Abstract
A paradigm shift is taking place in orthopaedic and reconstructive surgery from using medical devices and tissue grafts to a tissue engineering approach that uses biodegradable scaffolds combined with cells or biological molecules to repair and/or regenerate tissues. One of the potential benefits offered by solid free-form fabrication technology (SFF) is the ability to create scaffolds with highly reproducible architecture and compositional variation across the entire scaffold, due to its tightly controlled computer-driven fabrication. In this review, we define scaffold properties and attempt to provide some broad criteria and constraints for scaffold design in bone engineering.We also discuss the application-specific modifications driven by surgeon's requirements in vitro and/or in vivo. Next, we review the current use of SFF techniques in scaffold fabrication in the context of their clinical use in bone regeneration. Lastly, we comment on future developments in our groups, such as the functionalization of novel composite scaffolds with combinations of growth factors; and more specifically the promising area of heparan sulphate polysaccaride immobilization within the bone tissue engineering arena.
Keywords: tissue engineering, scaffolds, rapid prototyping, composites, mesenchymal stem cells, growth factors, heparan sulphate
Introduction
Using a hierarchical framework, tissue engineering can be subdivided into different strategies or concepts (Fig. 1). One strategy is purely cell-based involving the transplantation of autologous or allogeneic cell suspensions or cell-sheets that are injected and/or transplanted to a defect site or an injured tissue. Another strategy utilizes biomolecules (growth factors, completely lyophilized cell fractions, peptides, polysaccharides, etc.) aimed at delivering cues to the cells of the host tissue. Other methods are based on the use of different types of matrices (hydrogels, microspheres/beads, etc.) in combination with cells and/or biomolecules. The fourth and most frequently applied strategy focuses on seeding and culturing specific cell types in 3-D environments that closely mimic natural extracellular matrix. Such 3-D environments are specifically configured as cellular solids and referred to as 'scaffolds' (Fig. 2) in tissue engineering nomenclature.
1A–C.
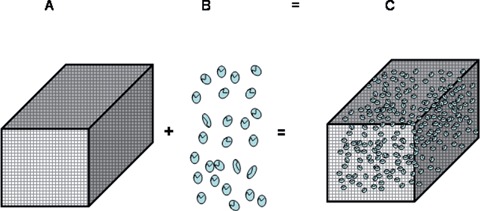
A paradigm shift is taking place in orthopaedic and reconstructive surgery from using medical devices and tissue grafts to engineering a tissue engineered construct (C) that uses biodegradable scaffolds (A) combined with cells (B) or biological molecules (B#) to repair and/or regenerate tissues.
2.
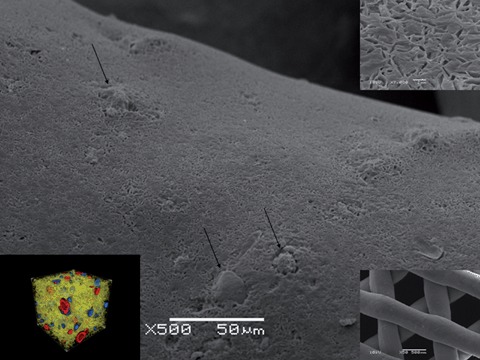
The tissue engineering laboratory at NUS did design, fabricate and characterize composite scaffolds made of mPCL- CaP by FDM in vitro (Ho ST, Hutmacher DW. A comparison of micro CT with other techniques used in the characterization of scaffolds. Biomaterials2006; 27:1362–1376, Lam CXF, Teoh SH, Hutmacher DW. Comparison of Degradation of PCL & PCL-TCP scaffolds in Alkaline Medium. Polymer International. 2006.0 and in vivo (Zhou YF, Chou AM, Li ZM, Hutmacher DW, Sae-Lim V, Lim TM. Combined marrow stromal cell sheet techniques and high strength biodegradable composite scaffolds for engineered functional bone grafts. Biomaterials. 2007; 28:814–24) Polymer/CaP composites confer favourable mechanical and biochemical properties, including strength viathe ceramic phase, toughness and plasticity viathe polymer phase, more favourable degradation and resorption kinetics, and graded mechanical stiffness. In these scaffolds, ceramic phase was homogenously distributed in the matrix (arrows, inset left corner) as well as exposed on the surface (Figure A, arrows highlight CaP particles on surface). Contact angle measurements showed that such composite surfaces were more hydrophilic and that the degradation kinetics were accelerated 3 to 4 times compared to PCL alone.
It can be argued that the 'scaffold-based tissue engineering concept' was introduced in the mid- 1980s when Dr Joseph Vacanti of the Children's Hospital approached Dr Robert Langer of MIT with an idea to design scaffolds for cell delivery as opposed to seeding cells onto or mixing cells into naturally occurring matrices with physical and chemical properties that are difficult to manipulate [1]. Today's concepts of scaffold- and matrix-based tissue engineering involve the combination of a scaffold with cells and/or biomolecules that promote the repair and/or regeneration of tissues [2]. These tissue- engineered constructs (TEC) are under intense investigation and various approaches and strategies are continually being developed. However, despite intense efforts, the ideal scaffold/cell or scaffold/neotissue construct (even for a specific tissue type) has yet to be developed. Certain minimum requirements are essential when developing TECs that must address the biochemical as well as chemical and physical properties of native tissue. Of these requirements, biocompatibility, vascularization [3, 4] and chemotaxis are vital. The scaffold must also be nonimmunogenic and free from prions involved in disease transmission and it must also possess suitable architectural qualities that are easily reproducible. There are also sterilization and delivery issues to contend with. Lastly, one must consider the temporal and spatial variations in some of these factors both in vitro and/or in vivo[5].
Despite rapid advancements in the fabrication of TECs, tissue engineering is still a long way off matching Natures' ability to grow and repair tissues and organs. Hence, tissue engineering still has room for improvement, nevertheless, from an engineering perspective the past decade has seen significant advances in scaffold design and fabrication [6]. Starting with simple foams and fibers, porosity and pore interconnectivity has quickly become a central theme, with pore dimensions and material properties being vital in promoting cell seeding, migration, proliferation and the de novo production of extra-cellular matrix. Significant advances have also been made in the incorporation of bioactive molecules into scaffolds, specifically in the area of bone engineering (Figs 3 and 4).
