Abstract
Neural tube defects (NTDs) are complex congenital malformations resulting from incomplete neurulation in embryo. Despite surgical repair of the defect, most of the patients who survive with NTDs have a multiple system handicap due to neuron deficiency of the defective spinal cord. In this study, we successfully devised a prenatal surgical approach and transplanted mesenchymal stem cells (MSCs) to foetal rat spinal column to treat retinoic acid induced NTDs in rat. Transplanted MSCs survived, grew and expressed markers of neurons, glia and myoblasts in the defective spinal cord. MSCs expressed and perhaps induced the surrounding spinal tissue to express neurotrophic factors. In addition, MSC reduced spinal tissue apoptosis in NTD. Our results suggested that prenatal MSC transplantation could treat spinal neuron deficiency in NTDs by the regeneration of neurons and reduced spinal neuron death in the defective spinal cord.
Keywords: neural tube defects, prenatal treatment, mesenchymal stem cell, in utero MSCs transplantation, neuron regeneration, neurotrophic factors
Introduction
Neural tube defects (NTDs) are complex congenital malformations resulting from incomplete neurulation during the 4th week of gestation in human. Spina bifida and anencephaly are the most common and severe forms of NTD affecting about 1 in 2000 live births worldwide [1]. In China, approximately 80,000 to 100,000 newborns each year are diagnosed to have NTDs [2]. Nowadays, many babies could survive with spina bifida attributable to improvements in medical and surgical management and care. However, most of these patients who survive with NTDs have multiple system handicap and limited life expectancy [3-5]. Prenatal repair of NTDs in foetuses has been shown to reduce the incidence of shunt-dependent hydrocephalus, as well as hindbrain herniation in some patients [3, 4, 6–8]. However, foetal surgical repair of the defects did not improve neurological outcome of patients [4]. Individuals with lumbosacral spina bifida continue to experience motor and sensory dysfunction of lower limbs, dysregulated anal and urethral sphincters after birth [9].
Foetal cellular therapy is a treatment option for a variety of birth defects. There are several perceived advantages of in utero cell transplantation: (i) the rapid growth of the foetus provides opportunity for engraftment and expansion of grafted cells, (ii) the immature immune system of the foetus would not reject the transplanted foreign cells and (iii) early treatment of disease is beneficial or critical for effective treatment [10]. Foetal cellular therapy has been employed in the treatment of congenital haematologic disorders and immunodeficiency disease [11-13], and also in experimental myelomeningocele (MMC) foetal lamb model [14].
Our previous studies have revealed that sensory and motor neuron deficiency is a primary anomaly coexisting with the spinal malformation in foetal rats with spina bifida aperta. The intrinsic neuron deficiency of the spinal cord could explain the observation that surgical repair of the unclosed neural tube fails to improve neural functions [15-17]. Taken together all these suggest that neuron replacement therapy basis on neuron regeneration or cell replacement of the defective spinal cord would be a promising approach to achieve better functional outcome in spina bifida patients.
Mesenchymal stem cells (MSCs) are multipotent stem cells and could differentiate into different cell types such as skeletal muscle, vascular, neuron, astrocyte, intestinal, bone and liver cells in response to different factors [18, 19]. A number of studies indicated that MSCs trans-differentiated into neurons both in vitro and in vivo[20-24]. In particular, MSCs are advantageous over other types of stem cells such as neural stem cell (NSC) in neuro-regenerative medicine due to their convenient isolation, lack of significant immunogenicity permitting allogenic transplantation without immunosuppressive drugs, lack of ethical controversy and their potentials to differentiate into tissue-specific cell types with trophic activity [18]. Spina bifida develops starting from the 4th week of gestation, and it can be diagnosed with prenatal α-fetoprotein screening and ultrasonography before the 12th week of gestation [4, 25]. Prenatal diagnosis of spinal bifida allows early intervention, which could ameliorate the aggravation of the deficits [26]. In this study, we evaluated for the first time the therapeutic potentials of a combination of surgical repair and in utero MSCs transplantation in the treatment of spina bifida in a rat model.
Materials and methods
Experiment animals
Outbred Wister rats of 10–12 weeks of age (250–300 gm) and 4 weeks (about 100 gm) of age were purchased from animal centre of China Medical University. The appearance of vaginal plugs in the female rat the morning after mating was timed as the embryonic day 0 (E0). Spina bifida aperta were induced with a single intragastric retinoic acid (140 mg/kg body weight; Sigma-Aldrich, St. Louis, MO, USA) administration on E10 as previously described [27]. All the animal experiments were performed with the approval obtained from the ethics committee of China Medical University.
Isolation, culture expansion, in vitro neuronal differentiation and transfection of bone marrow-derived MSCs
MSCs were isolated from 4-week-old Wister rat following previously published protocol [22]. Rat MSCs were cultured in DMEM/F12 (Gibco, Brooklyn, NY, USA) supplemented with 10% foetal bovine serum (FBS; Hyclone, Shrewsbury, NJ, USA) and 100 IU/ml penicillin to 100 μg/ml streptomycin (Gibco) on 25 cm2 tissue culture flasks (BD Biosciences, Franklin Lakes, NJ, USA). Primarily isolated MSCs were defined as P0, at confluency, cells were passaged (1 in 2 dilution) with fresh medium, and MSCs were cultured till passage 15, different passage MSCs were used for transplantation. Cultured MSCs expressed CD90, CD44, CD73 and CD29 but not CD34 and CD45 as revealed by flow cytometry using specific antibodies (551401, 550974, 551123, 562154, 560932, 554878; BD Biosciences) following previously published methods [28]. CD90 expression was used to estimate the purity of MSCs. For in vitro neural differentiation, P3-P5 MSCs were treated with BDNF alone (10 ng/ml) or BDNF (10 ng/ml) with retinoic acid (0.5 μM) for 14 days [29]. Twenty-four hours before transplantation, MSCs were transfected with eGFP expression adeno-5 vector (SinoGenoMax.Co., Ltd, Beijing, China) (100 pfu/1 cell), for the visualization of MSCs after transplantation into rat foetus. Before transplantation, cells were trypsinized, centrifuged, resuspended in small aliquot of fresh medium, GFP positive cell numbers were counted, the final concentration were adjusted to give a serial concentration ranged from 7000 to 35,000 cells/μl.
