Abstract
Santiago Ramón y Cajal discovered a new type of cell related to the myenteric plexus and also to the smooth muscle cells of the circular muscle layer of the intestine. Based on their morphology, relationships and staining characteristics, he considered these cells as primitive neurons. One century later, despite major improvements in cell biology, the interstitial cells of Cajal (ICCs) are still controversial for many researchers. The aim of study was to perform an immunohistochemical and ultrastructural characterization of the ICCs in the rabbit duo-denum. We have found interstitial cells that are positive for c-Kit, CD34 and nestin and are also positive for Ki67 protein, tightly associated with somatic cell proliferation. By means of electron microscopy, we describe ICCs around enteric ganglia. They present triangular or spindle forms and a very voluminous nucleus with scarce per-inuclear chromatin surrounded by a thin perinuclear cytoplasm that expands with long cytoplasmic processes. ICC processes penetrate among the smooth muscle cells and couple with the processes of other ICCs located in the connective tissue of the circular muscle layer and establish a three-dimensional network. Intercellular con-tacts by means of gap-like junctions are frequent. ICCs also establish gap-like junctions with smooth muscle cells. We also observe a population of interstitial cells of stellate morphology in the connective tissue that sur-rounds the muscle bundles in the circular muscle layer, usually close to nervous trunks. These cells establish different types of contacts with the muscle cells around them. In addition, the presence of a single cilium show-ing a structure 9 + 0 in an ICC is demonstrated for the first time. In conclusion, we report positive staining c-kit, CD34, nestin and Ki 67. ICCs fulfilled the usual transmission electron microscopy (TEM) criteria. A new ultrastructural characteristic of at least some ICCs is demonstrated: the presence of a single cilium. Some populations of ICCs in the rabbit duodenum present certain immunohistochemical and ultrastructural characteristics that often are present in progenitor cells.
Keywords: interstitial cells of cajal, adult stem cells, pluripotent stem cells, intestine, single cilium
Introduction
Ramón y Cajal discovered the interstitial cells when he studied the intestinal plexi of amphibians [1] and several mammals [2]. Dogiel (1985) confirmed this finding and called them interstitial cells of Cajal (ICCs) (‘Cajal’ sche zellen'). Cajal reported that these cells presented two different morphologies: fusiform and triangular or stellate. He defined the main morphologic characteristics present in both type of cells: a very voluminous nucleus, scant perinuclear cytoplasm and long cytoplasmic prolongations. He indicated that these cells are more abundant in young animals, and emphasized their staining affinities, similar to neurons when stained with methylen blue and the double impregnation Golgi method. ICCs are distributed in close relationship to the intestinal nervous plexi and also in the connective tissue that surrounds the smooth muscle cells of the circular muscle layer of the intestine. He also drew with great precision the networks that these cells constitute and suggested possible contacts with the membrane of muscle cells. Due to all these characteristics, Cajal considered them undoubtedly as nervous elements.
Several studies have increased our knowledge of the immunohistochemistry and ultrastructural features of ICCs [3–5] as well as the relationships that these cells establish with the surrounding elements [6]. According to the currently prevailing concept, ICCs are heterogeneous cells, composed of fibroblast-like, and/or muscle-like cells interposed between nerve and muscle cells [7]. From the functional point of view, ICCs are considered to be the origin of the slow electric waves of the gastrointestinal tract (pacemakers) and to be modulators in enteric neurotransmission [8–10]. However, Min in a recent review pointed out a strong need to clarify some of the current concepts about ICC [7].
Cajal also observed ICCs in relationship to vessels and pancreatic parenchyma, postulating their presence in different glands [11]. Recently, interstitial Cajal-like cells have also been described in the pancreas [12] and in different organs: myocardium [13], urethra [14], bladder [15, 16], urether [17], blood vessels [18] and human myometrium [19], in all cases with sympathetic innervations. These findings may question the functional role of these cells [20].
Recent studies have considered the possibility that ICCs present with certain plasticity and even may suffer transdifferentiation in other cell types [21]. Popescu et al. 2005 [22–24] analysed the different functions that ICC-like cells may develop. They suggested that these cells might play a role as uncommitted progenitor cells.
The mesenchymal origin of ICCs has been established by several authors [25–29], demonstrating that they do not derive from the neural crest. This fact seems to disagree with the original hypothesis of Cajal, who considered ICCs to be primitive neurons. However, recent studies have shown that after the emigration of neural crest cells an additional population of cells emigrates from the cranial neural tube. These cells originate in the ventral part of the hindbrain, emigrate through the site of attachment of the cranial nerves, and colonize a variety of developing structures of the gastrointestinal tract, where they differentiate into neurons, glia and ICCs. This cell population has been named the ventrally emigrating neural tube (VENT). ICCs in anterior gut, therefore, develop from two sources of cells, the mesodermal mesenchymal cells and VENT [30]. The aim of this study was to characterize ICCs in the rabbit anterior intestine by means of immunohistochemical and ultrastructural techniques. The presence of a single cilium is demonstrated. The functional role of this single cilium remains to be established.
Material and methods
The investigation was performed according to the European Community guidelines for animal ethical care and the Guide for care and use of laboratory animals published by the US National Institute of Health (NIH publication N° 85-23, revised 1985).
Normal adult rabbits (Orictolagus cuniculus) weighing between 800 and 900 grams (six males and four females) were used. They were housed in temperature controlled rooms (20 ± 1°C), and natural light, and had free access to standard food and tap water. The rabbits were sacrificed by cervical dislocation, under ether anaesthesia. After the extraction of the intestine, duodenum fragments were immersed in the specific fixative for each of the techniques described below.
