Abstract
Macropinocytosis defines a series of events initiated by extensive plasma membrane reorganization or ruffling to form an external macropinocytic structure that is then enclosed and internalized. The process is constitutive in some organisms and cell types but in others it is only pronounced after growth factor stimulation. Internalized macropinosomes share many features with phagosomes and both are distinguished from other forms of pinocytic vesicles by their large size, morphological heterogeneity and lack of coat structures. A paucity of information is available on other distinguishing features for macropinocytosis such as specific marker proteins and drugs that interfere with its mechanism over other endocytic processes. This has hampered efforts to characterize the dynamics of this pathway and to identify regulatory proteins that are expressed in order to allow it to proceed. Upon internalization, macropinosomes acquire regulatory proteins common to other endocytic pathways, suggesting that their identities as unique structures are short-lived. There is however less consensus regarding the overall fate of the macropinosome cargo or its limiting membrane and processes such as fusion, tubulation, recycling and regulated exocytosis have all been implicated in shaping the macropinosome and directing cargo traffic. Macropinocytosis has also been implicated in the internalization of cell penetrating peptides that are of significant interest to researchers aiming to utilize their translocation abilities to deliver therapeutic entities such as genes and proteins into cells. This review focuses on recent findings on the regulation of macropinocytosis, the intracellular fate of the macropinosome and discusses evidence for the role of this pathway as a mechanism of entry for cell penetrating peptides.
Keywords: cell penetrating peptides, endocytosis, endosomes, lysosomes, macropinocytosis, membrane ruffling, octaarginine, phagocytosis, sorting, Rab, TAT
Introduction
Most eukaryotic cells have an impressive capacity to apportion a fragment of their plasma membrane for invagination into enclosed structures that are then pinched off to form intracellular vesicles. The internalized membrane and cargo is then delivered to a number of different intracellular destinations such as lysosomes and the Golgi or recycled back to the plasma membrane. This process is generally termed endocytosis and is required for processes such as nutrient uptake and degradation, down-regulation of activated receptor signalling and antigen processing in the immune response [1]. Spatial organization of the endomembrane system and directed transport on endocytic pathways are both controlled by the cell cytoskeleton that typically controls centripetal movement to a perinuclear region that also contains lyso-somes, recycling endosomes, the microtubule organizing centre, centrosome and the Golgi apparatus [2, 3]. It is now known that a number of different internalization mechanisms exist, including uptake via clathrin coated pits, caveolae and other poorly characterized processes that do not seem to rely on either clathrin or caveolae [4–8] (Fig. 1).
1.
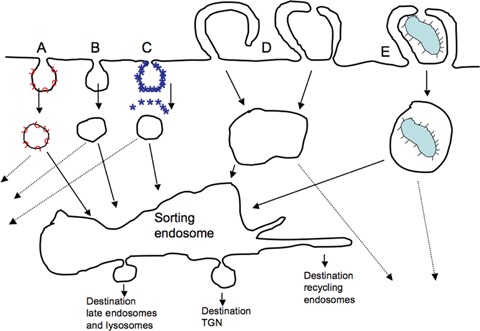
Endocytic pathways. A, caveolae delivering to caveosomes; B, clathrin independent endocytosis, C, clathrin coated uptake D, macropinocytosis showing macropinosome formation via fusion of ruffles with each other or the plasma membrane, E, phagocytosis. The contribution of a common early endosome in linking these pathways and partitioning membranes, proteins and cargo, over delivery to distinct classes of sorting endosomes as depicted by the dashed lines is unknown.
Phagocytosis is confined to specific cell types such as macrophages and dendritic cells and the same is true about macropinocytosis, a process that shares many features with phagocytosis – see below. Phagocytosis has been extensively characterized at morphological and molecular levels [9] but the lack of recent reviews detailing macropinocytosis is a reflection of our poor understanding of unique molecular features that may govern this event, and in many cell types, its exact physiological role over other endocytic processes [10–12]. Given the fact that pathogens are master highjackers of other endocytic pathways including phagocytosis, it is not surprising that macropinocytosis is also utilized by microorganisms to gain access to the cells. Examples include Salmonella, Listeria and adenoviruses and macropinocytosis inducing toxins have also been described [13–17].
In Dictyostelium, macropinocytosis, demonstrated by the formation of circular ruffles or crowns, accounts for most of fluid phase uptake and scanning electron microscopy (SEM) images of the projecting circular ruffles demonstrate the extensive reorganization of the plasma membrane that is required to form macropinosomes in this organism and in mammalian cells [18–20]. The distinct structures of these circular ruffles compared with other types of regulated and constitutive ruffling suggest macropinocytosis represents several distinct processes controlled via common and specific mediators.
A general feature of macropinocytosis and phago-cytosis is that the active portion of the plasma membrane is initially not involved in invagination but rather in an actin-dependent protrusion of the plasma membrane to the external milieu. There is presumably no regulation regarding the size or morphology of the enclosed macropinosome but in the case of phago-cytosis, as previously noted, this is largely pre-determined by the shape of the enveloped entity [12]. Common to both, however, is that the formed macropinosome or phagosome may be several micrometers in diameter; significantly larger than structures formed from other endocytic invaginations that rarely exceed 150 nm.
Membrane ruffling and the formation of the macropinosome
Macropinocytosis, as a form of pinocytosis, was initially described by Warren Lewis in 1931 [21]. He elegantly described his ‘Motion picture’ account of the appearance of ‘waving sheets’, macropinosome formation, traffic and subsequent shrinkage in rat macrophages. Much later, the prominence of macropinocytosis was shown to be greatly enhanced by the addition of growth factors and a large portion of what is now known about this process in macrophages was gained from experiments performed in cells incubated with macrophage-colony stimulating factor (M-CSF). More recently, macropinocytosis has been shown to be prominent in cell types that do not phagocytose, but this is mostly a feature of their response to growth factor stimulation. These include human epidermoid carcinoma (A431) cells after stimulation with epidermal growth factor (EGF) [22] and polarized Madin Darby Canine Kidney epithelial cells following hepatocyte growth factor/scatter factor (HGF/SF) stimulation [19]. Constitutive macropinocytosis has been observed in dendritic cells [23, 24] and NIH3T3 fibroblasts expressing a ruffling kinase [25] but whether this is a required cellular activity in non-phagocytic cells is unclear.
The membrane ruffling and macropinocytosis that immediately follows the addition of growth factors is accompanied by a rapid increase in uptake of markers for fluid phase endocytosis [26]. Presumably this is because of the engulfment of relatively large volumes of extracellular material into these enlarged macropinosomes. Markers such as fluorescent dextrans for fluorescent microscopy and horseradish peroxidase for electron microscopy are shown to be contained in relatively few numbers of large, non-coated structures whose intracellular fate is discussed below. Cells infected with Adenovirus type 2 for 10 min show extensive leading-edge ruffling; prominent also are filopodia in close proximity to these ruffles and the uptake of fluid phase markers, as opposed to clathrin coated vesicles is increased [17]. Similarly, treatment of the same cell line with EGF results in an extremely rapid (30 s) increase in fluid phase uptake accompanying extensive membrane ruffling [26, 27]; the resulting increase in fluid phase uptake is often used as a defining feature of this process. Within minutes of growth factor activation, cells boast the appearance of ruffles that cover the entire surface of the cell including the leading edge where they manifest as lamellipodia [28, 29]. The appearance and morphology of these is quite different to the circular ruffles, prominent in Dictyostelium (phacocytic cups) and HGF-SF stimulated epithelial cells [19, 20, 30, 31].