3.
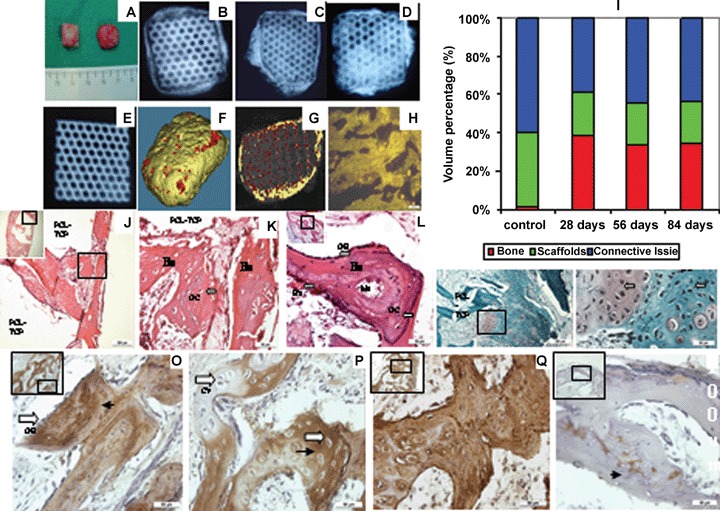
Constructs with BMSC sheet-scaffolds (mPCL-TCP) were implanted into nude rat, harvested after 28, 56 and 84 days. (A) Gross appearance of BMSC sheet-scaffolds constructs with osteogenic induction (Right) and non-induction (control) (Left) after 4 weeks. (B) X-ray detected bone like tissue formation in 28 day (C) 56 day (D) 84 day implants. (E) X-ray image of constructs without cell seeding (Control). (F) Micro-CT demonstrated the overall highly mineralized tissue similar to cortical (golden) and cancellous (red) bone in implanted constructs after 28 day. (G) Micro-CT images disclosed the hard tissue formation within constructs. Mineralized tissue with similar density with cancellous bone was detected (darker areas) while the lighter colour represented cortical bone. (H) Fluorescence was detected on the formed bone tissue after 28 days; the fluorescence came from the cFDA labeled BMSC. (I) Micro-CT quantification of implant tissue compositions depicted substantial bone formation in the induction group, accounting for 40% total volume for the 28, 56 and 84 day implantation, while the control group formed only connective tissue. (J) H&E staining shows lamellar bone-like tissue formed in both outer part and interior of constructs after 28 days. (K) High magnification image shows well organized lamellar bone like tissue (Bo) with distinct osteocytes located within bone tissue. (L) The typical osteoblasts (OB, black arrow) located on the surface of neo mineralized tissue with marrow cavities and blood vessels in 56 days implants. (M) Safranin-O staining demonstrated that hypertrophic chondrocytes (white arrow) were observed in 56 day implants in very low numbers. High magnification shows the chondrocytes (white arrow) with weak safranin-O staining surrounded by the osteocyte (black arrow). (N) OCN staining. Strong signals (arrow head) were detected on neo mineralized tissue while very weak to no-signals were detected on chondrocyte like cells (O). (P) Collagen type I staining. Extensive staining was detected on the neo mineralized tissue in constructs. (Q) Collagen type II staining. Limited signals (arrow head) were detected on chondrocyte like cells while no staining for the mineralized tissues. BV: blood vessel + red blood cells; Ma: marrow; OB: osteoblast; Bo: bone; OC: osteocyte; CY: chondrocyte. Scale bar: (J) 200μm; (H-Q) 50_m. Image reproduced from reference 11 with permission from Elsevier.
Native bone tissue is a major storage site for growth factors, especially for those characterized by their high affinity to heparin (heparin-binding growth factors [HBGFs]), such as fibroblast growth factors (FGFs), transforming growth factor-βs (TGF-βs), and bone morphogenic proteins (BMPs). BMPs are biologically active molecules capable of inducing new bone formation and have shown significant potential for clinical use in the repair of bone defects [7, 8]. Although there have been several successful studies using BMPs, there remain many unanswered questions such as patient site-specificity, for example, the ideal dose of BMP in an anterior cervical spine fusion may be quite different to that of a cranial defect. Carriers for BMP play an equally important role when it comes to the optimal delivery leading to predictable and functional bone regeneration [9]. For instance, a non-compressible scaffold has been shown to promote better fusion in the posterolateral spine than the clinically used collagen sponge.
In addition to the delivery of growth factors scaffold design is focusing on supporting the adhesion, growth and function of anchorage-dependent cell types by mimicking the interaction between cell surface receptors and extracellular matrix (ECM) molecules. This interaction is vital in regulating cellular functions including adhesion, survival, proliferation, migration and differentiation. The major focus in the literature has been on the integrin family of receptors that interact with a wide variety of ECM proteins [10]. More recently, heparan sulphate proteoglycans (HSPGs) have been identified as having a role in cell adhesion. These interactions occur mainly through electrostatic interactions between the negatively charged HSPG and the cluster of positively charged amino acids within the binding domains of ECM proteins. HSPGs are abundant cell surface and ECM molecules that consist of a defining core protein (such as syndecan, glypican or perlecan) to which are attached highly sulphated glycosaminoglycan (GAG) side-chains of heparan sulphate (HS) [11] (Fig. 5).
5.
