition, pharmacological compounds, used in the treatment of cardiovascular disease, can reduce plasma MP concentrations. Nevertheless, numerous issues remain to be solved before MP measurement can be applied as routine biological tests to improve cardiovascular risk prediction. In particular, prospective studies to identify the predictive values of MPs in pathologies such as cardiovascular diseases are needed to demonstrate whether MPs are useful biomarkers for the early detection of the disease and its progression.
Keywords: microparticles, biomarkers, cardiovascular diseases
Introduction
Cardiovascular diseases remain an important cause of morbi-mortality. Atherosclerosis, which predisposes to cardiovascular disorders, is often accompanied by endothelial dysfunction and associated endothelium injury [1]. Endothelial cells produce chemoattractant factors, which induce monocyte recruitment and infiltration in the neo-intima. There, monocytes differentiate into macrophages and capture oxidized low-density lipoproteins (LDLs) infiltrated from the circulation to the neo-intima, leading to the formation of inflammatory foam cells. Pathology progresses with exacerbated macrophage accumulation, with smooth muscle cell proliferation and migration from the media to the intima, collagen production and lesion calcification. Finally, erosion and rupture of the plaque induce thrombus formation, causing arterial occlusion, culminating in the acute clinical event. Atherosclerosis develops silently for many years before clinical manifestations occur. However, early detection of atherosclerotic lesion formation may allow measures to prevent the progression of the pathology towards clinical events. As imaging techniques do not allow early detection and cannot be used routinely, identification of circulating biomarkers is of particular importance to predict cardiovascular disease risk and pathology prognosis [2].
Cell apoptosis, inflammatory activation and cellular stress in general [3] occurring during atherosclerosis development induce the formation of MPs. Microparticles are small vesicles, between 0.1 and 1 μm in diameter. They are present in plasma of healthy individuals and their concentrations change in various clinical conditions [4]. Microparticle concentrations are increased in patients with cardiovascular risk factors and after cardiovascular events. Moreover, certain pharmacological treatments, used to treat cardiovascular diseases, lower plasma MP concentrations. Consequently, MPs emerge as cardiovascular disease markers of particular interest. The pathophysiological effects of MPs in vivo are still poorly understood. However, studies performed with MPs generated in vitro from cell lines or isolated ex vivo have shown that they can influence various processes involved in atherogenesis, such as endothelial function, angiogenesis, inflammation and thrombosis, suggesting that MPs are not only markers, but also actors in cardiovascular diseases.
The purpose of this review is to report the current knowledge regarding the role of MPs in cardiovascular diseases. First, we will describe the mechanisms of MP formation, MP structure, and MP preparation and quantification. Subsequently, we will discuss qualitative and quantitative differences between MPs and their association with cardiovascular risk factors and diseases, as well as their pathophysiological effects in atherogenesis. Finally, we will discuss data showing MP regulation by pharmacological treatments.
Microparticle formation, structure and composition
Microparticle formation
During their lifespan, cells are submitted to stimuli which can trigger MP shedding, a physiological mechanism leading to the evasion of “eat-me” signals, hence preventing cell recognition and phagocytosis by macrophages. This process is exacerbated under conditions such as inflammation, apoptosis or other cellular stresses, which enhance MP production. Although the formation process of MPs remains to be completely elucidated, it is clear that MP formation and shedding involve reorganization of membrane phospholipid distribution, with outer leaflet exposure of phosphatidylserine (PS), and modification of the cell architecture, with the disruption of cytoskeleton organization. Several studies report the existence of vesicles which are similar in size as MPs but which do not expose PS in the outer leaflet [5-8]. The nature and the mechanisms of formation of these vesicles are poorly understood, but could be due to cytoskeleton cleavage with maintenance of the asymmetric phospholipid distribution in the plasma membrane.
The mechanisms involved in cellular MP release are summarized in Figure 1. In quiescent cells, the distribution of phospholipids in the bilayer is asymmetric, with aminophospholipids (PS, phosphatidylinositol) localized in the inner leaflet, whereas neutral phospholipids (phosphatidylcholine, phosphatidylethanolamine) are in the outer leaflet. This asymmetric distribution is under the control of three proteins. The aminophospholipid translocase (or flippase) is an ATP-dependent protein driving the transport of aminophospholipids from the outer to the inner leaflet. This transporter is inhibited by high levels of calcium [9]. The floppase is also an ATP-dependent protein, allowing transport of phospholipids from the inner to the outer leaflet [10]. Finally, the scramblase induces random movements of phospholipids inside the membrane and is activated by increased levels of calcium [11]. In the absence of stimuli, at normal calcium concentrations, only the flippase is active, allowing localization of aminophospholipids in the inner leaflet. Increased intracellular calcium following cell stimulation inhibits flippase, whereas it activates floppase and scramblase, inducing movement of aminophospholipids from the inner leaflet to the outer leaflet. The role of scramblase in this process has been illustrated in patients with Scott syndrome who have a mutation in a scramblase encoding gene, in whom vesiculation of cells is impaired inducing coagulation defects [12]. In addition to modifying the activity of proteins maintaining phospholipid bilayer asymmetry, the increase of intracellular calcium activates proteases, such as calpain, which trigger cytoskeleton reorganization and/or destruction. Involvement of calpain in platelet vesiculation has been shown by inhibition of this protein which decreases MP release [13]. Treatment with cytochalasin D, which inhibits actin polymerization, also decreases MP formation [14]. Caspases are also involved in MP release. In the Jurkat T cell line, caspase-3 mediates the cleavage of ROCK I, a Rho-kinase, leading to MP release during apoptosis [15]. In endothelial cells, thrombin induces a specific pathway of endothelial vesiculation involving nuclear factor (NF)-κB and TRAIL, leading to ROCK II activation by caspase 2 [16]. Thus, modifications of the cytoskeleton, associated with the accumulation of phospholipids in the outer leaflet, cause a membrane blebbing that leads to MP shedding from the cell.
Fig 1.
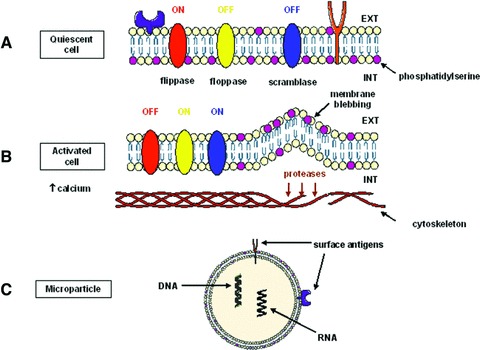
Schematic representation of the mechanisms of MP formation. (A) In quiescent cells, only flippase is active, allowing PS localization in the inner leaflet. (B) Increased calcium concentrations activate floppase and scramblase, and cytoskeleton reorganization. (C) MPs expose PS and contain proteins and nucleic acids from the cells of origin.
Various stimuli induce MP release from cells in vitro. Inflammatory cytokines (i.e. tumour necrosis factor [TNF]), apoptosis inducers (i.e. staurosporin), transcription blockers (i.e. actinomycin D) or cellular stress inducers (i.e. hydrogen peroxide, ultraviolet light or serum deprivation) induce MP generation in cell lines [17-20]. By contrast, little is known on the mediators and the mechanisms behind MP generation in vivo.
Microparticle composition and structure
Microparticles are constituted of material from their cells of origin, the exposure of PS on the external surface being a general characteristic of MPs.
