Abstract
Sirtuins (a class III histone deacetylase) have emerged as novel targets for cancer therapy. Salermide, a reverse amide compound that inhibits Sirtuin 1 (Sirt1) and Sirtuin 2 (Sirt2), has been shown to induce apoptosis in human cancer cells. The mechanism underlying cellular apoptotic signalling by salermide remains unclear. In this study, we show that salermide up-regulates the expression of death receptor 5 (DR5) in human non-small cell lung cancer (NSCLC) cells. Blocking DR5 expression by gene silencing technology results in a decrease in activated forms of several pro-apoptotic proteins (caspase-8, caspase-9, caspase-3, PARP). Increasing DR5 protein expression correlates with salermide-induced apoptosis in human NSCLC cells. We discovered that IRE-1α, Bip, activating transcription factor 3 (ATF4), activating transcription factor 3 (ATF3) and C/EBP homologous protein (CHOP) are induced by salermide, which suggests that DR5-dependent apoptosis is induced by endoplasmic reticulum stress. Moreover, knockdown of Sirt1 and Sirt2 expression resulted in up-regulation of ATF4, CHOP and DR5. Transfected NSCLC cells with ATF4, ATF3 or CHOP siRNA results in a decline in pro-apoptotic proteins (such as caspase-8, caspase-9, caspase-3 and PARP) despite salermide treatment. We demonstrate that salermide induces expression of ATF4, and ATF4 up-regulates ATF3 and subsequently modulates CHOP. This suggests that DR5 is modulated by the ATF4-ATF3-CHOP axis in NSCLC after Sirt1/2 inhibition or salermide treatment. This study highlights the importance of DR5 up-regulation in apoptosis induced by Sirt1/2 inhibition and elucidates the underlying mechanism in human NSCLC cells.
Keywords: salermide, death receptor 5 (DR5), ATF3, ATF4, CHOP, endoplasmic reticulum (ER) stress, Sirt1/2
Introduction
Recently, class I and II histone deacetylases (HDACs) have been identified as important regulators of growth in cancer cells. Thus, inhibitors of class I and II HDACs have emerged as targets for anticancer therapy, and several novel HDAC inhibitors have reached clinical trials [1]. In contrast to class I and II HDAC inhibitors, class III HDACs have been identified as having a dual role in both blocking and promoting tumour development. One sub-class of class III HDACs, nicotinamide adenine dinucleotide-positive (NAD+)-dependent class III HDACs (also termed Sirtuins) have been shown to induce and block apoptosis in neoplastic cells given the appropriate triggers [2]. Salermide has emerged as a novel HDAC inhibitor that inhibits Sirtuin 1 (Sirt1) and Sirtuin 2 (Sirt2) and results in programmed cell death [3].
Tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) can trigger apoptosis in a broad spectrum of cancer cells. Death receptor 5 (DR5) is one of TRAIL receptors and plays an important role in initiating the apoptotic cascade when cells are stressed such as exposure to chemotherapeutic agents [4]. Once DR5 is activated by TRAIL, the death receptor trimerizes and recruits Fas-associated death domain (FADD) protein and procaspase-8, both of which rapidly form a death-inducing signalling complex (DISC) [5, 6]. Caspase-8 cleaves caspase-3, which activates downstream effectors that initiate cellular apoptosis (extrinsic apoptosis pathway). In addition, activated caspase-8 cleaves Bid and the by-product tBid activates caspase 9, which also results in cellular apoptosis (intrinsic or mitochondrial apoptosis pathway) [7].
In times of cellular stress, DR5 expression can be induced without ligand–receptor interactions, resulting in a ligand-independent activation of the death receptor-mediated apoptotic signalling pathway [8, 9]. Some of the most powerful inducers of DR5 transcription that circumvent TRAIL–DR5 interactions include high local concentrations of SP1, AP1, p53, NF-κB,YY1 and C/EBP homologous protein [CHOP; also known as growth arrest and DNA damage gene 153 (GADD153); Ref. 10]. CHOP is one of the most potent inducer of DR5 and downstream apoptosis, and CHOP is frequently released during the endoplasmic reticulum (ER) stress response [11]. CHOP is typically undetectable in physiological conditions; however, it is dramatically increased during periods of ER stress, resulting in cell cycle arrest and ultimately apoptosis [12, 13].
Both activating transcription factor 3 (ATF3) and activating transcription factor 4 (ATF4) belong to the activating transcription factor/cyclic AMP response element binding protein (ATF/CREB) family of basic region-leucine zipper (bZip) transcription factors [14, 15]. ATF3 is an adaptive-response gene that participates in cellular processes by activating or repressing specific gene expression [14]. Whereas previous studies have revealed that ATF4 plays an important role in regulating CHOP [10], it is still unclear weather ATF3 also plays an important role in CHOP, thereafter DR5 regulation.