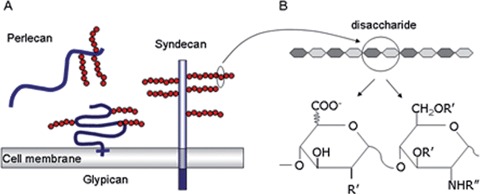
HSPG classifications and HS structure. (A) Heparan sulfate proteoglycans can be broadly classified as glypicans, perlecans and syndecans, depending on their distribution within and around the cell. Glypicans are attached to the cell membrane by GPI anchors, whereas syndecans are transmembrane proteins. Perlecans are actively secreted into the pericellular matrix and are predominantly found in the basement membrane. (B) Heparan sulfate is composed of repeating disaccharide units of glucuronic acid and glucosamine of varying length that can be modified at irregular intervals along the chain. These modifications cluster the sulfation patterns on the HS chain into discrete protein-binding domains and ultimately lead to different growth factor-binding specificities of different HS species. Copyright permission granted from Nature.
The importance of HS in mediating cell responses has also been shown with heparinase/heparitinase and sodium chlorate treatments. Heparinase cleaves HS chains into inactive disaccharide and tetrasaccharide components that have been shown to inhibit FGF2-mediated smooth muscle cell proliferation in injured carotid arteries [12]. Several in vitro studies have also cultured cells in media supplemented with chlorate to study the role of sulphated GAGs in different cell types [13]. Sodium chlorate (NaClO3) imparts its effects by inhibiting ATP-sulphurylase, the first enzyme in the synthesis of 3′-phosphoadenyl 5′-phosphosulphate (PAPS), a high-energy sulphate donor in biological reactions. The mitogenic response of cells to FGF2 is inhibited when cells are cultured in the presence of 30 mM NaClO3[14]. Such data has helped establish the idea that HS does not only bind a ligand for delivery to its cognate receptor, but must also itself bind to the receptor [15], in order to initiate signal transduction [16]; a powerful molecule indeed.
Numerous growth and adhesive proteins contain heparin-binding domains that specifically interact with HS. In all cases, the binding to HS modifies their biochemical properties and biological activity. Thus understanding the specific nature of the cell surface receptor-ECM interactions may provide a foundation for developing functional biomaterials designed to promote cell adhesion and growth. Indeed, the attachment of adhesive-peptide ligands mimicking heparin-binding domains to biomaterial surfaces has been shown to promote cell attachment [17]. Based on the above background, the authors of this review began a collaboration in 2004 with the aim of developing a bone engineering platform technology that combines novel composite scaffolds that mimic the host tissue matrix with growth factor potentiating HS sugar isoforms (Fig. 6A and B).
6A.
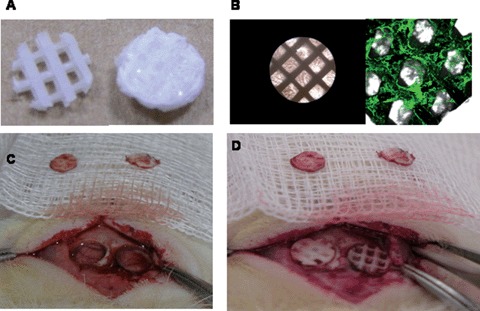
The ability of HS to regulate a suite of endogenous factors involved in the repair process of bone makes it an attractive therapeutic agent compared to the application of a single growth factor, e.g. BMP. We studied the bone regeneration potential of mPCL/TCP-Col1 scaffolds doped with 5 (left) and 30 (right) microgram HS in critical sized rat cranial defects. (A) mPCL-TCP scaffold (5mm diameter and 1mm thick, 0-90o lay-down pattern, 70% porosity) before treatment, left, and after lyophilization of 350 mg of rat tail collagen type 1, right. (B) Light microscopy showing the scaffold, left, and immunofluorescence showing the distribution of the HS within the lyophilised collagen. (C) Two full-thickness critical bone defects (5mm in diameter) were created in the rat parietal bone. Bone chips are shown. (D). Implantation of mPCL/TCP-Col1 scaffolds doped with 5 and 30 microgram HS into the defects.
6B.
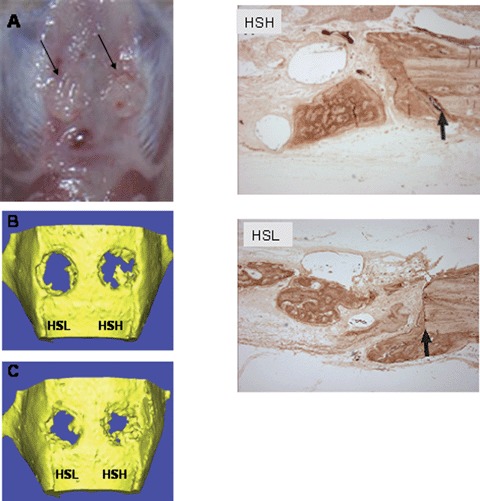
(A) Macroscopically observation of the defects sites shows excellent integration of the constructs with the host tissue (arrows). A smooth and non- fibrous integration could be observed for all groups at all time points. μCT scanning of the skull defect showing the progressive bone formation at 1 month (B) and 2 month (C) months with 5 microgram (HSL) and 30 microgram HS (HSH). Higher HS doses shows more bone formation in the defect site. (D) Antibody staining with a bone specific marker, namely osteocalcin (arrows show interface between host bone and newly formed bone) shows a stronger signal from the HSH group when compared to HSL group.
Scaffolds for bone engineering fabricated by solid free-form fabrication
As discussed above, a conceptual shift is taking place in orthopaedic and reconstructive surgery from using synthetic implants and tissue grafts to a tissue engineering approach that uses biodegradable scaffolds integrated with biological cells or molecules to regenerate tissues. This new paradigm requires scaffolds that balance temporary mechanical function with morphological properties (pore architecture, size and interconnectivity) to aid biological delivery and tissue regeneration [18]. Scaffold fabrication is a significant hurdle. Complex scaffold architecture designs generated using hierarchical image-based or CAD techniques usually cannot readily be built using conventional techniques. Instead, such scaffold architectures must be built using layer-by-layer manufacturing processes known collectively as solid free-form fabrication (SFF). A number of review articles [2, 6] and book chapters have reviewed and compared SFF scaffold fabrication methods so this review will concentrate on how scaffolds have per- formed clinically in bone engineering, and future directions for their use in regenerative medicine.