Immunocytochemistry: DAKO EnVision® method
The rabbit duodenum specimens were tested using the following antibodies: In order to determine the ganglion neurons of enteric plexus, we used neuron specific enolase (NSE) 1:200 (DAKO, Glostrup, Denmark). To characterize ICC: c-Kit (CD117), 1:500 (DAKO, Glostrup, Denmark); CD34 1:150 (DAKO, Glostrup, Denmark) and Nestin 1:100 (Rat-401; S Hockfield. Hybridoma Bank. University of Iowa). CD117 + CD34 comprise progenitor cells and their precursor of all haematopoietic cell lineages. In order to detect the presence of cells during division we used Ki-67 1:100 (DAKO, Glostrup, Denmark), a nuclear antigen which recognizes proliferation cells at all stages of the cell cycle.
The pieces are fixed for 6 hrs in 10% neutral phosphate-buffered formalin. Tissues are dehydrated using graded alcohols, cleared with xylene and embedded in paraffin wax. To minimize antigen denaturalization, tissues should not be exposed to temperatures higher than 60°C during processing. For increased tissue section adhesion, sections of 5μm. are collected in poly-L coated slides (DAKO S3003). The tissue sections were deparaffinated in xylene (10 min. twice) and rehydrated in a graded ethanol series up to distilled water.
For heat-induced antigen retrieval, the samples were treated for 2 min in an autoclave at 121°C containing 10% citrate buffer (Dako S2031). After washing twice with PBS (pH 7.4) for 3 min, the sections were treated with endogenous peroxidase blocking (Dako S2001) for 20 min, and washed twice in distilled water and PBS for 3 min. The blocking was repeated twice. The tissue sections with the primary antibodies were incubated for 30 min in a dark chamber, and afterwards washed twice in phosphate buffer saline (PBS) for 3 min. The tissue sections was covered with a peroxidase-based visualization kit (Dako K5007) for 30 min and then washed in PBS for 5 min. To confirm the presence of immunocomplexes, 3,3′-diaminobenzidine (DAB) was used as chromogene. The samples were washed twice in distilled water, contrasted with Mayer's haematoxylin for 1 min, rinsed gently with tap water, dehydrated in a graded series of ethanol, cleared in xylene and cover slipped with DPX. Digital microscope images were captured by means of an Olympus BX 51 microscope. Negative controls were made by omission of the primary antibody.
Electron microscopy
Tissue samples (about 1.5 mm3) were fixed in 2% glutaraldehyde (in 0.1 M cacodylate buffer), pH 7.3, for 4 hrs, at 4°C. Later they were washed twice with the same buffer for 10 min. Post-fixation was performed in 1% osmium tetroxide in 0.1 M cacodylate buffer for 2 hrs at 4°C. The samples were dehydrated in graded acetone (30%, 50%, 70% with 2% uranylacetate, 90%, 100%). After immersion in propylene oxide (three times for 10 min each), the samples were immersed in a mixture (1:1) of propylene oxide and Epon 812 resin overnight, and embedded in Epon 812. Semi-thin sections were cut and stained with 1% toluidine blue. Specific areas were chosen for ultrathin sectioning. Sections were cut using an ultramicrotome (Reichert-Jung, Austria). Ultrathin sections (50 nm) were collected on copper grids, counter-stained for 10 min with 1% uranyl acetate and Reynold's lead citrate, and then observed in a Jeol 1010 electron microscope at an acceleration voltage of 60 kV (Jeol, Tokyo, Japan).
Results
By means of immunohistochemical techniques, the ganglia in the myenteric plexus of the rabbit duodenum were characterized. The neurons of these ganglia are reactive for the neuron-specific enolase (Fig. 1A). Distributed around these ganglia there are interstitial cells that are positive for the c-Kit (Fig. 1B). In this location, specific antibodies were applied against c-Kit (Fig. 1C), CD34 (Fig. 1D) y Ki-67 (Fig. 1E) in serial sections and it was found that these interstitial cells are immunoreactive against these three markers. The observation of the preparations permitted the recognition of a positive reaction in the same cells. The Ki67 antibody (proliferation marker) reveals the nuclei of the cells, and that is why the marks are slightly displaced in Figure 3E. Some c-Kit positive cells contact by means of their cytoplasmic processes, following the connective tissue that separates the muscle bundles in the circular muscle layer (Fig. 1F). In this location, nestin positive immunoreactivity was found in cells with long cytoplasmic prolongations stained brown (Fig. 1G).
1.