There is no consensus as to the proportion of membrane ruffles that end up as macropinosomes [11], but the formation of an enclosed macropinosome is more than likely a result of fusion of the protruding structures or back-fusion of a protrusion with an unruffled section of the plasma membrane (Fig. 1). The resulting macropinosomes are, predictably, highly heterogeneous in size and morphology. A requirement for actin and the actin polymerization machinery on ruffling and phagocytosis including Rho family members and their upstream effectors such as Ras, PLC and PI 3-kinase, activated themselves by receptor tyrosine kinases, suggest that both processes are similarly organized. Unsurprisingly both processes are inhibited by actin-disrupting agents such as cytochalasin A [20]. There is also considerable overlap relating to the requirements of proteins regulating these two processes with those shown to be involved in promoting cell motility [32].
A number of studies have shown, in different cell types, that activation of the non-receptor kinase v-Src oncogene promotes macropinocytosis [33–37]. The original demonstration of v-Src effects showed that its kinase activity led to a constitutive formation of macropinosomes (in the absence of external growth factor), a 2-fold increase in fluid phase uptake and a less significant, 1.3-fold, increase in transferrin uptake [37]. This small increase in transferrin uptake compared with much higher stimulation of fluid phase endocytosis, up to tenfold, is common to many studies and suggests that transferrin receptors may be sequestered away from the forming macropinosome. There is also some recent evidence for this from experiments in Dictyostelium, showing that this organism excluded some but not all plasma membrane proteins away from the forming macropinocytic cup [38]. These studies suggest that the composition of the macropinocytic cup and nascent macropinosome is regulated, defining the later as a unique endocytic organelle rather than just an enclosed fragment of the original plasma membrane.
As mentioned earlier, once growth of the protrusion is finished there will be a requirement for a membrane fusion event to close leading edges to fashion the macropinosome (Fig. 1). The identities of several proteins and lipids that mediate membrane fusion on the endocytic and secretory pathway are now known but to date there have been no reports on the specific requirements for a ruffle–ruffle or ruffle–plasma membrane fusion protein. The fungal metabolite wortmannin inhibits lipid kinases that phosphorylate phosphatidylinositol (PI) at the 3 position;it is a prominent inhibitor of signalling emanating from the canonical PI 3-kinase that generates PI (3, 4, 5) P3 from PI(4, 5)P2. Nanomolar concentrations of wortmannin inhibit fluid phase uptake and the homotypic fusion of early endosomes [39, 40] and subsequent studies revealed that a number of PI 3-kinase variants were involved in endocytic traffic with the class III variant hVPS34, that specifically phosphorylates PI, being prominent on endosomal membranes and microdomains that are subsequently enriched in its product PI(3)P [41–43].
In macrophages, wortmannin also affected macropinocytosis and phagocytosis but the inhibitory step was in the closure of ruffles or phagocytic cups rather than the formation of membrane protrusions that were still clearly observed by SEM [18]. Whether this was solely due to the known effects of PI 3-kinase activity on the actin cytoskeleton or whether there is a separate requirement for a PI 3-kinase in a fusogenic event to generate an enclosed macropinosome is currently unknown. A second class III PI 3-kinase inhibitor, 3-methyladenine, does not impede macropinosome formation or internalization in EGF-stimulated A431 cells, but inhibited homotypic macropinosome–macropinosome fusion [44], suggesting that endosome fusion and macropinosome fusion share a requirement for PI(3)P.
Rabs and macropinocytosis
Rab proteins are small GTPases that control multiple membrane trafficking events in the cell. Approximately twelve Rab members have now been located on endocytic structures and several have been implicated in regulating the dynamics of distinct endocytic processes [45, 46]. One of these is Rab5 and represents one the most studied Rab variants that controls several endocytic processes including invagination at the plasma membrane, endosomal fusion, motility and signalling. Transfection of cells with a constitutively active Rab5 leads to the formation of swollen endosomes not dissimilar in appearance to early macropinosomes [47], and increased expression of Rab5 together with an active form of Ras promotes the formation of circular ruffles [48].
In agreement for a Rab5 role in circular ruffling, transfection of cells with the dominant negative Rab5 mutant (S34N) inhibited circular but not cell edge ruffling [48]. Rab5 involvement in macropinocytosis was further demonstrated when one of its effectors, Rabankyrin-5, was found to promote macropinocytosis and partial silencing of Rabankyrin-5 diminished EGF-stimulated fluid phase uptake [49]. Before assigning a role for Rab5 in ruffling and macropinocytosis it is noted that transfection of cells with Rab5 S34N has effects on numerous endocytic pathways thus similar effects may be observed in cells expressing other early endocytic variants such as Rab21 and Rab22a that give similar endocytic defects when their GTP-binding mutants are over-expressed [45]. Thus these effects on ruffling may point towards downstream effects of perturbation of the endocytic pathways in general rather than pointing to Rab5 as being absolutely required for ruffling. siRNA analysis and silencing all three Rab5 variants would point further to an absolute requirement for this protein in macropinosome formation and traffic. A second Rab protein, Rab34, has also been implicated in the formation of ruffles and macropinocytosis; similarly, the effects of this protein were shown in over-expressed systems or cells expressing Rab34 mutants [50].
ADP-ribosylation factors (ARFs) are also small GTPases that function in membrane traffic [51]. One variant, ARF6, is localized to the plasma membrane and in conjunction with the actin cytoskeleton and its exchange factors and activators, acts as a prominent ruffling factor regulating macropinocytosis, cell adhesion and migration [52, 53].
The fate of the macropinosome on the endocytic pathway
The impressive capacity of the cell to internalize its plasma membrane is somewhat overshadowed by its ability to later sort components on the endocytic pathway to a number of cellular destinations. Plasma membrane derived vesicles rapidly fuse with sorting endosomes (often termed early endosomes) that represent a tuboreticular network; indeed the entire endocytic pathway can be similarly defined [54]. It is currently unknown how many types of sorting endosomes exists within a single cell or the extent of mixing of membrane and cargo that then occurs between different pathways. There is now evidence to suggest that molecules entering via clathrin coated vesicles may be sorted before fusing with early endosomes or even prior to completion of the budding step to generate the clathrin coated vesicle [55–57].
The fate of a protein entering these sorting compartments is in part determined by both the nature of its lipid environment and by sorting signals it possesses on its cytoplasmic and transmembrane domain(s). It is in the interest of the cell to ensure that some proteins such as activated receptors are trafficked to the degradative lysosomes and that others such as the transferrin receptor are recycled back to the plasma membrane for another round of function. To fulfil this requirement, it organizes on endosomal membranes the formation of retrieval and sorting multiprotein complexes such as Endosomal Sorting Complexes Required for Transport (ESCRT) and retromer, that either interact with the cytoplasmic portions of endocytosed molecules, with distinct lipids on the endosomal membrane or with endosomal located regulatory proteins. The reader is referred to these reviews on specific aspects of the regulation of sorting and retrieval on the endocytic pathway [58–62].
Fluorescence and electron microscopy studies show early macropinosomes as large, uncoated vacuole like structures identified most commonly with an internalized fluid phase tracer such as dextran or horseradish peroxidase. Numerous groups have attempted to map the route the macropinosome and/or its associated cargo traverses downstream of the plasma membrane and from data review it is unlikely that they mature, in all cell types, via a common pathway to a single pre-defined location [12]. The intracellular dynamics of macropinosomes was first extensively studied in EGF stimulated A431 fibroblasts [22, 63] and macrophages [64–66] and later reviewed [12]. In macrophages, the macropinosomes followed a somewhat conventional centripetal route to tubular lysosomes;as determined by acquisition and loss of classical marker proteins such as the transferrin receptor and Rab7, identifying early and late endosomes, respectively. There were many similarities to this process when macropinosomes were studies in Dicyostelium [67] or in mammalian cells transfected with GFP-c-Src [34].
