Abstract
We previously identified a novel angiogenic peptide, AG30, with antibacterial effects that could serve as a foundation molecule for the design of wound-healing drugs. Toward clinical application, in this study we have developed a modified version of the AG30 peptide characterized by improved antibacterial and angiogenic action, thus establishing a lead compound for a feasibility study. Because AG30 has an α-helix structure with a number of hydrophobic and cationic amino acids, we designed a modified AG30 peptide by replacing several of the amino acids. The replacement of cationic amino acids (yielding a new molecule, AG30/5C), but not hydrophobic amino acids, increased both the angiogenic and the antimicrobial properties of the peptide. AG30/5C was also effective against methicillin-resistant Staphylococcus aureus (MRSA) and antibiotic-resistant Pseudomonas aeruginosa. In a diabetic mouse wound-healing model, the topical application of AG30/5C accelerated wound healing with increased angiogenesis and attenuated MRSA infection. To facilitate the eventual clinical investigation/application of these compounds, we developed a large-scale procedure for the synthesis of AG30/5C that employed the conventional solution method and met Good Manufacturing Practice guidelines. In the evaluation of stability of this peptide in saline solution, RP-HPLC analysis revealed that AG30/5C was fairly stable under 5°C for 12 months. Therefore, we propose the use of AG30/5C as a wound-healing drug with antibacterial and angiogenic actions.
Keywords: angiogenesis, antimicrobial, translational research, drug development
Introduction
Antimicrobial peptides are small proteins that are used by the innate immune system to combat bacterial infections in multicellular eukaryotes. The emergence of drug-resistant bacteria has driven the extensive investigation of these peptides as a potential source of new antimicrobial drugs that could complement current antibiotic regimes [1, 2]. In general, antimicrobial peptides are characterized by a net positive charge and an amphipathic three-dimensional structure that gives the peptides an electrostatic affinity to the outer leaflet of the microbial membrane, mediated by lipid molecules on bacterial surfaces [3]. This affinity leads to binding, disruption of the membrane and microbial cell death [4]. More than 100 antimicrobial peptides have been discovered in animals ranging from insects to humans; however, the development of antimicrobial peptide-based therapies has mainly been restricted to topical or local treatments because their activity is suppressed in serum [3-5].
We recently developed a novel antimicrobial peptide, named AG30, with angiogenic properties potentially similar to those of LL37 or PR39 [6]. Because both angiogenic and antimicrobial effects are beneficial in the process of wound healing, we initiated the clinical development process using AG30 as a foundation compound to develop novel topically applied wound-healing drugs. The development of a peptide-drug based topical treatment has several attractive features: (1) the drug is delivered directly to the wound; (2) high local tissue levels can be achieved through application of aggressive doses; and (3) the short half life of peptides, resulting from their degradation by peptidases, reduces their toxicity. Historically, similar cationic antimicrobial peptides, such as pexiganan, iseganan and omiganan, failed to pass the phase II or III trials in topical applications or systemic applications. Therefore, in this study, we developed the load compound based on a feasibility study using the foundation compound, AG30. Using both in silico and functional analyses, we produced a modified version of the AG30 peptide that may elicit more potent angiogenic and antimicrobial effects. As initial steps in the investigation of the potential clinical applications of this modified AG30 peptide, we developed a cost-effective production process and evaluated its efficiency using animal models.
Material and methods
Cell migration assay and tube formation assays and cell growth assay
Human aortic endothelial cells (HAEC, passage 3) were purchased from Clonetics Corp. (Palo Alto, CA, USA) and maintained in endothelial basal medium (EBM-2) that was supplemented with 5% foetal bovine serum (FBS) and endothelial growth supplement as described previously [6]. Cells were incubated at 37°C in a humidified atmosphere of 95% air–5% CO2 with exchange of medium every 2 days.
Human aortic endothelial cells migration was assayed using a modified Boyden chamber, as previously described [7]. Human aortic endothelial cells tube formation assays were conducted in triplicate in 24-well plates using an Angiogenesis Kit (Kurabo, Osaka, Japan) according to the manufacturer’s instructions, as previously described [6].
Human epidermal keratinocytes cell line (HaCaT cells) were maintained with DMEM supplemented with 10% FBS and 10 μg/ml Gentamicin. We used the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H- tetrazolium] assay for cell growth. Second day after stimulation with AG30/5C, 10 μl of Cell Riter 96 One Solution Reagent (Progema, Medison, WI, USA) was added to each well, and absorbance at 490 nm was measured [6].
Peptide design and synthesis and circular dichroism (CD) spectroscopy analysis
All peptides used in this experiment were purchased from the Peptide Institute, Inc. (Osaka, Japan). LL37 was synthesized as described previously [8]. The Boman Index was used to predict the function of the peptides [9]. We used the AGADIR program (http://www.embl-heidelberg.de/ExternalInfo/serrano/agadir/agadir-start.html) to predict the structure. Circular dichroism data were acquired with a Jasco J-820 spectrophotometer using a 1-mm-path-length cuvette at 20°C [10]. Spectra were collected for samples containing 0.3 μg/ml peptide in 20 mM phosphate buffer at pH 7.5, with and without the addition of 30% or 60% trifluoroethanol (TFE) [11].
Measurement of MICs against P. aeruginosa, S. Aureus and Candida
The minimum inhibitory concentrations (MICs, expressed as μg/ml) of modified AG30 and LL37 for Psudomonas aeruginosa (P. aeruginosa, ATCC27853), Staphylolcoccus aureus (S. aureus, ATCC29213) and Candida albicans (Candida, ATCC90028) and methicillin-resistant S. aureus (MRSA) were defined as the lowest concentration of peptide that inhibited visible bacterial growth after incubation for 16 hrs at 37°C with vigorous shaking, as previously described [6]. This experimental protocol was approved by the bio-safety committee at the Osaka University Graduate School of Medicine.