In this study, we hypothesize that salermide induces apoptosis in cancer cells by up-regulating DR5 expression. We have discovered a novel pathway that elucidates salermide activation of DR5 through the ATF4-ATF3-CHOP signalling axis. Our findings thus highlight a potential mechanism that has not been previously described, and this pathway explains the effects of class III HDAC inhibitors and the complex interaction with ATF4, ATF3, CHOP and DR5.
Materials and methods
Reagents
Purified salermide powder was purchased by Cayman Chemical (Ann Arbor, MI, USA). It was dissolved in DMSO for a concentration of 10 mmol/l and aliquots were stored at −80°C. Stock solutions were diluted to the desired final concentrations with growth medium prior to use. Rabbit polyclonal anti-DR5 antibody was purchased from ProSci (Poway, CA, USA). Mouse monoclonal anti-caspase-3 was purchased from Imgenex (San Diego, CA, USA). Rabbit anti-IRE-1α, rabbit anti-Bip, rabbit anti-caspase-8, anti-caspase-9 and anti-poly (ADP-ribose) polymerase (PARP) were purchased from Cell Signaling Technology (Danvers, MA, USA). Mouse monoclonal anti-β-actin and anti-GAPDH antibody was purchased from Sigma-Aldrich (St. Louis, MO, USA). Mouse monoclonal anti-CHOP, polyclonal anti-ATF4 and anti-ATF3 antibodies were obtained from Santa Cruz (Santa Cruz, CA, USA).
Cell lines and cell culture
The human tumour cell lines H1792, H157 (mutant type p53, mt p53), H1299, Calu-1 (p53 null) and H460, A549, H1650 (wild-type p53, wt p53) were grown in monolayer culture in RPMI 1640 supplemented with 5% foetal bovine serum at 37°C in a humidified atmosphere consisting of 5% CO2 and 95% air.
Cell survival assay
Cell survival was estimated by sulforhodamine B (SRB) assay as described earlier [4]. Cells were cultured in 96-well cell culture plates, treated on the second day with the agents indicated, and then subjected to staining by use of 0.4% (w/v) sulforhodamine B solution. The cell viability was determined using a spectrophotometric plate reader at the wavelength of 500 nm.
Western blot analysis
Whole cell protein lysates were prepared and analysed by Western blot as described previously [4]. Briefly, whole cell protein lysates (50 μg) were electrophoresed through 12% denaturing polyacrylamide slab gels, and the protein bands were transferred to a polymer of vinylidene fluoride (PVDF) membrane (Bio-Rad, Hercules, CA, USA) by electroblotting. The blots were probed or re-probed with the appropriate primary antibodies, blots were incubated with the secondary antibodies (Bio-Rad), and then antibody binding was detected by the ECL system, according to the manufacturer’s protocol. Western blot bands were quantified by the software SensiAnsys Series Gel Documentation and Analysis System. Induction fold is normalized with internal control (β-actin or GAPDH).
Gene silencing using small interfering RNA
Cells were seeded in six-well plates, and the following day, cells were transfected with indicated siRNA (DR5, ATF4, ATF3 and CHOP) when the cell density reached 40–60% confluence. Silencing RNA target sequences for DR5, CHOP and ATF4 have been previously described [10]. We chose the ATF3 siRNA target sequence 5′-GCACCTCUGCCACCGGATG-3′ [16]. The target sequence of Sirt1 and Sirt2 siRNA are 5′-CTGGAGCTGGGGTGTCTGT-3′ and 5′-GCGCGTTTCTTCTCCTGTA-3′, respectively. Cells were treated with the indicated compounds for the appropriate time based on observation. Cells were harvested and prepared for Western blot or SRB assay or Annexin V-PE/7-AAD staining and flow cytometry analysis.
Apoptosis assays
Apoptosis was evaluated by Annexin V staining using Annexin V-PE/7-AAD apoptosis detection kit purchased from BD Biosciences (San Jose, CA, USA) following the manufacturer’s instructions. Caspase activation was detected by Western blot.