In contrast to observations in macrophages, there was little evidence of co-localization of EGF-induced A341 macropinosomes with other endocytic markers such as transferrin, neither were macropinocytic cargo delivered to lysosomes in these cells [22]. In a separate study, the transferrin receptor was found to be enriched, via exocytosis, on plasma membrane ruffles of stimulated cells thus suggesting that membrane for ruffles and possibly macropinosome formation was provided in part from the endosomal pathway [29]. Early macropinosomes are however often noted to be devoid of transferrin and/or the transferrin receptor, strengthening the idea that sorting is occurring at the plasma membrane during ruffling and macropinosome formation, and that distinct lipid domains contribute to generate ruffles and macropinosomes.
More recent experiments in A431 cells demonstrated a higher degree of co-localization of EGF, and transferrin in macropinosomes but the fraction of EGF on enlarged structures or ruffles seemed to be significantly higher than transferrin, that was predominantly localized to more widely distributed smaller vesicles [49]. Evidence for macropinosomes being, at least at some stage, unique organelles comes from tracer co-localization experiments showing homotypic, i.e. macropinosome-macropinosome fusion but there was little evidence of macropinosomes fusing with other endocytic structures [22]. In Dictyostelium, the situation was somewhat different as much smaller endosomes appeared to contribute further to internalized macropinosomes suggestive of heterotypoic fusion; homotypic fusion was also prominent [68].
The use of alternative markers of endocytic pathways has recently enhanced our knowledge of the trafficking of macropinosomes though, as expected there is some discrepancy as to whether they progress as distinct entities. Antibodies recognizing the early endosomal autoantigen 1 protein EEA1 are now commonly used to label early endosomes in mammalian cells. On these structures, EEA1 binds to PI(3)P via its FYVE domain, falls off the endoso-mal membrane in the presence of wortmannin [69], binds other regulatory proteins such as Rab5 and regulates early endosome fusion and sorting [70–72]. Expression of Rabankyrin-5 in A431 cells resulted in the formation of swollen vesicles that were later defined as macropinosomes and EGF stimulated cells also sequestered endogenous Rabankyrin-5 on these structures [49]. The larger macropinosomes also harboured Rab5 but did not contain EEA1, however smaller structures that appeared to be more mature macropinosomes contained all three proteins. Earlier studies showed that EEA1 transiently associated with macropinosomes as 5 min macropinosomes were essentially EEA1 negative and then over a 25 min period EEA1 labelling increased and then decreased as the macropinosomes evolved; in agreement with previous studies there was no evidence of the progression of macropinosomes to typical late endosomes and lysosomes [73]. In dendritic cells, early macropinosomes (<4min) were depleted of early endocytic markers but then acquired EEA1 and the transferrin receptor, however, the macropinocytic cargo did not then co-localize with markers of late endosomes and lysosomes [74]. In a separate study, also in A431 cells, immature macropinosomes were labelled with early endocytic markers EEA1 and Rab5 and also Rab7, a classical marker of late endo-somes and lysosomes [75]. These studies were however performed in Rab7 over-expressing cells, that in the absence of stimulus, often display swollen structures, reminiscent of macropinosomes [76]. This suggests that macropinosomes rapidly develop classical early endosome characteristics before developing into late endocytic structures or diminishing in size and loosing their identity via membrane retrieval.
A role for nexins in macropinosome sorting
A member of the sorting nexin (SNX) family of proteins was recently shown be involved in macropinosome maturation [75]. SNXs comprise a family of approximately 30 peripheral membrane proteins that are characterized by having a SNX phox homology (PX) domain that bind most notably to PI(3)P [77, 78]; a number have therefore been located on endosomal structures. A least seven members also contain a Bin/Amphiphysin/Rvs (BAR) domain that is thought to aid in the binding of the PX domain to membrane but also to sense membrane curvature [79].
SNX5 was shown to co-localize with EEA1 on microdomains of macropinosomes but was also apparent in tubular extensions that seemed to emanate from these structures [75]; tubulation of macropinosomes was noted earlier in mammalian and amoeba cells [22, 68]. This suggest that tubulation may be one mechanism that helps retrieve membrane back to the plasma membrane, resulting in the shrinkage of the macropinosome that was noted in the first demonstration of macropinocytosis [21]. SNX5 and other sorting proteins may be working in conjunction with microtubules that ran in parallel to the tubular membranes and also appeared to organize themselves as a mesh around the macropinosome [75]. Similar conclusions come from research in Dictyostelium showing that microtubules were closely associated with macropinocytic vacuoles and tubular extensions emanating from them; macropinosome fusion was also dependent on microtubules [68]. There may therefore be a requirement for alternate protein complexes akin to ESCRT and retromer to retrieve and sort macropinosome membrane and cargo.
Whether the formed tubules recycle back to the plasma membrane to replete lost membrane is unknown but studies in dendritic cells suggest that retrieval may rather be a function of the regulated exocytosis of structures, enlargeosomes, that fuse with the plasma membrane in a calcium-dependent fashion [74]. The enlargeosome was initially described as being a unique vesicular compartment, showing no co-localization with conventional endo-lysosomal markers but containing the protein Desmoyokin-AHNAK, that was recruited, with the enlargeosome, to the plasma membrane. This exo-cytic response to macropinocytosis in dendritic cells is therefore quite different to that described in EGF-stimulated cells where transferrin positive vesicles served the function of enlargeosomes [29]. There is also some evidence for the involvement of the endo-plasmic reticulum in the traffic of material entering via macropinocytosis, or in providing membrane for macropinosome formation [80].
Cell penetrating peptides as vectors for drug delivery
Our understanding of disease processes will grow exponentially now that the human genome is unravelled and that tools are available to more easily study differences in expression and sequence of genes and proteins. In the wake of this is an increasing demand for the design of therapeutic entities to reverse disease-causing events at the intracellular level. This calls for targeting organs and cells, and third-order targeting of intracellular targets in the cytosol or in membrane bound compartments [81]. In the realm of gene delivery the target is most often the nucleus but it is becoming apparent that a number of other organelles are potential drug delivery targets. Endocytic pathways provide highly efficient routes that enable drug delivery researchers the opportunity to introduce a macromolecular entity across the plasma membrane [82], however the likelihood exists that a significant fraction, perhaps all, of the therapeutic payload will be recycled or will end up degraded in lysosomes. Holy Grails in drug delivery research are molecules that somehow promote the escape of therapeutic entities such as genes and proteins from early stages of the endocytic pathway. A wide variety of strategies have been designed including the use of bioresponsive agents that modify themselves and the endosome environment in the decreasing pH that accompanies transit in the endo-lysosomal system, through to the use of domains of proteins, often from pathogens, that have been shown to translocate through biological membranes. These include the hundereds of amino acid sequences that are now classified as cell penetrating peptides (CPP) or protein transduction domains and the reader is referred here to reviews in an edition of Advanced Drug Delivery Reviews dedicated to protein and peptide-mediated translocation [83].