Microparticles of different origins may differ in their phospholipid content. For example, the phospholipid composition of MPs isolated from blood of healthy donors consists, for the majority, of phosphatidylcholine (about 60%), sphingomyelin (∼20%), phosphatidylethanolamine (∼10%) and PS (∼5%) [21].
Microparticles could express inflammation markers, growth factors and metalloproteinases (MMP) [22]. Platelet and monocyte-derived MPs express tissue factor (TF) which together with the PS exposure plays a role in their pro-coagulant properties.
Initially, MPs were considered to be microvesicles free of nucleic acids [23]. However, recent studies have identified the presence of DNA and RNA in MPs generated in vitro from Jurkat and HL-60 cells [24, 25], and miRNA and pre-miRNA in MPs and exosomes from human mesenchymal cells and embryonic stem cells [26, 27].
Lipid, phospholipid and protein composition of MPs vary according to the cell origin, but interestingly, the stimulus of their formation can also modify MP composition. For example, endothelial cells submitted to apoptosis (by growth factor depletion) or activation by TNF in vitro release quantitatively and qualitatively distinct MPs [28]. Proteomic analysis has revealed that the spectrum of proteins found in MPs released in vitro from cultured cells depends at least in part on the stimulus used to induce their formation [29]. The identification of proteins at the MP surface allows cell origin determination. During blebbing, cell surface remodelling can lead to enrichment and/or elimination of various components from the cell. Therefore, MPs from the same cell origin can differ with respect to surface antigens, and some MPs may not express the surface antigens of the mother cell.
These components, phospholipids, proteins and, possibly, nucleic acids are responsible for the biological properties of MPs, as further described.
Microparticle isolation, preparation and measurement
The methods of preparation and measurement of MPs in biological samples vary among different research teams, and need standardization for MPs to become useful as disease biomarkers. We summarize here the main methods to prepare and quantify MPs. However, new methodologies of quantification of MPs are applied to this research field which have the potential to be used in the future (for review, see Ref. [30]).
Microparticle preparation
For MP quantification, cell-free samples need to be prepared before analysis. Microparticles can be measured in plasma, after removing platelets, and can be also isolated from tissues or cell culture supernatants.
Pre-analytical treatment of plasma
Generally, blood samples are collected on citrate, and kept at room temperature to avoid platelet lysis leading to subsequent errors in MP quantification. In the literature, only few reports address the needle size used for blood collection. Tushuizen et al. used a 19G needle [31]. Microparticles are analysed in platelet-free plasma (PFP) to avoid artefacts due to platelet activation. Therefore, blood is submitted to a first centrifugation at low speed (∼1500 χg) to eliminate red blood cells and leukocytes, and to a second centrifugation at high speed (∼13,000 χg) to eliminate platelets. Platelet-free plasma samples are rapidly frozen and stored at −80°C until analysis. However, certain authors consider the supernatant of whole blood centrifuged at low speed as PFP [32, 33].
The blood collection and sample processing methods have a strong impact on qualitative and quantitative MP analysis. The specific conditions used to analyse MPs in PFP vary considerably among research laboratories and standardization is crucial for MP assays to be useful for clinical diagnosis [34]. Pre-analytical variables relate to the venipuncture method by which the sample is obtained, the anti-coagulant used, the centrifugation speed to obtain PFP and finally whether the samples are analysed fresh or after freezing. Examples of protocols for plasma preparation before MP analysis in different cardiovascular clinical studies are summarized in Table 1.
Table 1.
Examples of blood processing methods used to prepare MPs before quantification
| Centrifugation conditions | Storage | MP quantification method | Reference |
|---|---|---|---|
| 1550 χ g, 20 min., 20°C | Liquid nitrogen frozen and storage at χ80°C | Flow cytometry | [31] |
| 1500 χ g, 15 min. + 12,000 χ g, 2 min. | Storage at −80°C | ELISA | [49] |
| 500 χ g, 15 min. + 9500 χ g, 5 min. | Storage at −80°C | Flow cytometry | [47] |
| 1500 χ g, 10 min. | Flow cytometry | [33] | |
| 1500 χ g, 15 min. + 13,000 χ g, 2 min., 20°C | Storage at −80°C | Flow cytometry | [50] |
| 1550 χ g, 20 min. + 2 χ 17,570 χ g, 30 min., 20°C | Liquid nitrogen frozen and storage at χ80°C | Flow cytometry | [51] |
| 1500 χ g, 20 min., 20°C | Storage at χ80°C | Flow cytometry | [32] |
| 1550 χ g, 20 min. + 2 χ 17,570 χ g, 30 min., 20°C | Liquid nitrogen frozen and storage at χ80°C | Flow cytometry | [54] |
| 160 χ g, 10 min. + 1000 χ g, 6 min. | Not communicated | Flow cytometry | [53] |
| 160 χ g, 10 χ min. + 1000 χ g, 8 min. | Not communicated | Flow cytometry | [56] |
| 13,000 χ g, 2 min. | Not communicated | Flow cytometry | [57] |
Microparticle isolation from tissues and fluids
In the cardiovascular system, MPs are present within the atherosclerotic plaques where they constitute the main reservoir for TF activity, promoting coagulation after plaque erosion and rupture [35]. Moreover, plaque MPs, harbouring proteolytic, angiogenic and inflammatory effectors, are potential actors in plaque vulnerability and lesion complications. Microparticles have been extracted from atherosclerotic plaques for qualitative and quantitative analysis. For these studies, MPs are extracted after tissue homogenization and centrifugation to eliminate tissular and cellular debris, followed by successive centrifugations to obtain MPs [35, 36]. Microparticles have also been isolated by successive centrifugation steps from synovial and vitreous fluid from patients with inflammatory arthritis or diabetes [37, 38]. In mice, MPs have also been isolated from ischaemic muscle, atherosclerotic plaques and liver after homogenization and successive centrifugations [39, 40].
Microparticle generation and isolation from cells in culture
To assess the pathophysiological properties of MPs in vitro, several studies have relied on the use of MPs obtained ex vivo from patient's blood. However, due to the limited amounts of MPs obtained ex vivo, many studies have used cells in culture treated with inflammatory or apoptotic stimuli to produce MPs in large quantities.
Cell lines display different sensitivities to apoptotic or inflammatory stimuli and thus differ in their ability to generate MPs. The most often used cell lines are human umbilical vein endothelial cells (HUVEC) and Jurkat cells (human lymphoid cells). Obviously, MPs can be produced and generated from all cell types. As previously mentioned (see section ‘Microparticle composition and structure’), numerous stimuli can be used to generate MP in vitro. In brief, MP-containing supernatant of treated cells is collected and centrifuged, once at low speed (∼1500 χg) to eliminate cells and once at high speed (∼20,000 χg) to pellet MPs. The MP pellet is recovered, generally in saline buffer solution, aliquoted and stored at −80°C before use. It is of importance to note that several authors used pellets obtained after centrifugation to 100,000 χg, a speed at which both MPs and exosomes (vesicles between 40 and 90 nm of diameter which do not bind annexin V) sediment [41]. Table 2 presents representative examples of protocols used to obtain MP preparations from cells in vitro.
Table 2.