Melt extrusion-based SFF systems provide a powerful instrument for the generation of scaffold platforms. Recent advances in both computational scaffold design and SFF have made it possible for tissue engineers to design and fabricate a whole range of new types of scaffolds. One of the major benefits is the flexibility to create scaffolds with highly reproducible architecture and compositional morphological variation across the entire matrix, due to the computer controlled fabrication process (Fig. 7). The applications of SFF technologies in scaffold fabrication are wide and varied, however, only a small number are have reached the clinical arena. An interdisciplinary group at the National University of Singapore has evaluated and patented the parameters necessary to process medical grade polycaprolactone (mPCL) and mPCL composites [PCL/HA, PCL/TCP, etc.] by fused deposition modelling (FDM). These socalled first-generation scaffolds have been studied for more than 5 years in a clinical setting and gained Food & Drug Administration (FDA) approval via a 510K application in 2006. Schantz et al.[19] has used mPCL scaffolds as burr whole plugs in a pilot study for cranioplasty. The clinical outcome after 12 months was positive, with all patients tolerating the implants with no adverse side effects reported. A functional and stable cranioplasty was observed in all cases. Today more then 200 patients have received burr hole plugs, scaffolds for orbital floor reconstruction (Fig. 4) or other cranioplasties.
7.
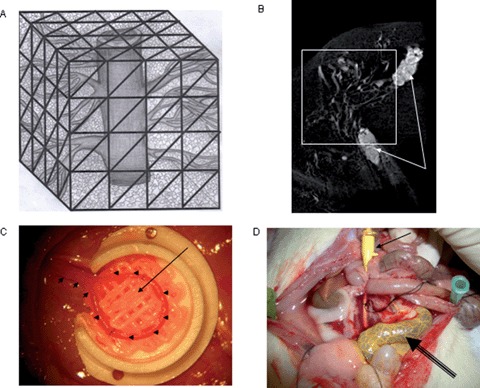
The majority of current tissue engineering approaches rely on the so called “extrinsic” mode of neovascularization. The neovascular bed originates from the periphery of the construct which should be implanted into a site of high vascularisation potential. Here recites a core limitation for transfer of tissue engineering models from the in vitro to the in vivo environment. Diffusion is the initial process involved, but it can only provide for cell nutrient supply and waste transport within a maximum range of 200 μm into the matrix. The survival of cells in the center of large cell containing constructs is therefore often limited by suboptimal initial vascularization. Cell labeling experiments have disclosed a considerable loss of osteoblasts within the first week following transplantation in porous cancellous bone matrices. Hence, reconstruction of small to moderate sized bone defects using extrinsic engineered bone tissues is technically feasible, and some of the currently developed concepts may represent alternatives to autologous bone grafts for certain clinical conditions, the reconstruction of large volume defects however remains challenging. Hence, reconstructive surgeons aim to generate so called “axially vascularized” tissues that can be transferred to the defect site using microsurgical techniques of vascular anastomosis. These tissues are immediately vascularized upon implantation into the defect as free flaps do Erol and Spira were the firast to report prefabrication of skin flaps by using an arteriovenous vessel which introduced this technique to the tissue engineering community. Most recently, the Horch/Kneser laboratory started collaboration with the Hutmacher laboratory to use the by AV-loop model (Figure C, small arrows) in combination with a composite scaffold (Figure C, long arrow shows with contrast agent filled loop, highly vascularized composite scaffold is found quadrant)) which is manufactured by a melt extrusion based SFF technique. Micro CT analysis (Figure B) of the with a construct agent perfused vascular network (Figure D, arrow show perfusion site with Microfil filled needle, double line arrow shows with Microfil filled vascular loop with caplillary network) of the engineered construct revealed a highly vascular zed construct.
4.
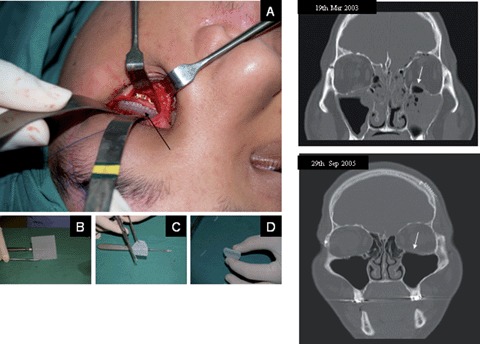
The intended clinical use defines the desired properties of engineered bone substitutes. Defects of load-bearing long bones, for instance, require constructs with high mechanical stability whereas initial plasticity is not essential. On the other hand for craniofacial applications, e.g. orbital floor fractures, moldable scaffolds (A–D) are favorable. Depending on the implantation site, initial vascularization is essential for enhanced engraftment and prevention of infections. Mechanical stability, osteoconductivity by attracting and stimulating bone-forming cells of the host bone, and ease of handling have been well balanced in the design of mPCL scaffold sheets in order to properly meet the clinician's needs. The clinical follow up 2.5 years postsurgery (lower CT image) of a patient receiving a mPCL scaffold (defect site shown in upper CT image) for the reconstruction of a orbital floor fracture defect showed complete bone regeneration of the defect site (arrow).
The second generation scaffolds produced by FDM for bone engineering are based on composites and have been evaluated in vitro[20] and in vivo[5]. mPolymer/CaP composites confer favourable mechanical and biochemical properties, including strength via the ceramic phase and toughness and plasticity via the polymer phase, they also possess more favourable degradation and resorption kinetics, and graded mechanical stiffness. In these scaffolds, the ceramic phase was homogenously distributed in the matrix as well as exposed on the surface. Contact angle measurements have shown that such composite surfaces were more hydrophilic and that the degradation kinetics were seen to be accelerated three to four times compared to PCL alone. Biochemical advantages include improved cell seeding, and enhanced control and/or simplification of the incorporation and immobilization of biological factors, such as BMP's [21]. Most recently the group at the National University of Singapore have undertaken several studies utilizing mPCL/CaP composite scaffolds in large animal models (Fig. 8A and B).
8A.
