Abstract
Physiological polyamines are ubiquitous polycations with pleiotropic biochemical activities, including regulation of gene expression, cell proliferation and modulation of cell signalling. Reports that the polyamines with cytoprotective activities were induced by diverse stresses raised the hypothesis that physiological polyamines may play a role in inducing stress response. In a wide range of organisms, physiological polyamines were not only induced by diverse stresses, such as reactive oxygen species (ROS), heat, ultraviolet (UV) and psychiatric stress but were able to confer beneficial effects for survival. Recent biochemical and genetic evidences show that polyamines can function as an ROS scavenger, acid tolerance factor and chemical chaperone, and positive regulators for expression of stress response genes which may explain their protective functions against diverse stresses. Taken together, these data suggest that physiological polyamines can function as primordial stress molecules in bacteria, plants and mammals, and may play an essential role in regulation of pathogen-host interactions.
Keywords: physiological polyamines, stress molecule, tolerance, pathogen-host interaction
Introduction
Physiological polyamines, such as putrescine, spermidine and spermine are ubiquitous aliphatic polycations synthesized endogenously in all organisms; their metabolic pathways are conserved, with minor variation, from bacteria to plants and animals. Polyamines can regulate the biochemical activity of various macromolecules, including DNA, RNA and proteins by binding to them. Suggested pleiotropic biological functions for polyamines include regulation of transcription and translation [1–4], and cell proliferation [5–7]. In addition, complex regulatory mechanisms for the induction and the homeostasis of polyamines, such as metabolic regulation of polyamines and regulation of gene expression of the proteins responsible for polyamine metabolism were also reported [8]. However, still it remains to be largely unknown about how physiological polyamines exert their biochemical activities and what physiological function they serve. Increasing reports showing that the polyamines are induced in response to diverse physico-biochemical stresses, including osmolarity, heat, reactive oxygen species (ROS), ultraviolet (UV), and psychiatric stress in a wide range of organisms raise the question whether physiological polyamine can function as a conserved stress molecule. Little study has been done to address whether physiological polyamines are induced directly to give tolerance in response to stresses or how the polyamines can exert any direct protective activities against stress. This review is aimed to answer whether physiological polyamines can function as stress molecules and how polyamines mediate a stress response to diverse physiological and physical stresses. Finally, divergent physiological functions of the polyamines in terms of stress molecule in pathogen-host interactions will be discussed.
Metabolism and transport of physiological polyamines
Physiological polyamines conserved in both prokaryotes and eukaryotes, including putrescine, spermidine and spermine, are synthesized from two primary precursors, L-arginine and L-methionine as depicted in Figure 1. Ornitine and S-adenosylmethionine (AdoMet) are formed from these amino acids through action of arginase and methionine adenosyltransferase (MAT), respectively. Putrescine is synthesized from ornitine by ornitine decarboxylase (ODC); in plants and some bacteria it can be synthesized alternatively via agmatine formed by arginine decarboxylase (ADC). Gamma-aminobutyric acid (GABA), an important neurotransmitter, is formed through decarboxylation of glutamate by glutamate decarboxylation (GDC) or through catabolic degradation of putrescine by diamine oxidase and GABA dehydrogenase. Despite having only a single cationic charge, GABA seems also to play a polyamine-like role. Cadaverine is a bacterial polyamine formed from lysine by lysine decarboxylase (LDC). Decarboxylated AdoMet (DcAdoMet) is formed through decarboxylation of AdoMet catalysed by AdoMet decarboxylase (AdoMetDC). Transfer of an aminopropyl group from DcAdoMet to putrescine and spermidine is catalysed by spermidine synthase and spermine synthase to yield spermidine and spermine, respectively.
1.
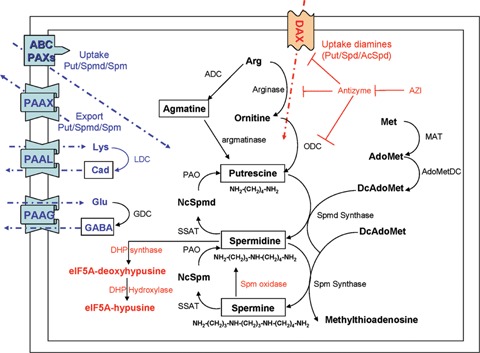
Metabolic pathways of physiological polyamines in prokaryotes and eukaryotes. Eukaryote- and prokaryote-specific pathways are represented in red colour and blue one, respectively. ODC, ornitine decarboxylase; LDC, lysine decarboxylase; GDC, glutamine decarboxylase; ADC, arginine decarboxylase; AZI, antizyme inhibitor; AdoMET, S-adenosylmethionine; DcAdoMET, decarboxylated AdoMET; Spmd, spermidine; Spm, spermine; Cad, cadaverine; GABA, γ-aminobutyric acid; PAO, polyamine oxidase; SSAT, spermidine/spermine N1-acetyltransferase; NcSpmd, N1-acetylspermidine; NcSpm, N1-acetylspermine, eIF5A, eukaryotic initiation factor 5A; PAAXs, polyamine antiporters; PAAL, Lys:Cad antiporter; PAAG, Glu:GABA antiporter; DAX, diamine transporter.
The first step in the catabolic interconversion of polyamines is catalysed by spermidine/spermine N1- acetyltransferase (SSAT) to form N1-acetylspermidine and N1-acetylspermine that is back converted to spermidine and spermine by polyamine oxidase (PAO), respectively. The PAO reaction also yields stoichiometrically 3-acetamidopropanal and H2O2. Acetylated polyamines seem to be the major form of polyamines exported in eukaryotic cells. Spermine can be directly converted to spermidine by spermine oxidase (SMO) in animal cells because spermine is a preferential substrate for SMO over acetylated spermine [9, 10]. Spermidine also serves as a precursor for the synthesis of hypusine, an unusual amino acid residue in eukaryotic initiation factor 5A (eIF5A), which is essential for eukaryotic cell proliferation [5, 6].
In eukaryotes, regulation of polyamines levels is governed primarily by activity of ODC which catalyses the first step in the synthesis of canonical polyamines. Concentration levels of polyamines are tightly controlled by transcriptional regulation and rapid post-translational degradation, by antizyme (AZ) and antizyme inhibitor (AZI), and by exportation of the acetylated polyamines from the cells. ODC is known to be inactivated by proteasomal degradation through heterodimer formation with AZ which is synthesized through polyamine-mediated frame-shifting of the AZ gene [4]. On the other hand, in prokaryotes polyamines levels are regulated primarily by transcriptional regulation of enzymes catalysing the first step in polyamine synthesis, such as μLDC, glutamate decarboxylase (GDC), ADC, and ODC, and by corresponding antiporters that are responsible for exporting polyamines as shown in Figure 1. Export of excess accumulated polyamines may be mediated in a membrane potential-dependent manner by polyamine antiporters (PAAXs), such as the Lys:cadaverine antiporter and the Glu:γ-aminobutyric acid antiporter, since they are induced by the polyamines and have a high Km values (<100 μM) for polyamines export. Uptake of the polyamines is carried out primarily by an ABC-type synporter with a low Km value (few μM) for polyamine uptake [11]. Although a mammalian polyamine transporter has not been identified, pharmacological studies showed that polyamines may be transported by diamine transporters (DAX) with broad specificity [12, 13].
Are physiological polyamines stress molecules?
Organisms respond continuously to environmental changes and evolutionary pressure selects those most prone to developing appropriate adaptive systems. The stringent response is a conserved adaptive regulatory system in prokaryotes. Under conditions of amino acid or carbon starvation, most prokaryotes and several plants rapidly turn off expression of unstringent genes for survival and upregulate genes involved in protecting from starvation and maintenance of dormancy. These responses are mediated by induction of hyperphosphorylated guanosine nucleotides, ppGpp and pppGpp through an uncharged tRNA-sensing mechanism and sigmaS-dependent gene expression, respectively [14–16]. In eukaryotes, unoccupied tRNAs (utRNAs) and unfolded proteins in the endoplasmic reticulum (ER) increase during starvation or ER stress and suppress cap-dependent translation of many mRNAs; however, mRNAs involved in protective response to the stresses are activated via phosphorylation of eIF2α by eIF2α family kinases, including protein kinase R (PKR) and PKR-like ER kinase (PERK) [17, 18]. This conserved response to ER stress is called as the unfolded protein response (UPR) and constitutes an integral adaptive system in eukaryotes.
The basic principle underlying polyamine adaptive responses appears to be shared by the prokaryotic stringent response and the eukaryotic UPR. When subjected to starvation, ppGpp and adenosine triphosphate (ATP)-dependent proteases, Lon and Clp induced by utRNA or unfolded proteins stimulate induction of polyamine synthesis by liberating free amino acid substrates through the action of the proteases on ribosomal proteins. Increases in the levels of polyamines induce positive feedback control for the synthesis of polyamines [19, 22], and subsequently turn on multiple stress-related regulons and turn off general gene expression as depicted in Figure 2. Proposed mechanisms for polyamine modulated gene expression include chromatin remodelling via DNA methylation and histone acetylation [23, 24], induction of structural changes in DNA, tRNA or mRNA [25–27], and regulation of transcriptional regulators such as Myc, Oct-1, and enhancer binding protein (EBP) [28, 29]. Polyamines in low concentration usually stimulate gene expression, but elevation of polyamines levels above certain concentrations could exert an inhibitory effect by differential interaction with target molecule. For example, binding of estrogen receptor or EBP to respective cognate DNA binding elements was stimulated by sub-millimolar concentrations of putrescine or spermine which induced DNA conformational change and polyamines in millimolar concentrations inhibited EBP binding to target DNA [28, 30–32]. Concentration-dependent differential effects of polyamines on the initiation and elongation of protein synthesis in a cell-free system was also reported [33]. The effective concentrations of polyamines (sub-mM to mM) in regulating gene expression in response to stresses seem to be physiologically relevant since a substantial fraction of putrescine and spermidine (< sub-mM) exist in free form even in unstressed cells, though most of spermine exists in RNA- and DNA-bound forms. It was reported that there remained 0.2 mM of free spermidine (15% of total spermidine) and 12.5 mM of putrescine (38% of total putrescine) in lymphocyte and Escherichia coli, respectively, based on calculations with binding constants of each polyamine to target macromolecules and the cellular concentration of the macromolecules under physiological concentrations of K+ and Mg2+[34, 35]. In addition, weak ionic interaction of polyamines with RNA, DNA, proteins and anionic phospholipids (Ks > 5 × 10−4 M) can increase the local concentrations of polyamines in the vicinity of the interacting molecules; thus, direct protective functions of polyamines such as radical scavenging may be facilitated.
2.
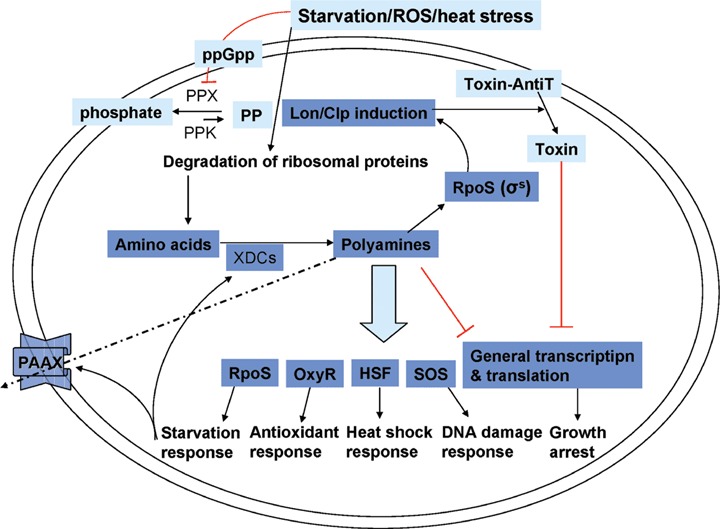
Schematic model for the stress response mediated by physiological polyamines. In response to diverse stresses, levels of physiological polyamines levels are up regulated by positive feedback regulation, which activates stress-related regulons represses general gene expression. AntiT, antitoxin; PP, polyphosphate; PPX, exopolyphosphatase; PPK, polyphosphate kinase; HSF, heat shock transcription factor; PAAXs, polyamine antiporters; XDCs, amino acid decarboxylases.