Results
Salermide induces concentration- and time-dependent apoptosis in human lung cancer cell lines
To identify whether salermide induces apoptosis in human NSCLC cells, we tested this agent in vitro in H1792, H157 (mt p53); H1299, Calu-1 (p53 null); H460, A549 and H1650 (wt p53) cell lines. Cells were treated with 12.5, 25 or 50 μmol/l of salermide for 48 hrs. Cell death was quantitated by SRB assays (Fig. 1A), showing salermide induced growth inhibition in a concentration-dependent manner in tested cell lines. Western blot analysis revealed that salermide (20–60 μmol/l) effectively induced the cleavage of caspase-8, caspase-9, caspase-3 and PARP (Fig. 1B and C). Compared with other cell lines detected, H1650 was particularly sensitive to salermide-induced apoptosis because apoptosis in H1650 cells occurred at lower concentration (20 μmol/l; Fig. 1A) and shorter time interval (24 hrs; Fig. 1B). We also noted H460 cells appeared more resistant to salermide than other cell lines. Because H157 cells appeared to represent the typical cellular response to salermide, we chose H157 cell line to perform our time course experiment. It showed that apoptosis in H157 cells occurred at 24 hrs, and it peaked at 48 hrs. This data confirm that salermide induces apoptosis in a concentration- and time-dependent manner in human lung cancer cell lines.
Fig 1.
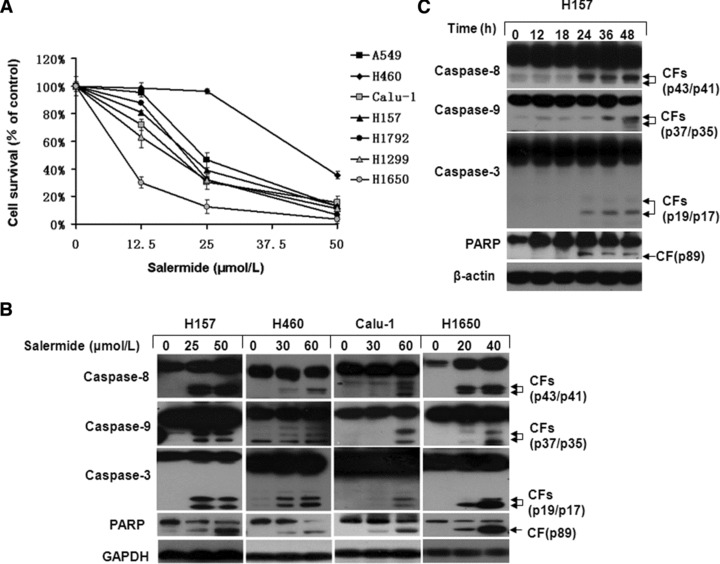
Salermide triggered apoptosis in a concentration- and time-dependent manner. The indicated cell lines were seeded in 96-well microtitre plates and treated with the given concentration of salermide for 48 hrs. (A) Live cell number was estimated using SRB assay for calculation of cell survival. Points: mean of four replicate determinations; bars: S.D. (B) Cells were treated with indicated concentrations of salermide for 48 hrs (H157, H460 and Calu-1) or 24 hrs (H1650) and harvested for Western blot analysis. (C) H157 cells were treated with 50 μmol/l salermide for various lengths of time. Both attached and suspended cells were harvested for Western blot analysis; CF: cleaved form.
Salermide up-regulates DR5 expression in lung cancer cells
To understand the mechanism of salermide-induced apoptosis, we examined several relevant genes and proteins in the apoptosis pathway. Treatment of H157, H460 and Calu-1 cells with salermide for 48 hrs increased both isoforms of DR5 expression (Fig. 2A). DR5 mainly consists of two isoforms, which differ by 29 amino acids [17]. We also treated H157 and Calu-1 cancer cells using salermide for various lengths of time. We found that the increase of DR5 occurred at 12 hrs and was sustained for up to 48 hrs (Fig. 2B). As shown in Figure 2C, in H460 and Calu-1 cells, the basal level of DR5 and salermide-induced DR5 decreased with transfection of DR5 siRNA. Western blot analysis also demonstrated that salermide-induced cleavage of caspase-8, caspase-9, caspase-3 and PARP in cells transfected with control siRNA but not in DR5 siRNA-transfected cells (Fig. 2C). Furthermore, DR5 knockdown protected cells from salermide-induced cell killing when compared to cells transfected with control siRNA (Fig. 2D). In summary, salermide induces apoptosis via up-regulation of DR5.
Fig 2.

DR5 was induced by salermide in human NSCLC cells. Cells were treated with indicated concentrations of salermide for 48 hrs (A) or with 50 μmol/l (H157) and 60 μmol/l (Calu-1) salermide for the indicated times (B) and then subjected to preparation of whole-cell protein lysates. The given proteins were detected using Western blot analysis and quantified using SensiAnsys software, bars: S.E.M. *P < 0.05, **P < 0.01 as compared with control. Silencing of DR5 expression was done by DR5 siRNA transfection in H460 and Calu-1 cells. Forty-eight hours after transfection, cells were treated with 40 μmol/l salermide. Whole-cell protein lysates were harvested for Western blot analysis (C). After DR5 siRNA transfection in H460 and Calu-1 cells in 96-well plate for 24 hrs, the cells were subject to cell viability measurements by SRB assay (D).
