Abstract
Raft-dependent endocytosis is in large part defined as the cholesterol-sensitive, clathrin-independent internalization of ligands and receptors from the plasma membrane. It encompasses the endocytosis of caveo-lae, smooth plasmalemmal vesicles that form a subdomain of cholesterol and sphingolipid-rich lipid rafts and that are enriched for caveolin-1. While sharing common mechanisms, like cholesterol sensitivity, raft endocytic routes show differential regulation by various cellular components including caveolin-1, dynamin-2 and regulators of the actin cytoskeleton. Dynamin-dependent raft pathways, mediated by caveolae and morphologically equivalent non-caveolin vesicular intermediates, are referred to as caveolae/raft-dependent endocytosis. In contrast, dynamin-independent raft pathways are mediated by non-caveolar intermediates. Raft-dependent endocytosis is regulated by tyrosine kinase inhibitors and, through the regulation of the internalization of various ligands, receptors and effectors, is also a determinant of cellular signaling. In this review, we characterize and discuss the regulation of raft-dependent endocytic pathways and the role of key regulators such as caveolin-1.
Keywords: raft-dependent endocytosis, caveolin-1, caveolae, cholesterol, cellular signaling
Introduction
Identified in the 1950s, caveolae are 50–80 nm diameter plasma membrane invaginations that are morphologically distinct from clathrin-coated pits [1]. Caveolae are cholesterol- and sphingolipid-rich and considered a subdomain of plasma membrane microdomains or lipid rafts. Lipid rafts have been defined as ‘small (10–200 nm) heterogeneous membrane domains enriched in sterol and sphingolipids that are involved in the compartmentalization of various cellular processes’[2]. Multiple studies have described the role of caveolae and rafts in the endocytosis of various ligands (for reviews see refs. [3–5]). Several raft-dependent pathways have been described and raft ligands are quite liberal in their selectivity for a particular route of entry into the cell. The extent and nature of raft-dependent endocytosis is regulated by various cellular components that include caveolin-1 (Cav1), cholesterol and dynamin as well as regulators of the actin cytoskeleton.
Cav1 is the major component of caveolae and its expression is essential for the formation of caveolar vesicles. An absence of caveolae is noted in cells that do not express Cav1 and its reintroduction into these cells induces caveolae formation at the plasma membrane [6]. Two other proteins of the same family also exist. Caveolin-2 (Cav2) facilitates but is not essential for caveolae formation [7–9]. Caveolin-3 (Cav3) is specifically expressed in muscle [10]. Cav1 is a scaffolding protein that oligomerizes at the plasma membrane [11]. Caveolae are highly immobile at the plasma membrane [12] and Cav1 has been proposed to be a negative regulator of raft-dependent endocytosis [13, 14]. However, upon activation by SV40, Cav1 mobility at the cell surface is greatly increased [15]. There is also evidence for raft-dependent endocytic pathways independent of Cav1 that are mediated by distinct carrier vesicles [16–19]. In this review, we will characterize the various raft-dependent endocytic pathways and discuss their regulation.
Raft-dependent endocytosis encompasses various pathways
Internalization of molecules via clathrin-coated pits is the best studied endocytic pathway [20]. Various other pathways, commonly referred to as clathrin-independent, have been identified and are under intense investigation. Some of these pathways are cholesterol-sensitive and therefore considered to be raft-mediated. It is important to recognize that clathrin-mediated endocytosis is also sensitive to acute depletion of cholesterol [21, 22] and that raft recruitment has been shown to precede clathrin-dependent endocytosis for EGFR, BCR and anthrax toxin [23–25]. In addition, macropinocytosis, involving Rac1-dependent membrane ruffling at the plasma membrane, can be cholesterol-sensitive, potentially defining another dynamin-independent raft pathway [26, 27]. However, macropinocytosis has been shown to be dynamin-dependent in NIH-3T3 and HUVEC cells [28, 29]. The dynamin- and raft-dependence of macropinocytotic pathways may be cell type and cargo-specific. For the purposes of this review, raft-dependent endocytic pathways will be defined by their clathrin-independence and cholesterol-sensitivity and will not include macropinocytosis.
A characteristic of some of these raft-dependent pathways is their dependence on dynamin, a molecule involved in vesicular fission from the plasma membrane [30, 31]. The formation of dynamin-dependent smooth plasma membrane vesicles, or caveolar invaginations can occur both in the presence or absence of caveolins [13]. Similarities between the caveolae and non-caveolin dynamin-dependent raft endocytic pathways led us to refer to them inclusively as caveolae/raft-dependent endocy-tosis [14]. Dynamin-independent raft pathways have been described that are caveolin-independent and invoke tubular intermediates [16, 17]. While the heterogeneity of raft domains [32] is certainly indicative of higher orders of complexity and regulation of their endocytosis, to a large extent, and at least for now, raft-dependent endocytic pathways can be classified based on their caveolin- and dynamin-dependence (Fig. 1A). This classification is based on mechanistic similarities of the raft-dependent internalization of select ligands at the plasma membrane. Indeed, different cargoes that use similar raft endocytic mechanisms may be internalized via distinct raft domains and targeted to different intracellular sites [14, 33].