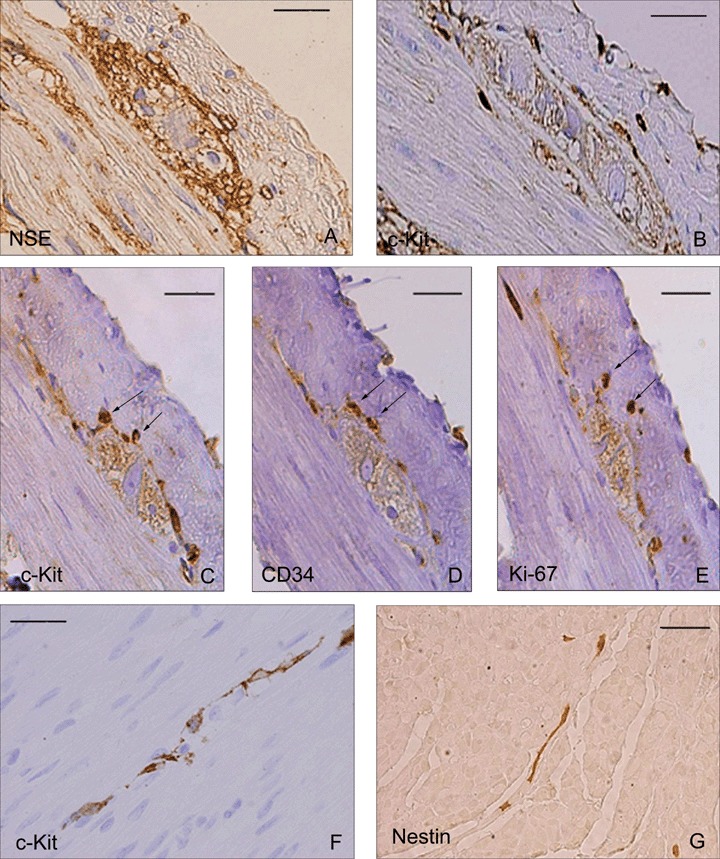
Rabbit duodenum. Immunohistochemistry, EnVision® method. (1A) NSE positive ganglion. (1B) ICC processes surrounding a ganglion in the myenteric plexus. (1C, D E) Serial sections of the same ganglion. (C) c-Kit. D: CD34. (E) Ki-67 labelled nucleus. The arrows point towards positive structures for the three markers. (1F) c-Kit positive ICC interconnected in the circular muscle coat. (1G) Nestin positive ICC in the connective tissue of the circular musculature. Scale bar = 20 μm.
3.

Junctions between two ICC and between two smooth muscle cells. (3A) TEM. Adherent junction between two processes of ICC. (3B) TEM. Desmosome-like junction between two smooth muscle cells in the rabbit duodenum. cv: caveolae, dc: dense bodies, mt: mitochondria, N: nucleus, Rer: rough endoplasmic reticulum. Scale bar = 200 nm.
TEM images showed the structure of ganglia in the myenteric plexus of the duodenum of Orictolagus cuniculus. Neurons surrounded by glial cells constitute compact ganglions. Thick axonal trunks constitute part of these ganglion structures. The nucleus of the neurons presents uniformly distributed chromatin, a thin frame of marginal heterochromatin and a prominent nucleolus (Fig. 2A). Around these ganglia, and separated by a margin of connective tissue, ICCs can be observed presenting triangular or fusiform shapes. The nucleus of these interstitial cells is very voluminous and is surrounded by a small perinuclear cytoplasm that expands with long prolongations. The most common cytological characteristics are: cisternae of smooth and rough endoplasmic reticulum, free ribosomes, dictyosomes of Golgi apparatus, numerous simple vesicles, several mitochondria, intermediate filaments and lysosomes. A basal lamina appears incomplete or absent. The cytoplasm of these cells presents a higher electron density than the cytoplasm of the surrounding muscle cells. The cytoplasmic processes of ICCs surround the external contours of the ganglia. In addition, these prolongations penetrate among the smooth muscle cells and couple with the prolongations of other ICCs located in the connective tissue of the circular muscle layer (Fig. 2A). ICCs of this location establish close intercellular contacts between themselves by means of different specialized areas of the plasmalemal membrane: invaginations to receive and surround the extreme of a prolongation of a nearby ICC (Fig. 2B), close contacts between the membranes of two ICC, presenting electrondense reinforcements (Fig. 3A); occasionally peg and socket junctions can be see although gap-like junctions are more frequent. In addition to constituting networks of interconnected cells, these cells establish contacts with smooth muscle cells by means of gap-like unions. Smooth muscle cells in the intestine wall contact with each other by means of multiple cellular junctions, characterized by an electron dense reinforcement immediately under the cell membrane in the zones of intercellular contacts, so-called ‘desmosome-like’ by some investigators. In the intercellular space, a mild electron density due to trans-membrane proteins can be observed (Fig. 3B).
2.
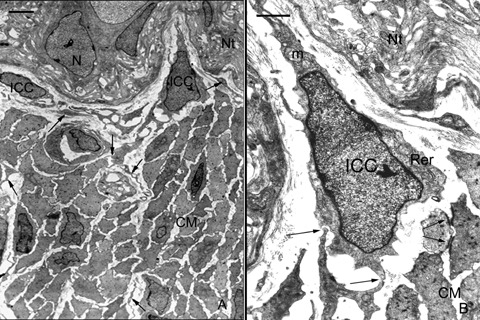
Interstitial cells in the myenteric plexus of rabbit duodenum. (2A) TEM. ICC in the limit between ganglia in the myenteric plexus and the circular muscle layer. Processes from these cells (arrows) extend among smooth muscle cells. Scale bar = 2 μm. (2B) TEM. Detail of 2A. ICC with triangular morphology, elongate nucleus and a scarce perinuclear cytoplasm. Junctions between this cell and a process of neighbour ICC (arrows). Desmosome-like junctions between two smooth muscle cells. CM: Circular muscular layer, ICC: interstitial cell of Cajal, m: mitochondria, N: neuron, Nt: Nervous trunk, Rer: rough endoplasmic reticulum. Scale bar = 1 μm.