Foetal surgery and microinjection
We developed a new approach to transplant cells into foetuses with combined techniques of foetal surgery, microinjection and microsurgery. Retinoic acid treated pregnant rats of E16, E17 or E18 were anaesthetized with pentobarbitone sodium (40 mg/kg body weight). An incision was made in the abdominal wall, and the uterine horn was exteriorized. To relieve uterine spasm, the uterus was covered with wet gauze immersed with warm physiologic saline and atropine (0.1 mg/kg body weight) was given intraperitoneally. Under the microscope the position of lumbosacral spine of foetus was identified through the wall of uterus. Then 7–0-nylon purse-string suture and a small incision were made on the wall of uterus. The amniotic sac was opened and the defective region of spinal cord was exposed. GFP transfected MSCs suspension (0.2 μl/injection site) was injected into the defective region of spinal cord with a micropipette (internal tip diameter 100 μm) connected to a Hamilton syringe. Micropipettes for injection were made from borosilicate glass capillaries (model GD-1; Narishige Scientific Instruments, Tokyo, Japan) by using a micropipette puller (model PB-7; Narishige Scientific Instruments). After MSCs injection, the foetuses were returned to the uterus, and the wound of the uterus was closed (Fig. 1). In average, two to three foetuses could be injected in one dam without compromising the survival of the foetuses. The pregnant rats recovered from the anaesthesia within 1 hr and were returned to their home cage.
Fig 1.
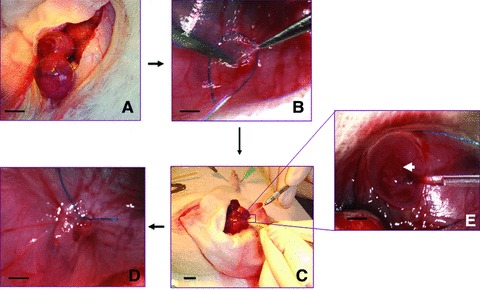
Foetal surgery and microinjection. (A) An incision was made in the abdominal wall to exteriorize the uterine horn. (B) Lumbosacral spine of foetus was identified through the wall of uterus under microscope, a 7-0-nylon purse-string suture and a small incision were made on the wall of uterus. (C) The amniotic sac was opened and the defective region of spinal cord was exposed. GFP transfected MSCs were injected into the defective region of spinal cord with a micropipette connected to a Hamilton syringe. (D) After MSCs injection, the foetuses were returned to the uterus, and the wound of the uterus was closed. (E) Enlarged view of (C), arrow indicates the defective spinal cord. (A, C) Scale bar: 5 mm. (B, D, E) Scale bar: 2 mm.
Survived cell observation and counting after transplantation
The pregnant rats on E20 were reanaesthetized with an overdose of pentobarbitone sodium, and the foetuses that had been transplanted were harvested by caesarean section. Foetuses were perfused transcardially with 15 ml physiologic saline followed by 25 ml 4% paraformaldehyde. The lumbosacral spinal column containing muscle, spinal cord and subcutaneous tissue was dissected and post-fixed in the same fixative for 24 hrs at 4°C, followed by cryopreservation in 20% sucrose for 24 hrs. The spinal column were then sectioned into 30 μm serial transverse sections, and all GFP positive MSCs in the spinal column were observed and counted under fluorescence microscope (Nikon, Tokyo, Japan). All the sections were also stained with DAPI, and only those GFP positive cells that contained a nucleolus were counted to avoid the same GFP cell being counted more than once. The MSCs survival rates were calculated as the average of survived cell numbers/totally injected cell numbers/spinal cord. Image was taken with C1 confocal microscope (Nikon, Tokyo, Japan) or fluorescence microscope connected to a CCD camera (Nikon). Sections with GFP positive MSCs observed were marked and kept at –80°C in the dark for further immunofluorescence or TUNEL study.
Immunofluorescence
To evaluate the differentiation potentials of transplanted MSCs in the spinal column, sections were analysed by immunofluorescence using antibodies against neuron, glia and GFP as previously described [30]. Primary antibodies used were mouse anti-nestin (1:100) (MAB353; Millipore, Billerica, MA, USA), mouse anti-GFAP (1:200) (MAB3402; Millipore), mouse anti-p75 NGFR (MAB365; Millipore), mouse anti-neurofilament (1:500) (ab24570; Abcam, Cambridge, MA, USA), mouse anti-myoD (1:100) (554130; BD Biosciences), rabbit anti-BDNF (1:50) (bs-0248R; Bios, China), rabbit anti-NGF (1:50) (bs-0067R; Bios, China). In brief, after antigen retrieval, sections were blocked with PBS containing 10% FBS and 0.1% Triton x-100, the specimen was incubated with primary antibodies in combination with rabbit anti-GFP (1:200) or mouse anti-GFP in case of BDNF and NGF staining (1:200) (AG279, AG281; Beyotime Institute of Biotechnology, China) in 10% FBS-PBST for overnight at 4°C. After primary antibodies incubation, sections were washed three times with PBS followed by incubation with Alexa Fluor 488-conjugated goat anti-rabbit IgG antibody (Invitrogen, Carlsbad, CA, USA) and TRITC-conjugated goat anti-mouse IgG (AP124R; Millipore) in 10% FBS-PBST for 1 hr at room temperature. After washing, sections were stained with DAPI (C1002; Beyotime Institute of Biotechnology), then mounted with anti-fade mounting medium (P0126; Beyotime Institute of Biotechnology). To determine the percentage of MSC differentiation, all GFP immuno-positive cells and all GFP immuno-positive cells that were also immuno-positive for the above neuron/glia marker on each section were counted.
For cell staining, microscopic slide with MSCs seeded 24 hrs before were fixed with 4% paraformaldehyde for 30 min., and were analysed by immunofluorescence. Ten (40χ magnification) non-contiguous and non-overlapping random chosen microscopic fields distributed evenly across the slide were examined, the number of MSCs expressing the specific neuron/glia marker was counted by observers who were not informed of the identities of the experiments. The percentages of MSCs expressing specific marker were determined and reported as the number of positive cells/total cells.
TUNEL analysis
TUNEL analysis and immunofluorescence for GFP was performed using In Situ Cell Death Detection Kit (Roche, Indianapolis, IN, USA) and with mouse anti-GFP antibody (Beyotime Institute of Biotechnology) according to manufacturer's protocol. Briefly, after antigen retrieval the sections were blocked with PBS containing 10% FBS and 0.1% Triton x-100, and incubated with anti-GFP antibody (1:200) at 4°C overnight. Then the sections were washed and incubated with TUNEL reaction mixture and TRITC-anti mouse IgG at 37°C for 1 hr. After washing, sections were stained with DAPI and mounted with anti-fade mounting medium. The images were taken with C1 confocal microscope (Nikon).