1.
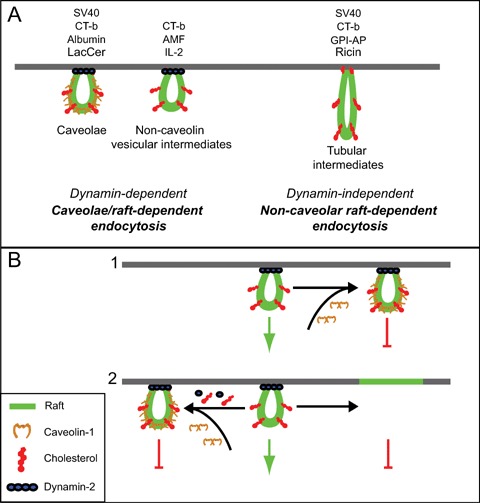
Raft-dependent endocytosis and its regulation by Cav1. (A) Several endocytic pathways are characterized as raft-dependent and mediate the uptake of various ligands, including but not limited to those indicated. These include dynamin-dependent pathways that invoke caveolae or non-caveolin vesicular intermediates and that can be referred to as caveolae/raft-dependent endocytosis [14]. Dynamin-independent pathways invoke non-caveolar tubular intermediates. While similar mechanisms control the uptake of the indicated raft-dependent ligands, they are not necessarily internalized by the same raft domains or follow similar intracellular targeting routes. (B) Cav1 may negatively regulate uptake via the dynamin-dependent, non-caveolin pathway by either stabilizing raft invaginations at the cell surface (1) or by sequestering key components, including cholesterol, dynamin and others, required for raft-dependent uptake (2). Cholesterol is not shown in the flat portion of the membrane to simplify the diagram. LacCer:lactosylceramide; CT-b: cholera toxin b subunit; GPI-AP: glycosylphosphatidylinositol-anchored proteins; AMF: autocrine motility factor; IL-2: interleukin-2; SV40:simian virus 40.
Some ligands enter the cell via a caveolae-dependent pathway. The simian virus SV40 follows a dynamin-dependent, caveolae-mediated pathway that targets a caveolin-positive endosome, the caveosome, before being delivered to the smooth endoplasmic reticulum [34]. When stimulated by SV40, caveolin, dynamin and actin are recruited sequentially to the caveolae [34]. The raft-dependent endocytic pathway of cholera toxin b-subunit (CT-b) has also been characterized as a dynamin-dependent, caveolar pathway [19, 35–37]. Albumin is internalized via a dynamin-dependent pathway that requires cave-olin [38]. In lymphocytes lacking Cav1, endocytosis of the interleukin-2 receptor occurs via a clathrin-independent, cholesterol-sensitive pathway that requires dynamin and is regulated by the RhoA GTPase [39]. In NIH-3T3 cells, the autocrine motility factor receptor is localized to caveolae and internalization of its ligand, AMF, is cholesterol and dynamin-dependent and negatively regulated by Cav1 expression [13, 40].
A raft-dependent, dynamin-independent pathway has also been described for CT-b and SV40 [16, 17] that exhibits similarity to a Cdc42-dependent pathway followed by GPI-anchored proteins (GPI-AP) and fluid phase markers [41]. In fibroblasts from Cav1 knockout mice, SV40 exploits an alternate, Cav1-independent pathway that is cholesterol and tyrosine kinase dependent but independent of clathrin, dynamin-2 and ARF6 [16]. A similar pathway has also been described for CT-b in Cav1−/− fibrob-lasts where it is ARF6-dependent [17]. This pathway invokes not caveolar invaginations but the formation of uncoated tubular endocytic structures and an intracellular dynamin-dependent step for delivery to endosomes and the Golgi apparatus [17]. Internalization of CT-b has also been shown to occur via a dynamin-independent pathway defined not by caveolin but by flotillin, another raft component [42].
