Abstract
Mucin glycoproteins are major secreted or membrane-bound molecules that, in cancer, show modifications in both the mucin proteins expression and in the O-glycosylation profile, generating some of the most relevant tumour markers in clinical use for decades. Thus far, the identification of these biomarkers has been based on the detection of either the protein or the O-glycan modifications. We therefore aimed to identify the combined mucin and O-glycan features, that is, specific glycoforms, in an attempt to increase specificity of these cancer biomarkers. Using in situ proximity ligation assays (PLA) based on existing monoclonal antibodies directed to MUC1, MUC2, MUC5AC and MUC6 mucins and to cancer-associated carbohydrate antigens Tn, Sialyl-Tn (STn), T, Sialyl-Lea (SLea) and Sialyl-Lex (SLex) we screened a series of 28 mucinous adenocarcinomas from different locations (stomach, ampulla of Vater, colon, lung, breast and ovary) to detect specific mucin glycoforms. We detected Tn/STn/SLea/SLex-MUC1 and STn/SLea/SLex-MUC2 glycoforms in $50% of the cases, with a variable distribution among organs. Some new glycoforms-T/SLea-MUC2, STn/T/SLea/SLex-MUC5AC and STn/T/SLea/SLex-MUC6-were identified for the first time in the present study in a variable percentage of cases from different organs. In conclusion, application of the PLA technique allowed sensitive detection of specific aberrant mucin glycoforms in cancer, increasing specificity to the use of antibodies either to the mucin protein backbone or to the O-glycan haptens alone.
Keywords: cancer biomarkers, mucins, glycosylation, post-translational modifications (PTMs), in situ proximity ligation assay (in situ PLA)
Introduction
Mucin glycoproteins are major secreted or membrane-bound molecules produced by glandular epithelia. Adenocarcinomas show characteristic changes both in the mucin proteins expression and in their O-glycosylation, generating some of the most relevant tumour markers and, notably, most of the tumour markers in clinical use [1]. Most cancer biomarker assays, including mucin and O-glycan based assays, take advantage of changes in expression and biodistribution of products derived from cancer cells. Most current cancer serum biomarker assays probe changes in levels of circulating mucins or O-glycoproteins, such as MUC16 (CA125) and MUC1 (CA15–3), or changes in specific O-glycans such as STn (CA72–4) and SLea (CA19–9). However, assays detecting mucins have low specificity because benign
conditions also result in enhanced levels. Assays detecting specific O-glycans, on the other hand, suffer from lack of organ specificity and sensitivity because aberrant O-glycans are found on many different mucins and glycoproteins in all carcinoma cells. We have tried to circumvent these problems by developing a number of monoclonal antibodies (MAbs) directed to specific cancer-associated glycoforms of mucins, and found that specific detection of, for example, Tn-MUC1 greatly enhance specificity for cancer by immunohistology [2]. This approach is a step forwards on previous observations that indicated that some MUC1 antibodies exhibited a glycosylation-dependent recognition of cancer cells [3] but where the exact reason for the cancer specificity was elusive. Another example is the generation of antibodies specific to a GalNAc-glycosylated MUC2 mucin glycopeptide that substantiates our capability to build glycoform-specific recognition tools [4]. Furthermore, recent approaches have shown, by development of a microarray platform of synthetic O-glycosylated peptides [5, 6], that antibody recognition of cancer mucins can be glycopeptide specific. Taken together, these evidences bring a new hope for the possibility of getting a better specificity using glycopeptides, instead of mucin proteins or glycans alone, as cancer biomarkers.
It would therefore be desirable to develop methods that allow for detection of specific proteins in combination with aberrant posttranslational modifications (PTMs), such as O-glycans, and might have a more general application than glycopeptide specific MAbs. A recently developed technique, in situ proximity ligation assay (in situ PLA) [7, 8], created the possibility for in situ glycopeptide identification, as we have recently shown by recognition of the MUC2-Sialyl-Tn(STn) glycopeptide in pre-neoplastic and neoplastic gastric lesions [9]. In this report, we extended this approach in search of different cancer-associated glycoforms of mucins by immunohistology in a series of mucinous adenocarcinomas from different locations-stomach, ampulla of Vater, colon, lung, breast and ovary-where we know, by definition, that more than 50% of the tumour is composed by extracellular mucins. In situ PLA was targeted to identify cancer-associated simple mucin-type carbohydrates Tn, STn and Thomsen-Friedenreich (T) and sialylated Lewis antigens Sialyl-Lewisa (SLea) and Sialyl-Lewisx (SLex) and carrier mucins MUC1, that is also shed from the cell surface, and secreted mucins MUC2, MUC5AC and MUC6. The results support the hypothesis that enhanced specificity for cancer can be achieved by probing combinations of proteins and their aberrant PTMs.
Materials and methods
Human tissue samples
Sections from cancer specimens were obtained from patients with mucinous adenocarcinomas undergoing surgery at Hospital S. João, Medical Faculty (Porto, Portugal) between 1991 and 2009. Tissue fragments were fixed in 10% formaldehyde and embedded in paraffin. Serial sections of 3 mm were cut and used for immunostaining and PLA. We evaluated 28 cases of mucinous adenocarcinomas of several organs-stomach (n = 4), ampulla of Vater (n = 2), colon (n = 7), lung (n = 4), breast (n = 6) and ovary (n = 5). Table S1 shows gender, age and stage of the cases (staging was according to Ref. 10). The use of retrospective samples where informed consent cannot be obtained is authorized for research studies by the Portuguese Law. All sections selected for immunohistochemistry/immunofluorescence and PLA were haematoxylin and eosin stained and slides scanned using a Zeiss Optical Microscope. The scanned images were used as a reference for co-localizing glycans and mucins expression before PLA.
Monoclonal antibodies, purification and conjugation with biotin and fluorescein isothiocyanate (FITC)
Monoclonal antibodies and their characteristics are listed in Table S2. Two antibodies were used for MUC2 detection-PMH1 (an IgM), which recognizes MUC2 tandem repeat with a minimum of 1 mol of GalNAc, and Ccp58 (an IgG1), which recognizes more immature MUC2. Detection of MUC2 was therefore not completely overlapping with the two antibodies (Table 1). Hybridoma culture of clones CLH2 and CLH5 was adapted to a serum free medium (Thermo Scientific HyClone ADCF-MAb), supplemented with 1% penicillin–streptomycin and with a sequential decrease in foetal bovine serum (FBS) concentration. Hybridoma supernatants were purified according to their isotype. For IgG antibodies, supernatants were purified in HiTrap Protein G sepharose columns (GE Healthcare, Amersham Biosciences, Üppsala, Sweden), according to the manufacturer’s protocol. Buffer exchange was performed in PD-10 Desalting Columns (GE Healthcare) to PBS (pH 7.4). IgM 3C9 antibody was purified in IgY anti-IgM coupled to sepharose beads columns. Purified immunoglobulins were quantified using a NanoDrop spectrophotometer ND-1000 (for IgGs) or BCA quantification assay (for IgM) using bovine gamma globulin standards (Thermo Scientific Pierce Protein Research, Rockford, IL, USA) for the calibration curve.