The most intensely studied CPPs include those derived from the transduction domains of the HIV transcription factor TAT (HIV-TAT peptide) or the Drosophila melanogaster homeobox protein Antennapedia (penetratin), together with synthetic oligomers of arginine (R4-R16). The sequences of a few of these are shown in Table 1 and common to many, but not to all, is the relatively high number of positive amino acids lysine and arginine over other amino acids. In view of the heterogeneity of their sequences and properties they are often further subclassified to arginine-rich, amphipathic, non-basic and more specific criteria [84, 85]. The charge on the cationic peptides allows them to interact strongly with negative charges on the plasma membrane and this interaction is reduced in cell lines with defects in heparan sulphate and glycosaminoglycan synthesis [86–88]. Beyond interacting with cells, a number of CPPs traverse biological membranes, be it alone or attached to much larger cargo ranging from small molecule drugs to antisense oligonucleotides, siRNA, plasmids, peptides and proteins [89–92]. They do not appear to be cell-type specific and their incorporation into targeting and intracellular delivery complexes show they hold great promise as agents for enhancing translocation across biological barriers. Their uptake mechanisms have therefore been studied in great detail with a view to the subsequent design of more efficient delivery and translocating systems.
1.
Sequences of the most studied CPPs with respect to endocytic uptake
| Peptide | Sequence |
|---|---|
| HIV TAT peptide | YGRKKRRQRRR |
| Octaarginine | RRRRRRRR |
| SynB1 | RGGRLSYSRRRFSTSTGR |
| Penetratin (Ant) | RQIKIWFQNRRMKWKK |
| Transportan | GWTLNSAGYLLGKINLKALAALAKKIL |
| HIV-gp41 fusion peptide | GALFLGFLGAAGSTMGA |
Initially there was agreement that a number of CPPs entered cells in a temperature independent manner and once inside they rapidly entered the nucleus. Later, a large part of their interaction with cells and nuclear delivery was revealed to be a product of fixation, and that a significant fraction of what was termed cell associated peptide was confined to the plasma membrane [93, 94]. All microscopy and cytometry experiments with these entities are now performed in live cells, and trypsinization and heparin washing is now commonly used to reduce plasma membrane binding prior to quantifying intracellular peptide.
Confocal microscopy highlights the fact that the peptides enter vesicular structures in a number of different cell types including the HeLa and A549 cells shown in Figure 2. But despite extensive study there is no consensus regarding the uptake mechanism and whether there is a preferred entry route. As previously mentioned, hundereds of CPPs have now been described and as there is no consensual sequence, it is unlikely that a common mechanism of entry exists and this is highlighted in a number of studies [87, 95–97]. Equally, any cargo that they are attached to could also influence cellular interaction, endocytosis and intracellular dynamics [96, 98, 99]. Focus here will be on experiments performed with fluorescent variants of HIV-TAT, oligoarginines and penetratin (Table 1).
2.
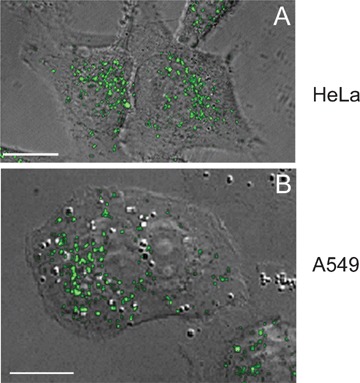
Localization of D-R8 in cells. HeLa (A) and A549 (B) cells were incubated for 1 hr at 37°C with 2 μM of the D-enantiomerof R8-Alexa488 peptide (RRRRRRRRGC-Alexa488) and immediately analysed by scanning confocal fluorescence microscopy combined with scanning Direct Interference Contrast. Shown are superimposed images from 25 to 30 sections through the Z-axis. The peptide-labelled vesicles in these cells are concentrated in a perinuclear region but it remains to be seen whether any are, or are derived from, macropinosomes. Previous studies in HeLa cells show a significant fraction of these to be late endosomes and lysosmes [110]. Scale bar 15 μM.
The case for and against macropinocytosis
In EGF stimulated A431 cells the increased fluid phase uptake that accompanies macropinocytosis was found to be inhibited by the ion-exchange inhibitor amiloride, however, endocytosis of the transferrin receptor was unaffected by this drug [63]. Similarly, the enhanced fluid phase uptake, driven by adenovirus-induced macropinocytosis was inhibited by 5-(N-ethyl-N-isopropyl) amiloride (EIPA), a more potent analogue [17]. Consequently, these agents are regularly used to define uptake via macropinocytosis, but amiloride and EIPA also strongly inhibit receptor-mediated uptake of albumin in renal proximal tubule-derived epithelial cells [100–102]. Here, it is unlikely that uptake is via macropinocytosis and albumin has been shown to enter cells via clathrin coated pits and caveolae [103–106]. To various extents, EIPA also inhibits the uptake of HIV-TAT peptide and oligoarginine peptides raising the possibility of a link between macropinocytosis and uptake of CPPs [87, 107, 108], and supporting previous studies showing amiloride inhibition on the uptake of a TAT peptide-Cre protein conjugate [109]. The microscopy studies to date show the peptides, as single fluorescent conjugates, labelling typical pinocytic vesicles and tubules (Fig. 2) as opposed to macropinocytic vacuoles but careful analyses of their location immediately after addition to cells, when macropinosomes may be much more pronounced, has yet to be performed. One reason for this is that their extensive binding to the plasma membrane makes interpretation at early time points quite difficult, especially when the amount of internalized peptide is low and thus the fluorescent signal weak. An exception to this rule are leukaemic cells such as K562 and KG1a that intriguingly show minimal plasma membrane binding of HIV-TAT or octaarginine peptides [110–112].
There is strong evidence that these peptides promote undefined actin rearrangements and this like membrane ruffling may be a Rac-dependent activity as both TAT peptide and octaarginine (R8) rapidly increased the levels of activated Rac and also induced the formation of lamellipodia [87]. In other studies CPPs HIV-TAT and nona-arginine induced the rapid internalization of the EGF- and tumour necrosis factor receptor [113] and it will be interesting to determine whether there is a coincidental increase in fluid phase endocytosis in these cells. Support for this comes from observations that the TAT–Cre conjugate and TAT peptide conjugated to a fusogenic peptide, enhanced fluid phase uptake of 70 KD dextran [109].
A number of studies have now shown that a fraction (up to 90%) of HIV-TAT and R8 peptide uptake at 37 C occurs independently of inhibition by ion-exchange inhibitors EIPA and amiloride [87, 108, 110, 111]. Uptake of R8 and HIV-TAT in leukaemia cells was relatively insentitive to EIPA but at concentrations higher than 50 μM a much higher fraction of the peptide was found in the cytosol [111]. In a separate study, the cytosolic delivery of oligoarginine and uptake of a CPP-immunogenic antigen chimera was unaffected by amiloride treatment [114, 115]. Amiloride and amiloride analogues have additional quite dramatic effects on the actin cytoskeleton and cell morphology [116], and their capacity to inhibit macropinocytosis may be mediated through actin. The sub-cellular distribution of conventional markers for early and late endo-somes, EEA1 and Lamp1, were also highly sensitive to amiloride and EIPA treatment [111] and of specific note for endocytosis studies is that inhibition of the Na+/H+ exchanger will also affect cellular pH levels. These agents have a plethora of other effects on cells [117] suggesting they should be used with great caution if forming the sole criteria for macropinocytosis. Studies proposing that uptake is via macropinocytosis are also contradictory to others that demonstrate peptide inhibition by agents that disrupt uptake by clathrin coated vesicles [88].
Downstream of the plasma membrane, HIV-TAT peptide and octaarginine co-localize with markers of early endosomes [94, 108, 118] but there is some controversy regarding the subsequent fate of the peptides;and this aspect of their intracellular dynamics is, as noted, is extremely important for drug delivery applications. Analysis using confocal microscopy is often difficult to interpret as the possibility exists that a proportion of the signal is emanating from partially degraded peptide, and if the pH-sensitive fluorophore fluorescein isothiocyanate is used as a label, then its emission will be significantly diminished within endosomes and especially lysosomes. Our studies suggest that a major fraction of octaarginine and HIV-TAT peptide in leukaemia and HeLa cells, is rapidly delivered to lysosomes [110], though other studies suggest that lysosomes are bypassed by a penetratin peptide that was trafficked from endosomes to the Golgi [119].