Mouse wound model and infection models
In the mice tail wound model, full-thickness wounds were made on the dorsal surface of mouse tails as previously described [12]. For wound-healing model, 9-week-old male C57BL/6 db/db mice were used in this study. We completely removed hair from the backs of mice using fine-tooth clippers and hair-removing cream 3 days prior to operation. After induction of anaesthesia, 15 mm punch biopsy wounds were made on the backs of each mouse (n = 10–12 in each group). After topical application of each peptide (100 μg/ml), the wounds were covered with a semi-permeable polyurethane dressing. Topical application of each peptide and measurements of wound areas were repeated on days 0, 2, 4, 7, 9, 11, 14 and 16. Samples were obtained 7 days after the operation. After fixation in cold acetone (at −20°C for 15 min.), capillary EC were identified by immunohistochemical staining using an antimouse PECAM mAb (Pharmingen, San Diego, CA, USA) and then counted in 10 independent views of each group [13]. After day 16, peptide application and film dressing were halted for 10 days, and hair growth was observed.
Similar to the mouse wound model, we made wounds on the backs of db/db mice (n = 6–8 in each group). We topically applied 100 μl of methicillin-resistant S. aureus on the wounds (1 χ 104 cfu/ml) with or without each peptide tested (100 μg/ml). The wounds were then covered with a semi-permeable polyurethane dressing. Topical application of each peptide and measurements of wound areas by taking pictures were repeated on days 0, 2, 4, 7, 9, 11, 14 and 16. All animal experiments were performed according to the Guidelines for Animal Experiments at the Osaka University Graduate School of Medicine.
Measurement of blood flow by Lase Doppler imaging
Measurement of blood flow with Laser Doppler imaging (LDI; Moor Instruments, Axminister, UK) was performed as described previously [13,
14]. Because Laser Doppler flow velocity correlates with capillary density, we measured the blood flow just below the skin by LDI. Consecutive measurements were obtained over the same regions of interest by averaging four sites around the wound.
Porcine wound-healing model
In the porcine wound model, full-thickness wounds were made on the side abdomen surface of pigs as modified previous method [15]. Three male NIBS minipigs (32.0, 32.1 or 33.5 kg) were used (NARC Corporation, Chiba). Pigs were pre-medicated with ketamine (500 mg/body) and isoflurane (2–4%) were administered for anaesthesia. We completely removed hair from the side abdomen of pigs using fine-tooth clippers and outlined the circle in a 25 mm diameter. Full-thickness excisional wounds were created in a uniform depth of 10 mm. We created four wounds on one side at intervals of 30 mm. After topical application (200 μl/site) of each peptide (100 μg/ml), the wounds were covered with a semipermeable polyurethane dressing. Topical application of each peptide was repeated on days 0, 2, 4, 6, 8 and 10. Samples were obtained 12 days after the operation. These frozen sections were stained with haematoxylin and eosin for overall morphological observation. After fixation in cold acetone (at −20°C for 15 min.), capillary EC were identified by immunohistochemical staining with von Willebrand factor (1:200 dilution; DAKO, Glostrup, Denmark) [16]. All animal experiments were performed according to the Guidelines for Animal Experiments at the Osaka University Graduate School of Medicine.
Statistical analysis
All values are expressed as means ± S.E.M. Analysis of variance with subsequent Fisher’s PLSD test analysis was used to determine the significance of differences in multiple comparisons.
Results
Development of a modified versions of the AG30 peptide
We previously identified a novel angiogenic peptide consisting of 30 amino acids, named AG30 [6]. As shown in Figure 1A, AG30 is a good foundation molecule for the development of wound-healing drugs, and this study may help to establish the feasibility of AG30-related lead compounds for clinical applications. The original AG30 molecule has an amphipathic structure; most of the positively charged amino acids are localized to one side of the molecule, while most of the hydrophobic amino acids are localized to the opposite side [6]. Notably, AG30 contains very few proline or glycine residues that would interrupt the α-helical structure. Thus, in our design strategy for developing modified AG30 molecules, we replaced several neutral amino acids (specifically proline, asparagine, asparagine acid and serine) with cationic or hydrophobic amino acids and added a capping structure (Ac-KLT on the C-terminus and KGI-amido on the N-terminus) to stabilize the helical structure [15] (Fig. 1B). The total net charge of each designed peptide is increased by adding cationic amino acids (AG30: +11, AG30/2C: +14, AG30/5C: +17, respectively).
Fig 1.