CT-b therefore provides an example of an endo-cytic ligand internalized by several pathways including clathrin-coated pits and both dynamin-dependent and independent raft pathways [43]. A recent study showed that 50% of CT-b enters the cell via clathrin-coated pits with the remainder internalized via dynamin-independent, caveolin-independent uncoated tubules. In the same study, the authors showed that about only 2% of the total pool of Cav1 positive caveolae contributes to the internalization of CT-b, suggesting that internalization of CT-b via caveolae represents only a minor contribution [17]. However, CT-b internalization was found to be deficient in immortalized Cav1−/− MEF-derived cell lines [44] contrasting with the demonstration that primary Cav1−/− MEFs show no difference in CT-B uptake compared to wild-type MEFs [17]. In HeLa cells, depletion of flotillin by siRNA prevents its uptake via a dynamin-independent route and switches it to a dynamin-dependent route [42]. Variable cell surface expression of the CT-b receptor, GM1 ganglioside, impacts on the extent of its raft-dependent endocytosis [45]. In addition, CT-b concentrations used vary significantly (from 0.05 to 10 μg/ml) between studies from different laboratories [17, 42, 45, 46]. Interestingly, in studies defining the dynamin-independent raft pathway, both CT-b and dextran concentrations were relatively low [17, 18]. Variable factors, ranging from expression of ligand receptors to raft components, may impact not only on the extent of CT-b uptake but also on its route of entry into different cells or clonal populations of the same cell type.
Raft-dependent endocytosis is therefore a highly complex process in which the same cargo can follow various entry routes and in which different cargo can use similar entry routes with different molecular reg-ulation. This complexity should not preclude efforts to classify these pathways based on common denomi-nators, as proposed in Figure 1A. Further characterization of the cargo-specificity and molecular regulation of raft-dependent pathways will lead to a better understanding of what are clearly intricate mechanisms regulated by multiple factors.
Cav1 and the regulation of raft-dependent endocytosis
Fluorescence recovery after photobleaching (FRAP) experiments have shown that movement of Cav1 at the cell surface is restricted by cortical actin as well as through interaction with the actin-binding protein filamin [12, 47]. Caveolar stability at the plasma membrane suggests that rapid, constitutive internalization and turnover of caveolae is unlikely to occur. Rapid, reversible budding of caveolae, or potocyto-sis, was originally suggested to regulate folate internalization [48]. More recently, TIRF microscopy was used to show that reversible caveolae budding is limited to the subplasma membrane region by the underlying actin cytoskeleton [15]. Disruption of the actin cytoskeleton induces rapid internalization of caveolar vesicles [49, 50]. Recruitment of SV40 to caveolae induces the transient, localized breakdown of the actin cytoskeleton [51]. Actin depolymerization also induces internalization of tight junction proteins via a caveolae-dependent pathway [52]. However, earlier work showed that disruption of the actin cytoskeleton by cytochalasin D in A431 cells inhibited alkaline phosphatase uptake via caveolae [37]. The submembrane actin cytoskeleton would therefore appear to be a critical regulator of the endocytic potential of caveolae.
Several raft-dependent endocytic pathways are regulated via Rho family GTPases. GPI-anchored proteins have been shown to be internalized via a Cdc42-regulated pathway that is independent of Rho and Rac [41]. RhoA regulates IL-2 receptor internalization [39] and CT-b endocytosis to the Golgi apparatus in Cos-1 cells is dependent on RhoG [53]. The Menkes disease ATPase (ATP7A) uptake can be inhibited by a Rac-1 dominant negative mutant [54]. Constitutively active Rac and RhoA also downregu-late clathrin-dependent endocytosis [55]. Differential expression and local activation of Rho family GTPases may be a key determinant of the cell type specificity of raft-dependent endocytosis.
Threshold levels of Cav1 and cholesterol regulate caveolae formation [56, 57]. Various cholesterol modulating agents, including methyl-β-cyclodextrin, nys-tatin and filipin, have been shown to inhibit both caveolae expression and raft-dependent endocytosis [13, 16, 57–60]. Caveolar endocytosis of various lig-ands can be significantly increased by addition of cholesterol or glycosphingolipid to human fibroblasts [60]. Using heterokaryons expressing both Cav1-GFP and Cav1-RFP, it has been shown that cholesterol depletion increased exchange between otherwise stable Cav1 positive structures [15]. Cav1 interacts directly with cholesterol [61, 62] and cholesterol levels in lipid raft fractions obtained from Cav1 expressing cells were 3–4-fold higher than in matched cells lacking Cav1 [63]. It is possible that Cav1 regulation of raft endocytosis is linked to its ability to sequester cholesterol in raft domains (Fig. 1B). In biological membranes, the ratio between cholesterol and phos-pholipids is maintained slightly below 1:1 [64]. When in excess and therefore free from interaction with phospholipids, cholesterol shows a higher chemical activity [65]. This pool has been described as active cholesterol [65–68]. Cav1 may therefore regulate cholesterol-dependent processes, such as raft endo-cytosis, through sequestration of active cholesterol.