These endocytosis studies must also be reviewed in parallel with those that clearly show energy independent uptake of CPPs through the plasma membrane [97, 98, 108, 110, 112]. Recent studies in leukaemia cells suggest that R8 enters cells efficiently at 4°C but at temperatures ≥12°C the same cell may internalize the peptide by endocytosis and direct plasma membrane translocation; the relative contribution of each is dependent on temperature and the peptide concentration [112]. This raises the possibility that entry into the cytosol from the extracellular media or the endo-lysosome lumen occurs via a similar concentration dependent mechanism (Fig. 3). Model membrane systems have also been utilized to investigate the binding and translocation capacities of a number of CPPs and highlight the dependency of peptide sequence and lipid composition of the membranes. Proposed models include those suggesting that CPPs are driven via a potential difference following membrane destabilization, that they induce the formation of inverted micelles or that they themselves mediate pore formation [84]. In view of the significant differences in sequences and properties of peptides included in the CPP family, there are likely to be more than one mechanism by which they traverse lipid barriers.
3.
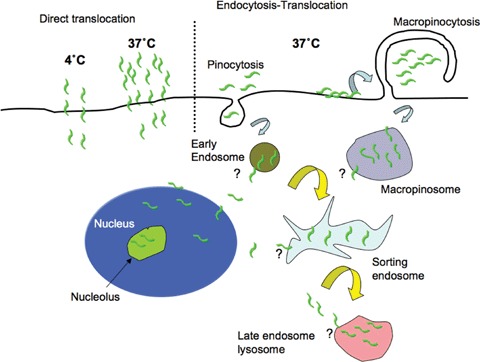
Proposed model for the cellular entry of cationic cell penetrating peptides HIV-TAT and R8. Uptake of the peptides occurs by at least two temperature and concentration dependent mechanisms [112]. At 4°C, entry can only occur via plasma membrane translocation. At 37°C and at high, threshold, extracellular concentration the peptide may enter the cell via a number of endocytic pathways and via direct plasma membrane translocation. A number of endocytic pathways including macropinocytosis caused by peptide induced plasma membrane ruffling is likely to be utilized. Escape from undefined endosomal compartments (?) could conceivably be the result of the accumulation of the peptide to a similar threshold concentration that promotes translocation across the plasma membrane.
Conclusions
Further studies of macropinocytosis in different cell types will undoubtedly shed more light on this poorly understood endocytic mechanism. The hunt will continue for unique markers or regulators of this process and more specific inhibitory drugs, and these will then allow for a better understanding of the factors governing the formation and maturation of the nascent macropinosome and its fate once released from the plasma membrane.
The jury is clearly still out with regards to whether the interaction of CPPs with cells induces a specific form of macropinocytosis and enhances internalization by one or more forms of endocytosis. At the relatively high concentrations (number of peptide molecules per cell) typically used to study these peptides by fluorescent microscopy (typically 1–10 μM with 1 × 105–5 × 105 cells) it is likely that the peptides enter through multiple pathways in the fluid phase and via non-specific adsorptive endocytosis. CPPs will undoubtedly pose many more fascinating questions as their intracellular dynamics is further studied. A more daunting challenge in drug delivery research is to identify the route of translocation of the active moiety, as opposed to what is seen through a microscope lens, that then mediates a biological response.
Acknowledgments
The work reported here was supported in part by the BBSRC and the Leukaemia Research Fund. The author would like to thank Professor Shiroh Futaki, University of Kyoto for supplying D-R8 and Catherine Watkins and John Hickey, Welsh School of Pharmacy for cell culture assistance to generate Fig. 2.
References
- 1.Marsh M. Endocytosis. Oxford: Oxford University Press; 2001. [Google Scholar]
- 2.Cole NB, Lippincott-Schwartz J. Organization of organelles and membrane traffic by microtubules. Curr Opin Cell Biol. 1995;7:55–64. doi: 10.1016/0955-0674(95)80045-x. [DOI] [PubMed] [Google Scholar]
- 3.Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol. 2006;7:897–908. doi: 10.1038/nrm2060. [DOI] [PubMed] [Google Scholar]
- 4.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 5.Johannes L, Lamaze C. Clathrin-dependent or not: is it still the question? Traffic. 2002;3:443–51. doi: 10.1034/j.1600-0854.2002.30701.x. [DOI] [PubMed] [Google Scholar]
- 6.Kirkham M, Parton RG. Clathrin-independent endo-cytosis:new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta. 2005;1746:349–63. doi: 10.1016/j.bbamcr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 8.Nichols B. Caveosomes and endocytosis of lipid rafts. J Cell Sci. 2003;116:4707–14. doi: 10.1242/jcs.00840. [DOI] [PubMed] [Google Scholar]
- 9.Jutras I, Desjardins M. Phagocytosis: at the crossroads of innate and adaptive immunity. Annu Rev Cell Dev Biol. 2005;21:511–27. doi: 10.1146/annurev.cellbio.20.010403.102755. [DOI] [PubMed] [Google Scholar]
- 10.Amyere M, Mettlen M, Van Der Smissen P, Platek A, Payrastre B, Veithen A, Courtoy PJ. Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int J Med Microbiol. 2002;291:487–94. doi: 10.1078/1438-4221-00157. [DOI] [PubMed] [Google Scholar]
- 11.Maniak M. Macropinocytosis. Oxford: Oxford University Press; 2001. [Google Scholar]
- 12.Swanson JA, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–8. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- 13.Fabbri A, Falzano L, Travaglione S, Stringaro A, Malorni W, Fais S, Fiorentini C. Rho-activating Escherichia coli cytotoxic necrotizing factor 1: macropinocytosis of apoptotic bodies in human epithelial cells. Int J Med Microbiol. 2002;291:551–4. doi: 10.1078/1438-4221-00166. [DOI] [PubMed] [Google Scholar]
- 14.Francis CL, Ryan TA, Jones BD, Smith SJ, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–42. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann C, Schmidt G. CNF and DNT. Rev Physiol Biochem Pharmacol. 2004;152:49–63. doi: 10.1007/s10254-004-0026-4. [DOI] [PubMed] [Google Scholar]
- 16.Kolb-Maurer A, Wilhelm M, Weissinger F, Brocker EB, Goebel W. Interaction of human hematopoietic stem cells with bacterial pathogens. Blood. 2002;100:3703–9. doi: 10.1182/blood-2002-03-0898. [DOI] [PubMed] [Google Scholar]
- 17.Meier O, Boucke K, Hammer SV, Keller S, Stidwill RP, Hemmi S, Greber UF. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J Cell Biol. 2002;158:1119–31. doi: 10.1083/jcb.200112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–60. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowrick P, Kenworthy P, McCann B, Warn R. Circular ruffle formation and closure lead to macropinocytosis in hepatocyte growth factor/scatter factor-treated cells. Eur J Cell Biol. 1993;61:44–53. [PubMed] [Google Scholar]
- 20.Hacker U, Albrecht R, Maniak M. Fluid-phase uptake by macropinocytosis in Dictyostelium. J Cell Sci. 1997;110(Pt 2):105–12. doi: 10.1242/jcs.110.2.105. [DOI] [PubMed] [Google Scholar]
- 21.Lewis WH. Pinocytosis. Bull. Johns Hopkins Hosp. 1931;49:17–23. [Google Scholar]