A population of interstitial cells can be observed of stellate morphology in the connective tissue that surround the muscle bundles in the circular muscle layer, usually close to nervous trunks. These cells present four or five long cytoplasmic processes in the same cross section (Fig. 4). These processes emerging from the cell body and extend through the connective tissue that separate the smooth muscle cells and establish contact with their membrane. Three type of contacts can be observed: small type-gap-like junctions, as both membranes present a clearly visible electron dense reinforcement (Fig. 4A); lateral contacts with small buttons in the muscle cell (Fig. 4B); and small invaginations in the membrane of the smooth muscle cells that receive a long dichotomous branching processes (Fig. 4C). An ICC can contact by means of gap-like junctions with several muscle cells at the same time (Fig. 4) and a single prolongation can contact simultaneously with two different muscle cells (Fig. 4D). Moreover, these prolongations extend around the nervous trunks and establish close contacts with some of the axons (Fig. 5), although ultra structural images of synapses were not verified. Some of these axons present varicosities that contain peptidergic- type vesicles (showing their typical size and electron dense content with a clear surrounding halo); there are frequently mixed-type varicosities as well (containing electron-clear vesicles of smaller size and peptidergic-type vesicles).
4.
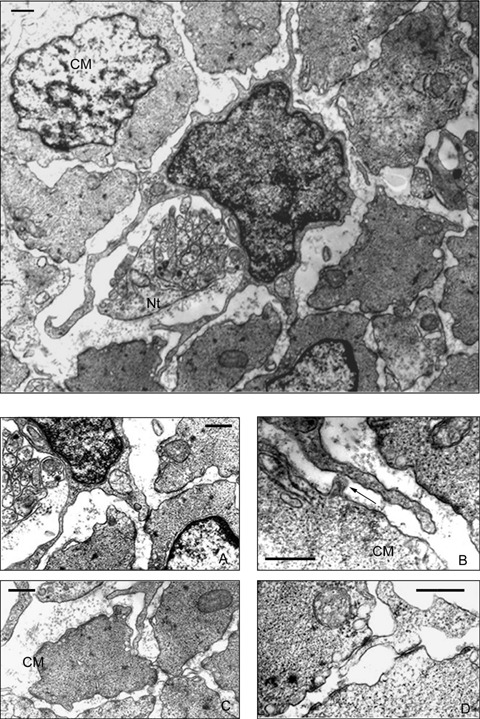
TEM. Intramuscular ICC showing four long prolongations in cross section, two of them surround a nervous trunk. Different types of contacts among processes of ICC are observed. (4A) Detail of Fig. 4. Small gap-like junction. (4B) Detail of Fig. 4. A small protrusion of a smooth muscle cell projects towards the prolongation of an ICC (arrow). (4C) Detail of Fig. 4. A prolongation of an ICC divides into two branches, one of which is received by a smooth muscle cell. (4D) A prolongation of an ICC establishes simultaneous contact with two smooth muscle cells by means of gap-like junctions. CM: Circular muscular layer, Nt: Nervous trunk. Scale bar = 500 nm.
5.

TEM. Nervous trunks close to an ICC. In the axons there can be observed microtubules and mitochondria. Some varicosities show peptidergic type vesicles with electron dense central core. CM: Circular muscle layer, ICC: Interstitial cell of Cajal, mt: mitochondria. Scale bar = 500 nm.
All the processes of ICCs present an electron dense cytoplasm, so that the organelles accumulate in the proximal and wider part of the long prolongations (Fig. 6A). At this level, mitochondria, small cisterns of the granular endoplasmic reticule and free polyribosomes can be seen (Fig. 6B). Initially we have not observed any ultra structural difference between ICC prolongations, so that we consider them as functionally equivalent.
6.
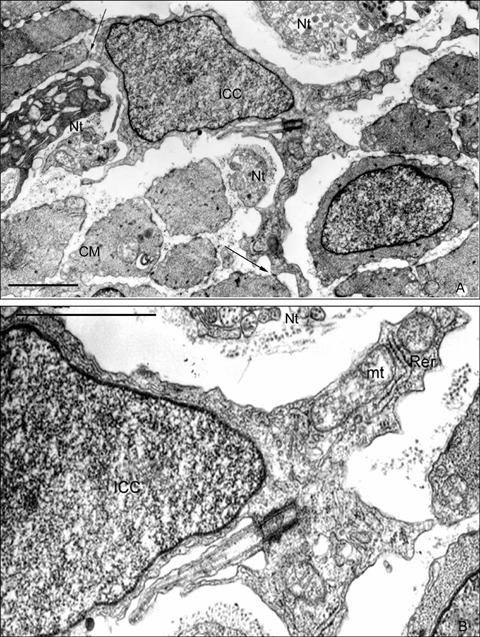
Presence of single cilium in ICC. (6A) TEM. Intramuscular ICC is close to nervous trunks. Processes from this ICC contact with the surrounding smooth muscle cells by a small gap-like junction (arrow). A single cilium can be observed in the wider cytoplasmic area. (6B) TEM. Detail of Fig. 6A. A centriole constitutes the basal corpuscle of a 9 + 0 structure single cilium. Cell organelles are concentrate in cytoplasm expanded regions. CM: Circular muscular layer, ICC: Interstitial cell of Cajal, mt: mitochondria, Nt: Nervous trunk, Rer: rough endoplasmic reticulum. Scale bar = 2 μm
It is especially interesting that the presence of a basal corpuscle that seems to guide the development and differentiation of a prolongation by means of polymerisation of microtubules can be observed in an ICC (Fig. 6A and B). This basal corpuscle constitutes a single cilium showing a 9 + 0 structure because it does not present the central pair of microtubules.