Caveolae budding from the plasma membrane and subsequent internalization requires dynamin II [36]. Expression of the K44A dynamin mutant increases the number of caveolae in caveolin-expressing NIH-3T3 cells as well as the formation of morphologically similar invaginations in Ras and Abl-transformed NIH-3T3 cells expressing little caveolin [13]. Indeed, caveolae-like structures in cells devoid of caveolin have been reported [69]. Several studies have shown that overexpression of Cav1 is associated with reduction, even inhibition of raft-dependent endocytosis [13, 17, 38, 46]. Cav1 overexpression was also found to inhibit the non-caveolar, dynamin-independent endocytosis of CT-b [17]. Reduction of Cav1 levels in mammary tumor-derived cell lines is associated with both increased plasma membrane mobility and raft-dependent uptake of CT-B to the Golgi apparatus. Interestingly, regulation of CT-b mobility and endocytosis in these cells occurred at Cav1 levels below the threshold for caveolae formation (Lajoie, Nim and Nabi, unpublished). This suggests that Cav1 may act indirectly to regulate raft-dependent endocytosis by impacting on the composition and endocytic potential of non-caveolar raft domains (Fig. 1B). Indeed, the idea of dynamic exchange between raft domains is consistent with the ability of raft components, such as Cav1 or flotillin, to impact on the raft-dependent endocytosis of select ligands by modulating the endocytic potential of distinct raft domains.
Signaling and raft-dependent endocytosis
Treatment of cells with tyrosine kinase inhibitors blocks caveolae endocytosis while addition of the phosphatase inhibitor okadaic acid triggers endocytosis [17, 37, 51, 70]. Indeed, the use of the non-specific tyrosine kinase inhibitor genistein is generally recognized as a selective inhibitor of raft-dependent endocytic pathways. Cav1 is phosphorylated by Src kinase at tyrosine 14 [71]; however, the role of Cav1 phosphorylation in raft endocytosis is still unclear. The predominant cellular location of tyrosine phos-phorylated Cav1 is in focal adhesions. Redistribution of tyrosine phosphorylated Cav1 from focal adhesions to caveolae upon cell detachment from the extracellular matrix triggers raft-dependent endocy-tosis and plasma membrane depletion of Rac [72]. Activation of v-Src in Rat-1 cells is responsible for Cav1 phosphorylation and is associated with loss of plasma membrane caveolae [73]. In addition, Cav1 phosphorylation on tyrosine 14 is associated with flattening, aggregation and fusion of caveolae vesicles [74]. However, in pancreatic cancer cells, EGF stimulation of Src-mediated Cav1 phosphorylation leads to a marked increase in the number of assembled caveolae at the cell surface [75]. Src kinase regulation of transcytosis of albumin across the endothelial cell monolayer is associated with Cav1 phosphorylation [70]. Src kinase activity is also required for stimulation of caveolae internalization by glycosphingolipids and cholesterol [60]. However, whether Cav1 tyrosine phosphorylation is a critical regulator of caveolae internalization remains to be determined.
SV40 recruitment to caveolae stimulates local tyrosine phosphorylation. Tyrosine phosphorylation inhibitors do not prevent SV40 recruitment to caveo-lae but do prevent recruitment of dynamin to caveolae suggesting that tyrosine phosphorylation is crucialfor dynamin-dependent caveolae budding [51]. Similarly, the Src-dependent internalization of albumin via a Gi-coupled pathway requires interaction of its receptor, gp60, with Cav1 [38, 76]. Dominant negative Src reduces phosphorylation of dynamin-2 and dynamin-2 association with Cav1 resulting in reduced albumin uptake [77]. This suggests that tyrosine phosphorylation regulates caveolar budding by controlling dynamin recruitment to caveolae. However, the requirement for tyrosine kinases in the raft-dependent uptake of AMF in cancer cells expressing low levels of Cav1 [78] and in the dynamin-independent raft uptake of SV40 in Cav1−/− cells [16] is indicative of further complexity for the role of tyrosine phosphorylation in raft-dependent endocytosis. A siRNA screening approach of kinase inhibitors identified a large group of 208 human kinases as regulators of SV40 entry and 39 of them were involved in caveolae/raft trafficking [79]. Application of a similar approach to other raft ligands may identify common and, potentially, distinct kinases that control raft-dependent endocytosis of various raft ligands.