Real time PCR analysis
Eleven spinal cords transplanted with MSCs (MSCs transplantation group), five spinal cords injected with fresh medium (blank injection group) and 12 spinal cords without injection (no injection group) of E20 foetus with spina bifida aperta were dissected, and total RNA were isolated with RNeasy mini kit (Qiagen, Hilden, Germany) according to manufacturer's instruction. For cultured cells, RNA was isolated from at least 5 χ 105 cells. All RNA were quantified, and RNA with A260 nm/A280 nm ratio less than 1.8 was discarded. Total RNA (2 μg) was reverse-transcribed at 37°C for 15 min., 85°C for 5 sec. and 4°C with random 6-mer oligos (50 pmol), oligo dT primer (25 pmol) and the PrimeScript RT reagent kit (Takara, Shiga, Japan) in 20 μl of reaction solution. RT–PCR products were diluted 1:10. Diluted RT–PCR products (2 μl) were subjected to quantitative real-time PCR using a SYBR® Premix Ex Taq™ II (Perfect Real Time) kit (Takara) in 20 μl of reaction solution containing 2μl cDNA templates, 0.4 μM of each primer and 10 μl of 2χSYBR Green Master Mix and brought to final volume with RNase-free water, the reaction were performed in triplicate in LightCycler® Capillaries (04929292001; Roche) with a Light Cycler 1.5 system (Roche). PCR was performed as follows: pre-denaturation at 95°C for 30 sec., 45 cycles of denaturation at 95°C for 5 sec., annealing at 55°C–63°C for 20 sec. Negative control (without reverse transcriptase) gave no signal. The relative level of gene expression for each sample was calculated using the 2−ΔΔct method, and expressed as a fold induction compared with no injection control from the same pregnant rats after #223;-actin normalization. GFP expression was analysed with Premix Ex Taq™ (Perfect Real Time) kit (Takara). For GFAP and neurofilament expression, PCR products were also analysed with 3% agarose gel. Detailed information of primers and probe used in this study were listed in Table 1.
Table 1.
Sequences of primers and probe used in this study
| Gene | Accession number | Primer sequences (5′-3′) /exon location (nt) | Annealing temperature (°C) | PCR product (bp) |
|---|---|---|---|---|
| BDNF | NM_012513 | Sense GTCACAGTCCTGGAGAAAGTC / 1156–1176 Antisense: GATTGGGTAGTTCGGCATT / 1304–1286 | 60 | 149 |
| NGF | XM_227525 | Sense: GCGTAATGTCCATGTTGTTC / 251–270 Antisense: GTTTAGTCCAGTGGGCTTCA / 373–354 | 58 | 104 |
| NT-3 | NM_031073 | Sense: CGTCCCTGGAAATAGTCATA / 255–274 Antisense: CACGGAGATAAGCAAGAAAT / 364–345 | 60 | 110 |
| Caspase-3 | NM_012922 | Sense: GGAACGAACGGACCTGTGG / 441–459 Antisense: CGGGTGCGGTAGAGTAAGC / 660–642 | 60 | 220 |
| Bcl-2 | NM_016993 | Sense: GAGCGTCAACAGGGAGATG / 705–723 Antisense: GAGACAGCCAGGAGAAATCA / 874–855 | 60 | 170 |
| GFAP | NM_017009 | Sense: GACTATCGCCGCCAACTGC / 845–863 Antisense: TGGTAACTCGCCGACTCCC / 969–951 | 62 | 125 |
| Nestin | NM_012987 | Sense: GGAGCAGGAGAAGCAAGGT / 931–949 Antisense: GGGTCCAGAAAGCCAAGAG /1116–1098 | 63 | 186 |
| NF* | NM_012607 | Sense: TGAGAAACACCAAATGGGAGA /1164–1184 Antisense: CAAAGCCAATCCGACACTC / 1298–1280 | 62 | 135 |
| #223;-actin | NM_031144 | Sense: GGAGATTACTCCCTGGCTCCTA / 1026–1048 Antisense:GACTCATCGTACTCCTGCTTGCTG/1175–1152 | 60 | 150 |
| GFP | HM771697 | Sense: CTTCAAGATCCGCCACAACATC / 7597–7618 Antisense: ACCATGTGATCGCGCTTCTC / 7761–7742 Hydrolysis probe: 5′- (FAM)-CGCCGACCACTACCAGCA GAACACC(Eclipse)-3′/ 7639–7663 | 60 | 165 |
Neurofilament gene heavy chain.
Statistical analysis
All analyses were performed in a double-blind manner. All values are expressed as mean ± SEM. Student's t-test and Bonferroni's test were used for single and multiple comparisons, respectively. Chi-square test was used for comparisons of foetuses survival rates. P values less than 0.05 were considered to be statistically significant.
Results
Culture, character and in vitro neuronal differentiation of MSCs
In culture, MSCs displayed fibroblast-like, spindle-shaped morphology. Cultured MSCs were CD90, CD44, CD29 and CD73 positive but negative for CD34 and CD45 (Fig. 2A). The purity of MSCs in culture was examined by CD90 expression. The purity of MSCs was enriched from 65% at P0 to 80% at P1, and >90% from P2 to P15 (Fig. 2B). No obvious morphological changes were observed within the culture period. We next examined nestin (neural precursor cell marker) and BDNF (brain-derived neurotrophic factor) expression in cultured MSCs (Fig. 2C). The percentages of P3-P6 MSCs expressing nestin and BDNF were around 3–4% (nestin, 3.01 ± 0.17%; BDNF, 4.25 ± 0.13%). The percentages of MSCs expressing these markers increased with prolonged culture (P10-P14) (nestin, 6.1 ± 0.76%; BDNF, 6.64 ± 0.69%) (Fig. 2D), which was indicative of increasing neuronal differentiation potential of cultured MSCs. Elevated BDNF expression in prolonged cultured MSCs was further confirmed by quantitative real time RT-PCR analysis (Fig. 2E). We also studied neural differentiation of MSCs in vitro. After treatment with BDNF (10 ng/ml) and retinoic acid (0.5 μM) for 14 days, MSCs expressed nestin, GFAP and neurofilament, indicated that MSCs differentiated into neural lineage (Fig. 2F). Real time PCR analysis showed weak GFAP and neurofilament expression in longer cultured MSCs, and the expression greatly increased in BDNF and retinoic acid treated MSCs compared with BDNF treatment only (Fig. 2G and H).
Fig 2.