Discussion
Our results demonstrate c-kit positivity in ICCs around the ganglions and the intramuscular ICCs, in agreement with the results obtained in several mammal species [31–33]. The staining pattern of CD34 was quite similar to that of c-Kit, but there appeared to be larger numbers of cells stained for CD34 than for c-Kit, suggesting that only some CD34 positive cells concomitantly express c-Kit. The frequency and anatomic location of the ICC identified under the electron microscope corresponded well to those c-Kit positive cells seen in immunostained sections.
Nestin belongs to the intermediate filaments (class VI) family, is regarded as a marker for progenitor cells in CNS [34, 35]. Nestin is associated with dynamic cell processes; the expression of nestin is usually associated with dividing or migrating cells [36]. In addition, nestin expression has been reported in Kit-immunoreactive ICC and in gastrointestinal stromal tumours (GIST) which are mesenchymal tumours thought to derive from the ICCs [37]. These results showing nestin immunoreactivity (-ir) in intramuscular ICC of the rabbit duodenum agree with the results found by Vanderwinden [38]. He performed a detailed analysis of high-resolution images and double immunohistochemical staining in the human small intestine and demonstrated that most myenteric and intramuscular ICCs were nestinir, while nestinir was not detected in ICCs in the longitudinal musculature or in the submuscular plexus. It is thought that nestin expression may indicate some functional subdivision in the subpopulations of ICC [38].
The myenteric plexus is endowed with a considerable ability of regeneration and plasticity [39]. Some recent reports suggest that the postnatal enteric nervous system contains multipotent stem cells which persist throughout life and are capable of differentiating to neurons, glia and other cell types [40, 41]. Suarez-Rodriguez [42] describes an in vitro culture system where the presence, replication and differentiation of nestin positive cells in the postnatal intestine are observed. The replication and differentiation pattern of these cells suggest that they are enteric neural stem cells. The location of these multipotent cells in the in vivo intestine is a subject of debate. Given that one subpopulation of ICCs is immunoreactive for nestin, could these cells be considered as candidates?
Although self-renewal is difficult to demonstrate in vivo, this study reveals by means of a proliferation marker (Ki-67) [43, 44] that there are cells in different phases of the cell cycle in the periganglionic region and in intramuscular connective tissue. These cells seem to present the same location of ICCs.
Cajal described in detail two different morphologies in the ICCs of the amphibian intestine [1] and in several mammals [2]. These morphologies agree with the morphologic types that we have found in the rabbit duodenum: fusiform and triangular or stellate. Stellate ICCs are located mainly in the circular muscle layer. On the other hand, ICCs that spread around the mienteric plexus present triangular or fusiform shapes. Komuro [45] published one of the best morphologic studies on ICCs in the large intestine of the rabbit and he also found these two ICC morphologies. Our studies on the enteric nervous system have allowed us to confirm that, as in rabbits, there are only two morphological types of ICCs in Amphibians [46–48] and Reptiles [49, 50]. This seems to be a constant through the phylogenetic scale.
Min described in the human small intestine two morphological types of ICCs. He argues that the second cell type (type II) may represent a lower degree of differentiation than the cells defined as type I due to their ultrastructural characteristics [51]. Recent publications have showed evidences of plasticity in these cells [7, 52]. The ultrastructural characteristics that we have described are in agreement with the gold standard established criteria for positive diagnosis of ICCs [53]. Without any doubt, the phenotypical diversity of these cells should reflect in qualitative and quantitative variations in different organelles. For example, intramuscular ICCs show a large nucleus with scarce chromatin and a thin perinuclear cytoplasm, and relatively few cytoplasmic organelles. A voluminous nucleus in relation to the cytoplasm might suggest their capability to proliferate.
The presence of a primary cilium in ICC has been demonstrated for the first time. We would suggest that this is possibly the most relevant ultrastructural characteristic to be found in this study. The primary cilium is a single non-motile cilium derived from the parental centriole of the diplosomal pair that relocates to a position beneath the cell membrane after mitosis [54]. There are several structural differences between primary cilia and motile cilia: reduced length, lack of motility due to the absence of dynein arms and the lack of a central pair of microtubules in their axonemes. Several authors have reported the presence of a single cilium emerging from a basal centriole as an ultrastructural characteristic of precursor cells. It has also been published that neuronal precursors in the embryonic neuroepithelium [55–57] and primary precursors of new neurons in the adult avian brain [58] extend a single cilium as well.
In mammals, adult neurogenesis occurs at two principal sites: the subventricular zone of the lateral ventricles, which generates olfactory bulb neurons, and the hippocampus. Subventricular zone astrocytes are the in vivo primary precursors in this region and behave as stem cells in vitro. It is likely that sub-ventricular zone astrocytes divide asymmetrically to yield another subventricular zone astrocyte and a short-lived transit-amplifying progenitor. These sub-ventricular zone astrocytes have a short single cilium [59–61]. Thus, the existence of a single cilium characterizes the subventricular zone astrocytes in the central nervous system. In the same way, a single cilium could be a characteristic of precursor cells in the enteric nervous system. Recent findings reveal that the primary cilium is an antenna displaying specific receptors and relaying signals from these receptors to the cell body [62]. A second hypothesis is that they are involved in controlling the cell cycle [63]. Further studies on the functional role of this primary cilium present in ICCs may provide clues about the cell biology of enteric neurogenesis.