Examples of methods used to prepare MPs from cell cultures
| Cell type | Stimuli and treatment time | Centrifugation | Reference |
|---|---|---|---|
| RMVECs | Serum depletion, 2 hrs | 5000 χg, 10 min. + 100,000 χg, 2 hrs | [58] |
| CEM T | Actinomycin D 0.5 μg/ml, 24 hrs | 750 χg, 15 min. + 1500 χg, 5 min. + 3 χ 14,000 χg, 45 min. | [60] |
| HUVEC | Serum depletion, 4 hrs | 2000 χg, 10 min. + 100,000 χg, 2 hrs | [65] |
| Human platelet aggregates | Thrombin, collagen and calcium ionophore A23187, 10 min. | 1500 χg, 15 min. + 3 χ 13,000 χg, 45 min. | [67] |
| Human platelet aggregates | Treatment of platelets with thrombin, 5 min. | 100,000 χg, 1 hr | [66] |
| Human platelet aggregates | Treatment of platelets with thrombin, 5 min. | 100,000 χg, 1 hr | [69] |
| Jurkat | Staurosporine, etoposide, actinomycin D, TNF-α 24 hrs or UV light 280 nm, 10 min. | 1500 χg, 5 min. + 3 χ 100,000 χg, 20 min. | [77] |
| HUVEC | TNF-α 100 ng/ml, 48 hrs | 4300 χg, 5 min. + 3 χ 20,000 χg, 2 hrs | [81] |
RMVECs: rat microvascular endothelial cells; CEM T: human T cell lymphoblast–like cell line; HUVEC: human umbilical vein endothelial cells; HUT-78: lymphoma cell line derived from peripheral blood; Jurkat: lymphoma cell line derived from peripheral blood.
Qualitative and quantitative MP analysis
Microparticles can be measured using various methods and combinations thereof. Unfortunately, the analysis protocols are not standardized yet, rendering quantitative and/or qualitative MP analysis reported by different research laboratories difficult to compare.
Flow cytometry
The usual criteria to detect and quantify MPs by flow cytometry include sizes lower than 1 μm, and PS positivity, which is detected by binding of fluorescent labelled annexin V. Other criteria can be included both for quantitative and qualitative analyses such as expression of antigens characteristic of the cell of origin. For each cell type, various markers can be used and the choice differs considerably among laboratories. Moreover, as discussed in section ‘Pre-analytical treatment of plasma’, antigen distribution can differ between cell surface and MP surface. The most commonly used antigens are listed in Table 3. These criteria provide information about the presence of antigens at the surface, but not on their functionality. For example, TF may be detected on MPs using specific antibodies, but this does not indicate whether this TF is functionally active.
Table 3.
Non-exhaustive list of antigen markers used for MP cell origin determination
| Cell type | Antigen | Reference |
|---|---|---|
| Platelets | CD41 | [97] |
| CD42a | [98] | |
| CD42b | [99] | |
| CD61 | ||
| Endothelial cells | CD31* | [100] |
| CD62E | [101] | |
| CD34 | [39] | |
| CD51* | [102] | |
| CD105* | [103] | |
| CD144 | ||
| Erythrocytes | CD235a | [104] |
| Leukocytes | CD45 | [105] |
| Monocytes | CD14 | [106] |
| Granulocytes | CD66b | [101] |
| T lymphocytes | CD4 | [107] |
| CD8 | [107] |
These markers are not specific for endothelial cells. CD31 is also expressed on platelets, CD51 on platelets and macrophages and CD105 in activated monocytes/macrophages. For endothelial MP detection, markers should be combined to discriminate this population from other MPs (for example, platelet MPs are CD31+/CD41+ whereas endothelial MPs are CD31+/CD41−).
One major limit of flow cytometry is the detection of very small size MPs (less than 0.5 μm), not always feasible with the cytometers currently used. Particles with size inferior to the wavelength of the laser light used for detection may be undetectable. Therefore, there has been recent interest to apply other physical methods allowing detection of MPs with a higher sensitivity, such as novel generation cytometers (NAVIOS, which allow detection of particles with sizes more than 300 nm, and Apogee A50, which allow detection of particles with sizes more than 100 nm) or atomic force microscopy. However, these novel technologies are still in their infancy and further studies are needed to develop a gold standard method providing analysis of concentrations, size distribution and cellular origin. Interestingly, a very recent study, using SYTO 13, a fluorescent dye binding nucleic acids, used nucleic acids as parameter to detect MPs, which allowed identification of MPs with sizes less than 200 nm [25].
Although it is commonly accepted that MPs expose PS, which is detected by annexin V labelling, some studies have identified vesicles expressing specific markers of cellular origin, in the size range of MPs but not binding to annexin V [5-8]. Existence of MPs which do not expose PS is under debate. If such vesicles are considered to be MPs, measurement techniques involving annexin V binding (flow cytometry and ELISA assays) may not allow quantification of total MPs.
ELISA assays
ELISA is another method used to detect MPs. A 96-well microplate is coated with annexin V or with an antibody of interest for MP detection. After washing, a mix containing prothrombin, factor Xa, factor Va and calcium is introduced in the wells. PS on the MP surface allows activation of prothrombin to thrombin in the presence of factor Xa and factor Va. The generated amount of thrombin is measured with a specific chromogenic substrate [42].
Another method involves TF exposure on MPs. After MP fixation on annexin V–coated plates and washing, MPs exposing TF are revealed using a TF antibody coupled with peroxidase [43].
The advantages of ELISA are, first, the analysis of small MPs, not detectable with flow cytometry, and, second, the possibility of higher throughput than with flow cytometry. However, a particular attention should be paid to the MP preparation method, because ELISA does not include size as a criterion of measurement. ELISA thus does not allow discrimination of MPs from contaminating cells, exosomes or apoptotic bodies. Moreover, this method does not assess the concentration of MPs, but only quantifies PS content in the sample.
Microparticles as markers of cardiovascular diseases
Microparticles are detectable in plasma of healthy individuals. A large variation in the number and/or the type of MPs in terms of cell origin is observed in several pathologies associated with inflammation. Among them, cardiovascular diseases are of particular interest, because MPs may constitute interesting disease biomarkers.
Several studies have shown an association between the Framingham risk score, used to predict cardiovascular disease risk, and plasma concentrations of MPs from diverse cellular origins (leukocytes, platelets and endothelial cells) [44-46]. Addition of endothelial-derived MPs as parameter to the Framingham risk score model improved the prediction power of future cardiovascular events [46].
Correlations between MP concentrations in plasma and lifestyle factors increasing cardiovascular risk have been reported in several studies. Ingestion of two consecutive high-fat meals, separated by 4 hrs, which impairs endothelial function, increased oxidative stress and MP concentrations in young healthy men [31]. Passive smoking also alters endothelial function and increases endothelial cell MP concentrations [47].
Microparticle concentrations are also associated with features of the metabolic syndrome. Patients with hypertension have elevated levels of endothelial, platelet and monocyte MPs as compared to normotensive patients [33, 48]. In these patients, endothelial MP concentrations correlate strongly with systolic and diastolic blood pressure [33]. Obese women, without specific cardiovascular risk factors, displayed increased plasma MP concentrations, which correlated positively with body mass index (BMI) [49]. MP concentrations are elevated in type 1 and type 2 diabetic patients, but these patients display different patterns of MPs. In type 1 diabetes, total, platelet- and endothelial-derived MPs, displaying enhanced pro-coagulant activity, are elevated and correlate with HbA(1c) levels [50]. In type 2 diabetic patients, total MP concentrations are increased, but these MPs do not show enhanced pro-coagulant activity. Another study found a correlation of TF-exposing MPs with BMI, glycaemia, insulinemia and an inverse correlation with HDL-cholesterol in type 2 diabetic patients [51].