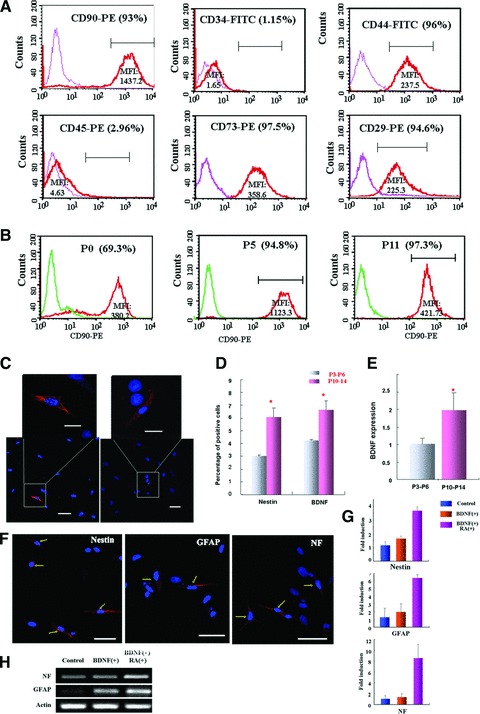
Characterization of cultured MSCs and in vitro differentiation of MSCs. (A) Surface marker expression was analysed by flowcytometry. MFI: median fluorescence intensity. (B) Percentages of MSCs in cultures expressing CD90. (C) Confocal images showing nestin and BDNF expression in cultured MSCs. Regions highlighted were magnified and shown above. Scale bar in lower panel: 50 μm; upper enlarged panel: 20 μm. (D) Quantitative analysis of nestin and BDNF expression in P3-P6 and P10-P14 MSCs. Percentages of MSCs expressing nestin and BDNF in P3-P6 and P10-P14 cultures were determined by the number of expressing cells/total cells in ten random microscopic fields. *P= 0.001 and **P= 0.02 compared with P3-P6 group. (E) Real time analysis of BDNF expression in cultured MSCs (P3-P6 and P10-P14). *P= 0.014 compared with P3-P6 group. (F) Neural marker expression in MSCs treated with BDNF and retinoic acid for 14 days. Arrows indicated positive cells. (G) Nestin, GFAP and neurofilament expression in BDNF and/or retinoic acid treated MSCs were also analysed with real time PCR. Values indicated fold induction compared with control cells. (H) PCR product of GFAP and neurofilament were analysed on 3% agarose gel. Control: cells cultured in normal condition for 14 days. BDNF (+): cells treated with BDNF (10 ng/ml) for 14 days. BDNF (+) RA (+): cells treated with BDNF (10 ng/ml) and retinoic acid (0.5 μM) for 14 days.
Survival of transplanted MSCs in foetal rat spinal column
A total of 86 pregnant rats received foetal surgery and microinjection (see Fig. 1 and Material and methods section) on E16, E17 and E18, and 5 died before E20, the other 81 were killed on E20 for spine sample collection. From the 81 pregnant rats, a total of 195 transplanted foetuses were harvested, and 152 foetuses (77.9%) were alive. The overall survival rates in different operation groups were tabulated (Table 2), and better survival was achieved in the operation groups performed on later development stages (E17 or E18) as compared with E16 group, but it did not reach a statistical significance (P= 0.67 E16 versus E17; P= 0.25 E16 versus E18). Lumbosacral spinal columns of 136 foetuses were sectioned, and spinal cords of the other 16 foetuses (including 5 foetuses received blank injection) were processed for RNA isolation. Transplanted MSCs (GFP positive) mainly located in the defective region of spinal cord and displayed different cell shapes such as round, long and spindle, or with long processes (Fig. 3A). In average, 22.8 ± 23.1% (n= 96) of transplanted MSCs survived in the spinal column. In addition, GFP cells were occasionally detected in regions away from the injection site including the normal spinal cord, muscle, subcutaneous tissues and ganglion (Fig. 3B). These GFP cells could represent transplanted cells those migrated out of the injection site and/or those delivered outside of the spinal cord during the prenatal cell delivery procedure.
Table 2.
Overall survival rate of foetal surgery and MSC transplantation
| E16 | E17 | E18 | |
|---|---|---|---|
| Total foetus injected | 120 | 52 | 23 |
| Alive foetuses after foetal surgery | 91 (75.8%) | 41 (78.8%) | 20 (87.0%) |
Fig 3.
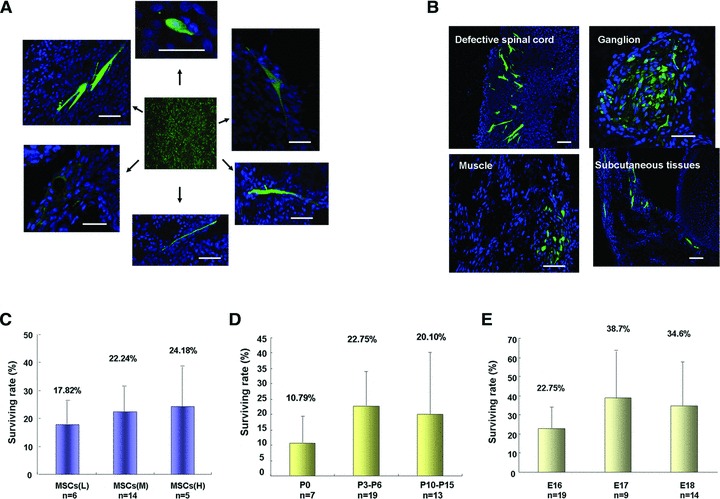
Survival of transplanted MSCs in rat foetus. (A, B) Representative confocal images of transplanted MSCs (GFP positive, green) survived on rat spinal column. Transplanted MSCs presented with various shapes (A) and were localized in different region of spinal column (B). Scale bar: 50 μm. (C–E) Effect of injection amount (C), cell passages (D) and development stages of foetuses (E) on transplanted MSCs survival. Data are shown as mean ± SEM, and numbers (n) of spinal column analysed were indicated under each column.
