Abstract
Inflammation is associated with both acute and chronic neurological disorders, including stroke and Alzheimer's disease (AD). Cytokines such as interleukin (IL)-1 have several activities in the brain both under physiological and pathophysiological conditions. The objective of this study was to evaluate consequences of the central blockade of IL-1 transmission in a previously developed transgenic mouse strain with brain-directed overexpression of human soluble IL-1 receptor antagonist (Tg hsIL-1ra). Effects on brain morphology and brain levels of the AD-related proteins β-amyloid precursor protein (APP) and presenilin 1(PS1), as well as the levels of IL-1β, IL-6 and tumour necrosis factor-α (TNF-α) were analysed in homozygotic and heterozygotic mice and wild type (WT) controls, of both genders and of young (30–40 days) and adult (13–14 months) age. A marked reduction in brain volume was observed in transgenic mice as determined by volumetry. Western blot analysis showed higher levels of APP, but lower levels of PS1, in adult animals than in young ones. In the cerebellum, heterozygotic (Tg hsIL-1ra+/−) mice had lower levels of APP and PS1 than WT mice. With one exception, there were no genotypic differences in the levels of IL-1β, IL-6 and TNF-α. The cytokine levels were generally higher in adult than in young mice. In conclusion, the chronic blockade of IL-1 signalling in the brain was associated with an atrophic phenotype of the brain, and with modified levels of APP and PS1. Brain-directed overexpression of hsIL-1ra was not followed by major compensatory changes in the levels of pro-inflammatory cytokines.
Keywords: amyloid precursor protein, brain volume, interleukin, mouse, presenilin, transgenic
Introduction
There is increasing evidence for interactions between the cells and signals of the immune system and the nervous system, both with regard to the regulation of physiological processes, and the involvement of inflammatory mechanisms in the pathophysiology of certain neurodegenerative disorders, including Alzheimer's disease (AD). Cytokines are pleiotropic, low molecular weight proteins that are involved in physiological and pathological processes, partly through their activities in the brain. Interleukin-1 (IL-1) is one of the most important proinflammatory cytokines in the immune response to infection, and is also implicated in neuroinflammation associated with several neurodegenerative disorders (see ref. [1]), such as cerebral ischaemia [2, 3] and AD [4]. In addition, IL-1 is involved in many physiological processes, including brain development [5], glucose and lipid metabolism, modulation of sleep and neuroendocrine regulation (see refs. [1, 6]), as well as in the modulation of learning and memory processes [7, 8]. The IL-1 system is composed of three major ligands, i.e. the agonists IL-1α and IL-1β and the endogenous receptor antagonist, IL-1ra. An intracellular response is initiated by the binding of IL-1α or IL-1β to the signalling receptor IL-1 receptor type I (IL-1RI), leading to its association with the IL-1R accessory protein (IL-1RAcP) [9]. IL-1ra acts as a selective, competitive antagonist of IL-1-induced activity, and binds to the signalling IL-1RI without the following association with IL-1RAcP [9]. Four isoforms of IL-1ra have been identified, a 17-kDa secretory form [10], and three intracellular forms of 18, 25 and 15 kDa, respectively [11], suggesting a complex regulation of IL-1 signalling. The expression of IL-1ra is induced in the brain in several models of neurological disease, such as upon peripheral administration of kainic acid (KA), as model for human temporal lobe epilepsy [12–15], and following cerebral ischaemia [16, 17]. Notably, peripheral administration of IL-1ra [18], and delivery of IL-1ra into the brain by means of adenoviral transfection [19, 20] provided neuroprotection in focal cerebral ischaemia in rodents. The recent report showing that IL-1ra may have agonistic activities in the hippocampus, independent of IL-1RI [21], can only stimulate the research in this area.
Inflammatory processes appear to play an important role in AD, but the cellular and molecular events of inflammation in relation to neuronal cell death are not fully elucidated. An increasing body of evidence suggesting the involvement of IL-1 in the pathogenesis of AD has accumulated in the latest years (for review see ref. [22]). Cytokines such as IL-1 and interleukin-6 (IL-6) have been shown to affect the synthesis, metabolism and secretion of β-amyloid precursor protein (APP) [23–26], the precursor for β-amyloid (Aβ) peptide, the main constituent in amyloid plaques. A recent study showed that IL-1β and TNF-α influence the metabolism of APP by stimulating γ-secretase cleavage [26]. Presenilin 1 (PS1) is part of the protein complex performing the γ-cleavage activity [27], and disease-causing mutations of PS1 have been found in several families with hereditary AD [28–30].