Table 1.
Expression of cancer-associated carbohydrates and mucins in mucinous adenocarcinomas from different locations
| Carbohydrate/mucin | Stomach (n = 4) n (%) | Ampulla (n = 2) n (%) | Colon (n = 7) n (%) | Lung (n = 4) n (%) | Breast (n = 6) n (%) | Ovary (n = 5) n (%) | Total (n = 28) n (%) |
|---|---|---|---|---|---|---|---|
| Tn | 4 (100) | 2 (100) | 7 (100) | 4 (100) | 6 (100) | 4 (80) | 27 (96) |
| STn | 4 (100) | 2 (100) | 7 (100) | 2 (50) | 3 (50) | 4 (80) | 22 (78) |
| T | 4 (100) | 2 (100) | 3 (43) | 3 (75) | 5 (83) | 2 (40) | 19 (68) |
| SLea | 3 (75) | 2 (100) | 5 (71) | 2 (50) | 2 (33) | 4 (80) | 18 (64) |
| SLex | 4 (100) | 2 (100) | 7 (100) | 4 (100) | 5 (83) | 5 (100) | 27 (96) |
| MUC1 | 4 (100) | 2 (100) | 6 (86) | 3 (75) | 6 (100) | 5 (100) | 25 (89) |
| MUC2 | 4 (100)* | 2 (100)* | 7 (100)* | 3 (75)* | 6 (100)* | 2 (40)* | 24 (86)* |
| 4 (100)† | 2 (100)† | 7 (100)† | 2 (50)† | 4 (67)† | 2 (40)† | 21 (75)† | |
| MUC5AC | 2 (50) | 1 (50) | 4 (57) | 2 (50) | 1 (17) | 5 (100) | 15 (54) |
| MUC6 | 0 (0) | 1 (50) | 1 (14) | 1 (25) | 2 (33) | 2 (40) | 7 (25) |
MUC2 mucin detected by PMH1 MAb.
MUC2 mucin detected by Ccp58 MAb.
CLH2, HMFG2, 1E3, TKH2, 3C9, CA19.9 and KM93 purified MAbs were conjugated to biotin using the (+)-Biotin N-hydroxysuccinimide ester reagent (Sigma-Aldrich, Co., St. Louis, MO, USA) in a ratio of 40 mg/mg antibody. To remove all the non-reacting biotin, the solution was purified in PD-10 Desalting Columns (GE Healthcare) and dialysed against PBS. Moreover, CLH2, CLH5, 1E3, TKH2 and CA19.9 purified MAbs were conjugated to FITC, isomer I 90% (Sigma-Aldrich). Briefly, 20 ml of 1M sodium bicarbonate buffer (pH 9.0) and FITC dissolved in DMSO (10 mg/ml) were added to 200 ml of a 2–10 mg/ml protein solution. The reaction was stirred, protected from light at room temperature, for 1 hr. To remove all the non-reacting FITC, the solution was purified using Amicon Ultra-0.5 10K spin filters (Millipore, Billerica, MA, USA), according to the manufacturer’s instructions, and recovered in PBS. NaN3 was added in a concentration of 0.03% to preserve the conjugated MAbs. The effectiveness of conjugation was evaluated by immunohistochemistry (biotinylated MAbs) and immunofluorescence (FITC-conjugated MAbs) in tissue samples.
Immunohistochemistry and immunofluorescence
Immunohistochemistry and immunofluorescence were performed to map, onto the scanned haematoxylin and eosin sections, the staining pattern for carbohydrates and mucins. For immunohistochemistry the avidin–biotin–peroxidase complex (ABC) method was used. Paraffin sections were dewaxed and rehydrated. Sections designed for neuraminidase treatment (necessary for recognition of MUC2 mucin using PMH1 MAb) were washed in PBS Tween 20 0.01% (PBS-T) and incubated with neuraminidase from Clostridium perfringens type VI (Sigma Chemical Co., St. Louis, MO, USA) diluted in 0.1M sodium acetate buffer (pH 5.5) to a final concentration of 0.1 U/ml, for 2 hrs at 378C, followed by washes in ice-cold water. Antigen retrieval for Ccp58 antibody was carried by microwave treatment in sodium citrate buffer (10 mM, pH 6.0) for 20 min. Sections for immunohistochemistry were treated with 3% hydrogen peroxide (H2O2) in methanol for 10 min., to block endogenous peroxidase. Then all sections were incubated for 20 min. with normal rabbit serum in PBS with 10% bovine serum albumin (BSA), to block non-specific staining. Excess normal serum was removed and replaced by specific primary antibody in the appropriate dilution in PBS with 5% BSA. Sections were incubated overnight at 48C. After washing, sections for immunohistochemistry were incubated with biotin-labelled secondary rabbit antimouse antibody (Dako, Glostrup, Denmark) diluted 1:100 in PBS with 5% BSA for 30 min. and with ABC kit (Vector Labs, CA, USA) for 30 min. Sections were stained for 3 min. with 0.05% 3,3’-diaminobenzidinetetrahydrochloride (DAB) containing 0.01% H2O2. Sections were counterstained with haematoxylin, dehydrated and mounted. Slides were examined using a Zeiss Optical Microscope. For immunofluorescence, sections were incubated with rabbit antimouse Ig FITC-labelled secondary antibody (Dako) diluted 1:70 in PBS with 5% BSA for 45 min., protected from light. Sections were washed two times for 5 min. in PBS-T. 49,6-diamidino-2-phenylindole (DAPI) was used as a nuclear counterstain and sections were mounted in Vectashield mounting media (Vector Labs). Samples were examined under a Leica DM2000 fluorescence microscope equipped with DAPI and FITC interference filters. Images were acquired using a Leica DFC340 FX camera and Leica Application Suit software. For biotinylated and FITC-conjugated MAbs, the secondary antibody was not added.
All series included positive controls: tissue sections from a breast and gastric carcinoma expressing all the mucin and carbohydrate antigens tested. Negative controls were performed by omitting primary antibodies or by using irrelevant primary antibodies in the same concentration as the test MAbs. Serial dilutions were performed to select optimal conditions for non-conjugated antibodies (Table S2). Cases were analysed for each MAb staining and regions of co-localization between glycans and mucins were identified on the scanned haematoxylin and eosin slides to select cases for PLA.