Increased concentrations of leukocyte-derived MPs correlate with the inflammatory marker CRP and metabolic syndrome features as well as the presence of atherosclerotic plaques detected by ultrasound analysis in asymptomatic patients [52]. Increased levels of endothelial CD144+ MP were observed in type 2 diabetic patients with non-calcified plaques [32]. Several studies have shown an increase of endothelial and platelet MPs in patients with acute coronary syndrome [53-55] or venous embolism [56]. Patients with peripheral arterial disease have increased platelet-derived MPs exposing P-selectin [54]. Correlations between MP concentrations and clinical parameters have been observed, such as between MPs exposing CD31 and coronary artery endothelial function in patients with CAD [57]. These results identify MPs as promising markers of cardiovascular diseases. Finally, it has been reported that MPs of distinct origin than those found in blood are detectable in atherosclerotic lesions, suggesting a local cell production of specific MPs [35]. MPs are more abundant in atherosclerotic plaques than in the blood, and present a more pronounced prothrombogenic activity [35].
Microparticles as actors in cardiovascular diseases
Atherosclerosis development culminates in artery occlusion when plaques rupture and thrombi are formed. Microparticles display several properties that can contribute to vascular disease initiation, progression and its clinical complications. Most studies analysing the biological effects of MPs in atherogenesis and its complications have been performed in vitro, using MPs produced in vitro from cells treated with inflammatory or apoptotic stimuli, or isolated ex vivo from blood or atherosclerotic plaques. The properties of these MPs are pleiotropic, and we report here a non-exhaustive list of these effects. It is noteworthy that MP properties highly differ according to two parameters: the cell type of origin and the stimulus used to generate them.
Microparticles in endothelial function and angiogenesis
Microparticles have different effects on endothelial cell function and angiogenesis depending on their cell of origin. Microparticles isolated from patients with myocardial infarction, as well as a mixture of MPs and exosomes from rat renal microvascular endothelial cells (RMVEC) which bear the NADPH oxidase p22(phox) subunit, impair vasorelaxation of rat aortic rings, associated with a reduction of nitric oxide (NO) production and an increased of superoxide anion production [58, 59]. Microparticles produced by treatment of T lymphocytes with the apoptotic agent actinomycin D increase the production of reactive oxygen species and decrease NO synthesis in Eahy 926 endothelial cells. This occurs via the phosphorylation of extracellular signal–regulated kinases (ERK1/2) and phosphoinositide 3-kinase (PI3K) [60]. However, MPs produced by treatment of the same T cell line with phytohaemagglutinin, phorbol-12-myristate-13 acetate and actinomycin D bear sonic hedgehog, thus inducing NO production and enhancing vasorelaxation depending also on the PI3K/ERK pathway [61]. Microparticles isolated from metabolic syndrome patients reduce NO production and impair endothelium-dependent relaxation when injected intravenously in mice probably via decreased endothelial NO synthase expression, decreased expression of the NADPH oxidase p47(phox) subunit in MPs and increased expression of superoxide dismutase in endothelial cells [62].
Endothelial MPs produced in vitro from HUVEC inhibit human mitral valve endothelial cell proliferation by modulating growth factor fibroblast growth factor and vascular endothelial growth factor signalling, whereas they stimulate HUVEC proliferation [63]. Microparticles isolated from human atherosclerotic plaques also increase HUVEC proliferation, via interaction of CD40 ligand on MPs and CD40 on endothelial cells [64]. A mixture of MPs and exosomes from HUVEC inhibit angiogenesis, whereas a mixture of MPs and exosomes from platelets promote angiogenesis in an in vitro Matrigel test using HUVEC [65, 66]. Moreover, MPs from platelets promote angiogenesis ex vivo on rat aortic rings [67]. Circulating MPs from mice increase bone marrow-derived endothelial progenitor cell differentiation, pro-angiogenic gene expression in vitro and angiogenesis in vivo, in a peroxisome proliferator–activated receptor α (PPAR-α)– dependent manner [68].
Microparticles in inflammation
As inflammation is an important contributor to atherogenesis, the effects of MPs on inflammatory processes have been investigated in detail.
An important step of atherogenesis involves adhesion of circulating monocytes on the vascular endothelium. A mixture of MPs and exosomes from platelets increase endothelial cell ICAM-1 and the adhesion of monocytic U937 cells to HUVEC [69]. However, MPs released from polymorphonuclear leukocytes bear annexin I, which inhibits the interaction between leukocytes and endothelial cells, a potentially anti-atherogenic effect [70].
Several studies show a role for MPs in stimulating pro-inflammatory cytokine production in vitro by endothelial cells (IL-6, MCP-1, iNOS, COX-2), via activation of the JNK1 and NF-κB pathways [71, 72], and by monocytes (TNF, interleukin-1β (IL-1β) and interleukin-1 receptor antagonist (IL-1Ra)) [73]. Arachidonic acid released from platelet-derived MPs increases COX-2 and ICAM-1 expression in endothelial cells, and COX-2 expression in monocytes via induction of protein kinase C (PKC) translocation from the cytosol to the membrane [74, 75].
Aminophospholipids located on the MP membrane can also enhance inflammation. These aminophospholipids are substrates of phospholipase A2, which catalyses the synthesis of lysophosphatidic acid (LPA), a mediator of inflammation [76].
Finally, MPs produced from Jurkat cells enhance MP production by a mouse macrophage cell line, which may exacerbate the inflammatory process [77].
Microparticles in thrombosis
PS and TF exposed on the MP surface are potent promotors of blood coagulation. PS exposure allows the assembly of the prothrombinase and tenase complexes. Monocyte MPs are a source of active TF which promotes thrombin generation via factor VII in vitro[78]. These MPs can fuse with the platelet membrane, transferring TF to the surface of platelets thus allowing thrombin formation [79]. MPs from pericardial blood of cardiac surgery patients promote thrombogenesis in vivo in rats via their TF activity [80]. Moreover, MPs can promote coagulation not only by exposition of TF and PS, but also by modulation of TF expression, as shown in human monocytic THP-1 cells [81].
P-selectin seems to play a crucial role in the effect of MPs on thrombosis. Indeed, interaction between platelet P-selectin and its ligand P-selectin glycoprotein ligand 1 on MPs contributes to thrombus development in mice [82]. P-selectin can also exacerbate the coagulation process by inducing MP formation, as shown in transgenic mice expressing high levels of soluble P-selectin which display elevated blood MP concentrations [83].
In conclusion, the roles of MPs in coagulation are multiple. By these properties, they can exacerbate thrombosis, and potentially precipitate the clinical complications of atherosclerosis.
Pharmacological modulation of plasma MP concentrations
Microparticles concentrations are elevated in patients with cardiovascular risk factors and disease. Moreover, MPs may actively modulate the process of atherogenesis. Therefore, measurement of MPs may be of pharmacological interest as activity markers in early clinical development or as efficacy markers in cardiovascular risk patients. A number of studies have reported the effect of certain drugs, used to decrease CV risk, on MP concentrations, suggesting that the beneficial effects of these drugs could, at least in part, be mediated via a reduction of MP concentrations.