Cav1 has a well-established scaffolding function implicated in the sequestration of cytokine receptors and lipid-anchored signaling intermediates as well as cholesterol [80, 81]. Sequestration of EGFR and TGF R to caveolae and interaction with Cav1 is associated with inhibition of signaling capacity [76, 82–84]. These studies were later confirmed when it was shown that Cav1 was able to induce sequestration of the receptor [85] and to directly bind EGFR [86, 87]. Moreover, the second cysteine region of EGFR contains sequences that target the receptor to caveolae/raft domains [88]. Upon stimulation with EGF, EGFR is no longer localized in low density raft fractions, consistent with its migration from caveolae to clathrin coat pits upon stimulation [83]. Alternatively, Cav1 may indirectly regulate EGFR signaling through regulation of the cholesterol content of lipid rafts [89]. When stimulated with a high EGF dose, EGFR is internalized via a caveolae/raft-dependent pathway associated with ubiquitination of the receptor [90]. Similarly, clathrin-dependent uptake of TGF R is associated with subsequent signaling events via Smad2 phosphorylation in EEA1-positive endo-somes while its caveolae/raft-dependent is associated with receptor degradation through binding to the smad7-smurf2 complex [82]. Raft-dependent endo-cytosis is therefore both regulated by and impacts on cell signaling.
Conclusion
Raft-dependent endocytosis includes various cholesterol-sensitive endocytic routes, distinct from clathrin-mediated endocytosis, which can be classified based on their dependence on Cav1 and dynamin. These pathways share sensitivity to cholesterol depletion as well as to other more selective regulators whose cell-specific expression may impact on the endocytic pathway followed by multiple raft-dependent ligands. By impacting indirectly on raft domain organization, various raft components, including cholesterol, Cav1 and flotillin, regulate raft-dependent endocytosis. Cav1 acts as a determinant of raft-dependent endocytosis by stabilizing rafts at the cell surface, via receptor recruitment or through sequestration of cholesterol and other critical determinants of raft-dependent endocytosis. Further study of raft-dependent endocytosis should lead to the further classification and identification of specific regulators of the endocytic potential of these varied pathways. Open questions that remain relate to the molecular regulation of raft-dependent endocytosis and how the heterogeneous composition of raft domains, including but not limited to cargo, impacts and determines their endocytic potential, mechanism of internalization and intracellular targeting.
References
- 1.Palade G. Fine structure of blood capillaries. J Appl Phys. 1953;24:1424. [Google Scholar]
- 2.Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–8. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Hommelgaard AM, Roepstorff K, Vilhardt F, Torgersen ML, Sandvig K, Van Deurs B. Caveolae: stable membrane domains with a potential for internalization. Traffic. 2005;6:720–4. doi: 10.1111/j.1600-0854.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- 4.Kirkham M, Parton RG. Clathrin-independent endocy-tosis:new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta. 2005;1745:273–86. doi: 10.1016/j.bbamcr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–20. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- 6.Fra AM, Williamson E, Simons K, Parton RG. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci USA. 1995;92:8655–9. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, Kohtz DS, Van Donselaar E, Peters P, Lisanti MP. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem. 1997;272:29337–46. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- 8.Lahtinen U, Honsho M, Parton RG, Simons K, Verkade P. Involvement of caveolin-2 in caveolar biogenesis in MDCK cells. FEBS Lett. 2003;538:85–8. doi: 10.1016/s0014-5793(03)00135-2. [DOI] [PubMed] [Google Scholar]
- 9.Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, Kneitz B, Hou H, Jr, Christ GJ, Edelmann W, Lisanti MP. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol. 2002;22:2329–44. doi: 10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Way M, Parton RG. M-caveolin, a muscle-specific caveolin-related protein. FEBS Lett. 1995;376:108–12. doi: 10.1016/0014-5793(95)01256-7. [DOI] [PubMed] [Google Scholar]
- 11.Monier S, Parton RG, Vogel F, Behlke J, Henske A, Kurzchalia TV. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell. 1995;6:911–27. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomsen P, Roepstorff K, Stahlhut M, Van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol Cell. 2002;13:238–50. doi: 10.1091/mbc.01-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le PU, Guay G, Altschuler Y, Nabi IR. Caveolin-1 is a negative regulator of caveolae-mediated endocyto-sis to the endoplasmic reticulum. J Biol Chem. 2002;277:3371–9. doi: 10.1074/jbc.M111240200. [DOI] [PubMed] [Google Scholar]