To improve the survival of MSCs in rat spinal column, we examined factors that may contribute to MSCs survival after transplantation. Firstly, we examined the impact of number of the injected cell. MSCs survival was found to be 17.82 ± 9.85% in the low injection group (L) (1000–2000 cells/injection/spinal cord), 22.24 ± 12.01% in the medium injection group (M) (2000–4000 cells/injection/spinal cord) and 24.28 ± 18.85% in the high injection group (H) (4000–6000 cells/injection /spinal cord). MSCs survival increased slightly if more cells were injected, but the increase did not reach a statistical significance (P= 0.99, L versus M and P= 0.86, L versus H) (Fig. 3C). Secondly, we investigated if prolonged culture of MSCs affects the survival of transplanted MSCs. P3-P6 and P10-P14 cells presented similar survival (22.75 ± 12.2% and 20.01 ± 22.5%), which was higher than that of P0 cells (10.79 ± 9.4%) (P= 0.22, P0 versus P3-P6 and P= 0.56, P0 versus P10-P14) (Fig. 3D). Thirdly, we evaluated the effects of the embryonic stages of recipients on the survival of transplanted MSCs. Less MSCs (22.75 ± 12.2%) survived in E16 foetal spinal column than in E17 (38.7 ± 25.1%) and E18 (34.6 ± 23.5%) group, again the differences did not reach a statistical significance (P=0.1, E16 versus E17 and P=0.18, E16 versus E18, respectively) (Fig. 3E).
Differentiation of transplanted MSCs in spinal column with spina bifida aperta
To ascertain if transplanted MSCs generated different types of cell in the recipient spinal column, we examined the expressions of specific cell markers in transplanted MSCs. During the 2–4 days of embryonic development, transplanted MSCs expressed markers for neural precursor cell (nestin), neuron (neurofilament), neurogliocyte (GFAP), motoneuron (P75NGFR) and myoblast (MyoD) (Fig. 4A). Systematic view of the images was shown in Figure S1. In line with the increasing neuronal differentiation potentials of MSCs in prolonged culture (Fig. 2D), prolonged cultured MSCs generated more neurons in the spinal column (Fig. 4B and Table 3). Development stages of the recipients also affected neural differentiation of MSCs (Fig. 4C). MSCs in E16 spinal column displayed significantly higher neuronal marker expression as compared with E18 group (Table 3), there is a tendency of higher neural maker expression of MSCs survived in E16 spinal column compared with E17 group, but the differences are not significant (P= 0.1, 0.16, 0.17 for nestin, GFAP and NF, respectively) (Table 3). Our results indicated that transplantation of MSCs into rat foetus on E16 favoured MSCs differentiation.
Fig 4.
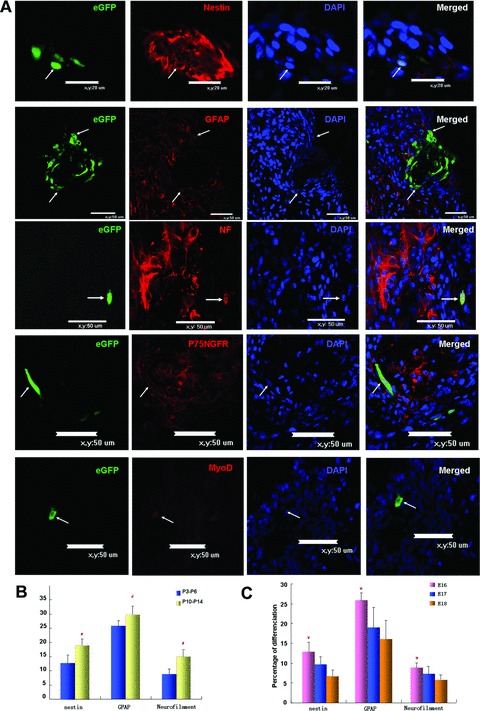
Differentiation of transplanted MSCs in spinal column. (A) Confocal images showing colocalization of GFP and cell-specific markers (red) (arrows). Scale bar: 20 μm for nestin expression; scale bar: 50 μm in all the other panels: (B) Quantitative analysis of nestin, neurofilament and GFAP expressing MSCs in rat foetal spinal column with P3-P6 and P10-P14 cultured MSCs. (C) Quantitative analysis of nestin, neurofilament and GFAP expression in MSCs transplanted in E16, E17 and E18 spinal column. Percentages of transplanted MSCs expressing these markers were determined as the number of double positive cells/total GFP positive cells.#P < 0.05 compared with P3-P6 group. *P < 0.05 compared with E18 group.
Table 3.
Effect of different development stages and MSCs passages on various neural markers expression in transplanted MSCs
| E16 (PC/total) | E17 (PC/total) | E18 (PC/total) | |||
|---|---|---|---|---|---|
| P3-P6 | P10-P14 | P3-P6 | P3-P6 | ||
| Nestin | 12.7%± 2.7% (P= 0.0094)* | 19.3%± 1.7% (P= 0.017)† | 9.23%± 2.1% | 6.61%± 1.5% | |
| Neurofilament | 8.94%± 1.1% (P= 0.01)* | 15.5%± 1.7% (P= 0.01)† | 7.25%± 1.5% | 5.85%± 1.1% | |
| GFAP | 25.5%± 2.1% (P= 0.038)* | 29.6%± 2.6% (P= 0.03)† | 18.99%± 5.1% | 16.3%± 4.5% | |
Compared with E18 group.
Compared with P3-P6 group.
Neurotrophic factors induction by MSCs transplantation
Besides neuron replacement, the neurotrophic factors induced by MSCs transplantation also play an important neuroprotective role in spina bifida aperta. After transplantation, 22.7% (69/304) and 21.6% (54/250) MSCs expressed BDNF and NGF, respectively, BDNF expression is much higher than before transplantation (Figs 5A and 2D). As shown in Figure 5B, immuno-reactivity of BDNF and NGF was localized to both the GFP immuno-positive transplanted cells, and in the adjacent host spinal cells. Taken all these suggested that transplanted MSCs not only expressed BDNF and NGF themselves, but also induced BDNF and NGF expression in the host spinal tissue. In MSCs transplantation group (successful transplantation was confirmed by real time analysis of GFP expression in dissected spinal cord), expression of neurotrophic factors in the spinal cord increased markedly (Fig. 5C). NGF and NT-3 expression were significantly higher in the transplanted groups (P 0.03 and 0.017 compared with blank injection group, respectively). BDNF expression was also elevated in MSCs transplantation group, although it was not significantly different from blank injection (P= 0.26), which could be attributable to the relatively high basal BDNF expression in the spinal tissue [31].
Fig 5.