Statins
Statins are hypolipidaemic drugs, which decrease plasma cholesterol concentrations (especially cholesterol in the highly atherogenic LDL fraction) by inhibiting HMG CoA reductase, the rate-limiting enzyme of cholesterol synthesis. Numerous clinical studies have shown beneficial effects of statins in cardiovascular disease prevention, not only by reducing cholesterol, but possibly also via their effects on endothelial function, vascular inflammation and platelet aggregation (for review, see Ref. [84]). Several studies show that statins reduce MP concentrations or modify their composition.
Association of simvastatin with losartan, an angiotensin II receptor antagonist used in the treatment of hypertension, decreased monocyte-, endothelial- and platelet-MP concentrations in patients with hypertension and type 2 diabetes [85, 86]. Pravastatin treatment for 8 weeks reduced fibrinogen receptor gpIIIa on platelet MPs in type 2 diabetes patients [87]. This receptor for fibrinogen plays an important role in thrombus formation and is present in platelet MPs, where its concentration depends on the stimulus which has induced MP formation. Reduction of this receptor on MPs could decrease thrombus formation and contribute to decreased cardiovascular events in diabetic patients. In patients displaying hyperlipidaemia and type 2 diabetes, pitavastatin alone did not reduce platelet MP concentrations in plasma. However, association with eicosapentaenoic acid (EPA), a fatty acid with anti-inflammatory effects [88], reduced platelet MPs to a larger extent than EPA alone [89]. Atorvastatin treatment (2 months) of patients with type 1 diabetes and dyslipidaemia reduced gpIIIa-, P-selectin- and TF-containing MPs [90].
These in vivo lowering effects of statins on circulating MPs could be, at least in part, explained by their anti-inflammatory properties. Moreover, it has been shown that fluvastatin prevents TNF-induced MP production by human coronary artery endothelial cells, by inhibiting the Rho kinase pathway which is involved in cytoskeleton reorganization and thus in MP formation [91].
Anti-oxidants
Oxidative stress triggers numerous deleterious processes in atherosclerosis, leading to endothelial dysfunction and platelet activation. A reduction of circulating MP levels by anti-oxidant treatment could reflect an improvement of endothelial function and a reduction of platelet activation. Interestingly, one study analysing the effects of treatment with vitamin C for 5 days has reported a decrease of endothelial and platelet MPs in diabetic and dyslipidaemic patients [92].
Peroxisome proliferator–activated receptor activators
Peroxisome proliferator–activated receptors are ligand-activated nuclear receptors regulating the expression of genes implicated in lipid and glucose metabolism, and inflammation. PPAR-α and PPAR-γ agonists are used in clinical practice to improve dyslipidaemia and type 2 diabetes, respectively [93, 94]. The PPAR-γ agonist pioglitazone reduced circulating endothelial MPs in patients with metabolic syndrome, independent of its effects on insulin sensitivity [95]. Another study reported that treatment with bezafibrate, a PPAR-pan agonist, which lowers plasma lipid concentrations and coagulation parameters such as fibrinogen, reduced platelet-derived MPs in patients with connective tissue diseases and secondary hyperlipidaemia caused by steroid treatment [96]. Pharmacological regulation of MPs has been studied mostly in human beings. Very recently, we used the apoE2KI mouse model of atherosclerosis as a preclinical pharmacological model to test the effects of PPAR-α activation on MP concentrations [40]. Our results showed that fenofibrate decreases MPs in atherosclerotic lesion as well as circulating MPs in a PPAR-α–dependent manner. This effect could be due to the anti-inflammatory properties of fenofibrate, which could decrease MP production.
Conclusion
This review summarized the current knowledge identifying cell-derived MPs as actors and/or markers in cardiovascular diseases. It is now increasingly admitted that MPs may contribute to the initiation, progression and clinical complications of this pathology. Further studies are needed to better delineate the pathophysiological properties of MPs. Second, MPs appear interesting biomarkers to predict cardiovascular disease risk. Nevertheless, numerous issues remain to be addressed before MP measurement can be applied as routine biological tests to improve cardiovascular risk prediction of patients, and the efficacy of pharmacological interventions. A biomarker test needs to comply different requirements, such as (1) facility to obtain patient samples in which the biomarker can be easily measured, (2) existence of a sensitive and reproducible assay involving a methodology applicable in numerous laboratories, with a cost per assay compatible with large-scale application, (3) providing additional information on the cardiovascular risk over the known risk factors (i.e. age, sex, LDL-cholesterol, etc.) or identifying patients at risk earlier than with the classical risk factors. Prospective studies showing the predictive value of MPs in pathologies, such as cardiovascular diseases, are needed to demonstrate whether these biomarkers will be useful in the early detection of disease and its progression.
To conclude, MPs can be considered as actors in atherosclerosis, and may become potentially interesting biomarkers in the future pending technological developments allowing easy and reproducible measurement.
Conflict of interest
The authors confirm that there is no conflict of interest.
References
- 1.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–41. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochholzer W, Morrow DA, Giugliano RP. Novel biomarkers in cardiovascular disease: update 2010. Am Heart J. 2010;160:583–94. doi: 10.1016/j.ahj.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension. 2006;48:180–6. doi: 10.1161/01.HYP.0000231507.00962.b5. [DOI] [PubMed] [Google Scholar]
- 4.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157–71. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Connor DE, Exner T, Ma D, et al. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb Haemost. 2010;103:1044–52. doi: 10.1160/TH09-09-0644. [DOI] [PubMed] [Google Scholar]
- 6.Sekula M, Janawa G, Stankiewicz E, et al. Endothelial microparticle formation in moderate concentrations of homocysteine and methionine in vitro. Cell Mol Biol Lett. 2011;16:69–78. doi: 10.2478/s11658-010-0040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macey MG, Enniks N, Bevan S. Flow cytometric analysis of microparticle phenotype and their role in thrombin generation. Cytometry B Clin Cytom. 2011;80:57–63. doi: 10.1002/cyto.b.20551. [DOI] [PubMed] [Google Scholar]
- 8.Amabile N, Gurin AP, Leroyer A, et al. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–8. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 9.Beleznay Z, Zachowski A, Devaux PF, et al. ATP-dependent aminophospholipid translocation in erythrocyte vesicles: stoichiometry of transport. Biochemistry. 1993;32:3146–52. doi: 10.1021/bi00063a029. [DOI] [PubMed] [Google Scholar]