The aim of the present study was to investigate the influence of IL-1ra on the brain volume and the levels of proteins involved in the neuropathology in AD, i.e. APP and PS1, using the transgenic mouse strain with brain-directed overexpression of human soluble IL-1ra (Tg hsIL-1ra) [31]. In view of the commonly occurring compensatory mechanisms in genetically modified animals, the brain levels of IL-1β, and other proinflammatory cytokines influenced by IL-RI-mediated activity, such as IL-6 [32] and tumour necrosis factor-α (TNF-α) [33, 34], are analysed in parallel to the transgenic expression of human IL-1ra.
Materials and methods
Animals
Tg hsIL-1ra was developed previously using C57B6/CBA (Charles River, Germany) as the background strain [31]. The promoter for murine glial fibrillary acidic protein (GFAP) was used to limit the expression of hsIL-1ra to the central nervous system (CNS).
Homozygotic (Tg hsIL-1ra+/+), heterozygotic (Tg hsIL-1ra+/−) and WT littermates of 30–40 days and 13–14 months were bred in the animal facility at Karolinska University Hospital Huddinge, and used for the studies on cytokine production. In addition, age- and gender-matched WT mice (C57B6/CBA, Charles River, Germany) obtained from the same provider as the animals used for generation of the transgenic mice were used for the morphological studies. The animals were housed under controlled conditions of temperature (21.0 ± 0.4°C), relative humidity (55–65%) and light-dark cycle (12:12 hrs, lights on at 06:00 hrs). Food and tap water were available ad libitum. The experimental procedures were approved by the Stockholm South local committee on ethics of animal experiments (S30–02).
Animal genotyping
Animal genotyping was performed on DNA extracted and purified with the GenElute kit (Sigma, St Louis, MO, USA) from tissue obtained from earmarking the animals. Polymerase chain reaction (PCR) was performed using specific primers (Thermo Electron, GmbH, Ulm, Germany) for hsIL-1ra (sense: 5’-CGACCCTCTGGGAGAAAATC-3’, anti-sense: 5’-CTCATCACCAGACTTGACAC-3’) and mouse β-actin (sense: 5’-AGGGAAATCGTGCGTGACAT- 3’, anti-sense: 5’-CATCTGCTGCAAGGTGGACA-3’, the latter used for normalising the levels of hsIL-1ra to a mouse house-keeping protein. Briefly, primers and sample DNA were added in equimolar amounts (0.2 μM), together with 0.2 μl Taq-polymerase, 0.2 μl Taq-antibody, 1.5 μl MgCl2, 5 μl reaction buffer, 50 μM dNTP (all from Invitrogen AB, Uppsala, Sweden) and distilled H2O, to a final volume of 50 μl. Amplification was performed in an Eppendorf Thermocycler as follows: (1) 94°C, 5 min; (2) 94°C, 30 sec; (3) X°C, 5 min; (4) 72°C, 1 min; (2) to (4) was repeated 11 times with X starting on 56°C, and lowered with 0.5°C each iteration until 51°C; (5) 94°C, 30 sec; (6) 51°C, 5 min; (7) 72°C, 1 min; (5) to (7) was repeated 15 times; (8) 72°C, 7 min; (9) 4°C. The PCR-product was analysed by electrophoresis in Tris-borate- ethylenediaminetetraacetic acid (EDTA)-buffered agarose gels (2% wt/vol). The gels were stained with ethidium bromide and scanned in ultraviolet light. There was no band at the size of hsIL-1ra in samples from the WT mice.
Morphological studies
Measurements of brain volume
Nineteen male 30–40 days old WT (n = 6), Tg hsIL-1ra+/− (n = 7) and Tg hsIL-1ra+/+ (n = 6), and 26 male 12 months old WT (n = 8), Tg hsIL-1ra+/− (n = 9) and Tg hsIL-1ra+/+ (n = 9) mice were used for the assessment of total brain volume, using the principle of communicating vessels. Briefly, we used a custom-made device, consisting of two plastic syringes, of 10 and 1 ml, respectively, connected with plastic tubing and filled with phosphate-buffered saline (PBS), 0.01 M, pH 7.4. The 10-ml syringe was positioned vertically and served as a container for the mouse brain, while the 1-ml syringe was positioned horizontally and used for reading the volume dislocated by the brain. Subsequently, each brain was transferred into a solution of 4% para-formaldehyde (PF) in 0.1 M Sörensen phosphate buffer, pH 7.4 and stored at 4°C until further processing.