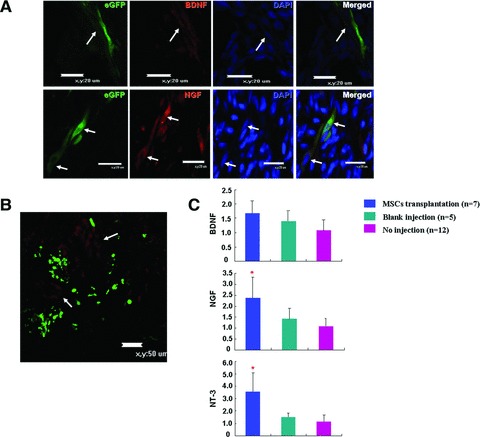
Neurotrophic factors induction by MSCs transplantation. (A) Confocal images showing transplanted MSCs expressed BDNF (red; upper) and NGF (red; lower) (arrows). Scale bar: 20 μm. (B) Confocal image showing NGF expression around the transplanted MSCs. Arrows indicated NGF positive cells (red) in the spinal tissue in adjacent to transplanted MSCs. Scale bar: 50 μm. (C) Real time analysis of neurotrophic factors expression in E20 spinal cords transplanted with MSCs (MSCs transplantation, n= 7), injected with same amount of medium (blank injection, n= 5) and without injection (no injection, n = 12). *P < 0.05 compared with blank injection.
Reduction of apoptosis after MSCs transplantation
Apoptosis plays an important role in the development of NTDs [32, 33]. We studied the apoptosis of whole spinal column after transplantation by TUNEL analysis. Apoptotic cells were frequently localized at the region of defective spinal cord. In contrast, apoptotic cells were rarely found at the defective spinal cord region around the transplanted MSCs (Fig. 6A). Apoptosis was not observed in transplanted MSCs. In addition, the expression of caspase-3 and Bcl-2 in transplanted spinal cord was analysed by real time PCR analysis. Caspase-3 expression was significantly lower in MSCs transplantation group as compared with blank injection group (P= 0.03) and no injection group while Bcl-2 expression was not significantly different in the three groups (Fig. 6B).
Fig 6.
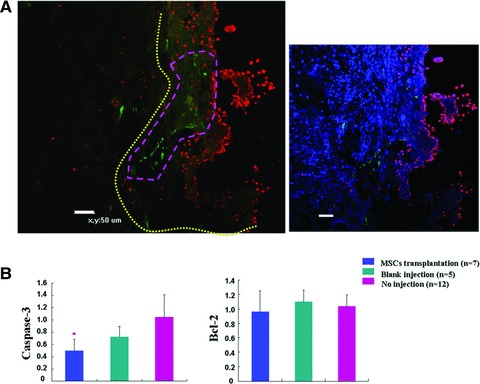
Reduction of apoptosis in the spinal column after MSCs transplantation. (A) Left: Representative confocal image showing decreased apoptosis around the transplanted MSCs. Yellow line demarcated the region of defective spinal cord, and purple line outlined the region where transplanted MSCs were localized. Apoptotic cells (red dots) were frequently localized in the defective spinal cord region away from the site of transplanted MSCs. In contrast, apoptotic cells were seldom found in the regions adjacent to the transplanted MSCs. Right: DAPI staining merged view of the left image. Scale bar: 50 μm. (B) Real time analysis of casapase-3 and Bcl-2 expression in E20 spinal cords transplanted with MSCs (MSCs transplantation, n= 7), injected with same amount of medium (blank injection, n= 5) and without injection (no injection, n= 12). Values presented fold expression differences compared with no injection group. P= 0.03 compared with blank injection.
Discussion
Congenital malformations affect approximately 5% of all live births every year [34,35]. Several of these are associated with in utero foetal demise, significant neonatal morbidity and mortality. Despite early diagnosis of the condition in most of the cases, treatment options for the affected foetuses are still limiting and not satisfactory. Today, open IUMR (in utero MMC repair) is being offered to selected mothers in several centres [15-17]. In this study, we examine the feasibilities and potentials of combination of surgical repair and in utero stem cell transplantation aiming at the attenuation of the progress of the malformation and possible neuron regeneration for the treatment of spina bifida.
It is apparent that the survival of the foetus and the mother is the first challenge in foetal surgery especially in earlier embryonic development stages as E16 and E17. Our new microsurgical and microinjection approach achieves over 75% survival of the operated foetuses and 94% survival of the mothers. Transplanted MSCs expressed markers of neural precursor cells, neurons and neurogliocytes in the spinal column of rats with spina bifida aperta. The highest neural differentiation (19.2% MSCs differentiated into neural precursor cells, 16% into neurons and 33% into neurogliocytes) was obtained with the transplantation of P10-P14 MSCs into E16 foetuses after 4 days (Fig. 4B and C, Table 3). MSCs have also been shown to give rise to neurons in the nervous system of adult or neonatal animals [20, 22, 40–42]. However, the efficiency of MSCs differentiation into neurons was reported to be only 3% at 28 days post-transplantation in adult mice [40]. The higher neural differentiation of MSCs in the foetal rat spinal column suggests that the rapidly growing foetal nervous system favours the differentiation of transplanted cells into neuronal lineage. Expression of neurotrophic factors from transplanted MSCs and from the surrounding rat spinal tissue was found associated with reduced apoptosis of the rat spinal tissue, which is indicative of neuroprotective effect of MSCs on neural tissue. In line with our observations, neuroprotective effect of transplanted MSCs has also been previously reported [23, 43, 44]. In addition to neuron replacement potential of MSCs, neuroprotective functions of transplanted MSCs is also critical in the success of therapy in the context of spina bifida aperta since apoptosis plays an important role in the development of NTDs. Reduction of apoptosis of the spinal tissue could be partly explained by inhibition of caspase-3 activity by neurotrophic factors secreted from the MSCs and the surrounding spinal tissue [45, 46]. In addition, Sadat et al. previously reported that adipose tissue-derived stem cells have a marked impact on anti-apoptosis through secreting IGF-I and VEGF, indicating there exits other stem cell sources than MSCs having anti-apoptotic effects [45].
Our data indicated that (i) MSCs differentiated into neurons in spinal cord, (ii) transplanted MSCs and the surrounding host spinal tissue secreted neurotrophic factors and (iii) apoptosis was reduced in the spinal tissues with transplanted MSCs. Functional outcome of prenatal MSCs transplantation especially in the recovery of sensorimotor functions has not been determined in present study due to the fact that those rat foetuses with spina bifida aperta died after birth. In future, we shall modify our surgical intervention so as to treat the foetuses at even earlier stages (E14 or E15) aiming to stop the damage progress of the unclosed neural tube at earlier stage of development so as to enhance the survival of MSCs implanted rats after birth, thus allowing assessment of the functional outcome of our treatment.