Conclusions
ICCs around the ganglia in the myenteric plexus and in the connective tissue of the muscular circular layer of the rabbit duodenum have been characterized. These cells are immunoreactive for the precursor cells markers: c-Kit, CD34 and nestin. In the same location there are Ki-67 positive cells with proliferative capability. The most typical ultrastructural characteristics are: a voluminous nucleus, scarce perinuclear chromatin, long cytoplasmic prolongations and also the unions that they establish with each other and with smooth muscular cells.
The presence of a single cilium, with the same characteristics of those neural precursor cells in the subventricular zone of the central nervous system has been demonstrated. We suggest that at least one subpopulation of ICCs in the rabbit duodenum presents some immunohistochemical and ultrastructural characteristics of progenitor cells.
Acknowledgments
This research has received financial support from Diputación General de Aragón (Aragón, Spain), Project B43 and Instituto Aragonés de Ciencias de la Salud (PAMER 2007).
References
- 1.Ramón y Cajal S. Nota sobre el plexo de Auerbach de la rana. Trabajos del Laboratorio de Histología de la Facultad de Medicina de Barcelona. 1892:23–8. [Google Scholar]
- 2.Ramón y Cajal S. Los ganglios y plexos nerviosos del intestino de los mamíferos y pequeñas adiciones a nuestros trabajos sobre la médula y gran simpático general. Imprenta y Librería de Nicolás Moya (Madrid) 1893:5–37. [Google Scholar]
- 3.Faussone-Pellegrini MS. Histogenesis, structure and relationship of interstitial cells of Cajal (ICC): from morphology to functional interpretation. Eur J Morphol. 1992;30:137–48. [PubMed] [Google Scholar]
- 4.Faussone-Pellegrini MS, Thuneberg L. Guide to the identification of interstitial cells of Cajal. Microsc Res Tech. 1999;47:248–66. doi: 10.1002/(SICI)1097-0029(19991115)47:4<248::AID-JEMT4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 5.Komuro T, Tokui K, Zhou DS. Identification of the interstitial cells of Cajal. Histol Histopathol. 1996;11:769–86. [PubMed] [Google Scholar]
- 6.Thuneberg L. One hundred years of interstitial cells of Cajal. Microsc Res Tech. 1999;47:223–38. doi: 10.1002/(SICI)1097-0029(19991115)47:4<223::AID-JEMT2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Min KW, Leabu M. Interstitial cells of Cajal (ICC) and gastrointestinal stromal tumor (GIST): facts, speculations, and myths. J Cell Mol Med. 2006;10:995–1013. doi: 10.1111/j.1582-4934.2006.tb00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thuneberg L. Interstitial cells of Cajal. In: Wood JD, editor. Hadbook of Physiology. The Gastrointestinal system I. Chapter 10. Bethesda, MD: American Physiological Society; 1989. [Google Scholar]
- 9.Ward SM, Sanders KM. Physiology and pathophysiology of the interstitial cells of Cajal: From bench to bedside I. Functional development and plasticity of interstitial cells of Cajal networks. Am J Physiol Gastrointest Liver Physiol. 2001;281:G602–11. doi: 10.1152/ajpgi.2001.281.3.G602. [DOI] [PubMed] [Google Scholar]
- 10.Ward SM, Sanders KM, Hirst GD. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol Motil. 2004;16:112–7. doi: 10.1111/j.1743-3150.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 11.Ramón y Cajal S. Terminación de los nervios y tubos glandulares del páncreas de los vertebrados. Trabajo del Laboratorio de la Facultad de Medicina de Barcelona. 1891:1–15. [Google Scholar]
- 12.Popescu LM, Hinescu ME, Ionescu N, Ciontea SM, Cretoiu D, Ardelean C. Interstitial cells of Cajal in pancreas. J Cell Mol Med. 2005;9:169–90. doi: 10.1111/j.1582-4934.2005.tb00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popescu LM, Gherghiceanu M, Hinescu ME, Cretoiu D, Ceafalan L, Regalia T, Popescu AC, Ardeleanu C, Mandache E. Insights into the interstitium of ventricular myocardium: interstitial Cajal-like cells (ICLC) J Cell Mol Med. 2006;10:429–58. doi: 10.1111/j.1582-4934.2006.tb00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pace-making cells in the rabbit urethra. J Physiol. 2000;526:359–66. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCloskey KD, Gurney AM. Kit positive cells in the guinea pig bladder. J Urol. 2002;168:832–6. [PubMed] [Google Scholar]
- 16.Davidson RA, McCloskey KD. Morphology and localization of interstitial cells in guinea pig bladder: structural relationship with smooth muscle and neurons. J Urol. 2005;173:1385–90. doi: 10.1097/01.ju.0000146272.80848.37. [DOI] [PubMed] [Google Scholar]
- 17.Pezzone MA, Watkins SC, Alber SM, King WE, De Groat WC, Chancellor MB, Fraser MO. Identification of c-kit- positive cells in the mouse ureter: the interstitial cells of Cajal of the urinary tract. Am J Physiol Renal Physiol. 2003;284:925–9. doi: 10.1152/ajprenal.00138.2002. [DOI] [PubMed] [Google Scholar]