- 22.Hewlett LJ, Prescott AR, Watts C. The coated pit and macropinocytic pathways serve distinct endo-some populations. J Cell Biol. 1994;124:689–703. doi: 10.1083/jcb.124.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norbury CC, Chambers BJ, Prescott AR, Ljunggren HG, Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Cell Biol. 1997;27:280–8. doi: 10.1002/eji.1830270141. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the man-nose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dharmawardhane S, Schurmann A, Sells MA, Chernoff J, Schmid SL, Bokoch GM. Regulation of macropinocytosis by p21-activated kinase-1. Mol Biol Cell. 2000;11:3341–52. doi: 10.1091/mbc.11.10.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haigler HT, McKanna JA, Cohen S. Rapid stimulation of pinocytosis in human carcinoma cells A-431 by epidermal growth factor. J Cell Biol. 1979;83:82–90. doi: 10.1083/jcb.83.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chinkers M, McKanna JA, Cohen S. Rapid rounding of human epidermoid carcinoma cells A-431 induced by epidermal growth factor. J Cell Biol. 1981;88:422–9. doi: 10.1083/jcb.88.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bretscher A. Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J Cell Biol. 1989;108:921–30. doi: 10.1083/jcb.108.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bretscher MS, Aguado-Velasco C. EGF induces recycling membrane to form ruffles. Curr Biol. 1998;8:721–4. doi: 10.1016/s0960-9822(98)70281-7. [DOI] [PubMed] [Google Scholar]
- 30.Blume-Jensen P, Claesson-Welsh L, Siegbahn A, Zsebo KM, Westermark B, Heldin CH. Activation of the human c-kit product by ligand-induced dimerization mediates circular actin reorganization and chemotaxis. EMBO J. 1991;10:4121–8. doi: 10.1002/j.1460-2075.1991.tb04989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mellstrom K, Heldin CH, Westermark B. Induction of circular membrane ruffling on human fibroblasts by platelet-derived growth factor. Exp Cell Res. 1988;177:347–59. doi: 10.1016/0014-4827(88)90468-5. [DOI] [PubMed] [Google Scholar]
- 32.Small JV, Stradal T, Vignal E, Rottner K. The lamel-lipodium: where motility begins. Trends Cell Biol. 2002;12:112–20. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- 33.Amyere M, Payrastre B, Krause U, Van Der Smissen P, Veithen A, Courtoy PJ. Constitutive macropinocytosis in oncogene-transformed fibrob-lasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol Biol Cell. 2000;11:3453–67. doi: 10.1091/mbc.11.10.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasahara K, Nakayama Y, Sato I, Ikeda K, Hoshino M, Endo T, Yamaguchi N. Role of Src-family kinases in formation and trafficking of macropinosomes. J Cell Physiol. 2007;211:220–32. doi: 10.1002/jcp.20931. [DOI] [PubMed] [Google Scholar]
- 35.Mettlen M, Platek A, Van Der Smissen P, Carpentier S, Amyere M, Lanzetti L, De Diesbach P, Tyteca D, Courtoy PJ. Src triggers circular ruffling and macropinocytosis at the apical surface of polarized MDCK cells. Traffic. 2006;7:589–603. doi: 10.1111/j.1600-0854.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 36.Veithen A, Amyere M, Van Der Smissen P, Cupers P, Courtoy PJ. Regulation of macropinocytosis in v-Src-transformed fibroblasts: cyclic AMP selectively promotes regurgitation of macropinosomes. J Cell Sci. 1998;111:2329–35. doi: 10.1242/jcs.111.16.2329. [DOI] [PubMed] [Google Scholar]
- 37.Veithen A, Cupers P, Baudhuin P, Courtoy PJ. v-Src induces constitutive macropinocytosis in rat fibroblasts. J Cell Sci. 1996;109(Pt 8):2005–12. doi: 10.1242/jcs.109.8.2005. [DOI] [PubMed] [Google Scholar]
- 38.Mercanti V, Charette SJ, Bennett N, Ryckewaert JJ, Letourneur F, Cosson P. Selective membrane exclusion in phagocytic and macropinocytic cups. J Cell Sci. 2006;119:4079–87. doi: 10.1242/jcs.03190. [DOI] [PubMed] [Google Scholar]
- 39.Clague MJ, Thorpe C, Jones AT. Phosphatidylinositol 3-kinase regulation of fluid phase endocytosis. FEBS Lett. 1995;367:272–4. doi: 10.1016/0014-5793(95)00576-u. [DOI] [PubMed] [Google Scholar]
- 40.Jones AT, Clague MJ. Phosphatidylinositol 3-kinase activity is required for early endosome fusion. Biochem J. 1995;311:31–4. doi: 10.1042/bj3110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clague MJ, Jones AT, Mills IG, Walker DM, Urbe S. Regulation of early-endosome dynamics by phos-phatidylinositol 3-phosphate binding proteins. Biochem Soc Trans. 1999;27:662–6. doi: 10.1042/bst0270662. [DOI] [PubMed] [Google Scholar]
- 42.Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier JM, Parton RG, Stenmark H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19:4577–88. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gillooly DJ, Raiborg C, Stenmark H. Phosphatidylinositol 3-phosphate is found in microdomains of early endosomes. Histochem Cell Biol. 2003;120:445–53. doi: 10.1007/s00418-003-0591-7. [DOI] [PubMed] [Google Scholar]
- 44.Araki N, Hamasaki M, Egami Y, Hatae T. Effect of 3-methyladenine on the fusion process of macropinosomes in EGF-stimulated A431 cells. Cell Struct Funct. 2006;31:145–57. doi: 10.1247/csf.06029. [DOI] [PubMed] [Google Scholar]
- 45.Simpson JC, Jones AT. Early endocytic Rabs:functional prediction to functional characterization. Biochem Soc Symp. 2005:99–108. doi: 10.1042/bss0720099. [DOI] [PubMed] [Google Scholar]
- 46.Somsel Rodman J, Wandinger-Ness A. Rab GTPases coordinate endocytosis. J Cell Sci. 2000;113:183–92. doi: 10.1242/jcs.113.2.183. [DOI] [PubMed] [Google Scholar]
- 47.Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–96. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lanzetti L, Palamidessi A, Areces L, Scita G, Di Fiore PP. Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature. 2004;429:309–14. doi: 10.1038/nature02542. [DOI] [PubMed] [Google Scholar]
- 49.Schnatwinkel C, Christoforidis S, Lindsay MR, Uttenweiler-Joseph S, Wilm M, Parton RG, Zerial M. The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms. PLoS Biol. 2004;2:E261. doi: 10.1371/journal.pbio.0020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun P, Yamamoto H, Suetsugu S, Miki H, Takenawa T, Endo T. Small GTPase Rah/Rab34 is associated with membrane ruffles and macropinosomes and promotes macropinosome formation. J Biol Chem. 2003;278:4063–71. doi: 10.1074/jbc.M208699200. [DOI] [PubMed] [Google Scholar]
- 51.D'Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–58. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 52.Donaldson JG. Multiple roles for Arf6:sorting, structuring, and signaling at the plasma membrane. J Biol Chem. 2003;278:41573–6. doi: 10.1074/jbc.R300026200. [DOI] [PubMed] [Google Scholar]
- 53.Sabe H. Requirement for Arf6 in cell adhesion, migration, and cancer cell invasion. J Biochem. 2003;134:485–9. doi: 10.1093/jb/mvg181. [DOI] [PubMed] [Google Scholar]
- 54.Marbet P, Rahner C, Stieger B, Landmann L. Quantitative microscopy reveals 3D organization and kinetics of endocytosis in rat hepatocytes. Microsc Res Tech. 2006;69:693–707. doi: 10.1002/jemt.20337. [DOI] [PubMed] [Google Scholar]