In situ PLA
In situ PLA was performed in sections where co-localization for each glycan–mucin combination was observed by immunohistochemistry and/or immunofluorescence. Deparaffinized tissue sections were pre-treated according to the immunohistochemistry/immunofluorescence protocol. For glycan–mucin combinations where MUC2 mucin was recognized by PMH1, neuraminidase treatment was carried out for only 1 hr at 378C at a final concentration of 0.1 U/ml, because further reaction times or enzyme concentrations abolish the majority of neuraminic acid present in sialylated carbohydrates as previously reported [9]. Then, sections were incubated for 30 min. with normal rabbit (for anti-biotin and anti-FITC secondary antibodies) or goat serum (for anti-IgG and anti-IgM secondary antibodies) in PBS with 10% BSA, to block non-specific staining. Excess normal serum was removed and replaced by specific primary antibodies in PBS with 5% BSA at the optimal dilution (Table S3). Sections were incubated overnight at 48C and unbound primary antibodies were removed. Oligonucleotide-conjugated PLA secondary probes were added in appropriate dilutions (Table S3) in PBS with 5% BSA and slides incubated in a pre-heated humid chamber for 1hr 45 min. at 378C. Anti-IgM and anti-IgG probes were prepared using AffiniPure Goat anti-mouse IgG or IgM (Jackson Immunoresearch, Laboratories, West Grove, PA, USA) secondary antibodies. Briefly, antibodies were concentrated to 100 mg/ml using Amicon Ultra-0.5 10K spin filters (Millipore) and activated with succinimidyl 6-hydrazinonicotinate acetone hydrazone conjugation reagent (SANH) (Nordic Biosite) using a ratio of 25 pmol SANH per 1 pmol of antibody, for 2 hrs at room temperature. Then antibodies were purified using Zebaspin columns and conjugated with the respective oligonucleotide sequence using 10 mM aniline (Sigma-Aldrich), overnight at 48C. The anti-IgG antibody was conjugated with a 5’aldehyde-modified minus probe (AAAAAAAAAAGACGCTAATAGTTAAGACGCTT; Trilink BioTechnologies, San Diego, CA, USA), while the anti-IgM antibody was conjugated with a 5’aldehyde-modified plus probe (AAAAAAAAAATATGACAGAACTAGACACTCTT; Trilink BioTechnologies). The conjugated probes were purified by high-performance liquid chromatography. For primary antibody pairs sharing the same isotype, we used haptenized probes (biotin- of FITC-conjugated). The PLA assays were performed with the DuoLink detection kit 613 (Olink AB, Uppsala, Sweden) according to the manufacturer’s instructions. Briefly, connector oligonucleotides were hybridized to probe pairs for 15 min. and circularized by ligation for 15 min. The sections were incubated with the processive w29 DNA polymerase for 90 min. to produce rolling circle products (RCPs). Rolling circle products were visualized with fluorescently labelled oligonucleotides and the sections counterstained with Hoechst 33342. Samples were examined under a Zeiss Imager.Z1 Axio fluorescence microscope equipped with DAPI, FITC and Texas Red filters. Proximity ligation assays products are seen as bright fluorescent dots. Images were acquired using a Zeiss Axio cam MRm and the AxioVision Rel. 4.8 software. The resulting images were modified using ImageJ as follows: background with radius 4 was subtracted from the red channel of the RGB images and a maximum filter with radius 1 was applied. The result was intensity-scaled to suit printing demands.
Proximity ligation assays was not performed for the Tn-MUC2 pair due to technical limitations. In fact, we were unable to use IgG isotype 1E3 MAb (anti-Tn) with the IgG anti-MUC2 Ccp58 because the concentration of Ccp58 was too low to permit conjugation of the latter. The alternative pair 1E3-PMH1 (PMH1 is an IgM) would require neuraminidase treatment, but even with 1 hr of neuraminidase 0.1 U/ml activity (sufficient for PMH1 epitope exposure), some STn was desialylated and exposed the Tn antigen (considered untrue Tn; data not shown). Neuraminidase treatment was necessary for PLAs using PMH1 antibody for recognition of MUC2 mucin, in reactions involving STn and SLea (Table S3). We have previously shown that under the working conditions neuraminidase did not affect STn recognition [9] and the same was observed for SLea (data not shown). Specificity of the PLA recognition was tested using recombinant glycopeptides of MUC2 and MUC5AC in Western blot PLA (Fig. S1). All series included a positive control for the PLA reaction: a section of gastric mucosa with intestinal metaplasia stained with STn–MUC2 pair as previously published [9].
Results
In situ PLA was performed after selection of the candidate mucin glycopeptides based upon co-localization observed by immunohistochemistry. Results from immunohistochemistry with different antibodies and co-localization data are shown in Tables 1 and 2, respectively. MUC1 and MUC2 mucin expression is predominant in the series of mucinous adenocarcinomas whereas Tn and SLex are the most frequently expressed cancer-associated glycans in the set of cases evaluated. Conversely, MUC6 is the less expressed mucin, while SLea is the less expressed glycan (Table 1).
Table 2.
Co-localization of expression of cancer-associated carbohydrates and mucins in mucinous adenocarcinomas from different locations
| Mucin–carbohydrate co-localization | Stomach (n = 4) n (%) | Ampulla (n = 2) n (%) | Colon (n = 7) n (%) | Lung (n = 4) n (%) | Breast (n = 6) n (%) | Ovary (n = 5) n (%) | Total (n = 28) n (%) |
|---|---|---|---|---|---|---|---|
| Tn-MUC1 | 4 (100) | 2 (100) | 5 (71) | 3 (75) | 5 (83) | 4 (80) | 23 (82) |
| Tn-MUC2* | 4 (100) | 2 (100) | 6 (86) | 3 (75) | 6 (100) | 2 (40) | 23 (82) |
| Tn-MUC5AC | 2 (50) | 1 (50) | 3 (43) | 2 (50) | 1 (17) | 3 (60) | 12 (43) |
| Tn-MUC6 | 0 (0) | 1 (50) | 0 (0) | 1 (25) | 2 (33) | 2 (40) | 6 (21) |
| STn-MUC1 | 4 (100) | 2 (100) | 6 (86) | 1 (25) | 3 (50) | 4 (80) | 20 (71) |
| STn-MUC2† | 4 (100) | 2 (100) | 7 (100) | 2 (50) | 3 (50) | 1 (20) | 19 (68) |
| STn-MUC5AC | 2 (50) | 1 (50) | 4 (43) | 2 (50) | 1 (17) | 3 (60) | 13 (46) |
| STn-MUC6 | 0 (0) | 1 (50) | 1 (14) | 1 (25) | 0 (0) | 1 (20) | 4 (14) |
| T-MUC1 | 4 (100) | 1 (50) | 1 (14) | 2 (50) | 4 (67) | 2 (40) | 14 (50) |
| T-MUC2* | 4 (100) | 0 (0) | 2 (29) | 2 (50) | 3 (50) | 0 (0) | 11 (39) |
| T-MUC5AC | 2 (50) | 1 (50) | 1 (14) | 2 (50) | 1 (17) | 1 (20) | 8 (28) |
| T-MUC6 | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 2 (33) | 1 (20) | 4 (14) |
| SLea-MUC1 | 3 (75) | 2 (100) | 4 (57) | 1 (25) | 2 (33) | 4 (80) | 16 (57) |
| SLea-MUC2† | 3 (75) | 2 (100) | 5 (71) | 2 (50) | 2 (33) | 2 (40) | 16 (57) |
| SLea-MUC5AC | 2 (50) | 1 (50) | 2 (29) | 1 (25) | 1 (17) | 4 (80) | 11 (39) |
| SLea-MUC6 | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 2 (40) | 3 (11) |
| SLex-MUC1 | 4 (100) | 2 (100) | 6 (86) | 3 (75) | 4 (67) | 4 (80) | 23 (82) |
| SLex-MUC2* | 4 (100) | 2 (100) | 6 (86) | 2 (50) | 2 (33) | 1 (20) | 17 (61) |
| SLex-MUC5AC | 2 (50) | 1 (50) | 4 (57) | 2 (50) | 1 (17) | 4 (80) | 14 (50) |
| SLex-MUC6 | 0 (0) | 1 (50) | 0 (0) | 1 (25) | 1 (17) | 2 (40) | 5 (18) |
Results refer to MUC2 mucin stained with Ccp58 MAb.