- 18.Harhun MI, Pucovsky V, Povstyan OV, Gordienko DV, Bolton TB. Interstitial cells in the vasculature. J Cell Mol Med. 2005;9:232–43. doi: 10.1111/j.1582-4934.2005.tb00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciontea SM, Radu E, Regalia T, Ceafalan L, Cretoiu D, Gherghiceanu M, Braga RI, Malincenco M, Zagrean L, Hinescu ME, Popescu LM. C-Kit immunopositive interstitial cells (Cjal-type) in human myometrium. J Cell Mol Med. 2005;9:407–20. doi: 10.1111/j.1582-4934.2005.tb00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huizinga JD, Faussone-Pellegrini MS. About the presence of interstitial cells of Cajal outside the musculature of the gastrointestinal tract. J Cell Mol Med. 2005;9:468–73. doi: 10.1111/j.1582-4934.2005.tb00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faussone-Pellegrini MS. Interstitial cells of Cajal: once negligible players, now blazing protagonists. Ital J Anat Embryol. 2005;110:11–31. [PubMed] [Google Scholar]
- 22.Popescu LM, Ciontea SM, Cretoiu D, Hinescu ME, Radu E, Ionescu N, Ceausu M, Gherghiceanu M, Braga RI, Vasilescu F, Zagrean L, Ardeleanu C. Novel type of interstitial cell (Cajal-like) in human fallopian tube. J Cell Mol Med. 2005;9:479–523. doi: 10.1111/j.1582-4934.2005.tb00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popescu LM, Gherghiceanu M, Cretoiu D, Radu E. The conective connection: interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells. J Cell Mol Med. 2005;9:714–30. doi: 10.1111/j.1582-4934.2005.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gherghiceanu M, Popescu LM. Interstitial Cajal-like cells (ICLC) in human resting mammary gland stroma. J Cell Mol Med. 2005;9:893–910. doi: 10.1111/j.1582-4934.2005.tb00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecoin L, Gabella G, Le Dourain N. Origin of the c-kit-positive interstitial cells in the avian bowel. Development. 1996;122:725–33. doi: 10.1242/dev.122.3.725. [DOI] [PubMed] [Google Scholar]
- 26.Young HM, Ciampoli D, Southwell BR, Newgreen DF. Origin of interstitial cells of Cajal of the mouse intestine. Dev Biol. 1996;180:97–107. doi: 10.1006/dbio.1996.0287. [DOI] [PubMed] [Google Scholar]
- 27.Young HM. Embryological origin of interstitial cells of Cajal. Microsc Res Tech. 1999;47:303–8. doi: 10.1002/(SICI)1097-0029(19991201)47:5<303::AID-JEMT1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 28.Wu JJ, Rothman TP, Gershon MD. Development of the interstitial cell of Cajal: origin, kit dependence and neuronal and nonneuronal sources of kit ligand. J Neurosci Res. 2000;59:384–401. doi: 10.1002/(SICI)1097-4547(20000201)59:3<384::AID-JNR13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Wallace AS, Burns AJ. Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res. 2005;319:367–82. doi: 10.1007/s00441-004-1023-2. [DOI] [PubMed] [Google Scholar]
- 30.Sohal GS, Ali MM, Farooqui FA. A second source of precursor cells for the developing enteric nervous system and interstitial cells of Cajal. Int J Dev Neurosci. 2002;20:619–26. doi: 10.1016/s0736-5748(02)00103-x. [DOI] [PubMed] [Google Scholar]
- 31.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–7. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torihashi S, Gerthoffer WT, Kobayashi S, Sanders KM. Identification and classification of interstitial cells in the canine proximal colon by ultrastructure and immunocytochemistry. Histochemistry. 1994;101:169–83. doi: 10.1007/BF00269542. [DOI] [PubMed] [Google Scholar]
- 33.Torihashi S, Ward SM, Nishikawa SI, Nishi K, Kobayashi S, Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine intestine. J Physiol. 1994;480:91–7. [Google Scholar]
- 34.Lendahl U, Zimmerman LB, McKey RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–95. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 35.Almazan G, Vela JM, Molina-Holgado E, Guaza C. Re-evaluation of nestin as a marker of oligodendrocite lineage cells. Microsc Res Tech. 2001;52:753–65. doi: 10.1002/jemt.1060. [DOI] [PubMed] [Google Scholar]
- 36.Sahlgren CM, Mikhailov A, Hellman J, Chou YH, Lendahl U, Goldman RD, Eriksson JE. Mitotic reorganization of the intermediate filament protein nestin involves phosphorylation by cdc2 kinase. J Biol Chem. 2001;276:16456–63. doi: 10.1074/jbc.M009669200. [DOI] [PubMed] [Google Scholar]
- 37.Tsujimura T, Makiishi-Shimobayashi C, Lundkvist J, Lendahl U, Nakasho K, Sugihara A, Iwasaki T, Mano M, Yamada N, Yamashita K, Toyosaka A, Terada N. Expression of the intermediate filament nestin in gastrointestinal stromal tumors and interstitial cells of Cajal. Am J Pathol. 2001;158:817–23. doi: 10.1016/S0002-9440(10)64029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanderwinden JM, Gillard K, DeLaet MH, Messam CA, Schiffmann SN. Distribution of the intermediate filament nestin in the mucularis propria of the human gastrointestinal tract. Cell Tissue Res. 2002;309:261–68. doi: 10.1007/s00441-002-0590-3. [DOI] [PubMed] [Google Scholar]
- 39.Hanani M, Ledder O, Yutkin V, Abu-Dalu R, Huang TY, Hartig W, Vannucchi MG, Faussone-Pellegrini MS. Regeneration of myenteric plexus in the mouse colon after experimental denervation with benzalkonium. J Comp Neurol. 2003;462:315–27. doi: 10.1002/cne.10721. [DOI] [PubMed] [Google Scholar]