- 10.Connor J, Pak CH, Zwaal RF, et al. Bidirectional transbilayer movement of phospholipid analogs in human red blood cells. Evidence for an ATP-dependent and protein-mediated process. J Biol Chem. 1992;267:19412–7. [PubMed] [Google Scholar]
- 11.Zwaal RF, Comfurius P, Bevers EM. Mechanism and function of changes in membrane-phospholipid asymmetry in platelets and erythrocytes. Biochem Soc Trans. 1993;21:248–53. doi: 10.1042/bst0210248. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki J, Umeda M, Sims PJ, et al. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468:834–8. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- 13.Shcherbina A, Remold-O'Donnell E. Role of caspase in a subset of human platelet activation responses. Blood. 1999;93:4222–31. [PubMed] [Google Scholar]
- 14.Dachary-Prigent J, Pasquet JM, Freyssinet JM, et al. Calcium involvement in aminophospholipid exposure and microparticle formation during platelet activation: a study using Ca2+-ATPase inhibitors. Biochemistry. 1995;34:11625–34. doi: 10.1021/bi00036a039. [DOI] [PubMed] [Google Scholar]
- 15.Sebbagh M, Renvoizé C, Hamelin J, et al. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–52. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- 16.Sapet C, Simoncini S, Loriod B, et al. Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood. 2006;108:1868–76. doi: 10.1182/blood-2006-04-014175. [DOI] [PubMed] [Google Scholar]
- 17.Pirro M, Schillaci G, Bagaglia F, et al. Microparticles derived from endothelial progenitor cells in patients at different cardiovascular risk. Atherosclerosis. 2008;197:757–67. doi: 10.1016/j.atherosclerosis.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Ullal AJ, Pisetsky DS. The release of microparticles by Jurkat leukemia T cells treated with staurosporine and related kinase inhibitors to induce apoptosis. Apoptosis. 2010;15:586–96. doi: 10.1007/s10495-010-0470-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolowos W, Gaipl US, Sheriff A, et al. Microparticles shed from different antigen-presenting cells display an individual pattern of surface molecules and a distinct potential of allogeneic T-cell activation. Scand J Immunol. 2005;61:226–33. doi: 10.1111/j.1365-3083.2005.01551.x. [DOI] [PubMed] [Google Scholar]
- 20.Martin S, Tesse A, Hugel B, et al. Shed membrane particles from T lymphocytes impair endothelial function and regulate endothelial protein expression. Circulation. 2004;109:1653–9. doi: 10.1161/01.CIR.0000124065.31211.6E. [DOI] [PubMed] [Google Scholar]
- 21.Weerheim AM, Kolb AM, Sturk A, et al. Phospholipid composition of cell-derived microparticles determined by one-dimensional high-performance thin-layer chromatography. Anal Biochem. 2002;302:191–8. doi: 10.1006/abio.2001.5552. [DOI] [PubMed] [Google Scholar]
- 22.Taraboletti G, D'Ascenzo S, Borsotti P, et al. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160:673–80. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simak J, Gelderman MP. Cell membrane microparticles in blood and blood products: potentially pathogenic agents and diagnostic markers. Transfus Med Rev. 2006;20:1–26. doi: 10.1016/j.tmrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Reich C, Pisetsky DS. The content of DNA and RNA in microparticles released by Jurkat and HL-60 cells undergoing in vitro apoptosis. Exp Cell Res. 2009;315:760–8. doi: 10.1016/j.yexcr.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Ullal AJ, Pisetsky DS, Reich C. Use of SYTO 13, a fluorescent dye binding nucleic acids, for the detection of microparticles in in vitro systems. Cytometry A. 2010;77:294–301. doi: 10.1002/cyto.a.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen TS, Lai RC, Lee MM, et al. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–24. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan A, Farber EL, Rapoport AL, et al. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS ONE. 2009;4:e4722–8. doi: 10.1371/journal.pone.0004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jimenez JJ, Jy W, Mauro LM, et al. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res. 2003;109:175–80. doi: 10.1016/s0049-3848(03)00064-1. [DOI] [PubMed] [Google Scholar]
- 29.Miguet L, Pacaud K, Felden C, et al. Proteomic analysis of malignant lymphocyte membrane microparticles using double ionization coverage optimization. Proteomics. 2006;6:153–71. doi: 10.1002/pmic.200500133. [DOI] [PubMed] [Google Scholar]
- 30.Yuana Y, Bertina RM, Osanto S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb Haemost. 2011;105:396–408. doi: 10.1160/TH10-09-0595. [DOI] [PubMed] [Google Scholar]
- 31.Tushuizen ME, Nieuwland R, Scheffer PG, et al. Two consecutive high-fat meals affect endothelial-dependent vasodilation, oxidative stress and cellular microparticles in healthy men. J Thromb Haemost. 2006;4:1003–10. doi: 10.1111/j.1538-7836.2006.01914.x. [DOI] [PubMed] [Google Scholar]
- 32.Bernard S, Loffroy R, Sérusclat A, et al. Increased levels of endothelial microparticles CD144 (VE-Cadherin) positives in type 2 diabetic patients with coronary noncalcified plaques evaluated by multidetector computed tomography (MDCT) Atherosclerosis. 2009;203:429–35. doi: 10.1016/j.atherosclerosis.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 33.Preston RA, Jy W, Jimenez JJ, et al. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension. 2003;41:211–7. doi: 10.1161/01.hyp.0000049760.15764.2d. [DOI] [PubMed] [Google Scholar]
- 34.Lacroix R, Robert S, Poncelet P, et al. Overcoming limitations of microparticle measurement by flow cytometry. Semin Thromb Hemost. 2010;36:807–18. doi: 10.1055/s-0030-1267034. [DOI] [PubMed] [Google Scholar]
- 35.Leroyer AS, Isobe H, Lesèche G, et al. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J Am Coll Cardiol. 2007;49:772–7. doi: 10.1016/j.jacc.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 36.Mayr M, Grainger D, Mayr U, et al. Proteomics, metabolomics, and immunomics on microparticles derived from human atherosclerotic plaques. Circ Cardiovasc Genet. 2009;2:379–88. doi: 10.1161/CIRCGENETICS.108.842849. [DOI] [PubMed] [Google Scholar]
- 37.Chahed S, Leroyer AS, Benzerroug M, et al. Increased vitreous shedding of microparticles in proliferative diabetic retinopathy stimulates endothelial proliferation. Diabetes. 2010;59:694–701. doi: 10.2337/db08-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berckmans RJ, Nieuwland R, Tak PP, et al. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum. 2002;46:2857–66. doi: 10.1002/art.10587. [DOI] [PubMed] [Google Scholar]
- 39.Leroyer AS, Ebrahimian TG, Cochain C, et al. Microparticles from ischemic muscle promotes postnatal vasculogenesis. Circulation. 2009;119:2808–17. doi: 10.1161/CIRCULATIONAHA.108.816710. [DOI] [PubMed] [Google Scholar]
- 40.Baron M, Leroyer AS, Majd Z, et al. PPARα activation differently affects microparticle content in atherosclerotic lesions and liver of a mouse model of atherosclerosis and NASH. Atherosclerosis. 2011;218:69–76. doi: 10.1016/j.atherosclerosis.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107:1047–57. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 42.Messer L, Alsaleh G, Freyssinet J, et al. Microparticle-induced release of B-lymphocyte regulators by rheumatoid synoviocytes. Arthritis Res Ther. 2009;11:R40–10. doi: 10.1186/ar2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee RD, Barcel DA, Williams JC, et al. Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity. Thromb Res. 2011 doi: 10.1016/j.thromres.2011.06.004. ; in press: doi:10.1016/j.thromres.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ueba T, Haze T, Sugiyama M, et al. Level, distribution and correlates of platelet-derived microparticles in healthy individuals with special reference to the metabolic syndrome. Thromb Haemost. 2008;100:280–5. [PubMed] [Google Scholar]