Results refer to MUC2 mucin stained with PMH1 MAb.
Simple mucin-type carbohydrate antigens (Tn, STn and T)
Tn antigen was expressed in 96% (27 of 28 cases), STn in 78% (22 of 28) and T in 68% of the cases (19 of 28) (Table 1).
Results from simple mucin-type carbohydrate–mucin glycopeptides evaluated by in situ PLA in mucinous carcinomas from different locations are summarized on Table 3.
Table 3.
Number and percentages of in situ PLA positive cases for simple mucin-type carbohydrate–mucin glycopeptides evaluated by in situ PLA in mucinous adenocarcinomas from different locations
| Carbohydrate–mucin | Stomach (n = 4) n (%) | Ampulla (n = 2) n (%) | Colon (n = 7) n (%) | Lung (n = 4) n (%) | Breast (n = 6) n (%) | Ovary (n = 5) n (%) | Total (n = 28) n (%) |
|---|---|---|---|---|---|---|---|
| Tn-MUC1 | 4 (100) | 2 (100) | 5 (71) | 3 (75) | 5 (83) | 4 (80) | 23 (82) |
| Tn-MUC2 | NA | NA | NA | NA | NA | NA | NA |
| Tn-MUC5AC | 2 (50) | 1 (50) | 1 (14) ↓ | 2 (50) | 1 (17) | 3 (60) | 10 (36) ↓ |
| Tn-MUC6 | 0 (0) | 1 (50) | 0 (0) | 1 (25) | 2 (33) | 2 (40) | 6 (21) |
| STn-MUC1 | 4 (100) | 2 (100) | 4 (57) ↓ | 1 (25) | 2 (33) ↓ | 3 (60) ↓ | 16 (57) ↓ |
| STn-MUC2 | 4 (100) | 2 (100) | 7 (100) | 2 (50) | 3 (50) | 1 (20) | 19 (68) |
| STn-MUC5AC | 2 (50) | 1 (50) | 0 (0) ↓ | 2 (50) | 1 (17) | 2 (40) ↓ | 8 (28) ↓ |
| STn-MUC6 | 0 (0) | 1 (50) | 1 (14) | 0 (0) ↓ | 0 (0) | 1 (20) | 3 (11) ↓ |
| T-MUC1 | 4 (100) | 1 (50) | 1 (14) | 2 (50) | 3 (50) ↓ | 1 (20) ↓ | 12 (43) ↓ |
| T-MUC2 | 3 (75) ↓ | 0 (0) | 2 (29) | 2 (50) | 3 (50) | 0 (0) | 10 (36) ↓ |
| T-MUC5AC | 2 (50) | 1 (50) | 1 (14) | 2 (50) | 1 (17) | 1 (20) | 8 (28) |
| T-MUC6 | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 2 (33) | 1 (20) | 4 (14) |
↓indicates a decrease in the number of PLA positive cases compared to the number of cases where co-localization was observed (Table 2).
NA: not evaluated.
Proximity ligation assays shows that MUC1 is the carrier of the Tn antigen in 82% (Fig. 1: PLA Tn-MUC1), MUC5AC in 36% and MUC6 in 21% of the cases (Fig. 2A and Table 3). Tn-MUC2 glycoform could not be evaluated (see Materials and Methods). MUC1 is a major carrier of the Tn antigen in cancers from all locations which fits with the high percentage of expression of both Tn and MUC1 (Table 1) and also to the number of cases where they co-localize (Table 2). In contrast, the lower percentage of cases where Tn is carried by MUC5AC and MUC6 seems to be in line with the lower percentage of positivity for the two mucins (Table 1).
Fig 1.
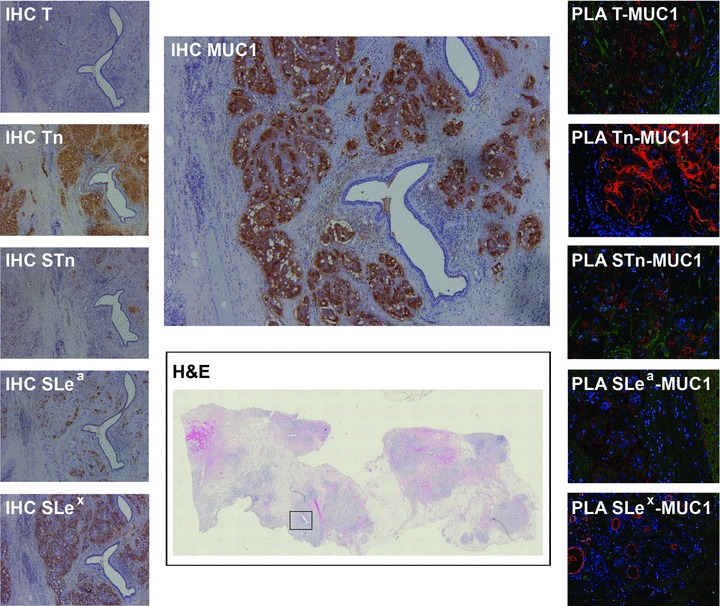
Mucinous breast carcinoma expressing MUC1 mucin and all simple mucin-type (Tn, STn, T) and sialylated Lewis antigens (SLea and SLex). All MUC1 glycoforms gave positive PLA signals. Marked in the haematoxylin and eosin image with a square is the specific area where all IHC and PLA images were obtained. Haematoxylin and eosin scanned image at 4x. Immunostained images at 50x magnification. PLA images at 200x magnification.