- 14.Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161:673–7. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tagawa A, Mezzacasa A, Hayer A, Longatti A, Pelkmans L, Helenius A. Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J Cell Biol. 2005;170:769–79. doi: 10.1083/jcb.200506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damm EM, Pelkmans L, Kartenbeck J, Mezzacasa A, Kurzchalia T, Helenius A. Clathrin- and caveolin-1-independent endocytosis:entry of simian virus 40 into cells devoid of caveolae. J Cell Biol. 2005;168:477–88. doi: 10.1083/jcb.200407113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkham M, Fujita A, Chadda R, Nixon SJ, Kurzchalia TV, Sharma DK, Pagano RE, Hancock JF, Mayor S, Parton RG. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol. 2005;168:465–76. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayor S, Rothberg KG, Maxfield FR. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science. 1994;264:1948–51. doi: 10.1126/science.7516582. [DOI] [PubMed] [Google Scholar]
- 19.Nichols BJ. A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat Cell Biol. 2002;4:374–8. doi: 10.1038/ncb787. [DOI] [PubMed] [Google Scholar]
- 20.Benmerah A, Lamaze C. Clathrin-coated pits:vive la difference? Traffic. 2007;8:970–82. doi: 10.1111/j.1600-0854.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- 21.Rodal SK, Skretting G, Garred O, Vilhardt F, Van Deurs B, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell. 1999;10:961–74. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci USA. 1999;96:6775–80. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abrami L, Liu S, Cosson P, Leppla SH, Van Der Goot FG. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J Cell Biol. 2003;160:321–8. doi: 10.1083/jcb.200211018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puri C, Tosoni D, Comai R, Rabellino A, Segat D, Caneva F, Luzzi P, Di Fiore PP, Tacchetti C. Relationships between EGFR signaling-competent and endocytosis-competent membrane microdomains. Mol Biol Cell. 2005;16:2704–18. doi: 10.1091/mbc.E04-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoddart A, Dykstra ML, Brown BK, Song W, Pierce SK, Brodsky FM. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity. 2002;17:451–62. doi: 10.1016/s1074-7613(02)00416-8. [DOI] [PubMed] [Google Scholar]
- 26.Grimmer S, Van Deurs B, Sandvig K. Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J Cell Sci. 2002;115:2953–62. doi: 10.1242/jcs.115.14.2953. [DOI] [PubMed] [Google Scholar]
- 27.Schneider B, Schueller C, Utermoehlen O, Haas A. Lipid microdomain-dependent macropinocytosis determines compartmentation of Afipia felis. Traffic. 2007;8:226–40. doi: 10.1111/j.1600-0854.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- 28.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116:1599–609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 29.Schlunck G, Damke H, Kiosses WB, Rusk N, Symons MH, Waterman-Storer CM, Schmid SL, Schwartz MA. Modulation of Rac localization and function by dynamin. Mol Biol Cell. 2004;15:256–67. doi: 10.1091/mbc.E03-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–34. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henley JR, Cao H, McNiven MA. Participation of dynamin in the biogenesis of cytoplasmic vesicles. Faseb J. 1999;13(Suppl 2):S243–7. doi: 10.1096/fasebj.13.9002.s243. [DOI] [PubMed] [Google Scholar]
- 32.Hancock JF. Lipid rafts:contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–62. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124:729–40. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3:473–83. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 35.Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol. 1998;141:101–14. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, Malik AB. Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J Cell Biol. 2000;150:1057–70. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell. 2001;7:661–71. doi: 10.1016/s1097-2765(01)00212-x. [DOI] [PubMed] [Google Scholar]
- 40.Benlimame N, Le PU, Nabi IR. Localization of autocrine motility factor receptor to caveolae and clathrin-independent internalization of its ligand to smooth endoplasmic reticulum. Mol Biol Cell. 1998;9:1773–86. doi: 10.1091/mbc.9.7.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2:411–23. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 42.Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 43.Torgersen ML, Skretting G, Van Deurs B, Sandvig K. Internalization of cholera toxin by different endo-cytic mechanisms. J Cell Sci. 2001;114:3737–47. doi: 10.1242/jcs.114.20.3737. [DOI] [PubMed] [Google Scholar]