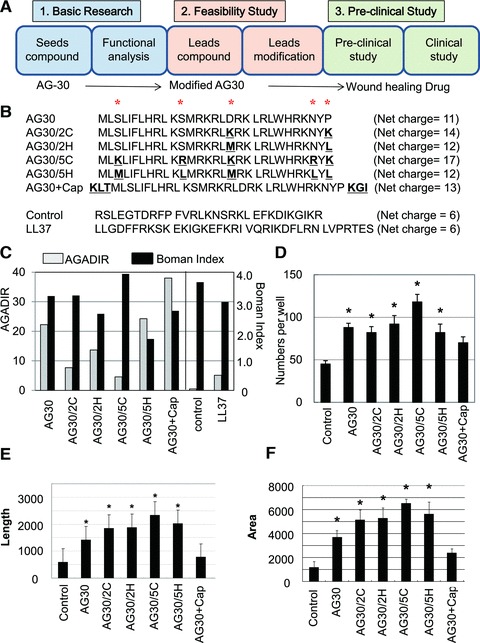
Several different modified AG30 peptides were created by replacing hydrophobic or cationic amino acids or adding capping structures. (A) Study design of this research. AG30 can be a good seed for wound-healing drug and this modified AG30 may correspond to be lead compounds as feasibility study toward clinical application (towards wound-healing drug). (B) Peptide sequences and net charge of modified AG30, control peptide and LL37. ‘*’ indicates positions where amino acids were replaced. ‘AG30/2C’ and ‘AG30/5C’ indicate the replacement of two or five cationic amino acid (from S, S, D, N, P to K, R, K, R, K, respectively). ‘AG30/2H’ and ‘AG30/5H’ indicate the replacement of two or five hydrophobic amino acids (from S, S, D, N, P to M, L, M, L, L, respectively). ‘AG30+Cap’ indicates the addition of a capping structure (Ac-KLT on the C-terminus and KGI-amide on the N-terminus). (C) In silico analysis of modified AG30. AGADIR (white bar) scores were calculated to predict the peptide structure and the Boman Index (black bar) was calculated to predict the pleiotropic effects of cationic peptides. (D) HAEC migration after treatment with modified AG30 for 24 hrs. (E, F) Tube formation after daily treatment with modified AG30 for 4 days; length and area were measured. N = 6 per group and duplicated. *P < 0.05 versus control, †P < 0.05 versus AG30.
We evaluated the potential activity of these candidate peptides using two different types of in silico analysis. To predict the structure, we used the AGADIR program, an algorithm based on the helix/coil transition theory that predicts the helical behaviour of monomeric peptides [17, 18]. In this analysis, the addition of five hydrophobic amino acids and a capping structure increased the score, although the addition of two or five cationic amino acids decreased the score (Fig. 1C). The Boman Index [9] was used to predict the function of the peptides; this index is calculated by adding the free energies of the side chains for transfer from cyclohexane to water and dividing that sum by the total number of residues. In this analysis, the addition of cationic amino acids gave a high score, while the addition of hydrophobic amino acids and a capping structure decreased the score (Fig. 1C).
Evaluation of modified AG30 peptides
As an initial evaluation of the designed peptides, we investigated their effects on the migration and tube forming ability of human aortic endothelial cells. In the migration assay, the addition of five cationic amino acids (i.e. compound AG30/5C) resulted in increased migration activity; however, the addition of a capping structure (i.e. compound AG30+Cap) resulted in a lower activity compared to AG30 (Fig. 1D). In the tube formation assay, the addition of cationic charged or hydrophobic amino acids strongly induced tube formation. However, the addition of a capping structure reduced this activity (Fig. 1E and F). We also examined the antibacterial effects of the designed peptides against P. aeruginosa, Candida and S. aureus. The addition of two or five cationic amino acids (i.e. compound AG30/2C or AG30/5C) increased the antibacterial activity against all bacteria, although the addition of five hydrophobic amino acids (i.e. compound AG30/5H) resulted in a lower antibacterial activity than AG30 (Fig. 2A–D). These results suggest that the addition of five cationic amino acids (i.e. compound AG30/5C) enhances both the angiogenic and the antimicrobial properties of the original AG30 molecule, a result that is compatible with the previously determined Boman Index scores.
Fig 2.
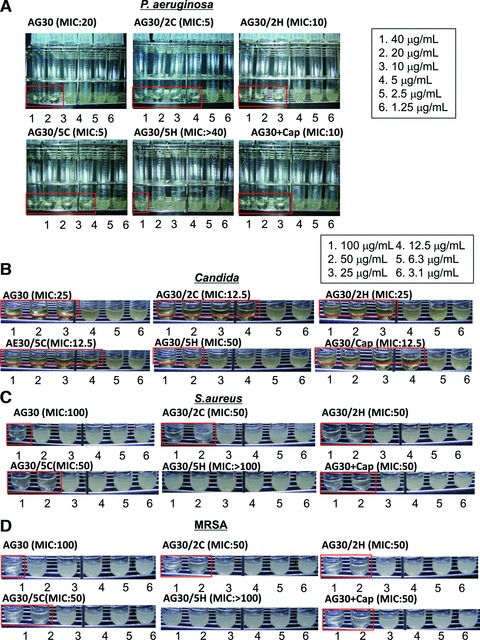
The antibacterial action of modified AG30. The antibacterial action of modified AG30 is evaluated to (A) P. aeruginosa (ATCC27853), (B) Candida (ATCC90028), (C) S. aureus (ATCC29213) and (D) MRSA. MIC assays were performed in the concentration range of 1.25–40 mg/ml of modified AG30, LL37 against P. aeruginosa and 3.1–100 mg/ml against Candida, S. aureus and MRSA. MIC was defined as the lowest peptide concentration, which prevented visible bacterial growth in the medium. Lower panel shows representative pictures of MIC assays with treatment of several peptides.