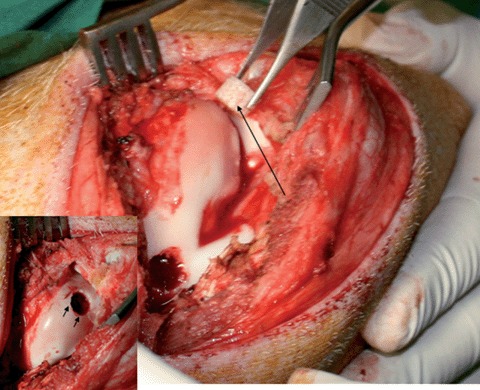
Implantation of a MSC loaded mPCL-CaP/PCL scaffold (long arrow) into a high load-bearing osteochondral defect (inset small arrows, defect size in the medial condyle diameter 5 mm, depth 8 mm) in a pig model.
8B.
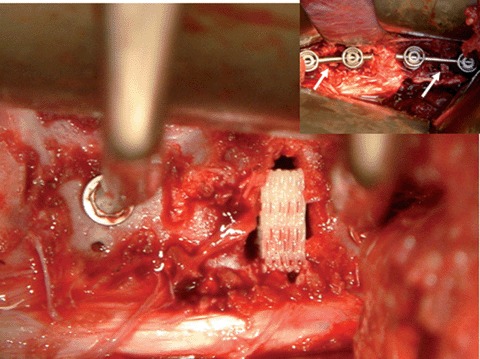
Implantation of a MSC loaded mPCL-CaP/scaffold (14×12×5 mm3) into a spinal fusion model (inset, disks were removed on level L1/L2 and L4/L5 and tissue engineered constructs were combined with internal fixation devices) in a 6 month old domestic pig.
Scaffolds combined with growth factors and other biologically active molecules
Orthopaedic, plastic and reconstructive surgery is beginning to use bone engineering as a preferential route to autologous or allogenic bone grafting. One treatment concept involves either doping a scaffold with growth factors (BMP, FGF, vascular endothelial growth factor [VEGF], etc.) or other biological molecules (HS, etc). In growth factor therapy, the drug delivery system should not only promote effective biological activity but should also limit spatial spread of the factor.
Many of the growth factors currently being investigated for fracture healing are HBGFs that are produced by the osteoblasts and stored within the matrix of bone. Of these, FGF-1 and FGF-2 have been extensively investigated as candidates for fracture healing. FGF-1 has been shown to aid in the bridging of a parietal bone critical defect [22] and to increase the bone-implant interface when used in combination with titanium-based scaffolds [23]. FGF-2 has been more extensively studied for its use in fracture healing, and it has been shown that daily, intravenous injections of FGF-2 for up to 2 weeks can enhance bone formation in rats [24–26]. Members of the TGF-β superfamily have also been shown to be successful in enhancing osteogenesis. Both TGF-β1 and –β2 are known to increase differentiation of committed osteoprogenitor cells, and are potent stimulators of bone repair in calvarial and long bone defects when administered alone [27] or in combination with either IGF-I or IGF-I/growth hormone (GH) [28]. In addition, growth factors can directly regulate the availability of other growth factors; FGFs have been shown to regulate the expression of VEGF and HGF, factors that are also mitogenic for osteoprogenitor cells [29]. Likewise, combined local delivery of IGF-I in combination with TGF-β1 in a hydrogel scaffold has been shown to significantly enhance bone formation in a rat tibial segmental defect over IGF-I alone [30] suggesting that perhaps the mode of delivery also affects the potential for growth factor-mediated bone healing.
Importantly, these findings clearly demonstrate that a universally applicable combination of growth factors has yet to be established for fracture therapy. HS by virtue of its binding to a multitude of growth factors normally present in the fracture haematoma, may represent an exciting avenue to augment fracture healing. Indeed, we have shown that a single application of HS at the time of injury is sufficient to enhance fracture repair, and is responsible for the up-regulation of endogenous growth factors, notably FGF-1, IGF-II, TGF- β1 and VEGF [31].
In addition to effective dose, incompatibility with sterilization procedures, poor thermal and pH tolerance and delivery regimes [32–34], growth factor efficacy in orthopaedics is complicated by virtue of their inherent instability. Growth factors are rapidly cleared in vivo as well as being highly susceptible to proteolytic degradation [35]. As such, large quantities of growth factors are usually required in order to achieve a successful outcome. Furthermore, the number of growth factors that are proto-oncogenic is increasing that is becoming an important translational problem [36]. Thus, the long-term use of growth factors as a therapy is still being debated. As such, an alternative, bioactive agent that can protect, localize and/or enhance the effects of exogenously applied growth factors in vivo would be a great advantage in orthopaedics as it would reduce the dosage of exogenous growth factors required. Furthermore, an agent that can be used independently to augment in vivo bone formation by harnessing the potential of endogenously produced growth factors would be even more therapeutically desirable.
Accelerated bone repair with growth factors, HS and HS-like molecules
Antagonism between FGF and BMP signalling is a repeating feature during new bone development. Recently, the use of BMP inhibitors together with FGF2 in the culture medium of human embryonic stem cells (hES) has been shown to facilitate the longterm maintenance of these cells in a pluripotent state [37]. Hence, the balance between FGF- and BMPmediated signalling is thought to impose a primary control over cell-fate decisions. As immunological rejection limits the use of allogenic or xenogenic bone grafting, there has been a significant shift within the field of orthopaedics towards bone engineering and its potentially more sophisticated techniques for promoting osteogenesis. One particular avenue our group is exploring is better harnessing the unique properties of growth factors by presenting them together with specific co-factors, such as the HS molecules together with osteoconductive scaffolds (Fig. 4A and B).