Foetal surgery and NSC delivery in experimental MMC foetal lamb model enhanced the survival and reduced the incidence of major paraparesis [14]. The transplanted NSCs remained as undifferentiated stem cells and produced neurotrophic factors in the recipient spinal cord. The beneficial effects of the NSC on the MMC foetal lamb was likely attributable to the production of neurotrophic factors from the transplanted NSC. It is still not known if transplanted NSC will differentiate into neurons in long term and replaced the missing neurons in the defective spinal cord.
In conclusion, we have established a new therapy for the potential treatment for spina bifida aperta, and this is the first attempt in the prenatal treatment of spina bifida aperta using MSCs transplantation. We provide evidence indicating that the transplanted MSCs could ameliorate sensory and motor neuron deficiency in spinal malformation by differentiation into neurons in situ and reduction of spinal neuron apoptosis. MSCs are multipotent and could differentiate into various cell types in addition to neurons. In future, the application of MSCs in cell replacement therapy for other congenital anomalies especially complicated malformations besides NTDs should be explored.
Acknowledgments
This study was supported by National Natural Foundation of China (Grant numbers: 81070538, 30872705, 30801242, 30371476).
Author contributions
H.L. designed, performed and analysed all of the experiments besides foetal operation. F.G. and L.M. performed cell culture, animal experiments and histology. J.J. and D.W. performed cell culture. J.M., M.J. and B.L. performed animal experiments. Y.F., L.W., W.W. and V.C.L. helped to write the manuscript. Z.Y. supervised the project, performed all the foetal operations and wrote the manuscript with H.L.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Supporting Information
Systematic view of the images shown in Figure 4A.(A–E) correspond to Figure 4A up to low panel. Boxedregion were enlarged in Figure 4A. Scale bars: 60 μm.
Fig. S2 Negative control performed for the staining shownin Figures 4 and 5. (A) Negative control using mouse IgG asfirst antibody. (B) Nestin, GFAP and NF expression innon-transplanted spinal column. (C) Negative control usingrabbit IgG instead of BDNF or NGF antibody.
References
- 1.Danzer E, Ernst LM, Rintoul NE, et al. In utero meconium passage in fetuses and newborns with myelomeningocele. J Neurosurg Pediatr. 2009;3:141–6. doi: 10.3171/2008.10.PEDS08199. [DOI] [PubMed] [Google Scholar]
- 2.Zhu L, Ling H. National Neural Tube Defects Prevention Program in China. Food Nutr Bull. 2008;29:S196–204. doi: 10.1177/15648265080292S123. [DOI] [PubMed] [Google Scholar]
- 3.Kohl T, Hering R, Heep A, et al. Percutaneous fetoscopic patch coverage of spina bifida aperta in the human-early clinical experience and potential. Foetal Diagn Ther. 2006;21:185–93. doi: 10.1159/000089301. [DOI] [PubMed] [Google Scholar]
- 4.Fichter MA, Dornseifer U, Henke J, et al. Foetal spina bifida repair-current trends and prospects of intrauterine neurosurgery. Foetal Diagn Ther. 2008;23:271–86. doi: 10.1159/000123614. [DOI] [PubMed] [Google Scholar]
- 5.Yuan Z, Cheng W, Hou A, et al. Constipation is associated with spina bifida occulta in children. Clin Gastroenterol Hepatol. 2008;6:1348–53. doi: 10.1016/j.cgh.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 6.ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 44, July 2003. (Replaces Committee Opinion Number 252, March 2001) Obstet Gynecol. 2003;102:203–13. [PubMed] [Google Scholar]
- 7.Hirose S, Farmer DL, Albanese CT. Foetal surgery for myelomeningocele. Curr Opin Obstet Gynecol. 2001;13:215–22. doi: 10.1097/00001703-200104000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Sutton LN. Foetal surgery for neural tube defects. Best Pract Res Clin Obstet Gynaecol. 2008;22:175–88. doi: 10.1016/j.bpobgyn.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rintoul NE, Sutton LN, Hubbard AM, et al. A new look at myelomeningoceles: functional level, vertebral level, shunting, and the implications for foetal intervention. Pediatrics. 2002;109:409–13. doi: 10.1542/peds.109.3.409. [DOI] [PubMed] [Google Scholar]
- 10.Muench MO, Barcena A. Stem cell transplantation in the fetus. Cancer Control. 2004;11:105–18. doi: 10.1177/107327480401100217. [DOI] [PubMed] [Google Scholar]
- 11.Roybal JL, Santore MT, Flake AW. Stem cell and genetic therapies for the fetus. Semin Foetal Neonatal Med. 2010;15:6. doi: 10.1016/j.siny.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Merianos D, Heaton T, Flake AW. In utero hematopoietic stem cell transplantation: progress toward clinical application. Biol Blood Marrow Transplant. 2008;14:729–40. doi: 10.1016/j.bbmt.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Muench MO. In utero transplantation: baby steps towards an effective therapy. Bone Marrow Transplant. 2005;35:537–47. doi: 10.1038/sj.bmt.1704811. [DOI] [PubMed] [Google Scholar]
- 14.Fauza DO, Jennings RW, Teng YD, et al. Neural stem cell delivery to the spinal cord in an ovine model of foetal surgery for spina bifida. Surgery. 2008;144:367–73. doi: 10.1016/j.surg.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Guan K, Li H, Fan Y, et al. Defective development of sensory neurons innervating the levator ani muscle in foetal rats with anorectal malformation. Birth Defects Res A Clin Mol Teratol. 2009;85:583–7. doi: 10.1002/bdra.20576. [DOI] [PubMed] [Google Scholar]
- 16.Yuan ZW, Lui VC, Tam PK. Deficient motor innervation of the sphincter mechanism in foetal rats with anorectal malformation: a quantitative study by fluorogold retrograde tracing. J Pediatr Surg. 2003;38:1383–8. doi: 10.1016/s0022-3468(03)00401-9. [DOI] [PubMed] [Google Scholar]
- 17.Jia H, Zhang K, Zhang S, et al. Quantitative analysis of sacral parasympathetic nucleus innervating the rectum in rats with anorectal malformation. J Pediatr Surg. 2007;42:1544–8. doi: 10.1016/j.jpedsurg.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 18.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–16. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Prockop DJ, Kota DJ, Bazhanov N, et al. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010;14:2190–9. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torrente Y, Polli E. Mesenchymal stem cell transplantation for neurodegenerative diseases. Cell Transplant. 2008;17:1103–13. doi: 10.3727/096368908787236576. [DOI] [PubMed] [Google Scholar]