In the structural analysis of the peptides using CD spectroscopy, all of the candidate peptides showed negligible helical structure in aqueous buffer; the addition of the helix-inducing cosolvent TFE (either 20% or 60%) resulted in the appearance of a clear helical component. This was demonstrated by the minimums at 208 and 220 nm and the increase of ellipticity at 200 nm, which resulted in the appearance of a clear helical component in all of the generated peptide. But the control peptide did not change upon the addition of TFE. We quantified the helical structure [19] formation tendency of each of the peptides, either with or without TFE (Fig. 3A–F). Unexpectedly, the addition of either five hydrophobic amino acids (i.e. compound AG30/5H) or a capping structure (i.e. compound AG30+Cap) did not increase the ratio of α-helical structures, and the addition of five cationic amino acids (i.e. compound AG30/5C) decreased the α-helical structure ratio in comparison to AG30. These results suggest that the ratio of α-helical structures upon the addition of TFE does not correspond to the AGADIR score and thus might not correspond to the angiogenic or antibacterial properties of each peptide. Similarly, the well-known antimicrobial peptide LL37 has a low AGADIR score but was found to possess an α-helical structure without addition of TFE. Furthermore, LL-37 has strong angiogenic effects and a high Boman Index score, indicating that the Boman index is more reliable for in silico screening in our study.
Fig 3.
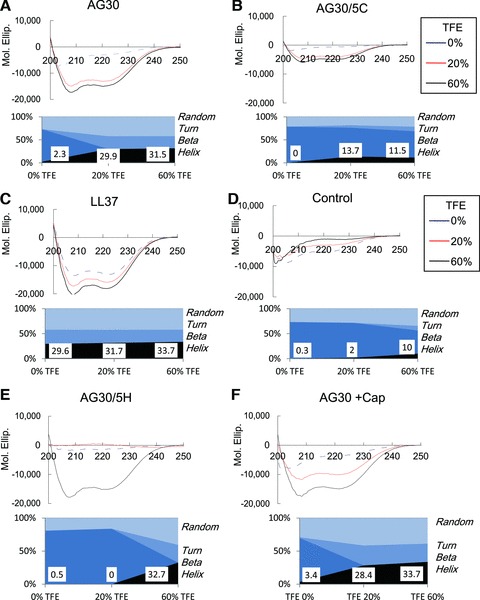
CD spectroscopy analysis. Modified AG30, LL37 and control peptide showed negligible helical structure in aqueous buffer, and the addition of the helix inducing cosolvent trifluoroethanol (20% or 60%) resulted in the appearance of a clear helical component, that demonstrated the minimum at 208 and 220 nm and the increase of ellipticity at 200 nm. In lower panel, the quantification of helical structure (Random, Turn, Beta, and Helix) was calculated, and the score of helix has been shown.
Antibacterial effects of modified AG30 (AG30/5C)
The advantage of using antimicrobial peptides as antibacterial agents is that bacteria are less likely to become resistant to these compounds in comparison to antibiotics. Indeed, the antibacterial effects (MICs) of AG30 and AG30/5C against MRSA were similar to their effects against methicillin-sensitive S. aureus (Fig. 2C and D).
Thus, we examined the MICs of AG30 and AG30/5C against antibiotic-resistant strains (A–E) of P. aeruginosa that were resistant to several types of antibiotics. Notably, AG30 and AG30/5C were both effective against antibiotic-resistant P. aeruginosa (Table 1). In this assay, AG30/5C also showed a more powerful antibacterial effect in comparison to the original AG30 peptide. Its antibacterial effects might occur through a ‘lytic’ mechanism that results from the attachment of cationic peptides to the bacterial membrane. To test this idea, we exogenously added cationic ions, such as CaCl2 or MgCl2, and examined whether the antibacterial effect of AG30/5C was inhibited. Indeed, the addition of CaCl2 or MgCl2 (both at 3 and 6 mM) completely blocked the antibacterial effects of AG30/5C (Table 2). These results suggest that AG30/5C kills bacteria through a lysis-based mechanism that may be different from the actions of other antibiotics.
Table 1.
MIC of AG30 or AG30/5C to antibiotic-resistant strain (A–E) of P. aeruginosa
| A | B | C | D | E | |
|---|---|---|---|---|---|
| AG30 | 20 | 10 | 40 | 20 | 10 |
| AG30/5C | 10 | 5 | 20 | 20 | 10 |
| CAZ | <8: S | <8: S | <8: S | <8: S | >32: R |
| CFPM | <8: S | <8: S | <8: S | <8: S | >16: R |
| CPZ | <16: S | 32 | <16: S | <16: S | >32: R |
| IPM/CS | <4: S | <4: S | <4: S | >8: R | <4: S |
| AZT | <8: S | >16: R | <8: S | <8: S | >16: R |
| TOB | >8: R | <4: S | >8: R | <4: S | <4: S |
| GM | >8: R | <4: S | >8: R | <4: S | 8 |
| FOM | >16: R | >16: R | >16: R | >16: R | >16: R |
| LVFX | <2: S | <2: S | <2: S | 4: S | >4: R |
| CPFX | <1: S | <1: S | <1: S | <1: S | >2: R |
The score shows MIC (mg/ml) for P. aeruginosa (A–E). R indicates resistance and S indicates sensitive to P. aeruginosa.CAZ: ceftazidime hydrate; CFPM: cefepime hidrochloride hydrate; CPZ: cefoperazone sodium; IPM/CS: imipenem/cilastatin sodium; AZT: aztreonam; TOB: tobramycin; GM: Gentamicin sulfate; FOM: fosfomycin sodium; LVFX: levofloxacin hydrate; CPFX: Ciprofloxacin hydrochloride.
Table 2.