Based on observations that stress-responsive genes were turned on by stress-induced polyamines described in sections below and gene expression was suppressed by elevated polyamine levels, stressinducible physiological polyamines may play a dual role by turning on stress-responsive genes and acting globally to shut down general genes. This may constitute a conserved basic adaptive stress response in living organisms. However, prolonged exposure to excess polyamines may cause direct or indirect detrimental effects on cell survival [36–39]; thus, strict regulation of cellular polyamine levels may critical for balancing a coping response to acute stresses and reduction of the toxicity induced by excess polyamines.
Data supporting the hypothesis that physiological polyamines induce genes protective against stresses have been reported, though the mechanism of how these simple polycations activate specific genes was not elucidated. Gene expression in response to heat shock was hampered in polyamine auxotropic E. coli, and was restored to normal by addition of putrescine [40]. Polyamine depletion by α-difluoromethylornithine (DFMO), a specific irreversible inhibitor of ODC in hepatocarcinoma cell lines, impaired not only induction of HSP70 mRNA following heat shock [41], but also DNA binding activity of heat shock transcription factor (HSF), a transcriptional activator for heat shock proteins (HSPs) [42]. In addition, recent reports showed that physiological polyamines were involved in regulation of rpoS, an alternative sigma factor gene, and subsequent expression of its gene product sigmaS which effects expression of stationary phase genes and serves as a global stress response switch. SigmaS levels were up-regulated by polyamine-mediated transcriptional activation and post-translational stabilization which induced sigmaS-dependent gene expression of stress response genes in E. coli under starvation conditions and during transition to stationary phase [43]. Moreover, H2O2-mediated induction of OxyR, a ROS-responsive transcription factor responsible for the induction of a series of antioxidant genes, was impaired in polyamine-deficient E. coli generated by genetic deletion of speABC, putrescine biosynthetic genes. Tolerance to hydrogen peroxide and induction of OxyR and its target genes, such as katG and ahpC, were recovered by exogenous application of putrescine or spermidine [44]. Thus, physiological polyamines can regulate transcription and translation of two main stress responsive regulons, OxyR and RpoS, in response to stress, though the detailed mechanism is not yet known. SOS genes such as recA and uvrA, which are involved in DNA repair, were effectively induced by spermine and less effectively by spermidine in a concentration-dependent manner in E. coli[45, 46]. The polyamines were shown to be essential for synthesis of ColE7, an E. coli nuclease-type colicin, and the rate of polyamine synthesis was directly related to the SOS response [47]. SOS induction of recA by exposure to UV-, gamma-irradiation and mitomycin C was reduced in polyamine-deficient E. coli, 3.0-, 2.5- and 4-fold lower, respectively, as compared to wild type [48].
An array of data show that physiological polyamines are induced in response to diverse physio-biochemical stresses, including acid, osmolarity, UV, ROS and heat, in a variety of organisms from bacteria to plants and mammals. In addition, reports have shown that induction of polyamines could exert protective effects on cells or organisms subjected to stresses, such as ROS [49–64], abnormal temperature [65, 66], pH [67, 68], heavy metals [69] and osmolarity [70–72]. Furthermore, down-regulation of polyamines by genetic interruption of polyamine synthesis caused a reduction in tolerance to stresses, while genetic elevation of polyamine synthetic enzymes such as ODC and ADC resulted in enhanced tolerance to stresses [71, 73–77].
Induction of a stress response was first observed in 1962, when heat stressing lead to chromosomal puffs in the salivary glands of Drosophilia lava. These anomalous puffs indicated that a group of proteins had been dramatically induced, which became known as HSPs. These proteins were found in all organisms and are more generally called stress proteins because they are induced in response to all known stresses. Stress proteins confer tolerance to stressors, and most act as molecular chaperones that assist refolding of damaged proteins or protect native proteins from damages. Here, physiological polyamines are presented as stress molecules, a general term describing both proteins and organic molecules induced in response to diverse stresses to provide protection against these stresses. In addition to direct protective activity such as ROS scavenging, acid tolerance and regulation of ion channels, polyamines in prokaryotes indirectly exerted protective functions against stresses by induction of stress responsive regulons by activation of RpoS, SoxR and SOS gene, but not RpoH, a well known HSF in prokaryotes. In eukaryotes, polyamines indirectly exert protective functions by activation of HSF. The polyamine-mediated induction of stressresponsive genes with concomitant repression of general gene expression may constitute a conserved stress-adaptive response. This hypothesis was further supported by the fact that polyamines are ubiquitous and abundant metabolites derived from amino acids, and can function as chemical chaperones. In addition, polyamine-mediated tolerance against stresses was demonstrated in a variety of genetically modified organisms overexpressing or lacking polyamine synthetic enzymes. Since ablations of polyamine synthesis impair cell survival under stressful conditions, induction of the polyamine-stress response seems to be essential in coping with the stresses. This along with induction of RpoH-mediated HSPs may constitute a main adaptive stress response in prokaryotes. In eukaryotes, the polyamine-stress response may function in limited situations as described in sections below. Because prolonged induction of polyamines may not give beneficial effect anymore due to toxicity and/or may not be attained effectively due to homeostatic regulation of their levels, the polyamine-stress response may function with limited efficacy in coping with chronic or repeated stresses. Regulation of a broad range of target genes via polyamines-mediated transcriptional cascades may be unsuitable for tight regulation of expression of respective target genes. Due to these limitations, eukaryotes may utilize an alternative version of stress response, such as the UPR. Pathways for negative metabolic regulation of induced polyamines (Fig. 1) and positive feedback induction of physiological polyamines in response to stresses (Fig. 2) would facilitate polyamine function as a stress molecule. We posit that polyamines have been exploited by organisms from bacteria to plants and animals as a primordial form of stress molecules. They are ubiquitous and abundant metabolites capable of regulating differential gene expression via homeostatic regulation of their cellular concentration in response to environmental changes including starvation.
Role of physiological polyamines in oxidative stress response
Organisms utilizing aerobic respiration and oxygenic photosynthesis for energy encounter oxidative challenge by ROS, and have diverse adaptive defence systems for ROS and/or reactive nitrogen species (RNS), including expression of ROS- and/or RNSscavenging enzymes, antioxidant metabolites and HSPs. Like starvation stress, oxidative stress is known to induce polyamines in a wide range of organisms. Cumulative data show that physiological polyamines can protect DNA from ROS damage in vivoand in vitro[49–51, 53, 78, 80–82]. Observation that DNA damage induced by ROS and 5,5′- dimethyl-1-pyroline-N-oxide (DMPO)-OH spin adduct formation of hydroxyl radical by DMPO were reduced by sub-millimolar concentrations of spermine in vitro suggested that polyamines could function as a direct radical scavengers [54]. Although excess polyamines can induce oxidative stress by ROS generation during catabolic oxidation of polyamines, a response which may occur under special conditions such as host defence [55, 83], in most cases induction of polyamine synthesis protected cells from oxidative stress in vivo[50, 51, 53, 56, 57, 76, 82].
Under oxidative stress, a conserved protective mechanism is switched on in a manner similar to that of the response to starvation stress. Upon exposure to oxidative stress, polyamine synthesis was induced and followed by selective expression in a polyamine concentration-dependent manner of a series of genes involved in protecting against ROS stress and repairing damage. In E. coli, physiological polyamines were shown to be required for transcription of the genes responsible for oxidative stress defence such as katG and katE genes, whose expression was regulated by the expression of oxyR and rpoS, respectively [44]. Moreover, the expression of not only rpoS, a transcriptional regulon responsive to starvation conditions, but also oxyR, a transcriptional regulon responsive to ROS, was also induced by polyamines. These selective transcriptional activation cascades along with direct antioxidant effects by polyamines can confer tolerance to oxidative stress.
Protective functions of polyamines against ROS stress were further demonstrated by recent independent studies in prokaryotes. Rapid killing in cultures under 95% O2/5% CO2 or H2O2 was observed in polyamine deficient mutants lacking putrescine and spermidine due to deletion of speABC genes [77]. Addition of polyamines, including cadaverine, putrescine and spermine, or introduction into cells of a plasmid harbouring the gene for superoxide dismutase (SOD) abolished oxygen toxicity. Sensitivity to paraquat, a known superoxide anion-generating chemical, was abolished more effectively by spermidine than putrescine. A key determinant for RNS stress resistance in many uropathogenic strains of E. coli was identified as CadC, a transcriptional activator for the cadB operon, by screening for uropathogenic E. coli transposon mutants unable to grow in the presence of acidified nitrite. Mutation of cadC, or its transcriptional targets cadB and cadA which encode lysine: cadaverine antiporter and LDC, respectively, resulted in significant loss of cadaverine production and increased sensitivity to acidified nitrite [58]. CadB expression, which was up-regulated by CadC in response to acid stress, was also shown to be up-regulated by SoxR in response to superoxide stress through binding of SoxR to the promoter region of the cadBA operon [63]. The protective role of cadaverine against ROS stress was also demonstrated by the induction of Mn-containing SOD (Mn-SOD) under ROS stress that was reduced by elevating gene dosage of cadBA; sensitivity to paraquatinduced oxidative stress was higher in a cadA mutant than in wild type.
Protective effects of physiological polyamines on oxidative stress were also observed in mammals. Ethanol-induced hepatotoxicity was decreased by GABA in a dose-dependent manner in rat hepatocytes which are known to express GABA synthetic enzymes and its tranporter. [64]. Addition of physiological polyamines also reversed ethanol-induced cytotoxicity that may be primarily a result of ethanolinduced ROS. Acute maternal hypoxia in rat was shown to cause an increase in ODC activity and polyamines levels in foetal brains [84, 85]. In addition, physiological concentrations of spermine (0.1 mM) prevented genistein-, glycyrrhetinic acid- and salicylate- induced mitochondrial swelling and membrane potential (Δψ) collapse caused by ROS stress in rat liver mitochondria, and protected rat liver from glutathione oxidation, lipid peroxidation and protein oxidation [86]. Spermine-mediated protective effects on mitochondria were further demonstrated by spermine suppression of ROS-induced glutathione efflux from liver mitochondria, probably by the protection against oxidation of critical thiol groups responsible for the mitochondrial pore opening [87]. A recent report showed that exogenous spermine and induction of endogenous spermine played roles in protecting Trypanosome cruzi insect stage epimastigotes, the causative parasite of Chagas'disease, from membrane lipid peroxidation by ROS. However, putrescine or cadaverine in T. cruzi had no effect on protection [60].