Salermide induces DR5 expression through a CHOP-dependent mechanism
We and others have demonstrated that CHOP can enhance DR5 expression through binding the DR5 promoter [10, 18]. In our system, salermide increased the expression of CHOP in H157 and Calu-1 cells at a moderate concentration (25–30 μmol/l; Fig. 3A). CHOP expression appeared at 12 hrs and was sustained up to 48 hrs (Fig. 3B). Moreover, CHOP siRNA transfection dramatically decreased salermide-induced CHOP expression as detected by Western blot analysis. Furthermore, salermide induction of caspase-8, caspase-9, caspase-3 and PARP cleavage was significantly suppressed in the cells transfected with CHOP siRNA (Fig. 3C). By blocking salermide-induced CHOP expression using CHOP siRNA, the level of DR5 protein expression accordingly decreased (Fig. 3C). We examined cell survival by SRB assay. Salermide (50–60 μmol/l) decreased viable H157 and Calu-1 cancer cells to less than 15%, while the percent of surviving cells was higher in CHOP siRNA-transfected cells (Fig. 3D). This supports our hypothesis that salermide induces CHOP-dependent DR5 expression.
Fig 3.

Up-regulation of CHOP and apoptosis-related proteins expression by salermide in human NSCLC cells. Cells were treated with the indicated concentrations of salermide for 48 hrs (A) or with 50 μmol/l (H157) or 60 μM (Calu-1) salermide for the indicated times (B). Whole-cell protein lysates were prepared from treated cells. CHOP was detected by Western blot analysis using CHOP-specific antibody and quantified by SensiAnsys software (*P < 0.05, **P < 0.01 versus control). H157 and Calu-1 cells were cultured in a six-well plate and on the second day transfected with control or CHOP siRNA. Twenty-four hours after the transfection, cells were reseeded in a six-well plate (C) or 96-well plates (D) and treated with 50 μmol/l (H157) or 60 μmol/l (Calu-1) salermide (C). The cells were harvested and apoptosis detected at the molecular level (C) or viability (D). Points: mean of four replicate determinations; bars: S.D.
Sirt1 and Sirt2 inhibition induces endopalsmic reticulum stress in lung cancer cells
CHOP up-regulation is an important activator of the ER stress pathway [19]. Therefore, we hypothesized that salermide could potentially induce ER stress in lung cancer cells. We examined several ER stress markers such as IRE-1α, Bip, EIF-2α and caspase 4 in H157 and Calu-1 cells. We found that salermide increased the expression of IRE-1α, Bip and phosphorylated EIF-2α, but decreased the levels of pro-caspase 4 (Fig. 4A). Time course in H157 cells showed that IRE-1α and Bip were up-regulated at 12 hrs and sustained for up to 48 hrs (Fig. 4B). Because salermide is the inhibitor of Sirt1 and Sirt2, we postulated Sirt1 and/or Sirt2 inhibition may induce ER stress. We performed several knockdown experiments in H157 cells using Sirt1 and Sirt2 siRNAs. As demonstrated in Figure 4C, Sirt1 or Sirt2 had limited effect on ER stress induction, while the combination of Sirt1 and Sirt2 knockdown markedly up-regulated ATF4 and CHOP, two important members of the ER stress pathway. Of note, DR5 expression was also up-regulated, and it gave rise to caspase 8 activation. In conclusion, salermide therapy can enhance the ER stress induced by Sirt1 and Sirt2 inhibition and can result in more potent DR5 induction and caspase 8 activation (Fig. 4C).
Fig 4.

Sirt1 and Sirt2 inhibition with salermide (A, B) or siRNA transfection (C) induces ER stress and DR5 up-regulation (C) in human NSCLC cells. H157 and Calu-1 cells were treated with 25/50 μmol/l salermide (H157) or 30/60 μmol/l salermide (Calu-1) for 48 hrs (A) or treated with 50 μmol/l salermide (H157) for the indicated hours (B). H157 cells were cultivated in a six-well plate and on the second day transfected with control, Sirt1, Sirt2 siRNA, respectively, or co-transfected with Sirt1 and Sirt2 siRNA. Cells were reseeded in a six-well plate on the third day (C) and treated with 50 μmol/L (H157) salermide for 48 hrs (C). Whole-cell protein lysates were harvested for Western blot analysis (A–C) and quantified using SensiAnsys software (A, B), *P < 0.05, **P < 0.01 as compared with control.