Effect of cationic ions or FBS on MIC of AG30 or AG30/5C to P. aeruginosa
| AG30 | AG30/5C | |
|---|---|---|
| No treat | 20 | 5 |
| +1 mM MgCl2 | 80 | 20 |
| +3 mM MgCl2 | >80: R | >80: R |
| +6 mM MgCl2 | >80: R | >80: R |
| +1 mM CaCl2 | 40 | 20 |
| +3 mM CaCl2 | >80: R | >80: R |
| +6 mM CaCl2 | >80: R | >80: R |
| +10% FBS | >80:R | >80:R |
The score shows MIC (mg/ml) for P. aeruginosa. R indicates resistance to P. aeruginosa (ATCC27853). FBS: foetal bovine serum.
Based on these results, we selected molecules to which five cationic amino acids has been added (i.e. compound AG30/5C) as lead compounds for the next stage of investigation and further evaluated the function of AG30/5C.
Development of a production system for modified AG30 (AG30/5C)
To facilitate the eventual clinical investigation/application of these compounds, we developed a large-scale procedure for the synthesis of AG30/5C that employed the conventional solution method and met Good Manufacturing Practice guidelines. The AG30/5C molecule was assembled from four segments, that is (1–10), (11–17), (18–23) and (24–30), as shown in Figure 4A. The carboxyl terminus of each of the segments was protected by a phenacyl ester (Pac) group, except for the C-terminal segment that was instead protected by a benzyl ester group. All of the side-chain functional groups were protected as follows: 2-chlorobenzyloxycarbonyl (ClZ) for Lys, benzyloxymethyl (Bom) for His, p-toluenesulfonyl (Tos) for Arg, formyl (CHO) for Trp and 2-bromobenzyloxycarbonyl (BrZ) for Tyr. Each segment was elongated stepwise using the EDC/HOBt coupling reagent method. During the chain assembly, the common starting material (i.e. Boc-Arg(Tos)-Leu-OPac) could be used for preparing segments (1–10), (11–17) and (18–23) because AG30/5C contains an Arg-Leu sequence at positions 9–10, 16–17 and 22–23. This facilitated the preparation of these segments. Before being subjected to segment condensation reactions, each segment was treated with zinc powder in acetic acid to remove the Pac group from the C-terminus. The C-terminal segment was elongated in a sequential manner with segments containing a free carboxylic acid at the C-terminus by applying the EDC/HOOBt method in DMF to obtain the fully protected AG30/5C peptide. After subsequent removal of the Nα-Boc group and the CHO group on Trp using TFA and 10% piperidine/DMF, all of the protecting groups were removed by HF/p-cresol (v/v, 85/15) in the presence of methoxyamine⋅HCl and 2-mercaptopyridine at −2°C to −5°C for 1 hr [17]. After conversion of the peptide from the HF form to the AcOH form using a Muromac (acetate form) column, the crude product was purified by ion-exchange chromatography on a CM-Sepharose column using a gradient of 20 mM AcONH4 buffer to 2 M NaCl/20 mM AcONH4 buffer (pH 5.0). This was followed by RP-HPLC on an YMC-Pak ODS column using a gradient of 20% CH3CN/0.1% TFA to 40% CH3CN/0.1% TFA. The purified product was passed successively through columns of Muromac (acetate form) and Sephadex G-10 using dilute acetic acid as an eluant, which was summarized in Figure 4B. We confirmed that the antibacterial and angiogenic functions of the peptides synthesized using the conventional solution method were similar to those synthesized by the previous solid method (data not shown). The advantages of this method include reduced synthesis cost and GMP grade applicability. Indeed, the cost to produce this peptide at a 10 g scale was roughly hundred thousand dollars, more than 10-fold reduction in price in comparison to the solid method. In addition, for GMP-scale procedures, each peptide-synthesis process is the same in the initial steps, and the final coupling reaction will be performed in the GMP facility. Based on this method, we will commence with large-scale production of this peptide for use in clinical trials.
Fig 4.
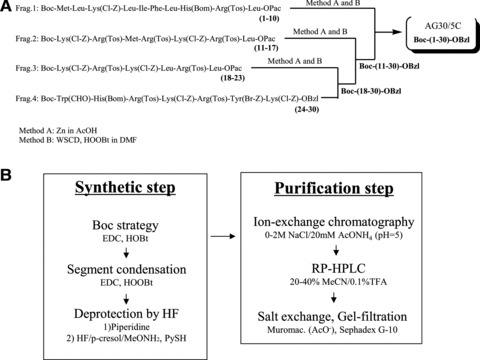
The scheme for the synthesis of AG30/5C. (A) The AG30/5C molecule was assembled from four segments, that is (1–10), (11–17), (18–23) and (24–30), and each segment was elongated stepwise using the EDC/HOBt method as coupling reagents. (B) A large-scaled procedure employing the conventional solution method that is applicable for Good Manufacturing Practice toward clinical application in both synthetic and purification steps. The crude product was purified by ion-exchange chromatograpy followed by RP-HPLC. The purified product was passed successively through columns of Muromac and Sephadex G-10.
We further examined the stability of this peptide in saline solution at both 5°C and room temperature. RP-HPLC analysis revealed that AG30/5C was fairly stable under 5°C for 12 months (96% stability), although it was less stable at room temperature (85% stability).
Effects of AG30/5C on murine and porcine wound models
In the process of wound healing, epidermal cell growth as well as angiogenesis is required to accelerate the time course of wound healing. Thus, we tested to examine the direct effect of AG30/5C on HaCaT cells. As shown in Figure 5A, the treatment of AG30/5C induced cell growth of HaCaT cells in a dose dependent manner. Thus, in the process of wound healing, AG30/5C can accelerate epithelialization as well as angiogenesis.