- 44.Sotgia F, Razani B, Bonuccelli G, Schubert W, Battista M, Lee H, Capozza F, Schubert AL, Minetti C, Buckley JT, Lisanti MP. Intracellular retention of glycosylphosphatidyl inositol-linked proteins in cave-olin-deficient cells. Mol Cell Biol. 2002;22:3905–26. doi: 10.1128/MCB.22.11.3905-3926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pang H, Le PU, Nabi IR. Ganglioside GM1 levels are a determinant of the extent of caveolae/raft-dependent endocytosis of cholera toxin to the Golgi apparatus. J Cell Sci. 2004;117:1421–30. doi: 10.1242/jcs.01009. [DOI] [PubMed] [Google Scholar]
- 46.Le PU, Nabi IR. Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endo-plasmic reticulum. J Cell Sci. 2003;116:1059–71. doi: 10.1242/jcs.00327. [DOI] [PubMed] [Google Scholar]
- 47.Stahlhut M, Van Deurs B. Identification of filamin as a novel ligand for caveolin-1:evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol Biol Cell. 2000;11:325–37. doi: 10.1091/mbc.11.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson RG, Kamen BA, Rothberg KG, Lacey SW. Potocytosis:sequestration and transport of small molecules by caveolae. Science. 1992;255:410–1. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- 49.Conrad PA, Smart EJ, Ying YS, Anderson RG, Bloom GS. Caveolin cycles between plasma membrane caveolae and the Golgi complex by micro-tubule-dependent and microtubule-independent steps. J Cell Biol. 1995;131:1421–33. doi: 10.1083/jcb.131.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mundy DI, Machleidt T, Ying YS, Anderson RG, Bloom GS. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J Cell Sci. 2002;115:4327–39. doi: 10.1242/jcs.00117. [DOI] [PubMed] [Google Scholar]
- 51.Pelkmans L, Puntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–9. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 52.Shen L, Turner Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell. 2005;16:3919–36. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prieto-Sanchez RM, Berenjeno IM, Bustelo XR. Involvement of the Rho/Rac family member RhoG in caveolar endocytosis. Oncogene. 2006;25:2961–73. doi: 10.1038/sj.onc.1209333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cobbold C, Coventry J, Ponnambalam S, Monaco AP. The Menkes disease ATPase (ATP7A) is internalized via a Rac1-regulated, clathrin- and caveolae-independent pathway. Hum Mol Genet. 2003;12:1523–33. doi: 10.1093/hmg/ddg166. [DOI] [PubMed] [Google Scholar]
- 55.Lamaze C, Chuang TH, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endo-cytosis by Rho and Rac. Nature. 1996;382:177–9. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- 56.Breuza L, Corby S, Arsanto JP, Delgrossi MH, Scheiffele P, Le Bivic A. The scaffolding domain of caveolin 2 is responsible for its Golgi localization in Caco-2 cells. J Cell Sci. 2002;115:4457–67. doi: 10.1242/jcs.00130. [DOI] [PubMed] [Google Scholar]
- 57.Hailstones D, Sleer LS, Parton RG, Stanley KK. Regulation of caveolin and caveolae by cholesterol in MDCK cells. J Lipid Res. 1998;39:369–79. [PubMed] [Google Scholar]
- 58.Parpal S, Karlsson M, Thorn H, Stralfors P. Cholesterol depletion disrupts caveolae and insulin receptor signaling for metabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. J Biol Chem. 2001;276:9670–8. doi: 10.1074/jbc.M007454200. [DOI] [PubMed] [Google Scholar]
- 59.Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–32. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma DK, Brown JC, Choudhury A, Peterson TE, Holicky E, Marks DL, Simari R, Parton RG, Pagano RE. Selective stimulation of caveolar endo-cytosis by glycosphingolipids and cholesterol. Mol Biol Cell. 2004;15:3114–22. doi: 10.1091/mbc.E04-03-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fielding PE, Russel JS, Spencer TA, Hakamata H, Nagao K, Fielding CJ. Sterol efflux to apolipoprotein A-I originates from caveolin-rich microdomains and potentiates PDGF-dependent protein kinase activity. Biochemistry. 2002;41:4929–37. doi: 10.1021/bi012091y. [DOI] [PubMed] [Google Scholar]
- 62.Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA. 1995;92:10339–43. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smart EJ, Ying Y, Donzell WC, Anderson RG. A role for caveolin in transport of cholesterol from endoplas-mic reticulum to plasma membrane. J Biol Chem. 1996;271:29427–35. doi: 10.1074/jbc.271.46.29427. [DOI] [PubMed] [Google Scholar]
- 64.Lange Y, Swaisgood MH, Ramos BV, Steck TL. Plasma membranes contain half the phospholipid and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts. J Biol Chem. 1989;264:3786–93. [PubMed] [Google Scholar]
- 65.McConnell HM, Radhakrishnan A. Condensed complexes of cholesterol and phospholipids. Biochim Biophys Acta. 2003;1610:159–73. doi: 10.1016/s0005-2736(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 66.Lange Y, Ye J, Steck TL. How cholesterol homeosta-sis is regulated by plasma membrane cholesterol in excess of phospholipids. Proc Natl Acad Sci USA. 2004;101:11664–7. doi: 10.1073/pnas.0404766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lange Y, Ye J, Steck TL. Activation of membrane cholesterol by displacement from phospholipids. J Biol Chem. 2005;280:36126–31. doi: 10.1074/jbc.M507149200. [DOI] [PubMed] [Google Scholar]