- 45.Chironi G, Simon A, Hugel B, et al. Circulating leukocyte-derived microparticles predict subclinical atherosclerosis burden in asymptomatic subjects. Arterioscler Thromb Vasc Biol. 2006;26:2775–80. doi: 10.1161/01.ATV.0000249639.36915.04. [DOI] [PubMed] [Google Scholar]
- 46.Nozaki T, Sugiyama S, Koga H, et al. Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart disease. J Am Coll Cardiol. 2009;54:601–8. doi: 10.1016/j.jacc.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 47.Heiss C, Amabile N, Lee AC, et al. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J Am Coll Cardiol. 2008;51:1760–71. doi: 10.1016/j.jacc.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 48.Nomura S, Kanazawa S, Fukuhara S. Effects of efonidipine on platelet and monocyte activation markers in hypertensive patients with and without type 2 diabetes mellitus. J Hum Hypertens. 2002;16:539–47. doi: 10.1038/sj.jhh.1001447. [DOI] [PubMed] [Google Scholar]
- 49.Goichot B, Grunebaum L, Desprez D, et al. Circulating procoagulant microparticles in obesity. Diabetes Metab. 2006;32:82–5. doi: 10.1016/s1262-3636(07)70251-3. [DOI] [PubMed] [Google Scholar]
- 50.Sabatier F, Darmon P, Hugel B, et al. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes. 2002;51:2840–5. doi: 10.2337/diabetes.51.9.2840. [DOI] [PubMed] [Google Scholar]
- 51.Diamant M, Nieuwland R, Pablo RF, et al. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation. 2002;106:2442–7. doi: 10.1161/01.cir.0000036596.59665.c6. [DOI] [PubMed] [Google Scholar]
- 52.Chironi GN, Simon A, Boulanger CM, et al. Circulating microparticles may influence early carotid artery remodeling. J Hypertens. 2010;28:789–96. doi: 10.1097/HJH.0b013e328335d0a8. [DOI] [PubMed] [Google Scholar]
- 53.Bernal-Mizrachi L, Jy W, Jimenez JJ, et al. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J. 2003;145:962–70. doi: 10.1016/S0002-8703(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 54.Van der Zee PM, Biró E, Ko Y, et al. P-selectin- and CD63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarction. Clin Chem. 2006;52:657–64. doi: 10.1373/clinchem.2005.057414. [DOI] [PubMed] [Google Scholar]
- 55.Mallat Z, Benamer H, Hugel B, et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–3. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- 56.Chirinos JA, Heresi GA, Velasquez H, et al. Elevation of endothelial microparticles, platelets, and leukocyte activation in patients with venous thromboembolism. J Am Coll Cardiol. 2005;45:1467–71. doi: 10.1016/j.jacc.2004.12.075. [DOI] [PubMed] [Google Scholar]
- 57.Werner N, Wassmann S, Ahlers P, et al. Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:112–6. doi: 10.1161/01.ATV.0000191634.13057.15. [DOI] [PubMed] [Google Scholar]
- 58.Brodsky SV, Zhang F, Nasjletti A, et al. Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Heart Circ Physiol. 2004;286:H1910–5. doi: 10.1152/ajpheart.01172.2003. [DOI] [PubMed] [Google Scholar]
- 59.Boulanger CM, Scoazec A, Ebrahimian T, et al. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation. 2001;104:2649–52. doi: 10.1161/hc4701.100516. [DOI] [PubMed] [Google Scholar]
- 60.Mostefai HA, Agouni A, Carusio N, et al. Phosphatidylinositol 3-kinase and xanthine oxidase regulate nitric oxide and reactive oxygen species productions by apoptotic lymphocyte microparticles in endothelial cells. J Immunol. 2008;180:5028–35. doi: 10.4049/jimmunol.180.7.5028. [DOI] [PubMed] [Google Scholar]
- 61.Agouni A, Mostefai HA, Porro C, et al. Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. FASEB J. 2007;21:2735–41. doi: 10.1096/fj.07-8079com. [DOI] [PubMed] [Google Scholar]
- 62.Agouni A, Lagrue-Lak-Hal AH, Ducluzeau PH, et al. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am J Pathol. 2008;173:1210–9. doi: 10.2353/ajpath.2008.080228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klinkner DB, Densmore JC, Kaul S, et al. Endothelium-derived microparticles inhibit human cardiac valve endothelial cell function. Shock. 2006;25:575–80. doi: 10.1097/01.shk.0000209558.69575.80. [DOI] [PubMed] [Google Scholar]
- 64.Leroyer AS, Rautou P, Silvestre J, et al. CD40 ligand + microparticles from human atherosclerotic plaques stimulate endothelial proliferation and angiogenesis a potential mechanism for intraplaque neovascularization. J Am Coll Cardiol. 2008;52:1302–11. doi: 10.1016/j.jacc.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 65.Mezentsev A, Merks R, O'Riordan E, et al. Endothelial microparticles affect angiogenesis in vitro: role of oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:H1106–14. doi: 10.1152/ajpheart.00265.2005. [DOI] [PubMed] [Google Scholar]
- 66.Brill A, Dashevsky O, Rivo J, et al. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67:30–8. doi: 10.1016/j.cardiores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 67.Kim HK, Song KS, Chung J, et al. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004;124:376–84. doi: 10.1046/j.1365-2141.2003.04773.x. [DOI] [PubMed] [Google Scholar]
- 68.Benameur T, Tual-Chalot S, Andriantsitohaina R, et al. PPARalpha is essential for microparticle-induced differentiation of mouse bone marrow-derived endothelial progenitor cells and angiogenesis. PLoS ONE. 2010;5:e12392–12. doi: 10.1371/journal.pone.0012392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barry OP, Praticò D, Savani RC, et al. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest. 1998;102:136–44. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dalli J, Norling LV, Renshaw D, et al. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008;112:2512–9. doi: 10.1182/blood-2008-02-140533. [DOI] [PubMed] [Google Scholar]
- 71.Mesri M, Altieri DC. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J Biol Chem. 1999;274:23111–8. doi: 10.1074/jbc.274.33.23111. [DOI] [PubMed] [Google Scholar]
- 72.Tesse A, Martnez MC, Hugel B, et al. Upregulation of proinflammatory proteins through NF-kappaB pathway by shed membrane microparticles results in vascular hyporeactivity. Arterioscler Thromb Vasc Biol. 2005;25:2522–7. doi: 10.1161/01.ATV.0000189298.62240.5d. [DOI] [PubMed] [Google Scholar]
- 73.Scanu A, Molnarfi N, Brandt KJ, et al. Stimulated T cells generate microparticles, which mimic cellular contact activation of human monocytes: differential regulation of pro- and anti-inflammatory cytokine production by high-density lipoproteins. J Leukoc Biol. 2008;83:921–7. doi: 10.1189/jlb.0807551. [DOI] [PubMed] [Google Scholar]
- 74.Barry OP, Kazanietz MG, Praticò D, et al. Arachidonic acid in platelet microparticles up-regulates cyclooxygenase-2-dependent prostaglandin formation via a protein kinase C/mitogen-activated protein kinase-dependent pathway. J Biol Chem. 1999;274:7545–56. doi: 10.1074/jbc.274.11.7545. [DOI] [PubMed] [Google Scholar]