Physiological polyamine-mediated acid stress response
Upon exposure to acid pH, organisms turn on pH homeostasis systems to cope with intracellular acidification that can cause enzyme inactivation and protein denaturation. Response to acid stress was studied most extensively in enteric bacteria, including E. coli, Shigella, Salmonellaand Vibrio, bacteria that survive an acidic environment en route to intestinal colonization. When Salmonella typhimurium was preincubated at pH 6, survival of adapted bacteria exposed to severe acid conditions (pH 3.3) increased 100-fold over that of unadapted bacteria. This acid tolerance response (ATR) required proteins induced during pre-adaption and during the acid response, including several HSPs [88]. ATR appears to be comprised of two components: an acid-inducible pH homeostasis system, and sigmaS-dependent induction of a series of acid-shock proteins. In addition, acid induction of the cadB A operon, which encodes a lysine:cadaverine antiporter and LDC was responsible for acid tolerance, as well as pH homeostasis under acidic pH in S. typhimurium[67]. E. coli uses two types of acid response systems to survive acidic environments. One is an oxidative or glucoserepressed system that is acid-induced during stationery phase in an RpoS- and cyclic AMP receptor protein (CRP)-dependent manner. The other is composed of three inducible polyamine synthesis and polyamine antiporter systems. These systems operate to consume protons with concomitant generation and export of polyamines, while co-importing the precursors of polyamines including CadB and CadA, GadA/GadB (GDC) and GadC (glutamate: γ-aminobutyricacid antiporter), and AdiA (ADC) and AdiC antiporters.
ATR by induction of polyamine synthetic enzymes and polyamine transporters appears to be conserved in a wide range of enteric bacteria, despite speciesdependent variable combinations of acid-inducible polyamine transporter systems. Mutational analysis of each acid-inducible polyamine transporter gene in Vibrio cholerae revealed that CadA, but not SpeF (ODC) or RpoS, plays a major role in ATR, although Vibrio cholerae had almost the same acid-inducible polyamine transporter systems as those in E. coli. In vivo competition assay using CadA- and SpeFmutant strains revealed that both CadA and SpeF were required for colonization of the intestine of suckling or adult mice [89]. Our recent report showed that Vibrio vulnificus expression of cadBA was regulated not only by acidic pH via CadC, a known pHdependent transcriptional activator [90], but also by SoxR, a ROS-induced transcription factor [63]. Thus, increases in the synthesis of cadaverine can impart tolerance to acidic pH, as well as ROS stress. The GadABC system was also shown to play a role in acid tolerance in E. coli O157 [91], Shigella flexneri[92,93], and Listeria monocytogenes[94]. Moreover, exogenous addition of polyamines, such as putrescine and spermidine restored GadA and GadB expression and acid tolerance in polyamine deficient E. coli mutants [19]. Thus, for acid tolerance expression of genes responsible for GABA synthesis is subjected to positive feedback regulation by end products, GadA or GadB. AdiA and AdiC also play a role in ATR of S. typhimurium[67] and E. coli[95, 97]. S. enterica serovar Typhimurium can display argininedependent acid tolerance, provided the cells are grown under anoxic conditions. Loss of acid tolerance was observed in mutants lacking adiA, adiC or adiY which encode an AraC-like regulator [98].
The mechanism by which physiological polyamines protect cells from acid stress is poorly understood, but polyamine-induced tolerance could be explained by several biochemical properties of the polyamines. Induction of polyamine antiporters and corresponding polyamine synthetic enzymes at acidic pH increases proton consumption in the cytoplasm. Protons are consumed during anabolic decarboxylation of amino acids in polyamine synthesis which protects the cell from acidification; subsequent antiporter-excretion of the polyamines protects against toxic polyamine accumulation [99, 100]. However, in conditions of very low pH, sustaining pH homeostasis by continuous proton consumption and membrane potential-coupled excretion of polyamines in the presence of their precursors is not feasible. For example, in stationary-phase E. coli cellular pH dropped to 4.5 under severe acid stress conditions (pH 2.5), and negative transmembrane potential changed to positive. Additional levels of protective actions may operate to maintain cellular pH above a given lower limit; for example, at pH 4 critical deformation in protein structures and loss of function may lead to cell death. In addition, secreted polyamines have been shown to directly inhibit porin through specific binding, which regulated outer membrane permeability and may contribute to acid tolerance [101–110]. Finally, chaperone activity of s and physiological polyamines may also contribute to acid stress protection, but not pH homeostasis, by facilitating folding of proteins denatured by acidic pH.
Physiological polyamines in osmotic stress response
Maintenance of cell turgor in response to fluctuations in external osmolarity is prerequisite for cell division, cell expansion and survival in living organisms. Upon exposure to hypoosmotic stress, cells use mechanosensitive channels in response to swelling to remove excess cytoplasmic solutes. Upon a shift to hyperosmotic stress, K+ uptake and glutamate synthesis in response to dehydration, followed by accumulation of compatible solutes, including proline, betained, ectoine and trehalose are induced in order to stabilize cell volume and protein folding.
Although mechanosensitive channels, potassium channels, and accumulation of compatible solutes by synthesis and uptake are primarily responsible for maintenance of cell turgor, physiological polyamines, small polycationic molecules, porins and aquaporins may also contribute. The question is raised whether polyamines in millimolar concentrations in the cytoplasm under conditions of osmotic stress may function as a compatible solute or a stabilizer for proteins and anionic phospholipids, in addition to regulating porin- and aquaporin-gating. In E. coli, hyperosmotic shift was shown to increase putrescine synthesis and subsequent secretion of the polyamines [111]. Induction of spermidine, spermine, ADC and AdoMET synthases by hyperosmotic stress was also observed in Synechocystis sp. PCC 6803 [112, 113]. Many authors have reported that in mammalian cells, hypoosmotic stress induced increases in polyamines such as putrescine [114–116]. Osmotonicity-dependent increases in polyamine levels in rat hepatoma were shown to be mediated by induction of ODC via rapid reduction in both level and stability of antizyme, a negative ODC regulator, in response to osmotic stress [117]. Moreover, physiological polyamines are known to directly regulate porin gating, which may facilitate maintenance of osmotic pressure [102, 106, 108]. Direct regulation of inward rectifier K+ channels by polyamines and specific regulation of aquaporins by diverse divalent cationic metals were shown [118, 119]. However, there have been few direct evidences supporting the hypothesis that polyamines can function in osmoregulation by regulating the channels involved in osmoregulation, such as mechanosensitive channels, K+-channels, and aquaporin.
In plants complex, osmoregulatory systems may exist as higher cell turgor in plants compared with bacteria or mammalian cells is maintained due to the presence of the cell wall. A number of recent reports demonstrated that physiological polyamines were commonly adopted in plants for protection against osmotic stress. While ODC is subject to extensive regulation via antizyme, and polyamines are commonly involved in the osmotic response in animals, ADCs seems to be used widely in plants and play a central role in exerting polyamine-mediated osmotic tolerance [120]. Higher putrescine levels established by overexpression of ADC in transgenic rice conferred drought tolerance. In addition, impairment of ADC in Arabidopsissubjected to osmotic shock lead to more serious cell injury than that in wild type. [121]. Genetic evidence suggested that ADC2, but not ADC1, was induced by osmotic stress and may also play a role in development [122, 123].
Physiological polyamines in neuronal stress response
In adult brain, early transient increase in polyamine metabolism, particularly putrescine synthesis, along with induction of universal stress proteins, is a common response to acute traumatic and emotional stresses [124]. Stress responses by physiological polyamines, a primordial form of stress molecule, were most widely observed in highly differentiated brain tissues in which cellular stress defence systems are not fully developed as a result of the functional complexity of neuronal cells. Severe traumatic stresses induced sustained activation of ODC in rodent brain, while transient activation of ODC was induced by acute, mild stresses. Putrescine levels were elevated (130–157% of the control) in all parts of the brain, with little or no reduction in spermidine and spermine levels, 24 hrs after subjecting mice to water-immersion or restraint stress [125, 126].
Studies with transgenic animals overexpressing ODC or SSAT have supported the idea that induction of putrescine, in response to these diverse neuronal insults is a neuroprotective adaptive response rather than a result of neuronal damage. Overexpression of ODC in transgenic mice showed elevation of putrescine in brain and significantly protected the transgenic animals from physical and chemical induced-seizure activity [127]; there was no sign of neuronal degeneration at 2 years of age in transgenic animals with life-long overexpressing ODC [128]. The elevated seizure threshold was neither due to changes in the major neurotransmitter amino acids, glutamate and GABA [127], nor due to elevation of free Mg2+-mediated N-methyl-D-aspartate (NMDA) blocking [129]. Substantial expansion of brain putrescine pools in transgenic rat or mouse overexpressing SSAT protected these animals from kainite-induced toxicity and pentylenetetrazolinduced seizure activity involving both tonic and clonic convulsions [130, 131]. Although the molecular mechanism of induced putrescine protection against neuronal damage is obscure, polyamine interaction with cationic channels, especially with NMDA receptor, may provide a clue. Prolonged activation of NMDA can lead to neuronal damage. Putrescine is believed to act as a weak NMDA receptor antagonist, while spermidine and spermine are agonists. Antagonistic activity of putrescine [132, 133] may block agonist activation of NMDA receptor and contribute to putrescine-mediated neuroprotection. This explanation was consistent with the observation that spatial learning and memory test performance was impaired in transgenic mice overexpressing ODC, as activation of NMDA is essential in the learning and memory process [127].
ODC was induced by amyloid beta (Aβ) and imparted protection against Aβ-induced toxicity which is presumed to be an important neurotoxic factor in Alzheimer's disease [134, 135]. Neurodegenerative disorders, such as Alzheimer's, Parkinson's and Huntington's diseases are characterized by the accumulation of intracellular toxic protein aggregates, Aβ, α-synuclein, and huntingtin, respectively. These protein aggregates are generated from misfolded proteins, which should have normally removed or refolded by stress proteins [136] or removed by stress-inducible autophagy, a cellular self-eating [137, 138]. Neuroprotective functions of physiological polyamines may facilitate protection of neuronal cells from cell damage along with stress proteins induced by UPR; thus, the polyamines may constitute integral components of a neuronal stressresponse system. This hypothesis was supported by findings that aliphatic diamines could function as chemical chaperones [139]. Further experiments to address whether accumulation of putrescine can function in protection from neurodegenerative diseases through chemical chaperone activity would be intriguing.
Both agmatine and spermine were shown to protect against ischaemic damages in vivo by diverse ways, including reducing the size of ischaemic infarcts and the loss of cerebellar neurons, and decreasing both nitric oxide synthase (NOS) and arginase activity [140–142]. In addition, agmatine was reported to show diverse neuroprotective activities, such as attenuation of neuronal death following excitotoxic spinal cord injury [143], prevention of neurotoxicity produced via NMDA or glutamate [144, 145], suppression of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) neurotoxicity [146], inhibition of inducible nitric oxide synthase (iNOS) [147] and reduction of hippocampus neuronal damage caused by glucocorticoids [75]. However, contradictory findings that the polyamines were implicated in the pathogenesis of diverse neurodegenerative diseases including ischaemia were also reported [148–150]. These conflicting results may be explained by the fact that oxidation of polyamines induced by traumatic stress can generate ROS, which can cause neuronal death, particularly in microglia that are sensitive to sub-micromolar concentrations of polyamines [59]. This explanation was supported by observations that MDL 72527, a specific inhibitor of PAO, reduced ischaemia -induced neurotoxicity, putrescine levels, and N1-acetylspermidine levels [149, 150]. However, more compelling evidence is needed to elucidate the mechanism and roles of physiological polyamines and their metabolites in neuronal stress responses, neuroprotection or neurotoxicity.