- 68.Puri V, Watanabe R, Dominguez M, Sun X, Wheatley CL, Marks DL, Pagano RE. Cholesterol modulates membrane traffic along the endocytic pathway in sphingolipid-storage diseases. Nat Cell Biol. 1999;1:386–8. doi: 10.1038/14084. [DOI] [PubMed] [Google Scholar]
- 69.Deckert M, Ticchioni M, Bernard A. Endocytosis of GPI-anchored proteins in human lymphocytes: role of glycolipid-based domains, actin cytoskeleton, and protein kinases. J Cell Biol. 1996;133:791–9. doi: 10.1083/jcb.133.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin tran-scytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem. 1997;272:25968–75. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- 71.Glenney JR., Jr Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem. 1989;264:20163–6. [PubMed] [Google Scholar]
- 72.Del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-Garcia A, Anderson RG, Schwartz MA. Phospho-caveolin-1 mediates inte-grin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–8. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ko YG, Liu P, Pathak RK, Craig LC, Anderson RG. Early effects of pp60(v-src) kinase activation on caveolae. J Cell Biochem. 1998;71:524–35. [PubMed] [Google Scholar]
- 74.Nomura R, Fujimoto T. Tyrosine-phosphorylated caveolin-1: immunolocalization and molecular characterization. Mol Biol Cell. 1999;10:975–86. doi: 10.1091/mbc.10.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Orlichenko L, Huang B, Krueger E, McNiven MA. Epithelial growth factor-induced phosphorylation of caveolin 1 at tyrosine 14 stimulates caveolae formation in epithelial cells. J Biol Chem. 2006;281:4570–9. doi: 10.1074/jbc.M512088200. [DOI] [PubMed] [Google Scholar]
- 76.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 77.Shajahan AN, Timblin BK, Sandoval R, Tiruppathi C, Malik AB, Minshall RD. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004;279:20392–400. doi: 10.1074/jbc.M308710200. [DOI] [PubMed] [Google Scholar]
- 78.Kojic LD, Joshi B, Lajoie P, Le PU, Leung S, Cox ME, Turbin DA, Wiseman SA, Nabi IR. Phosphoinositide-3-kinase, raft-dependent endocy-tosis of autocrine motility factor targets p-akt positive, caveolin-1 negative breast carcinoma cells Submitted.
- 79.Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;436:78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- 80.Liu J, Wang XB, Park DS, Lisanti MP. Caveolin-1 expression enhances endothelial capillary tubule formation. J Biol Chem. 2002;277:10661–8. doi: 10.1074/jbc.M110354200. [DOI] [PubMed] [Google Scholar]
- 81.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–22. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 82.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–21. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 83.Mineo C, Gill GN, Anderson RG. Regulated migration of epidermal growth factor receptor from caveo-lae. J Biol Chem. 1999;274:30636–43. doi: 10.1074/jbc.274.43.30636. [DOI] [PubMed] [Google Scholar]
- 84.Park WY, Park JS, Cho KA, Kim DI, Ko YG, Seo JS, Park SC. Up-regulation of caveolin attenuates epi-dermal growth factor signaling in senescent cells. J Biol Chem. 2000;275:20847–52. doi: 10.1074/jbc.M908162199. [DOI] [PubMed] [Google Scholar]
- 85.Matveev SV, Smart EJ. Heterologous desensitization of EGF receptors and PDGF receptors by sequestration in caveolae. Am J Physiol Cell Physiol. 2002;282:C935–46. doi: 10.1152/ajpcell.00349.2001. [DOI] [PubMed] [Google Scholar]
- 86.Cohen AW, Razani B, Schubert W, Williams TM, Wang XB, Iyengar P, Brasaemle DL, Scherer PE, Lisanti MP. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes. 2004;53:1261–70. doi: 10.2337/diabetes.53.5.1261. [DOI] [PubMed] [Google Scholar]
- 87.Cohen AW, Razani B, Wang XB, Combs TP, Williams TM, Scherer PE, Lisanti MP. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol. 2003;285:C222–35. doi: 10.1152/ajpcell.00006.2003. [DOI] [PubMed] [Google Scholar]
- 88.Yamabhai M, Anderson RG. Second cysteine-rich region of epidermal growth factor receptor contains targeting information for caveolae/rafts. J Biol Chem. 2002;277:24843–6. doi: 10.1074/jbc.C200277200. [DOI] [PubMed] [Google Scholar]
- 89.Westover EJ, Covey DF, Brockman HL, Brown RE, Pike LJ. Cholesterol depletion results in site-specific increases in epidermal growth factor receptor phos-phorylation due to membrane level effects. Studies with cholesterol enantiomers. J Biol Chem. 2003;278:51125–33. doi: 10.1074/jbc.M304332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci USA. 2005;102:2760–5. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]