- 55.Keyel PA, Mishra SK, Roth R, Heuser JE, Watkins SC, Traub LM. A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors. Mol Biol Cell. 2006;17:4300–17. doi: 10.1091/mbc.E06-05-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mundell SJ, Luo J, Benovic JL, Conley PB, Poole AW. Distinct clathrin-coated pits sort different G protein-coupled receptor cargo. Traffic. 2006;7:1420–31. doi: 10.1111/j.1600-0854.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 58.Bishop NE. Dynamics of endosomal sorting. Int Rev Cytol. 2003;232:1–57. doi: 10.1016/s0074-7696(03)32001-7. [DOI] [PubMed] [Google Scholar]
- 59.Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol. 2006;7:568–79. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- 60.Hurley JH, Emr SD. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct. 2006;35:277–98. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Urbe S. Ubiquitin and endocytic protein sorting. Essays Biochem. 2005;41:81–98. doi: 10.1042/EB0410081. [DOI] [PubMed] [Google Scholar]
- 62.Van Meer G, Sprong H. Membrane lipids and vesicular traffic. Curr Opin Cell Biol. 2004;16:373–8. doi: 10.1016/j.ceb.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 63.West MA, Bretscher MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J. Cell Biol. 1989;109:2731–9. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berthiaume EP, Medina C, Swanson JA. Molecular size-fractionation during endocytosis in macrophages. J Cell Biol. 1995;129:989–98. doi: 10.1083/jcb.129.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Racoosin EL, Swanson JA. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J Cell Sci. 1992;102:867–80. doi: 10.1242/jcs.102.4.867. [DOI] [PubMed] [Google Scholar]
- 66.Racoosin EL, Swanson JA. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J Cell Biol. 1993;121:1011–20. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rupper A, Lee K, Knecht D, Cardelli J. Sequential activities of phosphoinositide 3-kinase, PKB/Aakt, and Rab7 during macropinosome formation in Dictyostelium. Mol Biol Cell. 2001;12:2813–24. doi: 10.1091/mbc.12.9.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clarke M, Kohler J, Heuser J, Gerisch G. Endosome fusion and microtubule-based dynamics in the early endocytic pathway of dictyostelium. Traffic. 2002;3:791–800. doi: 10.1034/j.1600-0854.2002.31104.x. [DOI] [PubMed] [Google Scholar]
- 69.Patki V, Virbasius J, Lane WS, Toh BH, Shpetner HS, Corvera S. Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1997;94:7326–30. doi: 10.1073/pnas.94.14.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gillooly DJ, Simonsen A, Stenmark H. Cellular functions of phosphatidylinositol 3-phosphate and FYVE domain proteins. Biochem J. 2001;355:249–58. doi: 10.1042/0264-6021:3550249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mills IG, Jones AT, Clague MJ. Involvement of the endosomal autoantigen EEA1 in homotypic fusion of early endosomes. Curr Biol. 1998;8:881–4. doi: 10.1016/s0960-9822(07)00351-x. [DOI] [PubMed] [Google Scholar]
- 72.Mills IG, Jones AT, Clague MJ. Regulation of endo-some fusion. Mol Membr Biol. 1999;16:73–9. doi: 10.1080/096876899294788. [DOI] [PubMed] [Google Scholar]
- 73.Hamasaki M, Araki N, Hatae T. Association of early endosomal autoantigen 1 with macropinocytosis in EGF-stimulated A431 cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;277:298–306. doi: 10.1002/ar.a.20027. [DOI] [PubMed] [Google Scholar]
- 74.Falcone S, Cocucci E, Podini P, Kirchhausen T, Clementi E, Meldolesi J. Macropinocytosis:regulated coordination of endocytic and exocytic membrane traffic events. J Cell Sci. 2006;119:4758–69. doi: 10.1242/jcs.03238. [DOI] [PubMed] [Google Scholar]
- 75.Kerr MC, Lindsay MR, Luetterforst R, Hamilton N, Simpson F, Parton RG, Gleeson PA, Teasdale RD. Visualisation of macropinosome maturation by the recruitment of sorting nexins. J Cell Sci. 2006;119:3967–80. doi: 10.1242/jcs.03167. [DOI] [PubMed] [Google Scholar]
- 76.Bucci C, Thomsen P, Nicoziani P, McCarthy J, Van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–80. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carlton J, Bujny M, Rutherford A, Cullen P. Sorting nexins–unifying trends and new perspectives. Traffic. 2005;6:75–82. doi: 10.1111/j.1600-0854.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 78.Worby CA, Dixon JE. Sorting out the cellular functions of sorting nexins. Nat Rev Mol Cell Biol. 2002;3:919–31. doi: 10.1038/nrm974. [DOI] [PubMed] [Google Scholar]
- 79.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature:the amphiphysin BAR structure. Science. 2004;303:495–9. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 80.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc Natl Acad Sci USA. 2003;100:12889–94. doi: 10.1073/pnas.1735556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moghimi SM, Rajabi-Siahboomi AR. Recent advances in cellular, sub-cellular and molecular targeting. Adv Drug Deliv Rev. 2000;41:129–33. doi: 10.1016/s0169-409x(99)00060-5. [DOI] [PubMed] [Google Scholar]
- 82.Jones AT, Gumbleton M, Duncan R. Understanding endocytic pathways and intracellular trafficking:a prerequisite for effective design of advanced drug delivery systems. Adv Drug Deliv Rev. 2003;55:1353–7. doi: 10.1016/j.addr.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 83.Torchilin VP. Protein- and peptide-mediated transduction:mechanisms and implications for drug delivery. Adv Drug Del Rev. 2005;57:489–90. [Google Scholar]
- 84.Fischer R, Fotin-Mleczek M, Hufnagel H, Brock R. Break on through to the other side-biophysics and cell biology shed light on cell-penetrating peptides. Chembiochem. 2005;6:2126–42. doi: 10.1002/cbic.200500044. [DOI] [PubMed] [Google Scholar]
- 85.Wagstaff KM, Jans DA. Protein transduction: cell penetrating peptides and their therapeutic applications. Curr Med Chem. 2006;13:1371–87. doi: 10.2174/092986706776872871. [DOI] [PubMed] [Google Scholar]
- 86.Console S, Marty C, Garcia-Echeverria C, Schwendener R, Ballmer-Hofer K. Antennapedia and HIV transactivator of transcription (TAT) “protein transduction domains” promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J Biol Chem. 2003;278:35109–14. doi: 10.1074/jbc.M301726200. [DOI] [PubMed] [Google Scholar]
- 87.Nakase I, Tadokoro A, Kawabata N, Takeuchi T, Katoh H, Hiramoto K, Negishi M, Nomizu M, Sugiura Y, Futaki S. Interaction of arginine-rich peptides with membrane-associated proteoglycans is crucial for induction of actin organization and macropinocytosis. 2007;46:492–501. doi: 10.1021/bi0612824. [DOI] [PubMed] [Google Scholar]
- 88.Richard JP, Melikov K, Brooks H, Prevot P, Lebleu B, Chernomordik LV. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J Biol Chem. 2005;280:15300–6. doi: 10.1074/jbc.M401604200. [DOI] [PubMed] [Google Scholar]