ATF4 expression is prerequisite for salermide-induced DR5 expression
CHOP expression strongly depends on ATF4 [20]. We examined the expression of ATF4 when cancer cells were treated with salermide. H157 and Calu-1 cells were treated with increasing concentration of salermide for 48 hrs. By Western blot analysis, the level of ATF4 induced by salermide dramatically increased. The highest level of ATF4 was observed in concentrations ranging from 50 μmol/l salermide (H157 cells) to 60 μmol/l salermide (Calu-1 cells). Of note, 25 μmol/l salermide was sufficient to increase ATF-4 expression in H157 cells (Fig. 5A). ATF4 expression increased within 12 hrs of treatment in H157 and Calu-1 cells (Fig. 5B). We used ATF4 siRNA to block ATF4 expression and then examined cell sensitivity to salermide by Western blot. As shown in Figure 5C, transfection of ATF4 siRNA abrogated salermide-induced ATF-4 expression. The expression of salermide-induced apoptosis-related proteins (caspase-8, caspase-9, caspase-3 and PARP) was also suppressed in cells transfected with ATF4 siRNA. Specifically, DR5 levels were down-regulated in both control cells (from basal DR5 levels) or salermide-treated cells (from increased DR5 levels). Subsequent cell sensitivity to salermide treatment was significantly decreased in ATF4 siRNA-transfected cells in comparison to cells transfected with control siRNA (Fig. 5D). Together, these results indicate that salermide activates the ATF4–CHOP pathway, leading to DR5-dependent apoptosis.
Fig 5.

Salermide up-regulates ATF4 at expression level in human NSCLC cell lines. HI57 cells were treated with 25/50 μmol/l salermide and Calu-1 cells were treated with 30/60 μmol/l salermide for 48 hrs (A) or treated with 50 μmol/l (H157)/60μmol/l (Calu-1) salermide for indicated hours (B). (C) H157 and Calu-1 cells were cultured in a six-well plate and the next day were transfected with control (Ctrl) or ATF4 siRNA. Cells were divided into two wells the third day and treated with 50 μmol/l (H157)/60 μmol/l (Calu-1) salermide the fourth day for 48 hrs. Cells were subjected to preparation of whole-cell lysates for Western blot analysis (A–C). Western blot bands were quantified by SensiAnsys, *P < 0.05, **P < 0.01, compared to control (A, B). (D) The indicated transfectants were seeded in 96-well plates and treated with the various concentrations of salermide. After 48 hrs, the cells were subjected to the SRB assay for measurement of cell survival. Data are the average of four replicate determinations. Bars: ±S.D.
Salermide up-regulates ATF3, which leads to DR5-dependent apoptosis
We examined the downstream effects of salermide on ATF3 expression. By Western blot analysis, the level of ATF3 induced by salermide dramatically increased (Fig. 6A). ATF3 was up-regulated within 12 hrs in both H157 and Calu-1 cells (Fig. 6B). In H157 cells, the expression of ATF3 reached a peak at 48 hrs. In Calu-1 cells, protein expression peaked between 12 and 24 hrs (Fig. 6B). We silenced ATF3 gene expression by ATF3 siRNA. As presented in Figure 6C, DR5 expression was greatly diminished in cancer cells. ATF3 expression decreased in control cells (from basal levels) and salermide-treated cells (from elevated levels). As expected, the expression of DR5 and the cleaved forms including caspase-8, caspase-9, caspase-3 and PARP also declined. Cell survival curves showed that the rate of cell killing declined in ATF-3 siRNA-transfected cells (Fig. 6D). Collectively, salermide enhanced the level of ATF3 in a concentration- and time-dependent manner, demonstrating that salermide-induced DR5-dependent apoptosis requires up-regulation of ATF-3.
Fig 6.

Effects of salermide on the expression of ATF3- and DR5-dependent apoptosis in human NSCLC cell. The indicated cell lines were treated with the given concentrations of salermide for 48 hrs (H157, H460 and Calu-1) or 24 hrs (H1650). (B) H157 and Calu-1 cells were treated with 50 μmol/l (H157)/60 μmol/l (Calu-1) salermide for the given hours. Cells were subjected to preparation of whole-cell protein lysates. The given proteins were detected using Western blot analysis and evaluated by SensiAnsys. *P < 0.05, **P < 0.01 as compared with control (A, B). (C) H157 and Calu-1 cells were transfected as showed for 48 hrs, then cells were treated with 50 μmol/l (H157)/60 μmol/l (Calu-1) salermide for 48 hrs, the whole cells were harvested and molecular change about apoptosis were tested using Western blot. (D) The indicated cell lines were transfected with ATF3 siRNA and treated with the given concentrations of salermide. After 48 hrs, cell number was estimated using the SRB assay for calculation of cell survival. Points: mean of four replicate determinations; bars: S.D.