- 21.Brazelton TR, Rossi FM, Keshet GI, et al. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–9. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Wang XD, Chen G, et al. Study of in vivo differentiation of rat bone marrow stromal cells into schwann cell-like cells. Microsurgery. 2006;26:111–5. doi: 10.1002/micr.20184. [DOI] [PubMed] [Google Scholar]
- 23.Hofstetter CP, Schwarz EJ, Hess D, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99:2199–204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pisati F, Bossolasco P, Meregalli M, et al. Induction of neurotrophin expression via human adult mesenchymal stem cells: implication for cell therapy in neurodegenerative diseases. Cell Transplant. 2007;16:41–55. doi: 10.3727/000000007783464443. [DOI] [PubMed] [Google Scholar]
- 25.Blaas HG, Eik-Nes SH. Sonoembryology and early prenatal diagnosis of neural anomalies. Prenat Diagn. 2009;29:312–25. doi: 10.1002/pd.2170. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe M, Jo J, Radu A, et al. A tissue engineering approach for prenatal closure of myelomeningocele with gelatin sponges incorporating basic fibroblast growth factor. Tissue Eng Part A. 2010;16:1645–55. doi: 10.1089/ten.TEA.2009.0532. [DOI] [PubMed] [Google Scholar]
- 27.Danzer E, Schwarz U, Wehrli S, et al. Retinoic acid induced myelomeningocele in foetal rats: characterization by histopathological analysis and magnetic resonance imaging. Exp Neurol. 2005;194:467–75. doi: 10.1016/j.expneurol.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Cipriani P, Guiducci S, Miniati I, et al. Impairment of endothelial cell differentiation from bone marrow-derived mesenchymal stem cells: new insight into the pathogenesis of systemic sclerosis. Arthritis Rheum. 2007;56:1994–2004. doi: 10.1002/art.22698. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–56. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 30.Barile L, Cerisoli F, Frati G, et al. Bone marrow-derived cells can acquire cardiac stem cells properties in damaged heart. J Cell Mol Med. 2011;15:63–71. doi: 10.1111/j.1582-4934.2009.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura M, Bregman BS. Differences in neurotrophic factor gene expression profiles between neonate and adult rat spinal cord after injury. Exp Neurol. 2001;169:407–15. doi: 10.1006/exnr.2001.7670. [DOI] [PubMed] [Google Scholar]
- 32.Cecconi F, Piacentini M, Fimia GM. The involvement of cell death and survival in neural tube defects: a distinct role for apoptosis and autophagy? Cell Death Differ. 2008;15:1170–7. doi: 10.1038/cdd.2008.64. [DOI] [PubMed] [Google Scholar]
- 33.Massa V, Savery D, Ybot-Gonzalez P, et al. Apoptosis is not required for mammalian neural tube closure. Proc Natl Acad Sci U S A. 2009;106:8233–8. doi: 10.1073/pnas.0900333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pathak S, Lees C. Ultrasound structural foetal anomaly screening: an update. Arch Dis Child Foetal Neonatal Ed. 2009;94:F384–90. doi: 10.1136/adc.2008.137919. [DOI] [PubMed] [Google Scholar]
- 35.Romosan G, Henriksson E, Rylander A, et al. Diagnostic performance of routine ultrasound screening for foetal abnormalities in an unselected Swedish population in 2000–2005. Ultrasound Obstet Gynecol. 2009;34:526–33. doi: 10.1002/uog.6446. [DOI] [PubMed] [Google Scholar]
- 36.Farmer DL, von Koch CS, Peacock WJ, et al. In utero repair of myelomeningocele: experimental pathophysiology, initial clinical experience, and outcomes. Arch Surg. 2003;138:872–8. doi: 10.1001/archsurg.138.8.872. [DOI] [PubMed] [Google Scholar]
- 37.Johnson MP, Sutton LN, Rintoul N, et al. Foetal myelomeningocele repair: short-term clinical outcomes. Am J Obstet Gynecol. 2003;189:482–7. doi: 10.1067/s0002-9378(03)00295-3. [DOI] [PubMed] [Google Scholar]
- 38.Zambelli H, Barini R, Iscaife A, et al. Successful developmental outcome in intrauterine myelomeningocele repair. Childs Nerv Syst. 2007;23:123–6. doi: 10.1007/s00381-006-0169-5. [DOI] [PubMed] [Google Scholar]
- 39.Tulipan N, Sutton LN, Bruner JP, et al. The effect of intrauterine myelomeningocele repair on the incidence of shunt-dependent hydrocephalus. Pediatr Neurosurg. 2003;38:27–33. doi: 10.1159/000067560. [DOI] [PubMed] [Google Scholar]
- 40.Jendelova P, Herynek V, DeCroos J, et al. Imaging the fate of implanted bone marrow stromal cells labeled with superparamagnetic nanoparticles. Magn Reson Med. 2003;50:767–76. doi: 10.1002/mrm.10585. [DOI] [PubMed] [Google Scholar]
- 41.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711–6. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shyu WC, Chen CP, Lin SZ, et al. Efficient tracking of non-iron-labeled mesenchymal stem cells with serial MRI in chronic stroke rats. Stroke. 2007;38:367–74. doi: 10.1161/01.STR.0000254463.24655.14. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigues Hell RC, Silva Costa MM, Goes AM, et al. Local injection of BDNF producing mesenchymal stem cells increases neuronal survival and synaptic stability following ventral root avulsion. Neurobiol Dis. 2009;33:290–300. doi: 10.1016/j.nbd.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 44.Chen CJ, Ou YC, Liao SL, et al. Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol. 2007;204:443–53. doi: 10.1016/j.expneurol.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Sadat S, Gehmert S, Song YH, et al. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem Biophys Res Commun. 2007;363:674–9. doi: 10.1016/j.bbrc.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 46.Hirata Y, Meguro T, Kiuchi K. Differential effect of nerve growth factor on dopaminergic neurotoxin-induced apoptosis. J Neurochem. 2006;99:416–25. doi: 10.1111/j.1471-4159.2006.04006.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Systematic view of the images shown in Figure 4A.(A–E) correspond to Figure 4A up to low panel. Boxedregion were enlarged in Figure 4A. Scale bars: 60 μm.
Fig. S2 Negative control performed for the staining shownin Figures 4 and 5. (A) Negative control using mouse IgG asfirst antibody. (B) Nestin, GFAP and NF expression innon-transplanted spinal column. (C) Negative control usingrabbit IgG instead of BDNF or NGF antibody.