Moreover, the stress-inducibility of polyamines in brain is developmentally regulated, and its increase coincides with maturation of the hypothalamic-pituitary -adrenocortical (HPA) axis, a central component of the stress-response system [124]. Thus, induction of polyamines may be involved in the developmental maturation of HPA.
Physiological polyamines in other stress responses
Metabolic profiling analyses of the induction of acquired thermotolerance and freezing tolerance in response to heat shock and cold shock, respectively, revealed that the majority of heat-shock responses were shared with cold-shock responses in Arabidopsis. A co-ordinated increases in polyamine precursors in addition to an increase in the pool sizes of amino acids derived from pyruvate and oxaloacetate and compatible solutes during both heat and cold shock were observed [65].
Although few studies have been conducted on the temperature stress-induced polyamine response, recent findings showed that polyamines can function as chemical chaperones and shed light on the mechanism by which small polycations play a role in protection from cytotoxic stress. Essential structural determinants of diamines for suppressing thermal aggregation of lysozymes were determined. Small diamines including 1,5-diaminopentane were shown to be active, though 1,3-diaminopropane was most effective, whereas diamine derivatives with diols or monoamines had no protective effect [139]. Chemical chaperone activities of the polyamines can directly contribute to the protective function of these stress molecules by facilitating folding of denatured proteins generated by various stresses.
Solar ultraviolet B (UV-B) radiation induced cutaneous ODC in Ptch1+/− mice, in which patched (PTCH) was known to be a tumour-suppressor gene [151]. In addition, physiological polyamine response to UV exposure was studied in detail in tobacco cultivar [152]. An increase in polyamines, especially in putrescine levels, in thylakoid membranes upon elevated UVB exposure comprised one of the primary protective mechanisms in the photosynthetic apparatus of tobacco Bel B. The UVB sensitivity of tobacco cultivar Bel W3 was attributable to its inability to enhance putrescine levels in thylakoid membranes. After full adaptation to UVB including induction of carotenoids and additional flavonoids, the polyamine levels were reduced.
Upon exposure to heavy metals, such as Codmium, Copper, Nickel and Zinc, an array of amino acid derived metabolites, proline, betaine and polyamines accumulated to high millimolar concentrations in plants. The response molecules may have three major functions, namely metal binding, antioxidant defence and signalling [153]. In the leaves of Nymphoides peltatum, copper induced increases in putrescine levels and lowered spermidine and spermine levels. Exogenous application of spermidine or spermine markedly reversed the Copper-induced effects, including enhanced levels of proline, superoxide anion generation and Copper-induced lipid peroxidation [69].
Thus, physiological polyamines have been reported to be induced by a variety of stresses including radiation, heavy metal and emotional stress in addition to common stresses such as ROS, osmolarity, low pH and heat, which have been shown to confer tolerance against them. Herein, it was proposed that in response to physiological and physical stresses, physiological polyamines could function as a conserved primordial stress molecule in a wide range of organisms from bacteria to plants and animals.
Divergent roles of physiological polyamines in adapting to pathogen-host interaction
Once pathogens or parasites infect their host, both pathogen and host are mutually stimulated to produce a set of stress proteins including chaperones, a group of proteins with activities facilitating assembly and disassembly of macromolecular structures. Induction and/or secretion of stress proteins can modulate several levels of pathogen-host interaction, such as pathogen-host adhesion signalling, pathogen virulence and host pathogen defence systems [154].
Many pathogenic enteric bacteria pass through and survive acid environments in the process of colonization. Acid tolerance factors including polyamine synthetic enzymes may contribute to the virulence of these bacteria, particularly in acid sensitive V. cholera. In concerted action with speF-encoding inducible ODC, CadA was shown to be essential for colonization of the intestine of suckling and adult mice [89]. The role of CadA as a colonization factor may reflect its inducibility by acid and its protective function against the acid environments encountered during infection. In addition, RpoS, whose transcription is known to be induced by polyamines and low pH, was also shown to be an essential virulence factor, as demonstrated by rpoS null Salmonella attenuated for infection of mice through oral or intraperitoneal routes.
Physiological polyamines can modulate modified tRNA-mediated translational fidelity, maintenance of reading frame and efficiency for modified nucleosides such as queuosine or epoxy queusoine (Q) in the 3′, or wobble, position of an anticodon. VirF is an upstream regulator of virulence cascades, such as VirG and VirB in the Shigella. Putrescine or a combination of methionine and arginine restored VirF expression through modified tRNA-mediated translational control in Shigella flexneri, while expression of virulence genes including VirF was 10-fold reduced in tRNA modification-deficient mutant Shigelladue to the lack of epoxy Q in a subset of tRNA, when the bacteria were grown in minimal media [155].
PotD, a constitutive putrescine or spermidine transporter, was implicated in the pathogenicity of Streptococcus pneumoniae, a leading agent of bacterial pneumonia and meningitis [156]. Immunization with S. pneumoniae PotD abolished infection in a systemic mouse model [157]. There is no clear understanding of how PotD functions as an essential virulence factor, but recent findings [47] show that levels of PotD are increased by polyamine-mediated induction of ColE7. Polyamine-mediated induction of PotD, an ABC-type synporter responsible for preferential uptake of putrescine in low concentrations (few μM), can rapidly increase cellular polyamine concentrations sufficient to turn on virulence genes by importing extracellular putrescine and spermidine abundant in the host intestine and stomach.
On the other hand, results showing that polyamines can function as anti-virulent factors have also been reported. Pathoadaptive mutations in cadA were observed in pathogenic E. coli O111 producing Shigatoxin, and might be involved in the regulation of pathogenicity through adhesion to host cells [158]. The negative role of cadA in cell adhesion could reflect the fact that physiological polyamines regulate cytoskeletal structure adhesion by modulating F-actin stress fiber formation and the expression or function of RhoA [159, 160].
As described above, a number of bacteria including pathogens have inducible transport systems for excretion of accumulated excess polyamines that may be harmful to cells. Polyamines excreted during infection may affect infectivity of pathogens in several ways. Helicobacter pylori which induces an innate mucosal immune response and selectively colonizes the human stomach was shown to induce macrophage apoptosis by activation of inducible arginase followed by induction of ODC. Apoptosis of macrophage was also induced by exogenous polyamines [161]. Another virulence factor of secreted polyamines could be mediated by induction of polyamine oxidase 1 (PAO1) in Helicobacter pylori, which was demonstrated to cause H2O2 release and mitochondrial membrane depolarization [162]. The second pathological consequence of polyamine metabolism in H. pylori is gastric carcinogenesis which may be initiated through ROS stress induced by catabolic oxidation of spermine [163]. Polyamines excreted by a pathogen can also play a negative role in pathogenicity. Pathoadaptive mutation in cadA was observed in Shigella species evolved from nonpathogenic E. coli with intact cadA. Introduction of cadA into Shigella-inhibited Shigella induced transmigration of polymorphonuclear leucocytes (PMN) and exogenous cadaverine also blocked PMN transmigration and enterotoxicity induced by enterotoxins [164, 165].
The finding that blood forms of ODC null Trypanosoma brucei mutants could not proliferate without exogenous polyamines suggested that physiological polyamines may be essential for proliferation of the parasites, which render ODC and AdoMETDC important anti-parasitic drug targets. Inhibition of ADC by irreversible inhibitors was shown to reduce infection capacity of blood forms of Trypanosoma cruzi in mouse peritoneal macrophage and heart myoblasts [166]. ADC-generated argmatine could protect protozoan parasites in multiple ways. As iNOS was inhibited indirectly by agmatine aldehyde [147], agmatineinduced suppression of NOS would aid in survival of the parasite. Moreover, reactivation of ODC through denitroylation of cys360 [167] due to nitric oxide (NO) reduction could increase polyamine levels to support cell proliferation.
A number of reports suggested that physiological polyamines or their catabolic enzymes were exploited as a defence mechanism in plants with unique catabolic enzymes, particularly PAOs. The function of polyamine catabolism in plant defence systems is extensively reviewed elsewhere [168]. H2O2 generated by PAOs in plants is involved not only in induction of pathogen apoptosis, but also in cell wall strengthening during pathogen infection [169, 170]. In addition, induction of putrescine and ADC2, but not ADC1, by mechanical damage, methyl jasmonate and abscic acid in Arabidopsis suggested that physiological polyamines may be involved in wound response in plants that lack special mobilizing cells [171].
Conclusions
Physiological polyamines, ubiquitous polycations, were induced by diverse stresses, and induction of polyamines exerted multiple cytoprotective actions from ROS scavenging to polyamine-mediated induction of stress-responsive genes, though polyamines are simple aliphatic metabolites. This polyaminestress response may constitute an integral part of the stress response to a variety of stresses. As they may have evolved to play a role in maintaining cell function and viability, detailed knowledge of the molecular control mechanism for the cytoprotective functions of physiological polyamines may provide better ways for regulating pathophysiological conditions, including infection of pathogens and parasites, cancer and inflammation, and for generating environmentally adaptable plants to enhance crop production.
Acknowledgments
This work was supported by the 21C Frontier Microbial Genomics and Applications Center Program from Ministry of Science and Technology (MG05-0309-1-0), Korea.