The ATF4-ATF3-CHOP axis regulates DR5
We confirmed the effects of salermide on DR5, CHOP, ATF4 and ATF3 using flow cytometry analysis in NSCLC cells. The data showed that knocking down each protein in turn reduced the percent of apoptosis (Fig. 7A). Following this study, we validated the relationship amongst ATF4, ATF3 and CHOP in DR5-dependent cell apoptosis. Cells were transfected with ATF4, ATF3 or CHOP siRNA, respectively. Transfection with ATF4 siRNA in H157 and Calu-1 cells accordingly decreased the expression of ATF3, CHOP and DR5 from salermide-induced levels (Fig. 7B). Furthermore, by inhibiting ATF3 expression using siRNA, we detected that induction of ATF4 by salermide was unaffected, while the expression of CHOP and DR5 induced by salermide declined (Fig. 7C). Furthermore, in cells transfected with CHOP siRNA, salermide-induced expression including ATF4 and ATF3 was still unchanged, while the expression of DR5 declined (Fig. 7D). These results suggest that salermide triggers ER stress in human NSCLC cells, which mediates the induction of the ATF4-ATF3-CHOP axis that leads to DR5-dependent apoptosis.
Fig 7.

The relationship of ATF4, ATF3 and CHOP in NSCLC cells after salermide treatment. H157 and Calu-1 cells were transfected with control (Ctrl), ATF4, ATF3 and CHOP siRNA for 48 hrs and then treated with 25 μmol/l salermide (A) or 50 μmol/l (H157)/60 μmol/l (Calu-1) salermide (B–D) for 48 hrs. Apoptosis was measured by Annexin V-PE/7-AAD staining (A). In the Annexin V-PE/7-AAD assay, the percent positive cells in the upper right and lower right quadrants were added to yield the total of apoptotic cells. Whole-cell protein lysates were prepared from the aforementioned treatments for detection of the given proteins by Western blot analysis (B–D).
Discussion
To date, salermide has been demonstrated to lead to apoptosis by inhibiting SIRT1 and SIRT2 [3]; however, there are no known molecular mechanisms that explain how salermide triggers apoptosis in cancer cells. Our data show that salermide induces apoptosis through the ATF4-ATF3-CHOP axis, which in turn increases DR5 expression.
CHOP is known to enhance DR5 expression through binding the DR5 promoter in ER stress-induced apoptosis. DR5 up-regulation is attenuated if CHOP expression is blocked [10, 18]. Our work reveals that the basal level of DR5 and salermide-induced DR5 level decreases when CHOP is knocked down in H157 and Calu-1 cells. The level of other apoptosis-related proteins such as caspase-8, caspase-9, caspase-3 and PARP are simultaneously suppressed in cells transfected with CHOP siRNA. There are other known anti-cancer compounds, such as CDDO-Me, SCH66336 and celecoxib, which induce cancer cell death by activating the CHOP-DR5 apoptosis pathway [10, 21, 22]. It is well known that CHOP activation is regulated by at least four cis-acting elements in its promoter including AARE1, AARE2, ERSE1 and ERSE2. ATF4 binds to AARE1 and AARE2, and it plays an essential role in the regulation of CHOP transcription [23, 24]. The expression of CHOP decreases in cells transfected with ATF4 siRNA. This is in concordance with previous reports that ATF4 regulates CHOP. We found that in ATF4 siRNA transfected cells, DR5 expression was reduced to basal levels. Inhibiting ATF4 also blocked salermide-induced DR5 expression and activation of several apoptosis-related proteins (caspase-8, caspase-9, caspase-3 and PARP).
Activating transcription factor 3 (ATF3) belongs to the ATF/cAMP-response element-binding protein family of transcription factors and plays an important role in leading cancer cells to apoptosis [25]. ATF3 can induce apoptosis in ovarian cancer cells [26] and enhance etoposide- or camptothecin-induced apoptosis in HeLa cells [27]. Once cells are exposed to stressors, such as hepatotoxicity and DNA-damaging agents, ATF3 is rapidly up-regulated [28]. ATF3 binds to AARE, which is one of cis-acting elements of CHOP [23]. Our data demonstrate that salermide increases the expression of ATF3. We also show that a decrease in ATF3 levels reduces the expression of DR5 and activation of apoptosis-related proteins (caspase-8, caspase-9, caspase-3 and PARP). Because ATF3 and ATF4 can form a complex to promote the expression of NOXA [29], we speculate that salermide induces ATF4, and then ATF4 up-regulates ATF3. Subsequently, ATF3 and ATF4 form the complex and enhance the transcription of CHOP, leading to the DR5 induction. Anyway, how CHOP is regulated by ATF3 and ATF4 is an intriguing subject for further research.