To evaluate the suitable dose of AG30/5C using a wound model [12], we measured the blood flow of mouse tails 3 days after inducing a full thickness injury. The topical application of AG30/5C at a dose of either 10 or 100 μg/ml significantly increased blood flow in a dose-dependent manner (Fig. 5B and C). Based on these results, we evaluated the angiogenic effects of AG30/5C and LL37 in a diabetic mouse wound-healing model. Capillary density within the ischaemic thigh adductor skeletal muscles was analysed to obtain specific evidence of vascularity at the level of the microcirculation [13, 14]. In the analysis of angiogenesis, microcapillary density was significantly increased in the AG30/5C-treated group in comparison to the LL37-treated group (Fig. 5D and E). Interestingly, at day 28 the hair growth was frequently observed in the AG30/5C-treated group (Fig. 5C). This result may support the angiogenic effects of AG30/5C because perifollicular angiogenesis undergoes a hair-cycle-dependent expansion and degeneration stage [20]. We further evaluated the angiogenic property of AG30/5C by measuring the blood flow just below the skin around wound. The evaluation of blood flow is also useful to examine an angiogenic effect [18]. As shown in Figure 5F and G, AG30/5C or LL37 treatment significantly increased the blood flow around the wound, compared with control group. These results illustrate the angiogenic properties of AG30/5C in wound-healing models.
Fig 5.
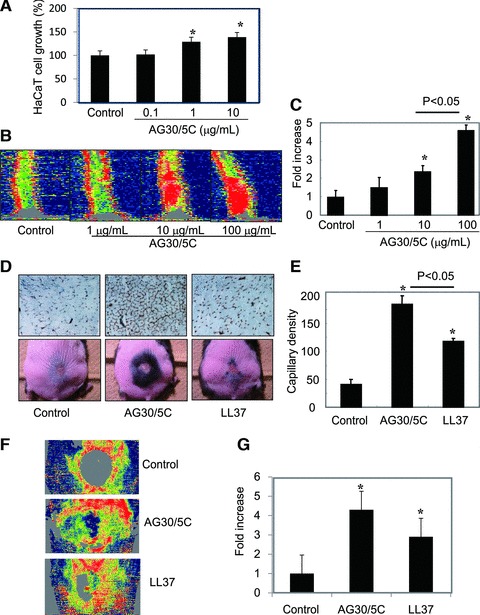
Evaluation of the angiogenic effects of AG30/5C in a wound-healing model. (A) Cell growth of HaCaT cells in the presence of AG30/5C (0, 0.1, 1 and 10 μg/ml). The quantification is presented as a percent increase. N = 8 per group and duplicated. (B, C) Blood flow was analysed by Laser Doppler Image in mouse tails three days after induction of full thickness injuries with topical application of AG30/5C (0, 1, 10 and 100 μg/ml). Low or no perfusion is displayed as dark blue, whereas the highest perfusion interval is displayed as red; the quantification is presented as a fold increase. N = 3–5 per group and duplicated. *P < 0.05 versus control. (D, E) Evaluation of wound healing in a diabetic mouse model treated with saline (control), AG30/5C or LL37. (D) The upper panel shows representative immunostains using anti-CD31 (PECAM) antibody (brown). The lower panel shows representative pictures after wound closure (day 24). Hair growth was observed around the edge of wound. (E) Capillary densities were quantified by cross section of ischaemic tissues immunostained with anti-CD31 (PECAM) antibody. N = 6–10 per group and duplicated. *P < 0.05 versus control. (F, G) Blood flow was analysed by LDI in mouse wound 3 days after operation with topical application of AG30/5C (100 μg/ml). Low or no perfusion is displayed as grey or dark blue, whereas the highest perfusion interval is displayed as yellow or red; the quantification is presented as a fold increase. N = 5–6 per group and duplicated. *P < 0.05 versus control.
Quantified analysis of the effect of AG30/5C in a wound-healing model
To investigate the possible clinical applications of AG30/5C, we quantified the effects of the peptide in a murine wound model. Under dressing conditions either with or without AG30/5C or LL37, the topical application of both compounds significantly decreased wound size at day 14 (Fig. 6A). Furthermore, three of the control-peptide-treated mice were afflicted with S. aureus infections during these experiments; in contrast, none of the mice in the AG30/5C- or LL37-treated groups became infected.
Fig 6.
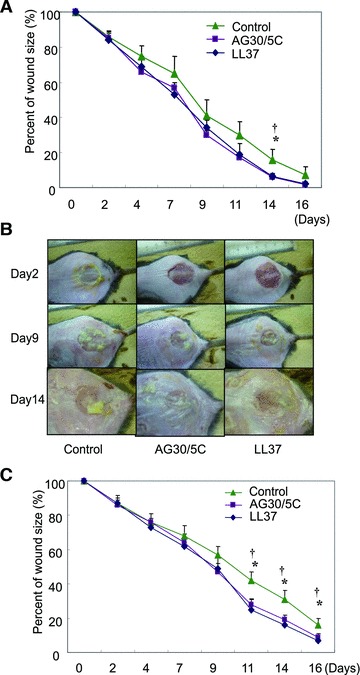
Evaluation of the effects of AG30/5C in a diabetic mouse wound-healing model with or without MRSA infection. (A) Time course of the quantification of wound size in diabetic mice with treatment of saline (control), AG30/5C or LL37 (100 μg/ml, respectively). N = 6–10 per group and duplicated. *P < 0.05 versus AG30/5C, †P < 0.05 versus LL37. (B, C) Evaluation of infectious wound healing in a diabetic mouse model treated with saline (control), modified AG30 or LL37. (B) Representative pictures of infectious wounds at days 2, 9 and 14. (C) Time course of the quantification of infectious wound size in diabetic mice treated with saline (control), AG30/5C or LL37 (100 μg/ml, respectively). N = 5–9 per group and duplicated. *P < 0.05 versus AG30/5C, †P < 0.05 versus LL37.