References
- 1.Henderson CM, Anderson CB, Howard MT. Antisense-induced ribosomal frameshifting. Nucleic Acids Res. 2006;34:4302–10. doi: 10.1093/nar/gkl531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Umekage S, Ueda T. Spermidine inhibits transient and stable ribosome subunit dissociation. FEBS Lett. 2006;580:1222–6. doi: 10.1016/j.febslet.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Stephenson AH, Christian JF, Seidel ER. Polyamines regulate eukaryotic initiation factor 4Ebinding protein 1 gene transcription. Biochem Biophys Res Commun. 2004;323:204–12. doi: 10.1016/j.bbrc.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 4.Childs AC, Mehta DJ, Gerner EW. Polyaminedependent gene expression. Cell Mol Life Sci. 2003;60:1394–406. doi: 10.1007/s00018-003-2332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SW, Mangold U, Waghorne C, Mobascher A, Shantz L, Banyard J, Zetter BR. Regulation of cell proliferation by the antizyme inhibitor: evidence for an antizyme-independent mechanism. J Cell Sci. 2006;119:2583–91. doi: 10.1242/jcs.02966. [DOI] [PubMed] [Google Scholar]
- 6.Dayoub R, Thasler WE, Bosserhoff AK, Singer T, Jauch KW, Schlitt HJ, Weiss TS. Regulation of polyamine synthesis in human hepatocytes by hepatotrophic factor augmenter of liver regeneration. Biochem Biophys Res Commun. 2006;345:181–7. doi: 10.1016/j.bbrc.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 7.Thomas T, Thomas TJ. Polyamine metabolism and cancer. J Cell Mol Med. 2003;7:113–26. doi: 10.1111/j.1582-4934.2003.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nisenberg O, Pegg AE, Welsh PA, Keefer K, Shantz LM. Overproduction of cardiac S-adenosylmethionine decarboxylase in transgenic mice. Biochem J. 2006;393:295–302. doi: 10.1042/BJ20051196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cervelli M, Polticelli F, Federico R, Mariottini P. Heterologous expression and characterization of mouse spermine oxidase. J Biol Chem. 2003;278:5271–6. doi: 10.1074/jbc.M207888200. [DOI] [PubMed] [Google Scholar]
- 10.Vujcic S, Diegelman P, Bacchi CJ, Kramer DL, Porter CW. Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem J. 2002;367:665–75. doi: 10.1042/BJ20020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igarashi K, Kashiwagi K. Polyamine transport in bacteria and yeast. Biochem J. 1999;344:633–42. [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Fernandez AJ, Rodriguez RA, Perez- Pertejo Y, Balana-Fouce R. Characterization of putrescine uptake in hamster amelanocytic melanoma AMEL-3 cells. Mol Cells. 2005;20:127–35. [PubMed] [Google Scholar]
- 13.O'Sullivan MC, Golding BT, Smith LL, Wyatt I. Molecular features necessary for the uptake of diamines and related compounds by the polyamine receptor of rat lung slices. Biochem Pharmacol. 1991;41:1839–48. doi: 10.1016/0006-2952(91)90122-l. [DOI] [PubMed] [Google Scholar]
- 14.Magnusson LU, Farewell A, Nystrom T. ppGpp: a global regulator in Escherichia coli. Trends Microbiol. 2005;13:236–42. doi: 10.1016/j.tim.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Artsimovitch I, Patlan V, Sekine S, Vassylyeva MN, Hosaka T, Ochi K, Yokoyama S, Vassylyev DG. Structural basis for transcription regulation by alarmone ppGpp. Cell. 2004;117:299–310. doi: 10.1016/s0092-8674(04)00401-5. [DOI] [PubMed] [Google Scholar]
- 16.Moris M, Braeken K, Schoeters E, Verreth C, Beullens S, Vanderleyden J, Michiels J. Effective symbiosis between Rhizobium etli and Phaseolus vulgaris requires the alarmone ppGpp. J Bacteriol. 2005;187:5460–9. doi: 10.1128/JB.187.15.5460-5469.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–33. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 18.Dever TE. Gene-specific regulation by general translation factors. Cell. 2002;108:545–56. doi: 10.1016/s0092-8674(02)00642-6. [DOI] [PubMed] [Google Scholar]
- 19.Jung IL, Kim IG. Polyamines and glutamate decarboxylase- based acid resistance in Escherichia coli. J Biol Chem. 2003;278:22846–52. doi: 10.1074/jbc.M212055200. [DOI] [PubMed] [Google Scholar]
- 20.Bryans M, Harley E, Gilmour SK. Elevated cellular polyamine levels enhance promoter activity in vivo. Biochem Biophys Res Commun. 1996;226:618–25. doi: 10.1006/bbrc.1996.1405. [DOI] [PubMed] [Google Scholar]
- 21.Panagiotidis CA, Huang SC, Canellakis ES. Posttranslational and transcriptional regulation of polyamine biosynthesis in Escherichia coli. Int J Biochem. 1994;26:991–1001. doi: 10.1016/0020-711x(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 22.Huang SC, Panagiotidis CA, Canellakis ES. Transcriptional effects of polyamines on ribosomal proteins and on polyamine-synthesizing enzymes in Escherichia coli. Proc Natl Acad Sci USA. 1990;87:3464–8. doi: 10.1073/pnas.87.9.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobbs CA, Paul BA, Gilmour SK. Deregulation of polyamine biosynthesis alters intrinsic histone acetyltransferase and deacetylase activities in murine skin and tumors. Cancer Res. 2002;62:67–74. [PubMed] [Google Scholar]
- 24.Stjernborg L, Heby O, Mamont P, Persson L. Polyamine-mediated regulation of S-adenosylmethionine decarboxylase expression in mammalian cells. Studies using 5′-([(Z)-4-amino-2-butenyl]methylamino)- 5′-deoxyadenosine, a suicide inhibitor of the enzyme. Eur J Biochem. 1993;214:671–6. doi: 10.1111/j.1432-1033.1993.tb17967.x. [DOI] [PubMed] [Google Scholar]
- 25.Igarashi K, Saisho T, Yuguchi M, Kashiwagi K. Molecular mechanism of polyamine stimulation of the synthesis of oligopeptide-binding protein. J Biol Chem. 1997;272:4058–64. doi: 10.1074/jbc.272.7.4058. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida M, Meksuriyen D, Kashiwagi K, Kawai G, Igarashi K. Polyamine stimulation of the synthesis of oligopeptide-binding protein (OppA). Involvement of a structural change of the Shine-Dalgarno sequence and the initiation codon aug in oppa mRNA. J Biol Chem. 1999;274:22723–8. doi: 10.1074/jbc.274.32.22723. [DOI] [PubMed] [Google Scholar]
- 27.Medina MA, Correa-Fiz F, Rodriguez-Caso C, Sanchez-Jimenez F. A comprehensive view of polyamine and histamine metabolism to the light of new technologies. J Cell Mol Med. 2005;9:854–64. doi: 10.1111/j.1582-4934.2005.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 28.Panagiotidis CA, Artandi S, Calame K, Silverstein SJ. Polyamines alter sequence-specific DNA-protein interactions. Nucleic Acids Res. 1995;23:1800–9. doi: 10.1093/nar/23.10.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gundogus-Ozcanli N, Sayilir C, Criss WE. Effects of polyamines, polyamine synthesis inhibitors, and polyamine analogs on casein kinase II using Myc oncoprotein as substrate. Biochem Pharmacol. 1999;58:251–4. doi: 10.1016/s0006-2952(99)00084-2. [DOI] [PubMed] [Google Scholar]
- 30.Lu B, Liang X, Scott GK, Chang CH, Baldwin MA, Thomas T, Benz CC. Polyamine inhibition of estrogen receptor (ER) DNA-binding and ligand-binding functions. Breast Cancer Res Treat. 1998;48:243–57. doi: 10.1023/a:1005949319064. [DOI] [PubMed] [Google Scholar]
- 31.Thomas T, Kulkarni GD, Gallo MA, Greenfield N, Lewis JS, Shirahata A, Thomas TJ. Effects of natural and synthetic polyamines on the conformation of an oligodeoxyRibonucleotide with the estrogen response element. Nucleic Acids Res. 1997;25:2396–402. doi: 10.1093/nar/25.12.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas T, Kiang DT. Modulation of the binding of progesterone receptor to DNA by polyamines. Cancer Res. 1988;48:1217–22. [PubMed] [Google Scholar]
- 33.Giannakouros T, Nikolakaki H, Georgatsos JG. Concentration-dependent effects of natural polyamines on peptide chain initiation and elongation in a cell-free system of protein synthesis. Mol Cell Biochem. 1990;99:9–19. doi: 10.1007/BF01261388. [DOI] [PubMed] [Google Scholar]
- 34.Miyamoto S, Kashiwagi K, Ito K, Watanabe S, Igarashi K. Estimation of polyamine distribution and polyamine stimulation of protein synthesis in Escherichia coli. Arch Biochem Biophys. 1993;300:63–8. doi: 10.1006/abbi.1993.1009. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe S, Kusama-Eguchi K, Kobayashi H, Igarashi K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J Biol Chem. 1991;266:20803–9. [PubMed] [Google Scholar]
- 36.Tome ME, Fiser SM, Payne CM, Gerner EW. Excess putrescine accumulation inhibits the formation of modified eukaryotic initiation factor 5A (eIF-5A) and induces apoptosis. Biochem J. 1997;328:847–54. doi: 10.1042/bj3280847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie X, Tome ME, Gerner EW. Loss of intracellular putrescine pool-size regulation induces apoptosis. Exp Cell Res. 1997;230:386–92. doi: 10.1006/excr.1996.3442. [DOI] [PubMed] [Google Scholar]
- 38.Brunton VG, Grant MH, Wallace HM. Mechanisms of spermine toxicity in baby-hamster kidney (BHK) cells. The role of amine oxidases and oxidative stress. Biochem J. 1991;280:193–8. doi: 10.1042/bj2800193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seiler N, Raul F. Polyamines and apoptosis. J Cell Mol Med. 2005;9:623–42. doi: 10.1111/j.1582-4934.2005.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miret JJ, Nainudel S, Goldemberg SH. Altered heat-shock response in polyamine-depleted bacteria. FEBS Lett. 1986;200:117–22. doi: 10.1016/0014-5793(86)80522-1. [DOI] [PubMed] [Google Scholar]
- 41.Desiderio MA, Tacchini L, Anzon E, Pogliaghi G, Radice L, Bernelli-Zazzera A. Effects of polyamine imbalance on the induction of stress genes in hepatocarcinoma cells exposed to heat shock. Hepatology. 1996;24:150–6. doi: 10.1002/hep.510240125. [DOI] [PubMed] [Google Scholar]
- 42.Desiderio MA, Dansi P, Tacchini L, Bernelli-Zazzera A. Influence of polyamines on DNA binding of heat shock and activator protein 1 transcription factors induced by heat shock. FEBS Lett. 1999;455:149–53. doi: 10.1016/s0014-5793(99)00873-x. [DOI] [PubMed] [Google Scholar]
- 43.Tkachenko AG, Shumkov MS. Role of putrescine in regulation of the sigmaS subunit of RNA polymerase in Escherichia coli cells on transition to stationary phase. Biochemistry. 2004;69:876–82. doi: 10.1023/b:biry.0000040219.33023.5a. [DOI] [PubMed] [Google Scholar]
- 44.Jung IL, Kim IG. Transcription of ahpC, katG, and katE genes in Escherichia coli is regulated by polyamines: polyamine-deficient mutant sensitive to H2O2-induced oxidative damage. Biochem Biophys Res Commun. 2003;301:915–22. doi: 10.1016/s0006-291x(03)00064-0. [DOI] [PubMed] [Google Scholar]
- 45.Oh TJ, Kim IG. The SOS induction of umu'-'lacZ fusion gene by oxidative damage is influenced by polyamines in Escherichia coli. Cell Biol Toxicol. 1999;15:291–7. doi: 10.1023/a:1007663701589. [DOI] [PubMed] [Google Scholar]