BMP/heparin mimetics have shown promise when delivered on collagen sponges.Now what is needed is the coupling of bioactive molecules to the next generation of scaffolding materials that will provide the key elements mentioned previously in this review.We are thus developing methodologies to engineer scaffolding devices that sustain tissue growth and maturation in the presence of functionalized growth factor/HS combinations. This is an important paradigm shift that we belief is achievable due to the resilience of HS to sterilization techniques, pH change, temperature and chemical solvents required for scaffold processing. Therefore, successful bone tissue engineering could be achieved through the delivery of bioactive growth factors in bioresorbable scaffolding matrices functionalized with HS. This method is particularly attractive as it can boost local osteoprogenitor cell recruitment, proliferation and differentiation.
GAG-based therapies are not a new approach to bone tissue engineering. HS-like molecules have been tested over the last decade for their ability to augment bone formation, as well as in the healing of other tissue types. Dextran derivatives substituted with carboxymethyl benzylamide sulfonate (CMDBS) have been shown to mimic heparin/HS by providing a somewhat similar protection and stabilization capability for growth factors as imparted by HS; albeit with less affinity. Otherwise known as 'regenerating agents' (RGTAs), these HS-like molecules have been shown to stimulate tissue repair in skin, bone, muscle and the cornea [38–44]. In all cases, these HSlike molecules are thought to exert their effect by increasing both cell proliferation and differentiation within the wound site, presumably by enhancing growth factor-mediated signals.
Although HS-like molecules have been shown to have the potential to accelerate fracture repair, HS molecules themselves are only now being used as a treatment therapy in bone. Jackson et al. (2006) [31] recently demonstrated that HS harvested from osteoblasts can increase trabecular bone volume by 20% following a once-off application into a rat middiaphyseal femoral fracture. Furthermore, HS supplementation was shown to increase the expression of alkaline phosphatase (ALP) and Runx2, as well as the expression of a number of HBGFs present within the callus [31].Together, these findings show that HS and HS-like molecules appear to have the remarkable potential to create an environment favourable to fracture repair. What is needed now is a better understanding of how best to deliver these potent molecules.
Due to the temporal pattern of growth factor expression during bone repair, we hypothesize that prolonged localized delivery of HS over the period of healing may further improve the effects of HS. We recently developed two techniques to prolong the delivery of HS [45, 46]. In the first study, HS was incorporated into electrospun poly(ɛ-caprolactone) (PCL) nanofibre mats. This produced fibres with smooth surfaces and no bead defects and was spun from polymer solutions with 8% w/v PCL in 7:3 dichloromethane:methanol solvent. Assessment of HS loading and imaging of fluorescently labelled HS showed a homogenous distribution throughout the fibre mats and a sustained release for up to 14 days. Importantly, the HS fibres did not induce an inflammatory response in macrophage cells in vitro and the released HS had sustained biological activity. In the later study, we encapsulated HS by the water-in oil-in water (W1/O/W2) technique using polycaprolactone (PCL) microcapsules and achieved similar biocompatibility and bioactivity results with a prolonged delivery of up to 40 days. Whilst promising in terms of HS release and bioactivity, these material constructs require extensive in vivo testing. Do they induce ectopic bone formation, can they be used to co-deliver important growth factors? Important questions that remain to be answered.
Conclusions
SFF techniques are experiencing increasing application in many biomedical fields, including regenerative medicine and tissue engineering. In general, scaffold-based tissue engineering requires a welldefined internal structure with interconnected porosity and SFF techniques allow us to design and fabricate such scaffolds. From a biological point of view, the designed scaffold should serve several functions, including (1) the ability to act as an immobilization site for transplanted cells, (2) the formation of a protective space to prevent unwanted tissue growth into the wound bed and allow healing with differentiated tissue, (3) directing the migration or growth of cells via surface properties of the scaffold and (4) directing migration or growth of cells via release of soluble molecules, such as growth factors, hormones and/or cytokines. Future work has to provide further compelling evidence that other SFF methods next to FDM offer the right balance of capability and practicality to be suitable for fabrication of materials in sufficient quantity and quality to move holistic tissue engineering technology platforms into a clinical application.
In addition to considerations of scaffold performance based on tissue engineering strategies, practical considerations of manufacture also arise. From a clinical point of view, it must be possible to manufacture scaffolds under good manufacturing practice (GMP) conditions in a reproducible and quality controlled fashion at an economic cost and speed. To move the current tissue engineering practices to the next frontier, some manufacturing processes will be required to accommodate the incorporation of cells and/or biological molecules during the scaffold fabrication process. These approaches are working towards the enablement of the tissue-engineered construct to not only have a controlled spatial distribution of cells and molecules, but also to possess a versatility of scaffold material and microstructure within one specifically designed and fabricated construct, for implantation in an intended anatomical site.
The development of an 'HS approach' for the control of progenitor phenotype has become more feasible in recent years as our understanding of HS cell biology improves. HSs are particularly attractive, as they potentiate the powerful effects of growth factors on cell recruitment, proliferation and differentiation, and do not depend on the synthesis of other co-factors or other specific activating agents. Thus they represent a novel approach for the control of cell phenotype. We would anticipate that HS, by virtue of its modulation of the biological activities of the FGFs and the BMPs, offers the possibility of intervening not just in embryonic growth, and stem and progenitor cell self-renewal, but also in stem cell-dependent wound healing and scaffold-based tissue engineering. Unlike other nucleic acid-based clinical approaches (e.g. virus and siRNA), therapeutic intervention may be particularly advantaged because HSs are chemically stable. Realization of the potential of HS for regenerative medicine will depend, however, on suitable delivery systems with its own intrinsic properties that mimic the host tissue architecture.
Acknowledgments
We thank Dr. Schantz and Dr. Lim for support with the clinical images.Work presented in this review was supported in part by the Sinagporean Biomedical Research Council (BMRC), contract grant number: BMRC 04/1/21/19/308 (R-397–000-613–305).