2 (A, B).
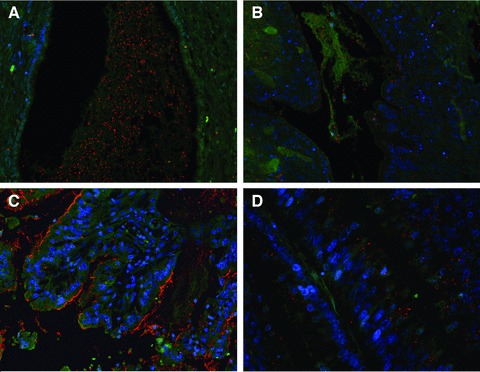
Mucinous ovarian carcinoma expressing Tn-MUC6 (A) predominantly at the extracellular mucin pools and SLea-MUC5AC glycoform (B) at cell cytoplasm and extracellular mucus. PLA images at 200x magnification. (C) Mucinous gastric carcinoma expressing STn-MUC1. PLA signals are seen at cell cytoplasm, apical membrane and extracellular mucus, at 200x magnification. (D) Mucinous colon carcinoma expressing Tn-MUC5AC at a perinuclear, Golgi-like, location, at 400x magnification. In this case, PLA signals suggest that the secreted MUC5AC had further processing of the Tn antigen which explains negativity by PLA at secreted mucus.
STn is carried by MUC1 in 57% (Fig. 1: PLA STn-MUC1 and Fig. 2C), MUC2 in 68% (Fig. 3: PLA STn-MUC2), MUC5AC in 28% and MUC6 in 11% of the cases (Table 3). MUC2 is therefore the major carrier of STn and, such as for Tn, a lower percentage of cases show STn carried by MUC5AC and MUC6 in line with the lower percentage of positivity for the two mucins (Table 1).
Fig 3.
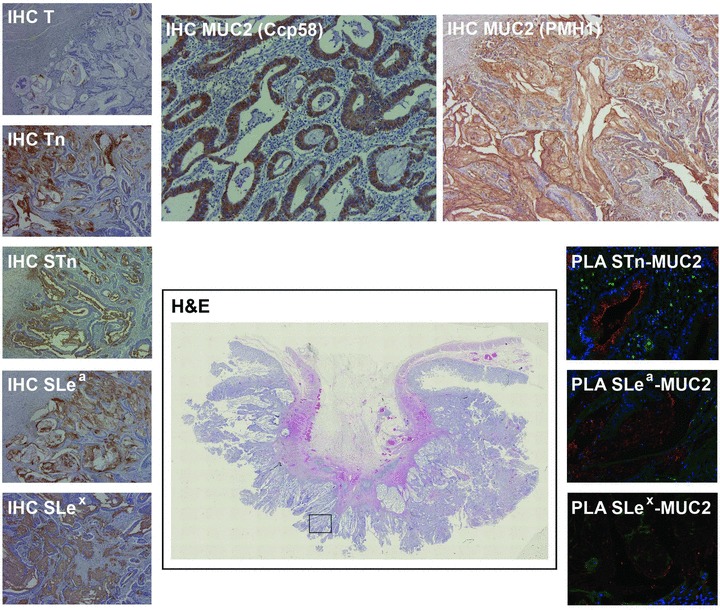
Mucinous colon carcinoma expressing MUC2 mucin and all simple mucin-type (Tn, STn, T) and sialylated Lewis antigens (SLea and SLex). All MUC2 glycoforms except T-MUC2 gave positive PLA signals. Marked in the haematoxylin and eosin image with a square is the specific area where all IHC and PLA images were obtained. Haematoxylin and eosin scanned image at 4x. Immunostained images at 50x magnification, except for Ccp58 staining, at 100x. PLA images at 200x magnification.
Finally, PLA shows that MUC1 is the carrier of the T antigen in 43% (Fig. 1: PLA T-MUC1), MUC2 in 36%, MUC5AC in 28% and MUC6 in 14% of the cases (Table 3).
Comparing PLA positivity with co-localization (Table 2) we observed that, in several cases, the number of PLA positive cases involving simple mucin-type carbohydrate antigens is lower than we would infer from co-localization (Tn-MUC5AC in colonic cases, STn-MUC1 in colonic, breast and ovarian cases, STn-MUC5AC in colonic and ovarian cases, STn-MUC6 in lung cases, T-MUC1 in breast and ovarian cases and T-MUC2 in gastric cases; Table 3). Finally, PLA signals were detected diffusely in the cytoplasm as well as in the extracellular mucus (Figs 1, 2A–C and 3) and, when involving MUC1, also at the cell membranes (Figs 1 and 2C). In some carcinomas, PLA positivity, for glycopeptides involving the Tn antigen, was confined to the perinuclear/Golgi area (Fig. 2D). These cases were considered negative.
Sialylated Lewis antigens (SLea and SLex)
SLea was expressed in 64% (18 of 28 cases) and SLex in 96% of the cases (27 of 28) (Table 1). Results from sialyl Lewis carbohydrate– mucin glycoforms evaluated by in situ PLA in mucinous carcinomas from different locations are summarized in Table 4.
Table 4.
Number and percentages of in situ PLA positive cases for sialyl Lewis carbohydrate–mucin glycopeptides evaluated by in situ PLA in mucinous adenocarcinomas from different locations
| Carbohydrate–mucin | Stomach (n = 4) n (%) | Ampulla (n = 2) n (%) | Colon (n = 7) n (%) | Lung (n = 4) n (%) | Breast (n = 6) n (%) | Ovary (n = 5) n (%) | Total (n = 28) n (%) |
|---|---|---|---|---|---|---|---|
| SLea-MUC1 | 3 (75) | 2 (100) | 4 (57) | 1 (25) | 2 (33) | 4 (80) | 16 (57) |
| SLea-MUC2 | 3 (75) | 2 (100) | 5 (71) | 2 (50) | 2 (33) | 1 (20) ↓ | 15 (54) ↓ |
| SLea-MUC5AC | 2 (50) | 1 (50) | 2 (29) | 1 (25) | 1 (17) | 3 (60) ↓ | 10 (36) ↓ |
| SLea-MUC6 | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 1 (20) ↓ | 2 (7) ↓ |
| SLex-MUC1 | 4 (100) | 2 (100) | 6 (86) | 3 (75) | 4 (67) | 4 (80) | 23 (82) |
| SLex-MUC2 | 4 (100) | 2 (100) | 5 (71) ↓ | 2 (50) | 2 (33) | 0 (0) | 15 (54) ↓ |
| SLex-MUC5AC | 2 (50) | 1 (50) | 3 (43) ↓ | 2 (50) | 1 (17) | 4 (80) | 13 (46) ↓ |
| SLex-MUC6 | 0 (0) | 1 (50) | 0 (0) | 1 (25) | 1 (17) | 2 (40) | 5 (18) |
↓indicates a decrease in the number of PLA positive cases compared to the number of cases where co-localization was observed (Table 2).