- 40.Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657–69. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bondurand N, Natarajan D, Thapar N, Atkins C, Pachnis V. Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development. 2003;130:6387–400. doi: 10.1242/dev.00857. [DOI] [PubMed] [Google Scholar]
- 42.Suarez-Rodriguez R, Belkind-Gershon J. Cultured nestin positive cells from postnatal mouse small bowel differentiate ex vivo into neurons, glia, and smooth muscle. Stem Cells. 2004;22:1373–85. doi: 10.1634/stemcells.2003-0049. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt MH, Broll R, Bruch HP, Finniss S, Bogler O, Duchrow M. Proliferation marker pKi-67 affects the cell cycle in a self-regulated manner. J Cell Biochem. 2002;87:334–41. doi: 10.1002/jcb.10302. [DOI] [PubMed] [Google Scholar]
- 44.Endl E, Gerdes J. The Ki-67 Protein: Fascinating forms and unknown function. Exp Cell Res. 2000;257:231–7. doi: 10.1006/excr.2000.4888. [DOI] [PubMed] [Google Scholar]
- 45.Komuro T. The interstitial cells in the colon of the rabbit. Cell Tissue Res. 1982;222:41–52. doi: 10.1007/BF00218287. [DOI] [PubMed] [Google Scholar]
- 46.Junquera C, Azanza MJ, Parra P, Peg MT, Garin P. The enteric nervous system of Rana ridibundastomach. Gen Pharmacol. 1986;17:597–605. doi: 10.1016/0306-3623(86)90102-3. [DOI] [PubMed] [Google Scholar]
- 47.Junquera C, Azanza MJ, Parra P, Peg MT, Garin P. The autonomic innervtion of Rana ridibunda intestine. Comp Biochem Physiol. 1987;87C:335–44. doi: 10.1016/0742-8413(87)90018-1. [DOI] [PubMed] [Google Scholar]
- 48.Junquera C, Azanza MJ, Parra P, Peg MT, Aisa J, Romero LM, Garin P. Intrinsic and extrinsic innervation of the amphibians esophagic myenteric plexuses. Histol Histopathol. 1988;3:115–24. [PubMed] [Google Scholar]
- 49.Martínez-Ciriano C, Junquera C, Castiella T, Gomez-Barrena E, Aisa J, Blasco J. Intrinsec innervation in the intestine of the lizzard Podarcis hispanica. Histol Histopathol. 2000;15:1093–105. doi: 10.14670/HH-15.1093. [DOI] [PubMed] [Google Scholar]
- 50.Junquera C, Martínez-Ciriano C, Castiella T, Serrano P, Aisa J, Calvo E, Lahoz M. Enteric plexus and interstitial cells of Cajal: interrelationship in the stomach of Podarcis hispanica (Reptilia). An ultra-structural study. Histol Histopathol. 2001;16:869–81. doi: 10.14670/HH-16.869. [DOI] [PubMed] [Google Scholar]
- 51.Min KW, Seo IS. Interstitial cells of Cajal in the human small intestine: Immunochemical and ultrastructural study. Ultrastructural Pathology. 2003;27:67–78. doi: 10.1080/01913120309927. [DOI] [PubMed] [Google Scholar]
- 52.Faussone-Pellegrini MS, Vannucchi MG, Ledder O, Huang TY, Hanani M. Plasticity of interstitial cells of Cajal: a study of mouse colon. Cell Tissue Res. 2006;325:211–7. doi: 10.1007/s00441-006-0174-8. [DOI] [PubMed] [Google Scholar]
- 53.Huizinga JD, Thuneberg L, Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal as targets for pharmacological intervention in gastrointestinal motor disorders. Trends Pharmacol Sci. 1997;18:393–403. doi: 10.1016/s0165-6147(97)01108-5. [DOI] [PubMed] [Google Scholar]
- 54.Rieder CL, Jensen CG, Jensen LC. The resorption of primary cilia during mitosis in a vertebrate (PtK1) cell line. J Ultrastruct Res. 1979;68:173–85. doi: 10.1016/s0022-5320(79)90152-7. [DOI] [PubMed] [Google Scholar]
- 55.Sotelo JR, Trujillo-Cenóz O. Electron microscope study on the development of ciliary components of the neural epithelium of the chick embryo. Z Zellforsch. 1958;49:1–12. doi: 10.1007/BF00335059. [DOI] [PubMed] [Google Scholar]
- 56.Cohen E, Meininger V. Ultrastructural analysis of primary cilium in the embryonic nervous tissue of mouse. Int J Dev Neurosci. 1987;5:43–51. doi: 10.1016/0736-5748(87)90047-5. [DOI] [PubMed] [Google Scholar]
- 57.Cohen E, Binet S, Meininger V. Ciliogenesis and centriole formation in the mouse embryonic nervous system. An ultrastructural analysis. Biol Cell. 1988;62:165–9. [PubMed] [Google Scholar]
- 58.Alvarez-Buylla A, García-Verdugo JM, Mateo A, Merchant-Larios H. Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J Neurosci. 1998;18:1020–37. doi: 10.1523/JNEUROSCI.18-03-01020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci. 1999;96:11619–24. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alvarez-Buylla A, García-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nature Reviews Neuroscience. 2001;2:287–93. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 61.Doetsch F. The glial identity of neural stem cells. Nature Neuroscience. 2003;6:1127–34. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 62.Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Current Opinion in Cell Biology. 2003;15:105–10. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 63.Tucker RW, Pardee AB. Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell. 1979;17:527–35. doi: 10.1016/0092-8674(79)90261-7. [DOI] [PubMed] [Google Scholar]