We also evaluated the antibacterial effects of AG30/5C using a murine infectious wound model. After topical treatment of MRSA on wounds, yellow pus was observed at day 2 and continuously increased over the duration of the experiment (Fig. 6B). We confirmed the presence of S. aureus in this yellow pus. Indeed, the infection delayed the time course of wound healing compared to a non-infectious wound-healing model. Importantly, co-treatment of either AG30/5C or LL37 attenuated the increase of yellow pus on the wounds (Fig. 6B) and significantly decreased wound size at days 11, 14 and 16 after treatment (Fig. 6C).
We further examined the effect of AG30/5C using a porcine wound model. After the topical application of both compounds every 2 days for 12 days under dressing condition, the diameter in the longitudinal views was evaluated as follows (control: 16.2 ± 0.5 mm, AG30/5C: 14.2 ± 0.5 mm, LL37: 15.4 ± 0.8 mm; Fig. 7A). In the evaluation of angiogenesis using a porcine wound model, microcapillary density was also significantly increased in both AG30/5C- and LL/37-treated groups in comparison to a control group (Fig. 7B and C). These results suggest that AG30/5C can potentially accelerate the wound-healing process.
Fig 7.
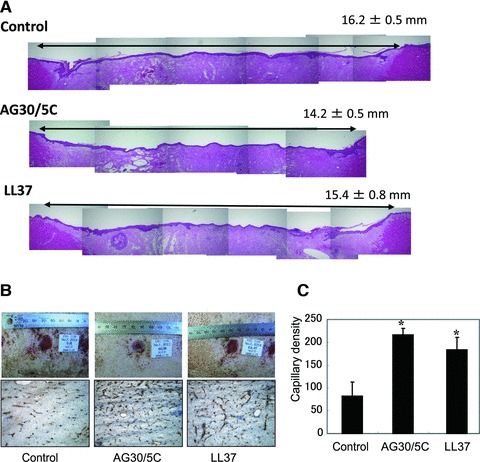
Evaluation of the effects of AG30/5C on a porcine wound-healing model. (A) Representative pictures of haematoxylin and eosin staining on full-thickness section with restored epithelium, treated with saline (control), AG30/5C or LL37 (100 μg/ml, respectively). In the upper right corner, means ± S.E.M. (mm) were shown. (B) The upper panel shows representative pictures of wound healing in each group (day 9). The lower panel shows representative immunostains using anti-vWF antibody (brown). (C) Capillary densities were quantified by cross section of ischaemic tissue immunostained with anti-vWF antibody. *P < 0.05 versus control. N = 6 per group and duplicated.
Discussion
In this study, we modified a previously described functional peptide, AG30, in an effort to develop novel wound-healing drugs that could potentially be used for clinical applications. Historically, many microbial peptides have failed to prove effective in clinical applications; this is likely because such small peptides can be easily degraded by peptidases in addition to their short half-lives.
This study is a follow-up to a previous feasibility study aimed at generating lead compounds from a foundation compound.
Short cationic amphipathic peptides with antimicrobial and/or immunomodulatory activities are important components of the innate immune response and provide a template for two separate classes of antimicrobial drugs. Rapid acting and potent antimicrobial peptides are promising targets for investigation in the development of clinical treatments. In our modifications of the foundation peptide, we addressed the net positive charge and the amphipathic three-dimensional structure of AG30, and designed to modulate the balance between a net charge and α-helical structure by replacing a few amino acids. As a result, AG30/5C, replaced of five cationic amino acids, exhibited increased angiogenic and antimicrobial activities. Although AG30/5C showed the lowest ratio of α-helical the structure is still helical, and the three-dimentional structure of AG30/5C showed the broad cationic peptide surface. Thus, we speculate that AG30/5C can keep the cationic surface, which leads to increased activity, even though α-helical structure is not tightly fixed. Balancing these two parameters, thus enabling the net positive charged surface to attach and accumulate at the cell surface, was critical for the activity of this peptide. Indeed, the antibacterial action of this peptide was antagonized by the addition of divalent cations such as Mg2+ and Ca2+. Some studies have modified antimicrobial peptides to develop new classes of antibiotics; however, this is the first report to modulate both antibacterial and angiogenic actions while maintaining activity and structure. The strategies and methods presented here could be useful in the further modifications of potential peptides in the early stages of clinical development.
Cationic antimicrobial peptides have a mixed history in clinical trials. The cationic peptides polymixin B and gramicidin S have long been used both in the clinic and as topical over-the-counter medicines. However, despite several clinical trials over the past ten years, the clinical success of cationic antimicrobial peptides has been limited [20]. To date, four cationic peptides have advanced into phase-III clinical trials. These peptides have been designed for treating impetigo and diabetic foot ulcers (the frog magainin derivative MSI-78; Pexiganan), oral mucosaitis (the pigprotegrin derivative IB-367; Iseganan), sepsis (the human bactericidal permeability protein derivative rBPI23; Neuprex) and catheter-associated infections (the cattle indolicidin variant CP-226; Omiganan). Among them, Omiganan has achieved statistically significant success both in reducing infections that tunnel in from the catheter insertion site and in reducing catheter colonization during phase IIa trials [21]. Importantly, all four peptides are derivatives that were generated from original cationic peptides. Thus, in this study, we employed a feasibility study to develop lead compounds. Using an animal wound-healing model, we utilized simple topical application without ointment or cream; this provided insight into the effectiveness of our peptide as a therapeutic drug and revealed some important limitations. To improve the effectiveness of the peptide as a therapeutic drug, it is possible that production of AG30/5C as an ointment or cream might enhance the local accumulation and structural stability of the peptide. The other aspect of peptide modification for eventual clinical development is to consider the cost of generating a peptide at the GMP level. In this study, we attempted to develop a large-scale procedure for the synthesis of AG30/5C that employed a conventional solution method applicable to Good Manufacturing Practice; importantly, we succeeded in generating a therapeutic peptide for use in pre-clinical trials. Furthermore, we tried to shorten the peptide while maintaining both its angiogenic and its antibacterial actions in an effort to minimize costs.