Proximity ligation assays shows that MUC1 and MUC2 are the major carriers of SLea in 57% and 54% of the cases, respectively (Fig. 1: PLA SLea-MUC1 and Fig. 3: PLA SLea-MUC2; Table 4). Both MUC1 and MUC2 are major carriers of SLea in gastric, ampullary and colonic carcinomas, whereas MUC1 predominates in the ovary and MUC2 in lung. MUC5AC is also the carrier of SLea (Fig. 2B) in a percentage $50% in gastric, ampullary and ovarian carcinomas and MUC6 is relevant only in ampullary cases.
MUC1 is the carrier of SLex in 82% (Fig. 1: PLA SLex-MUC1), MUC2 in 54% (Fig. 3: PLA SLex-MUC2), MUC5AC in 46% and MUC6 in 18% of the cases (Table 4). MUC1 is therefore the major carrier of SLex, followed by MUC2 and MUC5AC and, such as for most other carbohydrates, a lower percentage of cases show SLex carried by MUC6 in line with the lower percentage of positivity for this mucin (Table 1).
Comparing PLA positivity with co-localization (Table 2) we also observed that, in several cases, the number of PLA positive cases involving Lewis antigens is lower than we would infer from co-localization (SLea-MUC2/MUC5AC/MUC6 in ovarian cases and SLex-MUC2/MUC5AC in colon cases). Similar to the described above for simple mucin-type carbohydrate–mucin glycoforms, PLA signals were identified diffusely dispersed at the cancer cells cytoplasm and in extracellular mucus (Figs 1, 2B and 3) and, in the case of glycoforms involving MUC1, also at the cell membranes (Figs 1 and 2C).
Discussion
In this study we developed sensitive and specific PLA assays for detection of aberrant mucin glycoforms using existing MAbs to mucin protein backbones and O-glycan haptens, greatly extending information to the use of antibodies either to the mucin backbone or to the O-glycan haptens alone.
In accordance to many previous immunohistochemical studies, we observed that simple mucin-type carbohydrate antigens are expressed in a wide range of mucinous adenocarcinomas from different locations [11-17]. A major limitation of these cancer biomarkers is precisely due to their widespread expression in cancer. In situ PLA for specific mucin glycoforms showed that, with few exceptions, it is the mucin expression profile and not the carbohydrate biosynthetic profile that determines the mucin glycoforms biosynthesized in each case. In situ PLA goes beyond co-expression and even co-localization in that it shows with a high sensitivity the actual mucin glycopeptide biomarkers.
We identified Tn-MUC1 glycoform as one of the most frequent but also less tissue-specific cancer biomarker in the series of mucinous carcinomas (identically for SLex-MUC1). This is in agreement with a previous study [18], showing by SDS-PAGE that malignant effusions from breast, colon, gastric and ovarian cases have Tn antigen carried by MUC1. Our results also support the impressive cancer-specificity of MAb 5E5 generated for a MUC1-Tn glycopeptide epitope [2]. In fact, we could confirm a perfect fit between our Tn-MUC1 PLA results and immunostaining with 5E5 (data not shown). The Tn-MUC5AC and Tn-MUC6 glycoforms were also frequent, if we take into account the lower levels of expression of both mucins compared with MUC1—that is, it seems that if mucins are produced by cancer cells, there is a high probability that they carry Tn. Tn-MUC5AC glycoform is observed in $50% of carcinomas from stomach, ampulla of Vater, lung and ovary, but not in colon or breast carcinomas. Tn-MUC6 is only identified in a high percentage (50%) of cases from ampulla of Vater, with the limitation that only two carcinomas in this location were evaluated. In addition to MUC1, Freire et al. also identified MUC2, MUC5AC and MUC6 as mucin carriers for Tn antigen [18] and another study by the same group [19] identified MUC6 as a Tn carrier in the MCF-7 breast carcinoma cell line.
In our series, the major STn carrier was MUC2 mucin, despite MUC1 being expressed by a larger number of tumours than MUC2 (Table 1). Both STn-MUC2 and STn-MUC1 were identified in all gastric and ampullary carcinomas, whereas STn-MUC2 also gave positive signals in most ($50%) colonic, pulmonary and breast cases but not in ovarian cases, and STn-MUC1 was most prominent in colonic and ovarian cases (Table 3). We have recently identified, also using in situ PLA, the MUC2 mucin as a major carrier of STn in gastric carcinomas and pre-neoplastic lesions of intestinal metaplasia [9]. In the same work, we also showed that other mucins may be carriers of the STn structure, by lack of PLA signals in STn positive areas. However, the mucin carriers involved were not identified. Moreover, previous studies have pointed MUC1 mucin as a STn carrier by showing that gastric and breast cancer cell lines transfected with ST6GalNAc-I glycosyltransferase, the major enzyme responsible for STn biosynthesis [20], generate the STn-MUC1 glycoform [21, 22]. To a lesser extent, again due largely to their lower expression, MUC5AC and MUC6 are also STn carrier mucins ($50%) in gastric, ampullary and lung carcinomas and in ampullary carcinomas, respectively (Table 3). This new observation, that STn is carried by MUC5AC and MUC6 in human carcinomas, was not previously reported and adds new and very interesting glycopeptide biomarkers to our current panoply of choices.
T antigen had a more restricted profile of mucin carriers in the different locations, with a preference for the T-MUC1 combination, similar to Tn. The identification of T-MUC1 glycopeptide in cancer cells was previously performed with breast cancer cell lines [23]. Next in frequency was the T-MUC2 glycoform, a previously unreported glycopeptide combination that, together with MUC5AC and MUC6, are newly identified carriers of the T antigen in human carcinomas. Our data may help to better understand the relevance of mucin carriers in nipple aspirate fluid that recently was shown to be an important source for detection of T antigen in cancer patients. [24, 25]
Similar to simple mucin-type carbohydrate antigens, sialylated Lewis antigens are expressed in O-glycoptroteins at a wide range of adenocarcinomas from different locations [26, 27], and also on glycolipids and N-glycoproteins. As for other cancer-associated carbohydrates, this is a major limitation to their use in the clinical setting. Again, we observed by in situ PLA that it is the mucin expression profile and not the carbohydrate biosynthetic profile that largely determines the detected sialyl-Lewis-mucin glycoforms.