- 89.Deshayes S, Morris MC, Divita G, Heitz F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell Mol Life Sci. 2005;62:1839–49. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dietz GP, Bahr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol Cell Neurosci. 2004;27:85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 91.Henriques ST, Melo MN, Castanho MA. Cell-penetrating peptides and antimicrobial peptides: how different are they? Biochem J. 2006;399:1–7. doi: 10.1042/BJ20061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hudecz F, Banoczi Z, Csik G. Medium-sized peptides as built in carriers for biologically active compounds. Med Res Rev. 2005;25:679–736. doi: 10.1002/med.20034. [DOI] [PubMed] [Google Scholar]
- 93.Lundberg M, Johansson M. Positively charged DNA-binding proteins cause apparent cell membrane translocation. Biochem Biophys Res Commun. 2002;291:367–71. doi: 10.1006/bbrc.2002.6450. [DOI] [PubMed] [Google Scholar]
- 94.Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–90. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 95.Gammon ST, Villalobos VM, Prior JL, Sharma V, Piwnica-Worms D. Quantitative analysis of permeation peptide complexes labeled with Technetium-99m:chiral and sequence-specific effects on net cell uptake. Bioconjugate Chem. 2003;14:368–76. doi: 10.1021/bc0256291. [DOI] [PubMed] [Google Scholar]
- 96.Saalik P, Elmquist A, Hansen M, Padari K, Saar K, Viht K, Langel U, Pooga M. Protein cargo delivery properties of cell-penetrating peptides. A comparative study. Bioconjugate Chem. 2004;15:1246–53. doi: 10.1021/bc049938y. [DOI] [PubMed] [Google Scholar]
- 97.Thoren PE, Persson D, Isakson P, Goksor M, Onfelt A, Norden B. Uptake of analogs of penetratin, Tat(48–60) and oligoarginine in live cells. Biochem Biophys Res Commun. 2003;307:100–7. doi: 10.1016/s0006-291x(03)01135-5. [DOI] [PubMed] [Google Scholar]
- 98.Maiolo JR, Ferrer M, Ottinger EA. Effects of cargo molecules on the cellular uptake of arginine-rich cell-penetrating peptides. Biochim Biophys Acta. 2005;1712:161–72. doi: 10.1016/j.bbamem.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 99.Tunnemann G, Martin RM, Haupt S, Patsch C, Edenhofer F, Cardoso MC. Cargo-dependent mode of uptake and bioavailability of TAT-containing proteins and peptides in living cells. FASEB J. 2006;20:1775–84. doi: 10.1096/fj.05-5523com. [DOI] [PubMed] [Google Scholar]
- 100.Drumm K, Lee E, Stanners S, Gassner B, Gekle M, Poronnik P, Pollock C. Albumin and glucose effects on cell growth parameters, albumin uptake and Na+/H+-exchanger Isoform 3 in OK cells. Cell Physiol Biochem. 2003;13:199–206. doi: 10.1159/000072422. [DOI] [PubMed] [Google Scholar]
- 101.Gekle M, Drumm K, Mildenberger S, Freudinger R, Gassner B, Silbernagl S. Inhibition of Na+-H+ exchange impairs receptor-mediated albumin endocytosis in renal proximal tubule-derived epithelial cells from opossum. J Physiol. 1999;520:709–21. doi: 10.1111/j.1469-7793.1999.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gekle M, Freudinger R, Mildenberger S. Inhibition of Na+-H+ exchanger-3 interferes with apical receptor-mediated endocytosis via vesicle fusion. J Physiol. 2001;531:619–29. doi: 10.1111/j.1469-7793.2001.0619h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Monks J, Neville MC. Albumin transcytosis across the epithelium of the lactating mouse mammary gland. J Physiol. 2004;560:267–80. doi: 10.1113/jphysiol.2004.068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–20. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- 105.Schubert W, Frank PG, Razani B, Park DS, Chow CW, Lisanti MP. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J Biol Chem. 2001;276:48619–22. doi: 10.1074/jbc.C100613200. [DOI] [PubMed] [Google Scholar]
- 106.Yumoto R, Nishikawa H, Okamoto M, Katayama H, Nagai J, Takano M. Clathrin-mediated endocytosis of FITC-albumin in alveolar type II epithelial cell line RLE-6TN. Am J Physiol Lung Cell Mol Physiol. 2006;290:L946–55. doi: 10.1152/ajplung.00173.2005. [DOI] [PubMed] [Google Scholar]
- 107.Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J Control Release. 2005;102:247–53. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 108.Nakase I, Niwa M, Takeuchi T, Sonomura K, Kawabata N, Koike Y, Takehashi M, Tanaka S, Ueda K, Simpson JC, Jones AT, Sugiura Y, Futaki S. Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Mol Ther. 2004;10:1011–22. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 109.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–5. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 110.Al-Taei S, Penning NA, Simpson JC, Futaki S, Takeuchi T, Nakase I, Jones AT. Intracellular traffic and fate of protein transduction domains HIV-1 TAT peptide and octaarginine. Implications for their utilization as drug delivery vectors. Bioconjugate Chem. 2006;17:90–100. doi: 10.1021/bc050274h. [DOI] [PubMed] [Google Scholar]
- 111.Fretz M, Jin J, Conibere R, Penning NA, Al-Taei S, Storm G, Futaki S, Takeuchi T, Nakase I, Jones AT. Effects of Na+/H+ exchanger inhibitors on subcellular localisation of endocytic organelles and intracellular dynamics of protein transduction domains HIV-TAT peptide and octaarginine. J Control Release. 2006;116:247–54. doi: 10.1016/j.jconrel.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 112.Fretz MM, Penning NA, Taei SA, Futaki S, Takeuchi T, Nakase I, Storm G, Jones AT. Temperature, concentration and cholesterol dependent translocation of L- and D-octaarginine across the plasma and nuclear membrane of CD34 + leukaemia cells. Biochem J. 2007;403:335–42. doi: 10.1042/BJ20061808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fotin-Mleczek M, Welte S, Mader O, Duchardt F, Fischer R, Hufnagel H, Scheurich P, Brock R. Cationic cell-penetrating peptides interfere with TNF signalling by induction of TNF receptor internalization. J Cell Sci. 2005;118:3339–51. doi: 10.1242/jcs.02460. [DOI] [PubMed] [Google Scholar]
- 114.Batchu RB, Moreno AM, Szmania SM, Bennett G, Spagnoli GC, Ponnazhagan S, Barlogie B, Tricot G, Van Rhee F. Protein transduction of dendritic cells for NY-ESO-1-based immunotherapy of myeloma. Cancer Res. 2005;65:10041–9. doi: 10.1158/0008-5472.CAN-05-1383. [DOI] [PubMed] [Google Scholar]
- 115.Zaro J, Rajapaska T, Okamoto C, Shen W-C. Membrane transduction of oligoarginine in HeLa cells is not mediated by macropinocytosis. Mol Pharm. 2006;3:181–6. doi: 10.1021/mp0500869. [DOI] [PubMed] [Google Scholar]
- 116.Lagana A, Vadnais J, Le PU, Nguyen TN, Laprade R, Nabi IR, Noel J. Regulation of the formation of tumor cell pseudopodia by the Na+/H+ exchanger NHE1. J Cell Sci. 2000;113:3649–62. doi: 10.1242/jcs.113.20.3649. [DOI] [PubMed] [Google Scholar]
- 117.Kleyman TR, Cragoe EJ., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- 118.Potocky TB, Menon AK, Gellman SH. Cytoplasmic and nuclear delivery of a TAT-derived peptide and a beta-peptide after endocytic uptake into HeLa cells. J Biol Chem. 2003;278:50188–94. doi: 10.1074/jbc.M308719200. [DOI] [PubMed] [Google Scholar]
- 119.Fischer R, Kohler K, Fotin-Mleczek M, Brock R. A stepwise dissection of the intracellular fate of cationic cell-penetrating peptides. J Biol Chem. 2004;279:12625–35. doi: 10.1074/jbc.M311461200. [DOI] [PubMed] [Google Scholar]