Based on these results, we have now transitioned into a pre-clinical study that includes evaluations of toxicity at single or repeated doses, genotoxicity and examination using local stimulation while monitoring serum concentrations of AG30/5C. We will continue to propose AG30/5C as a wound-healing drug for the treatment of ischaemic ulcers of diabetes patients or peripheral arterial diseases.
Acknowledgments
We thank all the members of the Gene Therapy Science Laboratory for critical discussions during the course of this work. We also thank Yuichi Koga and Kazufumi Takano in Department of Material and Life Science, Osaka University for technical help in CD analysis. This work was supported by the National Institute of Biomedical Innovation and the Takeda Science Foundation.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–95. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 2.Hancock RE, Patrzykat A. Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr Drug Targets Infect Disord. 2002;2:79–83. doi: 10.2174/1568005024605855. [DOI] [PubMed] [Google Scholar]
- 3.Giangaspero A, Sandri L, Tossi A. Amphipathic alpha helical antimicrobial peptides. Eur J Biochem. 2001;268:5589–600. doi: 10.1046/j.1432-1033.2001.02494.x. [DOI] [PubMed] [Google Scholar]
- 4.Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66:236–48. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 5.Sorensen O, Bratt T, Johnsen AH, et al. The human antibacterial cathelicidin, hCAP-18, is bound to lipoproteins in plasma. J Biol Chem. 1999;274:22445–51. doi: 10.1074/jbc.274.32.22445. [DOI] [PubMed] [Google Scholar]
- 6.Nishikawa T, Nakagami H, Maeda A, et al. Development of a novel antimicrobial peptide, AG-30, with angiogenic properties. J Cell Mol Med. 2008;13:535–46. doi: 10.1111/j.1582-4934.2008.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rikitake Y, Hirata K, Kawashima S, et al. Involvement of endothelial nitric oxide in sphingosine-1-phosphate-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2002;22:108–14. doi: 10.1161/hq0102.101843. [DOI] [PubMed] [Google Scholar]
- 8.Koczulla R, von DegenfeldG, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–72. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boman HG. Antibacterial peptides: basic facts and emerging concepts. J Intern Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 10.Hunter HN, Jing W, Schibli DJ, et al. The interactions of antimicrobial peptides derived from lysozyme with model membrane systems. Biochim Biophys Acta. 2005;1668:175–89. doi: 10.1016/j.bbamem.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Jimenez MA, Evangelio JA, Aranda C, et al. Helicity of alpha(404–451) and beta(394–445) tubulin C-terminal recombinant peptides. Protein Sci. 1999;8:788–99. doi: 10.1110/ps.8.4.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho CH, Sung HK, Kim KT, et al. COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proc Natl Acad Sci USA. 2006;103:4946–51. doi: 10.1073/pnas.0506352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagami H, Maeda K, Morishita R, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542–7. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 14.Kunigiza Y, Tomita N, Taniyama Y, et al. Acceleration of wound healing by combined gene therapy of hepatocyte growth factor and prostacyclin synthase with Shima jet. Gene Ther. 2006;13:1143–52. doi: 10.1038/sj.gt.3302767. [DOI] [PubMed] [Google Scholar]
- 15.D’Andrea LD, Iaccarino G, Fattorusso R, et al. Targeting angiogenesis: structural characterization and biological properties of a de novo engineered VEGF mimicking peptide. Proc Natl Acad Sci USA. 2005;102:14215–20. doi: 10.1073/pnas.0505047102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azuma J, Taniyama Y, Takeya Y, et al. Angiogenic and antifibrotic actions of hepatocyte growth factor improve cardiac dysfunction in porcine ischemic cariomyopathy. Gene Ther. 2006;13:1206–13. doi: 10.1038/sj.gt.3302740. [DOI] [PubMed] [Google Scholar]
- 17.Munoz V, Serrano L. Elucidating the folding problem of helical peptides using empirical parameters. Nat Struct Biol. 1994;1:399–409. doi: 10.1038/nsb0694-399. [DOI] [PubMed] [Google Scholar]
- 18.Petukhov M, Munoz V, Yumoto N, et al. Position dependence of non-polar amino acid intrinsic helical propensities. J Mol Biol. 1998;278:279–89. doi: 10.1006/jmbi.1998.1682. [DOI] [PubMed] [Google Scholar]
- 19.Venyaminov S, Baikalov IA, Wu CS, et al. Some problems of CD analyses of protein conformation. Anal Biochem. 1991;198:250–5. doi: 10.1016/0003-2697(91)90421-o. [DOI] [PubMed] [Google Scholar]
- 20.Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107:409–17. doi: 10.1172/JCI11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Falla TJ. Antimicrobial peptides: therapeutic potential. Expert Opin Pharmacother. 2006;7:653–63. doi: 10.1517/14656566.7.6.653. [DOI] [PubMed] [Google Scholar]