We identified the SLea-MUC1 and SLea-MUC2 glycoforms as the most frequent but less tissue-specific cancer biomarkers carrying SLea in mucinous carcinomas. Previous studies have shown that MUC1 carrying SLea is identified in circulation in lung and breast carcinoma patients [28, 29] but also in pancreatic and colonic cell lines [30]. Despite being less frequent than SLea-MUC1 and SLea-MUC2, we also identified MUC5AC and MUC6 as mucin carriers of this glycan. For the first time, we determined that all secreted mucins evaluated (MUC2, MUC5AC and MUC6) are carriers of one of the most frequently used cancer biomarkers in the clinical field—CA19.9—irrespectively of the organ. Our results also raise the expectation that glycopeptides biomarkers will yield more specific targets for the identification of the cancer origin in the setting of a positive CA19.9 (SLea detection) serum assay. MUC1 is a predominant SLea carrier in gastric, ampullary, colonic and ovarian carcinomas, while SLea-MUC2 is predominant in stomach, ampulla, colon and lung. Moreover, SLea-MUC5AC is predominant in stomach, ampulla and ovary and SLea-MUC6 in ampullary cases. This needs to be confirmed in larger studies.
The identification of all studied mucins as carriers of SLex complements previous studies where only MUC1 [29-32] and MUC2 [32] were identified as putative SLex carriers. In contrast to our results, where SLex was found mostly on MUC1 in colon, lung, breast and ovary cases (Table 4), Hanski et al. [32] demonstrated that the SLex epitope is mostly associated with MUC2, rather than MUC1, in colon carcinomas.
All mucin glycoforms were identified diffusely in the cell cytoplasm and on secreted mucus and, in the case of MUC1, also at the cell membranes. In some cases, the PLA positivity was confined to the perinuclear/Golgi area, indicating that the glycopeptide may be further glycosylated before trafficking to the cell membrane or secretion and therefore it is not a bona fide cancer biomarker.
Although in situ PLA enhances the stringency in detection of mucin glycoforms, compared to co-localization, it has to be emphasized that the signal is dependent on proximal epitopes and not physical interaction, as is the case of other methods such as FRET. Therefore, proximity might, and may to some extent, mean exactly proximity and not ligation. Our arguments against this being a relevant source of false positives in our series is that in some cases, or at least in some foci, the identified glycopeptides were seen in the absence of the putatively confounding presence of other mucins and, also, that signals were so widespread and dense that this phenomenon has little plausibility. Also supporting the relevance of our findings is the observation that not all co-localization corresponded to the presence of PLA signals.
We have demonstrated an important new approach to identify targets for the development of specific biomarkers, expanding on previous work with mucins and O-glycans. Although organ-specific glycopeptide biomarkers were not identified, this observation may partly stem from our selection of a specific subtype of adenocarcinomas—mucinous adenocarcinomas-as study material. In fact, it may well be that some phenotypic homogeneity will be associated with a similar glycopeptide profile. Our finding that all mucins studied herein carry different carbohydrates raises the question if these carbohydrates are present on the same molecule. Hence one possibility ahead is to develop triple-binder reactions to detect, for example, a mucin with two different carbohydrates generating a ‘triplet’ biomarker, with increased specificity. [7, 33] Another issue that deserves further efforts is to develop glycopeptide specific antibodies in line with the Tn/STn-MUC1 specific MAb [2]. A major expansion of this study is the development of assays to detect the glycopeptides in circulation in serum assays. Finally, further studies should take into account the possibility that some of these glycopeptide biomarkers will also have a potential for prognostic evaluation.
In summary, our study in a limited set of mucinous adenocarcinomas from different locations, identified a set of cancer associated carbohydrate (both simple mucin-type and sialylated Lewis antigens) mucin carriers, thus adding new information to the field of cancer biomarkers. Despite the low number of cases, our results suggest that the newly identified glycopeptides will, in some cases, represent pan-carcinoma biomarkers but also, in other cases, tissue-specific cancer biomarkers.
Acknowledgments
The authors thank Sofia Santos and Emerson Bernardes for preparing the IgY columns for purification of IgM 3C9 MAb and Diana Campos for providing synthetic MUC5AC and MUC2 glycopetides. This work was supported by Fundação para a Ciência e a Tecnologia—FCT (PTDC/SAU-MII/64153/2006 and PIC/IC/82716/2007), EU-FP7-HEALTH-2007-A and Cancer Research UK.
Authors’ contributions
RP, ASC, OS and LD conceived and designed the experiments. RP, ASC, TC, AM and GP performed the experiments. RP, OS and LD analysed the data. GP, JMB, JTP, UM and HC contributed with reagents. OS gave important directions to the study. RP and LD wrote the paper. ASC, AM, JMB, JTP, CAR, RA, UM, HC and OS reviewed and revised the manuscript.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Reis CA, Osório H, Silva L, et al. Alterations in glycosylation as biomarkers for cancer detection. J Clin Pathol. 2010;63:611–8. doi: 10.1136/jcp.2009.071035. [DOI] [PubMed] [Google Scholar]
- 2.Srensen AL, Reis CA, Tarp MA, et al. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology. 2006;16:96–107. doi: 10.1093/glycob/cwj044. [DOI] [PubMed] [Google Scholar]
- 3.Burchell J, Gendler S, Taylor PapadimitriouJ, et al. Development and characterization of breast cancer reactive monoclonal antibodies directed to the core protein of the human milk mucin. Cancer Res. 1987;47:5476–82. [PubMed] [Google Scholar]
- 4.Reis CA, Srensen T, Mandel U, et al. Development and characterization of an antibody directed to an alpha-N-acetyl-D-galactosamine glycosylated MUC2 peptide. Glycoconj J. 1998;15:51–62. doi: 10.1023/a:1006939432665. [DOI] [PubMed] [Google Scholar]