- 75.Barry OP, Pratico D, Lawson JA, et al. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J Clin Invest. 1997;99:2118–27. doi: 10.1172/JCI119385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gräler MH, Goetzl EJ. Lysophospholipids and their G protein-coupled receptors in inflammation and immunity. Biochim Biophys Acta. 2002;1582:168–74. doi: 10.1016/s1388-1981(02)00152-x. [DOI] [PubMed] [Google Scholar]
- 77.Distler J, Huber LC, Hueber AJ, et al. The release of microparticles by apoptotic cells and their effects on macrophages. Apoptosis. 2005;10:731–41. doi: 10.1007/s10495-005-2941-5. [DOI] [PubMed] [Google Scholar]
- 78.Sturk-Maquelin KN, Nieuwland R, Romijn F, et al. Pro- and non-coagulant forms of non-cell-bound tissue factor in vivo. J Thromb Haemost. 2003;1:1920–6. doi: 10.1046/j.1538-7836.2003.00361.x. [DOI] [PubMed] [Google Scholar]
- 79.Scholz T, Temmler U, Krause S, et al. Transfer of tissue factor from platelets to monocytes: role of platelet-derived microvesicles and CD62P. Thromb Haemost. 2002;88:1033–8. [PubMed] [Google Scholar]
- 80.Biró E, Sturk-Maquelin KN, Vogel G, et al. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemost. 2003;1:2561–8. doi: 10.1046/j.1538-7836.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 81.Sabatier F, Roux V, Anfosso F, et al. Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood. 2002;99:3962–70. doi: 10.1182/blood.v99.11.3962. [DOI] [PubMed] [Google Scholar]
- 82.Falati S, Liu Q, Gross P, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197:1585–98. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.André P, Hartwell D, Hrachovinová I, et al. Pro-coagulant state resulting from high levels of soluble P-selectin in blood. Proc Natl Acad Sci USA. 2000;97:13835–40. doi: 10.1073/pnas.250475997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Devaraj S, Rogers J, Jialal I. Statins and biomarkers of inflammation. Curr Atheroscler Rep. 2007;9:33–41. doi: 10.1007/BF02693938. [DOI] [PubMed] [Google Scholar]
- 85.Nomura S, Shouzu A, Omoto S, et al. Losartan and simvastatin inhibit platelet activation in hypertensive patients. J Thromb Thrombolysis. 2004;18:177–85. doi: 10.1007/s11239-005-0343-8. [DOI] [PubMed] [Google Scholar]
- 86.Nomura S, Shouzu A, Omoto S, et al. Effects of losartan and simvastatin on monocyte-derived microparticles in hypertensive patients with and without type 2 diabetes mellitus. Clin Appl Thromb Hemost. 2004;10:133–41. doi: 10.1177/107602960401000203. [DOI] [PubMed] [Google Scholar]
- 87.Sommeijer DW, Joop K, Leyte A, et al. Pravastatin reduces fibrinogen receptor gpIIIa on platelet-derived microparticles in patients with type 2 diabetes. J Thromb Haemost. 2005;3:1168–71. doi: 10.1111/j.1538-7836.2005.01403.x. [DOI] [PubMed] [Google Scholar]
- 88.Wall R, Ross RP, Fitzgerald GF, et al. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–9. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 89.Nomura S, Inami N, Shouzu A, et al. The effects of pitavastatin, eicosapentaenoic acid and combined therapy on platelet-derived microparticles and adiponectin in hyperlipidemic, diabetic patients. Platelets. 2009;20:16–22. doi: 10.1080/09537100802409921. [DOI] [PubMed] [Google Scholar]
- 90.Tehrani S, Mobarrez F, Antovic A, et al. Atorvastatin has antithrombotic effects in patients with type 1 diabetes and dyslipidemia. Thromb Res. 2010;126:e225–31. doi: 10.1016/j.thromres.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 91.Tramontano AF, O'Leary J, Black AD, et al. Statin decreases endothelial microparticle release from human coronary artery endothelial cells: implication for the Rho-kinase pathway. Biochem Biophys Res Commun. 2004;320:34–8. doi: 10.1016/j.bbrc.2004.05.127. [DOI] [PubMed] [Google Scholar]
- 92.Morel O, Jesel L, Hugel B, et al. Protective effects of vitamin C on endothelium damage and platelet activation during myocardial infarction in patients with sustained generation of circulating microparticles. J Thromb Haemost. 2003;1:171–7. doi: 10.1046/j.1538-7836.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 93.Lefebvre P, Chinetti G, Fruchart J, et al. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116:571–80. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fiévet C, Staels B. Efficacy of peroxisome proliferator-activated receptor agonists in diabetes and coronary artery disease. Curr Atheroscler Rep. 2009;11:281–8. doi: 10.1007/s11883-009-0043-5. [DOI] [PubMed] [Google Scholar]
- 95.Esposito K, Ciotola M, Giugliano D. Pioglitazone reduces endothelial microparticles in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26 doi: 10.1161/01.ATV.0000231512.15115.25. [DOI] [PubMed] [Google Scholar]
- 96.Kagawa H, Nomura S, Nagahama M, et al. Effect of bezafibrate on soluble adhesion molecules and platelet activation markers in patients with connective tissue diseases and secondary hyperlipidemia. Clin Appl Thromb Hemost. 2001;7:153–7. doi: 10.1177/107602960100700213. [DOI] [PubMed] [Google Scholar]
- 97.Shet AS, Aras O, Gupta K, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–83. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 98.Nieuwland R, Berckmans RJ, McGregor S, et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95:930–5. [PubMed] [Google Scholar]
- 99.Feng B, Chen Y, Luo Y, et al. Circulating level of microparticles and their correlation with arterial elasticity and endothelium-dependent dilation in patients with type 2 diabetes mellitus. Atherosclerosis. 2010;208:264–9. doi: 10.1016/j.atherosclerosis.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 100.Bulut D, Tns H, Mgge A. CD31+/Annexin V+ microparticles in healthy offsprings of patients with coronary artery disease. Eur J Clin Invest. 2009;39:17–22. doi: 10.1111/j.1365-2362.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- 101.Joop K, Berckmans RJ, Nieuwland R, et al. Microparticles from patients with multiple organ dysfunction syndrome and sepsis support coagulation through multiple mechanisms. Thromb Haemost. 2001;85:810–20. [PubMed] [Google Scholar]
- 102.Arteaga RB, Chirinos JA, Soriano AO, et al. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol. 2006;98:70–4. doi: 10.1016/j.amjcard.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 103.Simak J, Holada K, Risitano AM, et al. Elevated circulating endothelial membrane microparticles in paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2004;125:804–13. doi: 10.1111/j.1365-2141.2004.04974.x. [DOI] [PubMed] [Google Scholar]
- 104.Van Beers EJ, Schaap M, Berckmans RJ, et al. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica. 2009;94:1513–9. doi: 10.3324/haematol.2009.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Enjeti AK, Lincz LF, Scorgie FE, et al. Circulating microparticles are elevated in carriers of Factor V Leiden. Thromb Res. 2010;126:250–3. doi: 10.1016/j.thromres.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 106.Aharon A, Tamari T, Brenner B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb Haemost. 2008;100:878–85. doi: 10.1160/th07-11-0691. [DOI] [PubMed] [Google Scholar]
- 107.Gris JC, Toulon P, Brun S, et al. The relationship between plasma microparticles, protein S and anticardiolipin antibodies in patients with human immunodeficiency virus infection. Thromb Haemost. 1996;76:38–45. [PubMed] [Google Scholar]