Our work reveals that blocking one of ATF4, ATF3, CHOP or DR5 expression inhibits both early and late apoptosis. Because CHOP, ATF4 and ATF3 play an important role in up-regulating DR5 expression, we sought to determine the relationship amongst them. By Western blot and RNA interference analysis, we discovered that by introducing salermide, ER stress-dependent apoptosis was activated and ATF4 expression was induced. ATF4 up-regulated ATF3, and ATF3 contributed to the activation of CHOP, which induced DR5.
CHOP and ATF4 have an important role in ER stress–mediated apoptosis [10]. Thus, we examined other ER stress markers such as IRE-1α, Bip, EIF2α and caspase 4 [30] to confirm that salermide can induce ER stress in lung cancer cells. Salermide treatment enhanced the expression of IRE-1α, Bip and phosphorylated EIF-2α and down-regulated the level of pro-caspase 4. This suggests that salermide triggers ER stress in lung cancer cells. Furthermore, when we inhibited the Sirt1 and/or Sirt2 expression to mimic salermide’s effect, we found knockdown of Sirt1 and Sirt2 expression resulted in up-regulation of ATF4, CHOP and DR5. This resulted in caspase 8 cleavage. In conclusion, Sirt1 and Sirt2 inhibition induces ER stress and DR5 up-regulation, which results in caspase 8 activation and subsequent apoptosis.
In summary, our work demonstrates that DR5 induction was modulated by the ATF3-ATF4-CHOP axis and elucidates a novel mechanism of apoptosis induced by Sirt1/2 inhibition or salermide as well as other chemotherapeutic agents in human NSCLC cells. This provides important mechanistic insight into using salermide in future clinical scenarios when it may synergize with current therapeutic strategies.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (30971479 and 31071215), the Independent Innovation Foundation of Shandong University (IIFSDU 2009JQ006), the Doctoral Fund of Ministry of Education of China (20090131110002), the Program for New Century Excellent Talents in University (NCET-10-0521) and the Shandong Natural Science Foundation (BS2009YY004, 2010GSF10218 and JQ201007).
Authors’ contribution
XL and LS conceived and designed the research study. GL, LS, XH and NZ performed the research. XL, GL and SS analysed the data and wrote the manuscript.
Conflict of interests
The authors confirm that there are no conflicts of interest.
References
- 1.Hoshino I, Matsubara H. Recent advances in histone deacetylase targeted cancer therapy. Surg Today. 2010;40:809–15. doi: 10.1007/s00595-010-4300-6. [DOI] [PubMed] [Google Scholar]
- 2.Neugebauer RC, Sippl W, Jung M. Inhibitors of NAD+ dependent histone deacetylases (sirtuins) Curr Pharm Des. 2008;14:562–73. doi: 10.2174/138161208783885380. [DOI] [PubMed] [Google Scholar]
- 3.Lara E, Mai A, Calvanese V, et al. Salermide, a Sirtuin inhibitor with a strong cancer-specific proapoptotic effect. Oncogene. 2009;28:781–91. doi: 10.1038/onc.2008.436. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Yue P, Zhou Z, et al. Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Natl Cancer Inst. 2004;96:1769–80. doi: 10.1093/jnci/djh322. [DOI] [PubMed] [Google Scholar]
- 5.Davis AR, Lotocki G, Marcillo AE, et al. FasL, Fas, and death-inducing signaling complex (DISC) proteins are recruited to membrane rafts after spinal cord injury. J Neurotrauma. 2007;24:823–34. doi: 10.1089/neu.2006.0227. [DOI] [PubMed] [Google Scholar]
- 6.Salvesen GS, Riedl SJ. Structure of the Fas/FADD complex: a conditional death domain complex mediating signaling by receptor clustering. Cell Cycle. 2009;8:2723–7. doi: 10.4161/cc.8.17.9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu W, Tiwary R, Li J, et al. Alpha-TEA induces apoptosis of human breast cancer cells via activation of TRAIL/DR5 death receptor pathway. Mol Carcinog. 2010;49:964–73. doi: 10.1002/mc.20681. [DOI] [PubMed] [Google Scholar]
- 8.Elrod HA, Sun SY. Modulation of death receptors by cancer therapeutic agents. Cancer Biol Ther. 2008;7:163–73. doi: 10.4161/cbt.7.2.5335. [DOI] [PubMed] [Google Scholar]
- 9.Sun SY. Chemopreventive agent-induced modulation of death receptors. Apoptosis. 2005;10:1203–10. doi: 10.1007/s10495-005-2274-4. [DOI] [PubMed] [Google Scholar]