- 5.Blixt O, Cló E, Nudelman AS, et al. A high-throughput O-glycopeptide discovery platform for seromic profiling. J Proteome Res. 2010;9:5250–61. doi: 10.1021/pr1005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wandall HH, Blixt O, Tarp MA, et al. Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Res. 2010;70:1306–13. doi: 10.1158/0008-5472.CAN-09-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Söderberg O, Gullberg M, Jarvius M, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 8.Weibrecht I, Leuchowius KJ, Clausson CM, et al. Proximity ligation assays: a recent addition to the proteomics toolbox. Expert Rev Proteomics. 2010;7:401–9. doi: 10.1586/epr.10.10. [DOI] [PubMed] [Google Scholar]
- 9.Conze T, Carvalho AS, Landegren U, et al. MUC2 mucin is a major carrier of the cancer-associated sialyl-Tn antigen in intestinal metaplasia and gastric carcinomas. Glycobiology. 2010;20:199–206. doi: 10.1093/glycob/cwp161. [DOI] [PubMed] [Google Scholar]
- 10.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer staging manual. 7th ed. New York: Springer-Verlag; 2010. [Google Scholar]
- 11.Itzkowitz SH, Yuan M, Montgomery CK, et al. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989;49:197–204. [PubMed] [Google Scholar]
- 12.Inoue M, Ton SM, Ogawa H, et al. Expression of Tn and sialyl-Tn antigens in tumour tissues of the ovary. Am J Clin Pathol. 1991;96:711–6. doi: 10.1093/ajcp/96.6.711. [DOI] [PubMed] [Google Scholar]
- 13.Tashiro Y, Yonezawa S, Kim YS, et al. Immunohistochemical study of mucin carbohydrates and core proteins in human ovarian tumours. Hum Pathol. 1994;25:364–72. doi: 10.1016/0046-8177(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 14.Werther JL, Rivera MacMurrayS, Bruckner H, et al. Mucin-associated sialosyl-Tn antigen expression in gastric cancer correlates with an adverse outcome. Br J Cancer. 1994;69:613–6. doi: 10.1038/bjc.1994.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terada T, Nakanuma Y. Expression of mucin carbohydrate antigens (T, Tn and sialyl Tn) and MUC-1 gene product in intraductal papillary-mucinous neoplasm of the pancreas. Am J Clin Pathol. 1996;105:613–29. doi: 10.1093/ajcp/105.5.613. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y, Schlag PM, Karsten U. Immunodetection of epithelial mucin (MUC1, MUC3) and mucin-associated glycotopes (TF, Tn, and sialosyl-Tn) in benign and malignant lesions of colonic epithelium: apolar localization corresponds to malignant transformation. Virchows Arch. 1997;431:159–66. doi: 10.1007/s004280050083. [DOI] [PubMed] [Google Scholar]
- 17.Ghazizadeh M, Ogawa H, Sasaki Y, et al. Mucin carbohydrate antigens (T, Tn, and sialyl-Tn) in human ovarian carcinomas: relationship with histopathology and prognosis. Hum Pathol. 1997;28:960–6. doi: 10.1016/s0046-8177(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 18.Freire T, Medeiros A, Reis CA, et al. Biochemical characterization of soluble Tn glycoproteins from malignant effusions of patients with carcinomas. Oncol Rep. 2003;10:1577–85. [PubMed] [Google Scholar]
- 19.Freire T, Bay S, von Mensdorff-PouillyS, et al. Molecular basis of incomplete O-glycan synthesis in MCF-7 breast cancer cells: putative role of MUC6 in Tn antigen expression. Cancer Res. 2005;65:7880–7. doi: 10.1158/0008-5472.CAN-04-3746. [DOI] [PubMed] [Google Scholar]
- 20.Marcos NT, Pinho S, Grandela C, et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004;64:7050–7. doi: 10.1158/0008-5472.CAN-04-1921. [DOI] [PubMed] [Google Scholar]
- 21.Pinho S, Marcos NT, Ferreira B, et al. Biological significance of cancer-associated sialyl-Tn antigen: modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 2007;249:157–70. doi: 10.1016/j.canlet.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Sewell R, Bäckstrm M, Dalziel M, et al. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumour-associated sialyl-Tn O-glycan in human breast cancer. J Biol Chem. 2006;281:3586–94. doi: 10.1074/jbc.M511826200. [DOI] [PubMed] [Google Scholar]
- 23.Powlesland AS, Hitchen PG, Parry S, et al. Targeted glycoproteomic identification of cancer cell glycosylation. Glycobiology. 2009;19:899–909. doi: 10.1093/glycob/cwp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar SR, Sauter ER, Quinn TP, et al. Thomsen-Friedenreich and Tn antigens in nipple fluid: carbohydrate biomarkers for breast cancer detection. Clin Cancer Res. 2005;11:6868–71. doi: 10.1158/1078-0432.CCR-05-0146. [DOI] [PubMed] [Google Scholar]
- 25.Deutscher SL, Dickerson M, Gui G, et al. Carbohydrate antigens in nipple aspirate fluid predict the presence of atypia and cancer in women requiring diagnostic breast biopsy. BMC Cancer. 2010;10:519. doi: 10.1186/1471-2407-10-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakamoto S, Watanabe T, Tokumaru T, et al. Expression of Lewisa, Lewisb, Lewisx, Lewisy, siayl-Lewisa, and sialyl-Lewisx blood group antigens in human gastric carcinoma and in normal gastric tissue. Cancer Res. 1989;49:745–52. [PubMed] [Google Scholar]
- 27.Zhou H, Schaefer N, Wolff M, et al. Carcinoma of the ampulla of Vater: comparative histologic/immunohistochemical classification and follow-up. Am J Surg Pathol. 2004;28:875–82. doi: 10.1097/00000478-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Inata J, Hattori N, Yokoyama A, et al. Circulating KL-6/MUC1 mucin carrying sialyl Lewisa oligosaccharide is an independent prognostic factor in patients with lung adenocarcinoma. Int J Cancer. 2007;120:2643–9. doi: 10.1002/ijc.22613. [DOI] [PubMed] [Google Scholar]
- 29.Sikut R, Zhang K, Baeckström D, et al. Distinct sub-populations of carcinoma-associated MUC1 mucins as detected by the monoclonal antibody 9H8 and antibodies against the sialyl-Lewis a and sialyl-Lewis x epitopes in the circulation of breast-cancer patients. Int J Cancer. 1996;66:617–23. doi: 10.1002/(SICI)1097-0215(19960529)66:5<617::AID-IJC6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 30.Burdick MD, Harris A, Reid CJ, et al. Oligosaccharides expressed on MUC1 produced by pancreatic and colon tumour cell lines. J Biol Chem. 1997;272:24198–202. doi: 10.1074/jbc.272.39.24198. [DOI] [PubMed] [Google Scholar]
- 31.Hanski C, Drechsler K, Hanisch FG, et al. Altered glycosylation of the MUC-1 protein core contributes to the colon carcinoma-associated increase of mucin-bound sialyl-Lewis(x) expression. Cancer Res. 1993;53:4082–8. [PubMed] [Google Scholar]
- 32.Hanski C, Hanski ML, Zimmer T, et al. Characterization of the major sialyl-Lex-positive mucins present in colon, colon carcinoma, and sera of patients with colorectal cancer. Cancer Res. 1995;55:928–33. [PubMed] [Google Scholar]
- 33.Schallmeiner E, Oksanen E, Ericsson O, et al. Sensitive protein detection via triple-binder proximity ligation assays. Nat Methods. 2007;4:135–7. doi: 10.1038/nmeth974. [DOI] [PubMed] [Google Scholar]