- 46.Oh TJ, Kim IG. The expression of Escherichia coli SOS genes recA and uvrA is inducible by polyamines. Biochem Biophys Res Commun. 1999;264:584–9. doi: 10.1006/bbrc.1999.1553. [DOI] [PubMed] [Google Scholar]
- 47.Pan YH, Liao CC, Kuo CC, Duan KJ, Liang PH, Yuan HS, Hu ST, Chak KF. The critical roles of polyamines in regulating ColE7 production and restricting ColE7 uptake of the colicin-producing Escherichia coli. J Biol Chem. 2006;281:13083–91. doi: 10.1074/jbc.M511365200. [DOI] [PubMed] [Google Scholar]
- 48.Kim IG, Oh TJ. SOS induction of the recA gene by UV-, gamma-irradiation and mitomycin C is mediated by polyamines in Escherichia coli K-12. Toxicol Lett. 2000;116:143–9. doi: 10.1016/s0378-4274(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 49.Ha HC, Yager JD, Woster PA, Casero RA., Jr Structural specificity of polyamines and polyamine analogues in the protection of DNA from strand breaks induced by reactive oxygen species. Biochem Biophys Res Commun. 1998;244:298–303. doi: 10.1006/bbrc.1998.8258. [DOI] [PubMed] [Google Scholar]
- 50.Muscari C, Guarnieri C, Stefanelli C, Giaccari A, Caldarera CM. Protective effect of spermine on DNA exposed to oxidative stress. Mol Cell Biochem. 1995;144:125–9. doi: 10.1007/BF00944391. [DOI] [PubMed] [Google Scholar]
- 51.Khan AU, Mei YH, Wilson T. A proposed function for spermine and spermidine: protection of replicating DNA against damage by singlet oxygen. Proc Natl Acad Sci USA. 1992;89:11426–7. doi: 10.1073/pnas.89.23.11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan NA, Havouis R, Quemener V, Moulinoux JP. Protein kinase C inhibitor (H-7) potentiates antiproliferative effects of a polyamine biosynthesis inhibitor. Anticancer Res. 1992;12:1223–6. [PubMed] [Google Scholar]
- 53.Tofilon PJ, Deen DF, Marton LJ. Polyamine depletion and drug-induced chromosomal damage: new results. Science. 1992;258:1378. doi: 10.1126/science.1455236. [DOI] [PubMed] [Google Scholar]
- 54.Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA., Jr The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci USA. 1998;95:11140–5. doi: 10.1073/pnas.95.19.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amendola R, Bellini A, Cervelli M, Degan P, Marcocci L, Martini F, Mariottini P. Direct oxidative DNA damage, apoptosis and radio sensitivity by spermine oxidase activities in mouse neuroblastoma cells. Biochim Biophys Acta. 2005;1755:15–24. doi: 10.1016/j.bbcan.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 56.Tkachenko A, Nesterova L, Pshenichnov M. The role of the natural polyamine putrescine in defense against oxidative stress in Escherichia coli. Arch Microbiol. 2001;176:155–7. doi: 10.1007/s002030100301. [DOI] [PubMed] [Google Scholar]
- 57.Ye B, Muller HH, Zhang J, Gressel J. Constitutively elevated levels of putrescine and putrescine-generating enzymes correlated with oxidant stress resistance in Conyza Bonariensis and Wheat. Plant Physiol. 1997;115:1443–51. doi: 10.1104/pp.115.4.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bower JM, Mulvey MA. Polyamine-mediated resistance of uropathogenic Escherichia coli to nitrosative stress. J Bacteriol. 2006;188:928–33. doi: 10.1128/JB.188.3.928-933.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takano K, Ogura M, Yoneda Y, Nakamura Y. Oxidative metabolites are involved in polyamineinduced microglial cell death. Neuroscience. 2005;134:1123–31. doi: 10.1016/j.neuroscience.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 60.Hernandez SM, Sanchez MS, De Tarlovsky MN. Polyamines as a defense mechanism against lipoperoxidation in Trypanosoma cruzi. Acta Trop. 2006;98:94–102. doi: 10.1016/j.actatropica.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Gerard F, Dri AM, Moreau PL. Role of Escherichia coli RpoS, LexA and H-NS global regulators in metabolism and survival under aerobic, phosphatestarvation conditions. Microbiology. 1999;145:1547–62. doi: 10.1099/13500872-145-7-1547. [DOI] [PubMed] [Google Scholar]
- 62.Hodges GR, Marwaha J, Paul T, Ingold KU. A novel procedure for generating both nitric oxide and superoxide in situ from chemical sources at any chosen mole ratio. First application: tyrosine oxidation and a comparison with preformed peroxynitrite. Chem Res Toxicol. 2000;13:1287–93. doi: 10.1021/tx0001272. [DOI] [PubMed] [Google Scholar]
- 63.Kim JS, Choi SH, Lee JK. Lysine decarboxylase expression of Vibrio vulnificus is induced by SoxR in response to superoxide stress. J Bacteriol. 2006;188:8586–92. doi: 10.1128/JB.01084-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Norikura T, Kojima-Yuasa A, Opare Kennedy D, Matsui-Yuasa I. Protective effect of gammaaminobutyric acid (GABA) against cytotoxicity of ethanol in isolated rat hepatocytes involves modulations in cellular polyamine levels. Amino Acids. 2007;32:419–23. doi: 10.1007/s00726-006-0381-3. [DOI] [PubMed] [Google Scholar]
- 65.Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, Guy CL. Exploring the temperature- stress metabolome of Arabidopsis. Plant Physiol. 2004;136:4159–68. doi: 10.1104/pp.104.052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen W, Nada K, Tachibana S. Involvement of polyamines in the chilling tolerance of cucumber cultivars. Plant Physiol. 2000;124:431–9. doi: 10.1104/pp.124.1.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park YK, Bearson B, Bang SH, Bang IS, Foster JW. Internal pH crisis, lysine decarboxylase and the acid tolerance response of Salmonella typhimurium. Mol Microbiol. 1996;20:605–11. doi: 10.1046/j.1365-2958.1996.5441070.x. [DOI] [PubMed] [Google Scholar]
- 68.Griswold AR, Jameson-Lee M, Burne RA. Regulation and physiologic significance of the agmatine deiminase system of Streptococcus mutans UA159. J Bacteriol. 2006;188:834–41. doi: 10.1128/JB.188.3.834-841.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X, Shi G, Xu Q, Hu J. Exogenous polyamines enhance copper tolerance of Nymphoides peltatum. J Plant Physiol. 2006;164:1062–70. doi: 10.1016/j.jplph.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Maiale S, Sanchez DH, Guirado A, Vidal A, Ruiz OA. Spermine accumulation under salt stress. J Plant Physiol. 2004;161:35–42. doi: 10.1078/0176-1617-01167. [DOI] [PubMed] [Google Scholar]
- 71.Kasinathan V, Wingler A. Effect of reduced arginine decarboxylase activity on salt tolerance and on polyamine formation during salt stress in Arabidopsis thaliana. Physiol Plant. 2004;121:101–7. doi: 10.1111/j.0031-9317.2004.00309.x. [DOI] [PubMed] [Google Scholar]
- 72.Chattopadhayay MK, Tiwari BS, Chattopadhyay G, Bose A, Sengupta DN, Ghosh B. Protective role of exogenous polyamines on salinity-stressed rice (Oryza sativa) plants. Physiol Plant. 2002;116:192–9. doi: 10.1034/j.1399-3054.2002.1160208.x. [DOI] [PubMed] [Google Scholar]
- 73.Capell T, Bassie L, Christou P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc Natl Acad Sci USA. 2004;101:9909–14. doi: 10.1073/pnas.0306974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chattopadhyay MK, Tabor CW, Tabor H. Polyamine deficiency leads to accumulation of reactive oxygen species in a spe2Delta mutant of Saccharomyces cerevisiae. Yeast. 2006;23:751–61. doi: 10.1002/yea.1393. [DOI] [PubMed] [Google Scholar]
- 75.Zhu MY, Wang WP, Bissette G. Neuroprotective effects of agmatine against cell damage caused by glucocorticoids in cultured rat hippocampal neurons. Neuroscience. 2006;141:2019–27. doi: 10.1016/j.neuroscience.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kasukabe Y, He L, Nada K, Misawa S, Ihara I, Tachibana S. Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol. 2004;45:712–22. doi: 10.1093/pcp/pch083. [DOI] [PubMed] [Google Scholar]
- 77.Chattopadhyay MK, Tabor CW, Tabor H. Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc Natl Acad Sci USA. 2003;100:2261–5. doi: 10.1073/pnas.2627990100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pedreno E, Lopez-Contreras AJ, Cremades A, Penafiel R. Protecting or promoting effects of spermine on DNA strand breakage induced by iron or copper ions as a function of metal concentration. J Inorg Biochem. 2005;99:2074–80. doi: 10.1016/j.jinorgbio.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Tkachenko AG, Pshenichnov MR, Nesterova L. Putrescine as a oxidative stress protecting factor in Escherichia coli. Mikrobiologiia. 2001;70:487–94. [PubMed] [Google Scholar]
- 80.Warters RL, Newton GL, Olive PL, Fahey RC. Radioprotection of human cell nuclear DNA by polyamines: radiosensitivity of chromatin is influenced by tightly bound spermine. Radiat Res. 1999;151:354–62. [PubMed] [Google Scholar]
- 81.Khan AU, Di Mascio P, Medeiros MH, Wilson T. Spermine and spermidine protection of plasmid DNA against single-strand breaks induced by singlet oxygen. Proc Natl Acad Sci USA. 1992;89:11428–30. doi: 10.1073/pnas.89.23.11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Snyder RD, Sunkara PS. Effect of polyamine depletion on DNA damage and repair following UV irradiation of HeLa cells. Photochem Photobiol. 1990;52:525–32. doi: 10.1111/j.1751-1097.1990.tb01795.x. [DOI] [PubMed] [Google Scholar]
- 83.Hosford ME, Muller JG, Burrows CJ. Spermine participates in oxidative damage of guanosine and 8- oxoguanosine leading to deoxyribosylurea formation. J Am Chem Soc. 2004;126:9540–1. doi: 10.1021/ja047981q. [DOI] [PubMed] [Google Scholar]
- 84.Longo LD, Packianathan S, McQueary JA, Stagg RB, Byus CV, Cain CD. Acute hypoxia increases ornithine decarboxylase activity and polyamine concentrations in fetal rat brain. Proc Natl Acad Sci USA. 1993;90:692–6. doi: 10.1073/pnas.90.2.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Packianathan S, Cain CD, Longo LD. Ornithine decarboxylase activity and polyamine concentrations in fetal rat brain: response to chronic hypoxic-hypoxia and/or carbon monoxide-hypoxia. Brain Res Dev Brain Res. 1994;83:138–41. doi: 10.1016/0165-3806(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 86.Sava IG, Battaglia V, Rossi CA, Salvi M, Toninello A. Free radical scavenging action of the natural polyamine spermine in rat liver mitochondria. Free Radic Biol Med. 2006;41:1272–81. doi: 10.1016/j.freeradbiomed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 87.Rigobello MP, Toninello A, Siliprandi D, Bindoli A. Effect of spermine on mitochondrial glutathione release. Biochem Biophys Res Commun. 1993;194:1276–81. doi: 10.1006/bbrc.1993.1961. [DOI] [PubMed] [Google Scholar]
- 88.Foster JW, Hall HK. Inducible pH homeostasis and the acid tolerance response of Salmonella typhimurium. J Bacteriol. 1991;173:5129–35. doi: 10.1128/jb.173.16.5129-5135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Merrell DS, Camilli A. The cadA gene of Vibrio choleraeis induced during infection and plays a role in acid tolerance. Mol Microbiol. 1999;34:836–49. doi: 10.1046/j.1365-2958.1999.01650.x. [DOI] [PubMed] [Google Scholar]