References
- 1.Vacanti CA. The history of tissue engineering. J Cell Mol Med. 2006;10:569–76. doi: 10.1111/j.1582-4934.2006.tb00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutmacher DW, Sittinger M, Risbud MV. Scaffoldbased tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004;22:354–62. doi: 10.1016/j.tibtech.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Bach AD, Arkudas A, Tjiawi J, Polykandriotis E, Kneser U, Horch RE, Beier JP. A new approach to tissue engineering of vascularized skeletal muscle. J Cell Mol Med. 2006;10:716–26. doi: 10.1111/j.1582-4934.2006.tb00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kneser U, Stangenberg L, Ohnolz J, Buettner O, Stern-Straeter J, Mobest D, Horch RE, Stark GB, Schaefer DJ. Evaluation of processed bovine cancellous bone matrix seeded with syngenic osteoblasts in a critical size calvarial defect rat model. J Cell Mol Med. 2006;10:695–707. doi: 10.1111/j.1582-4934.2006.tb00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y, Chen F, Ho ST, Woodruff MA, Lim TM, Hutmacher DW. Combined marrow stromal cell-sheet techniques and high-strength biodegradable composite scaffolds for engineered functional bone grafts. Biomaterials. 2007;28:814–824. doi: 10.1016/j.biomaterials.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 6.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–24. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 7.Kain MS, Einhorn TA. Recombinant human bone morphogenetic proteins in the treatment of fractures. Foot Ankle Clin. 2005;10:639–50. doi: 10.1016/j.fcl.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Govender S, Csimma C, Genant HK, Valentin- Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Borner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, Van Der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Ruter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123–34. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 9.De Long WG, Jr, Einhorn TA, Koval K, McKee M, Smith W, Sanders R, Watson T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89:649–58. doi: 10.2106/JBJS.F.00465. [DOI] [PubMed] [Google Scholar]
- 10.Cool SM, Nurcombe V. Heparan sulfate regulation of progenitor cell fate. J Cell Biochem. 2006;99:1040–51. doi: 10.1002/jcb.20936. [DOI] [PubMed] [Google Scholar]
- 11.Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–93. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 12.Kinsella MG, Irvin C, Reidy MA, Wight TN. Removal of heparan sulfate by heparinase treatment inhibits FGF-2-dependent smooth muscle cell proliferation in injured rat carotid arteries. Atherosclerosis. 2004;175:51–57. doi: 10.1016/j.atherosclerosis.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 13.Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991;252:1705–08. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- 14.Safaiyan F, Kolset SO, Prydz K, Gottfridsson E, Lindahl U, Salmivirta M. Selective effects of sodium chlorate treatment on the sulfation of heparan sulfate. J Biol Chem. 1999;274:36267–73. doi: 10.1074/jbc.274.51.36267. [DOI] [PubMed] [Google Scholar]
- 15.Guimond SE, Turnbull JE. Fibroblast growth factor receptor signalling is dictated by specific heparan sulphate saccharides. Curr Biol. 1999;9:1343–6. doi: 10.1016/s0960-9822(00)80060-3. [DOI] [PubMed] [Google Scholar]
- 16.McKeehan WL, Wang F, Kan M. The heparan sulfatefibroblast growth factor family: diversity of structure and function. Prog Nucleic Acid Res Mol Biol. 1998;59:135–76. doi: 10.1016/s0079-6603(08)61031-4. [DOI] [PubMed] [Google Scholar]
- 17.Zisch AH, Zeisberger SM, Ehrbar M, Djonov V, Weber CC, Ziemiecki A, Pasquale EB, Hubbell JA. Engineered fibrin matrices for functional display of cell membrane-bound growth factor-like activities: study of angiogenic signaling by ephrin-B2. Biomaterials. 2004;25:3245–57. doi: 10.1016/j.biomaterials.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–43. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 19.Schantz JT, Lim TC, Ning C, Teoh SH, Tan KC, Wang SC, Hutmacher DW. Cranioplasty after trephination using a novel biodegradable burr hole cover: technical case report. Neurosurgery. 2006;58 doi: 10.1227/01.NEU.0000193533.54580.3F. ONS-E176. [DOI] [PubMed] [Google Scholar]
- 20.Zhou YF, Sae-Lim V, Chou AM, Hutmacher DW, Lim TM. Does seeding density affect in vitro mineral nodules formation in novel composite scaffolds? J Biomed Mater Res. A. 2006;78:183–93. doi: 10.1002/jbm.a.30685. [DOI] [PubMed] [Google Scholar]
- 21.Rai B, Teoh SH, Hutmacher DW, Cao T, Ho KH. Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2. Biomaterials. 2005;26:3739–48. doi: 10.1016/j.biomaterials.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 22.Cuevas P, De Paz V, Cuevas B, Marin-Martinez J, Picon-Molina M, Fernandez-Pereira A, Gimenez- Gallego G. Osteopromotion for cranioplasty: an experimental study in rats using acidic fibroblast growth factor. Surg Neurol. 1997;47:242–6. doi: 10.1016/s0090-3019(96)00438-7. [DOI] [PubMed] [Google Scholar]
- 23.McCracken M, Lemons JE, Zinn K. Analysis of Ti- 6Al-4V implants placed with fibroblast growth factor 1 in rat tibiae. Int J Oral Maxillofac Implants. 2001;16:495–502. [PubMed] [Google Scholar]