- 10.Oh YT, Liu X, Yue P, et al. ERK/RSK signaling positively regulates death receptor 5 expression through co-activation of CHOP and Elk1. J Biol Chem. 2010;285:41310–9. doi: 10.1074/jbc.M110.153775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–9. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 12.Korfei M, Ruppert C, Mahavadi P, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:838–46. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiwary R, Yu W, Li J, et al. Role of endoplasmic reticulum stress in alpha-TEA mediated TRAIL/DR5 death receptor dependent apoptosis. PLoS One. 2010;5:e11865. doi: 10.1371/journal.pone.0011865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson MR, Xu D, Williams BR. ATF3 transcription factor and its emerging roles in immunity and cancer. J Mol Med. 2009;87:1053–60. doi: 10.1007/s00109-009-0520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su N, Kilberg MS. C/EBP homology protein (CHOP) interacts with activating transcription factor 4 (ATF4) and negatively regulates the stress-dependent induction of the asparagine synthetase gene. J Biol Chem. 2008;283:35106–17. doi: 10.1074/jbc.M806874200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turchi L, Aberdam E, Mazure N, et al. Hif-2alpha mediates UV-induced apoptosis through a novel ATF3-dependent death pathway. Cell Death Differ. 2008;15:1472–80. doi: 10.1038/cdd.2008.74. [DOI] [PubMed] [Google Scholar]
- 17.Screaton GR, Mongkolsapaya J, Xu XN, et al. TRICK2, a new alternatively spliced receptor that transduces the cytotoxic signal from TRAIL. Curr Biol. 1997;7:693–6. doi: 10.1016/s0960-9822(06)00297-1. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 19.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–30. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 20.Szegezdi E, Logue SE, Gorman AM, et al. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–5. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou W, Yue P, Khuri FR, et al. Coupling of endoplasmic reticulum stress to CDDO-Me-induced up-regulation of death receptor 5 via a CHOP-dependent mechanism involving JNK activation. Cancer Res. 2008;68:7484–92. doi: 10.1158/0008-5472.CAN-08-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun SY, Liu X, Zou W, et al. The farnesyltransferase inhibitor lonafarnib induces CCAAT/enhancer-binding protein homologous protein-dependent expression of death receptor 5, leading to induction of apoptosis in human cancer cells. J Biol Chem. 2007;282:18800–9. doi: 10.1074/jbc.M611438200. [DOI] [PubMed] [Google Scholar]
- 23.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–9. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 24.Averous J, Bruhat A, Jousse C, et al. Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. J Biol Chem. 2004;279:5288–97. doi: 10.1074/jbc.M311862200. [DOI] [PubMed] [Google Scholar]
- 25.Chen BP, Wolfgang CD, Hai T. Analysis of ATF3, a transcription factor induced by physiological stresses and modulated by gadd153/Chop10. Mol Cell Biol. 1996;16:1157–68. doi: 10.1128/mcb.16.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syed V, Mukherjee K, Lyons-Weiler J, et al. Identification of ATF-3, caveolin-1, DLC-1, and NM23-H2 as putative antitumorigenic, progesterone-regulated genes for ovarian cancer cells by gene profiling. Oncogene. 2005;24:1774–87. doi: 10.1038/sj.onc.1207991. [DOI] [PubMed] [Google Scholar]
- 27.Mashima T, Udagawa S, Tsuruo T. Involvement of transcriptional repressor ATF3 in acceleration of caspase protease activation during DNA damaging agent-induced apoptosis. J Cell Physiol. 2001;188:352–8. doi: 10.1002/jcp.1130. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, Li X, Guo B. KLF6 induces apoptosis in prostate cancer cells through up-regulation of ATF3. J Biol Chem. 2008;283:29795–801. doi: 10.1074/jbc.M802515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, Mora-Jensen H, Weniger MA, et al. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci U S A. 2009;106:2200–5. doi: 10.1073/pnas.0807611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hitomi J, Katayama T, Eguchi Y, et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and A{beta}-induced cell death. J Cell Biol. 2004;165:347–56. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]