- 90.Rhee JE, Kim KS, Choi SH. CadC activates pHdependent expression of the Vibrio vulnificus cadB A operon at a distance through direct binding to an upstream region. J Bacteriol. 2005;187:7870–5. doi: 10.1128/JB.187.22.7870-7875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Castanie-Cornet MP, Penfound TA, Smith D, Elliott JF, Foster JW. Control of acid resistance in Escherichia coli. J Bacteriol. 1999;181:3525–35. doi: 10.1128/jb.181.11.3525-3535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Biase D, Tramonti A, Bossa F, Visca P. The response to stationary-phase stress conditions in Escherichia coli: role and regulation of the glutamic acid decarboxylase system. Mol Microbiol. 1999;32:1198–211. doi: 10.1046/j.1365-2958.1999.01430.x. [DOI] [PubMed] [Google Scholar]
- 93.Waterman SR, Small PL. Identification of sigma Sdependent genes associated with the stationaryphase acid-resistance phenotype of Shigella flexneri. Mol Microbiol. 1996;21:925–40. doi: 10.1046/j.1365-2958.1996.00058.x. [DOI] [PubMed] [Google Scholar]
- 94.Cotter PD, Gahan CG, Hill C. A glutamate decarboxylase system protects Listeria monocytogenesin gastric fluid. Mol Microbiol. 2001;40:465–75. doi: 10.1046/j.1365-2958.2001.02398.x. [DOI] [PubMed] [Google Scholar]
- 95.Iyer R, Williams C, Miller C. Arginine-agmatine antiporter in extreme acid resistance in Escherichia coli. J Bacteriol. 2003;185:6556–61. doi: 10.1128/JB.185.22.6556-6561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gong S, Richard H, Foster JW. YjdE (AdiC) is the arginine: agmatine antiporter essential for argininedependent acid resistance in Escherichia coli. J Bacteriol. 2003;185:4402–9. doi: 10.1128/JB.185.15.4402-4409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hersh BM, Farooq FT, Barstad DN, Blankenhorn DL, Slonczewski JL. A glutamate-dependent acid resistance gene in Escherichia coli. J Bacteriol. 1996;178:3978–81. doi: 10.1128/jb.178.13.3978-3981.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kieboom J, Abee T. Arginine-dependent acid resistance in Salmonella enterica serovar Typhimurium. J Bacteriol. 2006;188:5650–3. doi: 10.1128/JB.00323-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Richard H, Foster JW. Escherichia coli glutamateand arginine-dependent acid resistance systems increase internal pH and reverse transmembrane potential. J Bacteriol. 2004;186:6032–41. doi: 10.1128/JB.186.18.6032-6041.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soksawatmaekhin W, Kuraishi A, Sakata K, Kashiwagi K, Igarashi K. Excretion and uptake of cadaverine by cadB and its physiological functions in Escherichia coli. Mol Microbiol. 2004;51:1401–12. doi: 10.1046/j.1365-2958.2003.03913.x. [DOI] [PubMed] [Google Scholar]
- 101.Aziz SM, Worthen DR, Yatin M, Ain KB, Crooks PA. A unique interaction between polyamine and multidrug resistance (P-glycoprotein) transporters in cultured Chinese hamster ovary cells transfected with mouse mdr-1 gene. Biochem Pharmacol. 1998;56:181–7. doi: 10.1016/s0006-2952(98)00132-4. [DOI] [PubMed] [Google Scholar]
- 102.Basle A, Delcour AH. Effect of two polyamine toxins on the bacterial porin OmpF. Biochem Biophys Res Commun. 2001;285:550–4. doi: 10.1006/bbrc.2001.5155. [DOI] [PubMed] [Google Scholar]
- 103.Bredin J, Simonet V, Iyer R, Delcour AH, Pages JM. Colicins, spermine and cephalosporins: a competitive interaction with the OmpF eyelet. Biochem J. 2003;376:245–52. doi: 10.1042/BJ20030814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.DelaVega AL, Delcour AH. Cadaverine induces closing of E. coli porins. EMBO J. 1995;14:6058–65. doi: 10.1002/j.1460-2075.1995.tb00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dela Vega AL, Delcour AH. Polyamines decrease Escherichia coli outer membrane permeability. J Bacteriol. 1996;178:3715–21. doi: 10.1128/jb.178.13.3715-3721.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iyer R, Delcour AH. Complex inhibition of OmpF and OmpC bacterial porins by polyamines. J Biol Chem. 1997;272:18595–601. doi: 10.1074/jbc.272.30.18595. [DOI] [PubMed] [Google Scholar]
- 107.Samartzidou H, Mehrazin M, Xu Z, Benedik MJ, Delcour AH. Cadaverine inhibition of porin plays a role in cell survival at acidic pH. J Bacteriol. 2003;185:13–9. doi: 10.1128/JB.185.1.13-19.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Samartzidou H, Delcour AH. Distinct sensitivities of OmpF and PhoE porins to charged modulators. FEBS Lett. 1999;444:65–70. doi: 10.1016/s0014-5793(99)00030-7. [DOI] [PubMed] [Google Scholar]
- 109.Tkachenko AG, Pozhidaeva ON, Shumkov MS. Role of polyamines in formation of multiple antibiotic resistance of Escherichia coli under stress conditions. Biochemistry. 2006;71:1042–9. doi: 10.1134/s0006297906090148. [DOI] [PubMed] [Google Scholar]
- 110.Iyer R, Wu Z, Woster PM, Delcour AH. Molecular basis for the polyamine-ompF porin interactions: inhibitor and mutant studies. J Mol Biol. 2000;297:933–45. doi: 10.1006/jmbi.2000.3599. [DOI] [PubMed] [Google Scholar]
- 111.Schiller D, Kruse D, Kneifel H, Kramer R, Burkovski A. Polyamine transport and role of potE in response to osmotic stress in Escherichia coli. J Bacteriol. 2000;182:6247–9. doi: 10.1128/jb.182.21.6247-6249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jantaro S, Maenpaa P, Mulo P, Incharoensakdi A. Content and biosynthesis of polyamines in salt and osmotically stressed cells of Synechocystis sp. PCC 6803. FEMS Microbiol Lett. 2003;228:129–35. doi: 10.1016/S0378-1097(03)00747-X. [DOI] [PubMed] [Google Scholar]
- 113.Shapiguzov A, Lyukevich AA, Allakhverdiev SI, Sergeyenko TV, Suzuki I, Murata N, Los DA. Osmotic shrinkage of cells of Synechocystis sp. PCC 6803 by water efflux via aquaporins regulates osmostress-inducible gene expression. Microbiology. 2005;151:447–55. doi: 10.1099/mic.0.27530-0. [DOI] [PubMed] [Google Scholar]
- 114.Poulin R, Wechter RS, Pegg AE. An early enlargement of the putrescine pool is required for growth in L1210 mouse leukemia cells under hypoosmotic stress. J Biol Chem. 1991;266:6142–51. [PubMed] [Google Scholar]
- 115.Poulin R, Coward JK, Lakanen JR, Pegg AE. Enhancement of the spermidine uptake system and lethal effects of spermidine overaccumulation in ornithine decarboxylase-overproducing L1210 cells under hyposmotic stress. J Biol Chem. 1993;268:4690–8. [PubMed] [Google Scholar]
- 116.Lovkvist-Wallstrom E, Stjernborg-Ulvsback L, Scheffler IE, Persson L. Regulation of mammalian ornithine decarboxylase. Studies on the induction of the enzyme by hypotonic stress. Eur J Biochem. 1995;231:40–4. [PubMed] [Google Scholar]
- 117.Mitchell JL, Judd GG, Leyser A, Choe C. Osmotic stress induces variation in cellular levels of ornithine decarboxylase-antizyme. Biochem J. 1998;329:453–9. doi: 10.1042/bj3290453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Williams K. Modulation and block of ion channels: a new biology of polyamines. Cell Signal. 1997;9:1–13. doi: 10.1016/s0898-6568(96)00089-7. [DOI] [PubMed] [Google Scholar]
- 119.Hedfalk K, Tornroth-Horsefield S, Nyblom M, Johanson U, Kjellbom P, Neutze R. Aquaporin gating. Curr Opin Struct Biol. 2006;16:447–56. doi: 10.1016/j.sbi.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 120.Alcazar R, Marco F, Cuevas JC, Patron M, Ferrando A, Carrasco P, Tiburcio AF, Altabella T. Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett. 2006;28:1867–76. doi: 10.1007/s10529-006-9179-3. [DOI] [PubMed] [Google Scholar]
- 121.Urano K, Yoshiba Y, Nanjo T, Ito T, Yamaguchi- Shinozaki K, Shinozaki K. Arabidopsis stressinducible gene for arginine decarboxylase AtADC2 is required for accumulation of putrescine in salt tolerance. Biochem Biophys Res Commun. 2004;313:369–75. doi: 10.1016/j.bbrc.2003.11.119. [DOI] [PubMed] [Google Scholar]
- 122.Soyka S, Heyer AG. Arabidopsis knockout mutation of ADC2 gene reveals inducibility by osmotic stress. FEBS Lett. 1999;458:219–23. doi: 10.1016/s0014-5793(99)01125-4. [DOI] [PubMed] [Google Scholar]
- 123.Hummel I, Gouesbet G, El Amrani A, Ainouche A, Couee I. Characterization of the two arginine decarboxylase (polyamine biosynthesis) paralogues of the endemic subantarctic cruciferous species Pringlea antiscorbutica and analysis of their differential expression during development and response to environmental stress. Gene. 2004;342:199–209. doi: 10.1016/j.gene.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 124.Gilad GM, Gilad VH. Overview of the brain polyamine-stress-response: regulation, development, and modulation by lithium and role in cell survival. Cell Mol Neurobiol. 2003;23:637–49. doi: 10.1023/a:1025036532672. [DOI] [PubMed] [Google Scholar]
- 125.Hayashi Y, Tanaka J, Morizumi Y, Kitamura Y, Hattori Y. Polyamine levels in brain and plasma after acute restraint or water-immersion restraint stress in mice. Neurosci Lett. 2004;355:57–60. doi: 10.1016/j.neulet.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 126.Gilad GM, Gilad VH. Polyamines in neurotrauma. Ubiquitous molecules in search of a function. Biochem Pharmacol. 1992;44:401–7. doi: 10.1016/0006-2952(92)90428-l. [DOI] [PubMed] [Google Scholar]
- 127.Halonen T, Sivenius J, Miettinen R, Halmekyto M, Kauppinen R, Sinervirta R, Alakuijala L, Alhonen L, MacDonald E, Janne J, et al. Elevated seizure threshold and impaired spatial learning in transgenic mice with putrescine overproduction in the brain. Eur J Neurosci. 1993;5:1233–9. doi: 10.1111/j.1460-9568.1993.tb00978.x. [DOI] [PubMed] [Google Scholar]
- 128.Lukkarainen J, Kauppinen RA, Koistinaho J, Halmekyto, Alhonen LM, Janne J. Cerebral energy metabolism and immediate early gene induction following severe incomplete ischaemia in transgenic mice overexpressing the human ornithine decarboxylase gene: evidence that putrescine is not neurotoxic in vivo. Eur J Neurosci. 1995;7:1840–9. doi: 10.1111/j.1460-9568.1995.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 129.Kauppinen RA, Halmekyto M, Alhonen L, Janne J. Nuclear magnetic resonance spectroscopy study on energy metabolism, intracellular pH, and free Mg2+ concentration in the brain of transgenic mice overexpressing human ornithine decarboxylase gene. J Neurochem. 1992;58:831–6. doi: 10.1111/j.1471-4159.1992.tb09332.x. [DOI] [PubMed] [Google Scholar]
- 130.Kaasinen K, Koistinaho J, Alhonen L, Janne J. Overexpression of spermidine/spermine N-acetyltransferase in transgenic mice protects the animals from kainate-induced toxicity. Eur J Neurosci. 2000;12:540–8. doi: 10.1046/j.1460-9568.2000.00940.x. [DOI] [PubMed] [Google Scholar]