- 24.Iwaniec UT, Mosekilde L, Mitova-Caneva NG, Thomsen JS, Wronski TJ. Sequential treatment with basic fibroblast growth factor and PTH is more efficacious than treatment with PTH alone for increasing vertebral bone mass and strength in osteopenic ovariectomized rats. Endocrinology. 2002;143:2515–26. doi: 10.1210/endo.143.7.8884. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, Hanada K, Tamura M, Shibanushi T, Nigi H, Tagawa M, Fukumoto S, Matsumoto T. Stimulation of endosteal bone formation by systemic injections of recombinant basic fibroblast growth factor in rats. Endocrinology. 1995;136:1276–84. doi: 10.1210/endo.136.3.7867582. [DOI] [PubMed] [Google Scholar]
- 26.Pun S, Florio CL, Wronski TJ. Anabolic effects of basic fibroblast growth factor in the tibial diaphysis of ovariectomized rats. Bone. 2000;27:197–202. doi: 10.1016/s8756-3282(00)00312-4. [DOI] [PubMed] [Google Scholar]
- 27.Joyce ME, Roberts AB, Sporn MB, Bolander ME. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990;110:2195–207. doi: 10.1083/jcb.110.6.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidmaier G, Wildemann B, Heeger J, Gabelein T, Flyvbjerg A, Bail HJ, Raschke M. Improvement of fracture healing by systemic administration of growth hormone and local application of insulin-like growth factor-1 and transforming growth factor-beta1. Bone. 2002;31:165–72. doi: 10.1016/s8756-3282(02)00798-6. [DOI] [PubMed] [Google Scholar]
- 29.Marie PJ. Fibroblast growth factor signaling controlling osteoblast differentiation. Gene. 2003;316:23–32. doi: 10.1016/s0378-1119(03)00748-0. [DOI] [PubMed] [Google Scholar]
- 30.Srouji S, Blumen feld I, Rachmiel A, Livne E. Bone defect repair in rat tibia by TGF-1 and IGF-1 released from hydrogel scaffold. Cell Tissue Bank. 2004;5:223–30. doi: 10.1007/s10561-004-0503-7. [DOI] [PubMed] [Google Scholar]
- 31.Jackson RA, Kumarasuriyar A, Nurcombe V, Cool SM. Long-term loading inhibits ERK1/2 phosphorylation and increases FGFR3 expression in MC3T3-E1 osteoblast cells. J Cell Physiol. 2006;209:894–904. doi: 10.1002/jcp.20779. [DOI] [PubMed] [Google Scholar]
- 32.Berscht PC, Nies B, Liebendorfer A, Kreuter J. Incorporation of basic fibroblast growth-factor into methylpyrrolidinone chitosan fleeces and determination of the invitro release characteristics. Biomaterials. 1994;15:593–600. doi: 10.1016/0142-9612(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 33.Westall FC, Rubin R, Gospodarowicz D. Brainderived fibroblast growth factor: a study of its inactivation. Life Sci. 1983;33:2425–9. doi: 10.1016/0024-3205(83)90636-7. [DOI] [PubMed] [Google Scholar]
- 34.Caccia P, Nitti G, Cletini O, Pucci P, Ruoppolo M, Bertolero F, Valsasina B, Roletto F, Cristiani C, Cauet G, et al. Stabilization of recombinant human basic fibroblast growth factor by chemical modifications of cysteine residues. Eur J Biochem. 1992;204:649–55. doi: 10.1111/j.1432-1033.1992.tb16678.x. [DOI] [PubMed] [Google Scholar]
- 35.Babensee JE, McIntire LV, Mikos AG. Growth factor delivery for tissue engineering. Pharm Res. 2000;17:497–504. doi: 10.1023/a:1007502828372. [DOI] [PubMed] [Google Scholar]
- 36.Hunter MG, Avalos BR. Granulocyte colony-stimulating factor receptor mutations in severe congenital neutropenia transforming to acute myelogenous leukemia confer resistance to apoptosis and enhance cell survival. Blood. 2000;95:2132–7. [PubMed] [Google Scholar]
- 37.Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 38.Papy-Garcia D, Barbosa I, Duchesnay A, Saadi S, Caruelle JP, Barritault D, Martelly I. Glycosaminoglycan mimetics (RGTA) modulate adult skeletal muscle satellite cell proliferation in vitro. J Biomed Mater Res. 2002;62:46–55. doi: 10.1002/jbm.10192. [DOI] [PubMed] [Google Scholar]
- 39.Blanquaert F, Saffar JL, Colombier ML, Carpentier G, Barritault D, Caruelle JP. Heparan-like molecules induce the repair of skull defects. Bone. 1995;17:499–506. doi: 10.1016/8756-3282(95)00402-5. [DOI] [PubMed] [Google Scholar]
- 40.Meddahi A, Caruelle JP, Gold L, Rosso Y, Barritault D. New concepts in tissue repair: Skin as an example. Diabetes & Metabolism. 1996;22:274–8. [PubMed] [Google Scholar]
- 41.Logeart-Avramoglou D, Jozefonvicz J. Carboxymethyl benzylamide sulfonate dextrans (CMDBS), a family of biospecific polymers endowed with numerous biological properties: a review. J Biomed Mater Res. 1999;48:578–90. doi: 10.1002/(sici)1097-4636(1999)48:4<578::aid-jbm26>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 42.Escartin Q, Lallam-Laroye C, Baroukh B, Morvan FO, Caruelle JP, Godeau G, Barritault D, Saffar JL. A new approach to treat tissue destruction in periodontitis with chemically modified dextran polymers. FASEB J. 2003;17:644–51. doi: 10.1096/fj.02-0708com. [DOI] [PubMed] [Google Scholar]
- 43.Lafont J, Blanquaert F, Colombier ML, Barritault D, Carueelle JP, Saffar JL. Kinetic study of early regenerative effects of RGTA11, a heparan sulfate mimetic, in rat craniotomy defects. Calcif Tissue Int. 2004;75:517–25. doi: 10.1007/s00223-004-0012-5. [DOI] [PubMed] [Google Scholar]
- 44.Berrada S, Amedee J, Avramoglou T, Jozefonvicz J, Harmand MF. Effect of a derivatized dextran on human osteoblast growth and phenotype expression. J Biomater Sci Polym Ed. 1994;6:211–22. doi: 10.1163/156856294x00329. [DOI] [PubMed] [Google Scholar]
- 45.Luong-Van E, Grondahl L, Chua KN, Leong KW, Nurcombe V, Cool SM. Controlled release of heparin from poly(epsilon-caprolactone) electrospun fibers. Biomaterials. 2006;27:2042–50. doi: 10.1016/j.biomaterials.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 46.Luong-Van E, Grondahl L, Nurcombe V, Cool S. In vitro biocompatibility and bioactivity of microencapsulated heparan sulfate. Biomaterials. 2007;28:2127–36. doi: 10.1016/j.biomaterials.2007.01.002. [DOI] [PubMed] [Google Scholar]