- 131.Kaasinen SK, Grohn OH, Keinanen TA, Alhonen L, Janne J. Overexpression of spermidine/spermine N1-acetyltransferase elevates the threshold to pentylenetetrazol-induced seizure activity in transgenic mice. Exp Neurol. 2003;183:645–52. doi: 10.1016/s0014-4886(03)00186-9. [DOI] [PubMed] [Google Scholar]
- 132.Williams K. Mechanisms influencing stimulatory effects of spermine at recombinant N-methyl-Daspartate receptors. Mol Pharmacol. 1994;46:161–8. [PubMed] [Google Scholar]
- 133.Williams K, Dawson VL, Romano C, Dichter MA, Molinoff PB. Characterization of polyamines having agonist, antagonist, and inverse agonist effects at the polyamine recognition site of the NMDA receptor. Neuron. 1990;5:199–208. doi: 10.1016/0896-6273(90)90309-4. [DOI] [PubMed] [Google Scholar]
- 134.Yatin SM, Yatin M, Aulick T, Ain KB, Butterfield DA. Alzheimer's amyloid beta-peptide associated free radicals increase rat embryonic neuronal polyamine uptake and ornithine decarboxylase activity: protective effect of vitamin E. Neurosci Lett. 1999;263:17–20. doi: 10.1016/s0304-3940(99)00101-9. [DOI] [PubMed] [Google Scholar]
- 135.Yatin SM, Yatin M, Varadarajan S, Ain KB, Butterfield DA. Role of spermine in amyloid betapeptide- associated free radical-induced neurotoxicity. J Neurosci Res. 2001;63:395–401. doi: 10.1002/1097-4547(20010301)63:5<395::AID-JNR1034>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 136.McClellan AJ, Tam S, Kaganovich D, Frydman J. Protein quality control: chaperones culling corrupt conformations. Nat Cell Biol. 2005;7:736–41. doi: 10.1038/ncb0805-736. [DOI] [PubMed] [Google Scholar]
- 137.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–9. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 138.Komatsu M, Kominami E, Tanaka K. Autophagy and neurodegeneration. Autophagy. 2006;2:315–7. doi: 10.4161/auto.2974. [DOI] [PubMed] [Google Scholar]
- 139.Okanojo M, Shiraki K, Kudou M, Nishikori S, Takagi M. Diamines prevent thermal aggregation and inactivation of lysozyme. J Biosci Bioeng. 2005;100:556–61. doi: 10.1263/jbb.100.556. [DOI] [PubMed] [Google Scholar]
- 140.Kim JH, Yenari MA, Giffard RG, Cho SW, Park KA, Lee JE. Agmatine reduces infarct area in a mouse model of transient focal cerebral ischemia and protects cultured neurons from ischemia-like injury. Exp Neurol. 2004;189:122–30. doi: 10.1016/j.expneurol.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 141.Gilad GM, Salame K, Rabey JM, Gilad VH. Agmatine treatment is neuroprotective in rodent brain injury models. Life Sci. 1996;58:41–6. doi: 10.1016/0024-3205(95)02274-0. [DOI] [PubMed] [Google Scholar]
- 142.Clarkson AN, Liu H, Pearson L, Kapoor M, Harrison JC, Sammut IA, Jackson DM, Appleton I. Neuroprotective effects of spermine following hypoxic- ischemic-induced brain damage: a mechanistic study. FASEB J. 2004;18:1114–6. doi: 10.1096/fj.03-1203fje. [DOI] [PubMed] [Google Scholar]
- 143.Fairbanks CA, Schreiber KL, Brewer KL, Yu CG, Stone LS, Kitto KF, Nguyen HO, Grocholski BM, Shoeman DW, Kehl LJ, Regunathan S, Reis DJ, Yezierski RP, Wilcox GL. Agmatine reverses pain induced by inflammation, neuropathy, and spinal cord injury. Proc Natl Acad Sci USA. 2000;97:10584–9. doi: 10.1073/pnas.97.19.10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhu MY, Piletz JE, Halaris A, Regunathan S. Effect of agmatine against cell death induced by NMDA and glutamate in neurons and PC12 cells. Cell Mol Neurobiol. 2003;23:865–72. doi: 10.1023/a:1025069407173. [DOI] [PubMed] [Google Scholar]
- 145.Wang WP, Iyo AH, Miguel-Hidalgo J, Regunathan S, Zhu MY. Agmatine protects against cell damage induced by NMDA and glutamate in cultured hippocampal neurons. Brain Res. 2006;1084:210–6. doi: 10.1016/j.brainres.2006.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gilad GM, Gilad VH, Finberg JP, Rabey JM. Neurochemical evidence for agmatine modulation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity. Neurochem Res. 2005;30:713–9. doi: 10.1007/s11064-005-6865-9. [DOI] [PubMed] [Google Scholar]
- 147.Satriano J, Schwartz D, Ishizuka S, Lortie MJ, Thomson SC, Gabbai F, Kelly CJ, Blantz RC. Suppression of inducible nitric oxide generation by agmatine aldehyde: beneficial effects in sepsis. J Cell Physiol. 2001;188:313–20. doi: 10.1002/jcp.1119. [DOI] [PubMed] [Google Scholar]
- 148.Takano K, Ogura M, Nakamura Y, Yoneda Y. Neuronal and glial responses to polyamines in the ischemic brain. Curr Neurovasc Res. 2005;2:213–23. doi: 10.2174/1567202054368335. [DOI] [PubMed] [Google Scholar]
- 149.Rao AM, Hatcher JF, Dogan A, Dempsey RJ. Elevated N1-acetylspermidine levels in gerbil and rat brains after CNS injury. J Neurochem. 2000;74:1106–11. doi: 10.1046/j.1471-4159.2000.741106.x. [DOI] [PubMed] [Google Scholar]
- 150.Dogan A, Rao AM, Hatcher J, Rao VL, Baskaya MK, Dempsey RJ. Effects of MDL 72527, a specific inhibitor of polyamine oxidase, on brain edema, ischemic injury volume, and tissue polyamine levels in rats after temporary middle cerebral artery occlusion. J Neurochem. 1999;72:765–70. doi: 10.1046/j.1471-4159.1999.0720765.x. [DOI] [PubMed] [Google Scholar]
- 151.Tang X, Kim AL, Feith DJ, Pegg AE, Russo J, Zhang H, Aszterbaum M, Kopelovich L, Epstein EH, Jr, Bickers DR, Athar M. Ornithine decarboxylase is a target for chemoprevention of basal and squamous cell carcinomas in Ptch1+/− mice. J Clin Invest. 2004;113:867–75. doi: 10.1172/JCI20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lutz C, Navakoudis E, Seidlitz HK, Kotzabasis K. Simulated solar irradiation with enhanced UV-B adjust plastid- and thylakoid-associated polyamine changes for UV-B protection. Biochim Biophys Acta. 2005;1710:24–33. doi: 10.1016/j.bbabio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 153.Sharma SS, Dietz KJ. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot. 2006;57:711–26. doi: 10.1093/jxb/erj073. [DOI] [PubMed] [Google Scholar]
- 154.Henderson B, Allan E, Coates AR. Stress wars: the direct role of host and bacterial molecular chaperones in bacterial infection. Infect Immun. 2006;74:3693–706. doi: 10.1128/IAI.01882-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Durand JM, Bjork GR. Putrescine or a combination of methionine and arginine restores virulence gene expression in a tRNA modification-deficient mutant of Shigella flexneri: a possible role in adaptation of virulence. Mol Microbiol. 2003;47:519–27. doi: 10.1046/j.1365-2958.2003.03314.x. [DOI] [PubMed] [Google Scholar]
- 156.Ware D, Jiang Y, Lin W, Swiatlo E. Involvement of potD in Streptococcus pneumoniae polyamine transport and pathogenesis. Infect Immun. 2006;74:352–61. doi: 10.1128/IAI.74.1.352-361.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shah P, Swiatlo E. Immunization with polyamine transport protein PotD protects mice against systemic infection with Streptococcus pneumoniae. Infect Immun. 2006;74:5888–92. doi: 10.1128/IAI.00553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Torres AG, Vazquez-Juarez RC, Tutt CB, Garcia- Gallegos JG. Pathoadaptive mutation that mediates adherence of shiga toxin-producing Escherichia coli O111. Infect Immun. 2005;73:4766–76. doi: 10.1128/IAI.73.8.4766-4776.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rao JN, Li L, Golovina VA, Platoshyn O, Strauch ED, Yuan JX, Wang JY. Ca2+-RhoA signaling pathway required for polyamine-dependent intestinal epithelial cell migration. Am J Physiol Cell Physiol. 2001;280:C993–1007. doi: 10.1152/ajpcell.2001.280.4.C993. [DOI] [PubMed] [Google Scholar]
- 160.Chen C, Young BA, Coleman CS, Pegg AE, Sheppard D. Spermidine/spermine N1-acetyltransferase specifically binds to the integrin alpha9 subunit cytoplasmic domain and enhances cell migration. J Cell Biol. 2004;167:161–70. doi: 10.1083/jcb.200312166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gobert AP, Cheng Y, Wang JY, Boucher JL, Iyer RK, Cederbaum SD, Casero RA, Jr, Newton JC, Wilson KT. Helicobacter pyloriinduces macrophage apoptosis by activation of arginase II. J Immunol. 2002;168:4692–700. doi: 10.4049/jimmunol.168.9.4692. [DOI] [PubMed] [Google Scholar]
- 162.Chaturvedi R, Cheng Y, Asim M, Bussiere FI, Xu H, Gobert AP, Hacker A, Casero RA, Jr, Wilson KT. Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J Biol Chem. 2004;279:40161–73. doi: 10.1074/jbc.M401370200. [DOI] [PubMed] [Google Scholar]
- 163.Xu H, Chaturvedi R, Cheng Y, Bussiere FI, Asim M, Yao MD, Potosky D, Meltzer SJ, Rhee JG, Kim SS, Moss SF, Hacker A, Wang Y, Casero RA, Jr, Wilson KT. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res. 2004;64:8521–5. doi: 10.1158/0008-5472.CAN-04-3511. [DOI] [PubMed] [Google Scholar]
- 164.Day WA, Jr, Fernandez RE, Maurelli AT. Pathoadaptive mutations that enhance virulence: genetic organization of the cadA regions of Shigella spp. Infect Immun. 2001;69:7471–80. doi: 10.1128/IAI.69.12.7471-7480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.McCormick BA, Fernandez MI, Siber AM, Maurelli AT. Inhibition of Shigella flexneri-induced transepithelial migration of polymorphonuclear leucocytes by cadaverine. Cell Microbiol. 1999;1:143–55. doi: 10.1046/j.1462-5822.1999.00014.x. [DOI] [PubMed] [Google Scholar]
- 166.Kierszenbaum F, Wirth JJ, McCann PP, Sjoerdsma A. Arginine decarboxylase inhibitors reduce the capacity of Trypanosoma cruzi to infect and multiply in mammalian host cells. Proc Natl Acad Sci USA. 1987;84:4278–82. doi: 10.1073/pnas.84.12.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bauer PM, Buga GM, Fukuto JM, Pegg AE, Ignarro LJ. Nitric oxide inhibits ornithine decarboxylase via S-nitrosylation of cysteine 360 in the active site of the enzyme. J Biol Chem. 2001;276:34458–64. doi: 10.1074/jbc.M105219200. [DOI] [PubMed] [Google Scholar]
- 168.Cona A, Rea G, Angelini R, Federico R, Tavladoraki P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006;11:80–8. doi: 10.1016/j.tplants.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 169.Rea G, Metoui O, Infantino A, Federico R, Angelini R. Copper amine oxidase expression in defense responses to wounding and Ascochyta rabiei invasion. Plant Physiol. 2002;128:865–75. doi: 10.1104/pp.010646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wisniewski JP, Rathbun EA, Knox JP, Brewin NJ. Involvement of diamine oxidase and peroxidase in insolubilization of the extracellular matrix: implications for pea nodule initiation by Rhizobium leguminosarum. Mol Plant Microbe Interact. 2000;13:413–20. doi: 10.1094/MPMI.2000.13.4.413. [DOI] [PubMed] [Google Scholar]
- 171.Perez-Amador MA, Leon J, Green PJ, Carbonell J. Induction of the arginine decarboxylase ADC2 gene provides evidence for the involvement of polyamines in the wound response in Arabidopsis. Plant Physiol. 2002;130:1454–63. doi: 10.1104/pp.009951. [DOI] [PMC free article] [PubMed] [Google Scholar]