Preparation of brain tissue samples
A total of 59 littermates at the age of 30–40 days (n = 29) including male WT (n = 6), Tg hsIL-1ra+/− (n = 5) and Tg hsIL-1ra+/+ (n = 6) mice, and female WT (n = 3), Tg hsIL- 1ra+/− (n = 7) and Tg hsIL-1ra+/+ (n = 2) mice, and at the age of 13–14 months (n = 30) including male WT (n = 4), Tg hsIL-1ra+/− (n = 5) and Tg hsIL-1ra+/+ (n = 5) mice and female WT (n = 4), Tg hsIL-1ra+/− (n = 6) and Tg hsIL-1ra+/+ (n = 6) mice, were used for ELISA and Western blot analysis. The animals were sacrificed by decapitation and the hippocampus, cerebellum, and parietal cortex, were rapidly dissected out on ice, frozen and stored at (80°C until further processing. The tissues were immersed in ice-cold lysis buffer containing 20 mM Tris-HCl, 137 mM NaCl, 2% Nonidet P-40, 2% Triton X and a 1% protease inhibitor cocktail (Sigma-Aldrich, Inc., St. Louis, MO, USA), and sonicated (Soniprep 150, MSE Ltd., Crawley, UK) in three cycles of 15-sec pulses with 20-sec cooling between pulses. The samples were centrifuged at 11.000 rpm for 10 min at 4°C and supernatants stored at –80°C. A bichinchoninic acid kit (Sigma-Aldrich) was used for protein determination.
Cytokine measurements
Levels of the transgene product (hsIL-1ra), and of murine (m) IL-1β, mIL-6 and mTNF-α, were analysed by ELISA Duoset Development kits (RD systems, Abingdon, UK) according to the manufacturer's protocol, in samples prepared (see above) of the hippocampus, parietal cortex and cerebellum from transgenic and WT mice.
Western blot analysis
Western blot analysis of APP and PS1 was performed on hippocampal and cerebellar samples from 48 littermates (also used for the determination of cytokine levels) at the age of 30–40 days (n = 24) including male WT (n = 5), Tg hsIL-1ra+/− (n = 4) and Tg hsIL-1ra+/+ (n = 5) mice and female WT (n = 3), Tg hsIL-1ra+/− (n = 5) and Tg hsIL-1ra+/+ (n = 2) mice, and at 13–14 months age (n = 24) including male WT (n = 4), Tg hsIL-1ra+/− (n = 4) and Tg hsIL-1ra+/+ (n = 4) mice and female WT (n = 4), Tg hsIL-1ra+/− (n = 4) and Tg hsIL-1ra+/+ (n = 4) mice. Each sample, containing 20-μg protein was loaded on SDS-PAGE 10% polyacrylamide gels. Proteins were transferred onto Hybond ECL nitrocellulose membranes (Amersham Biosciences, Little Chalfont, UK) by trans-blot electrophoretic transfer for 2 hrs at a constant current of 220 mA. After blocking for 30 min using 5% dried milk in Tris-buffered solution, the membranes were incubated over night with mouse monoclonal antibodies to APP at 1:1000 (22C11, reacting with the NH2-terminus; Chemicon, Temecula, CA, USA) or with rabbit antibodies to PS1 at 1: 2000 (reacting with the COOHterminus, Calbiochem, Darmstad, Germany). After washing in Tris-buffer containing 0.1% Tween-20 for 2 × 15 min, the membranes were incubated for 1.5 hrs with horseradish peroxidase (HRP)-linked anti-mouse IgG (1: 5000; Amersham Biosciences). Bound antibodies were detected by the enhanced chemiluminiscence (ECL) method (Amersham Biosciences), and exposure to Hyperfilm MP (Amersham Biosciences). The optical density (OD) was determined using the NIH IMAGE software, v.1.63. In order to ensure loading of equal amounts of protein, the membranes were stained with Ponceau S solution (Sigma-Aldrich).
Statistics
Two-way factorial ANOVA was used for analysis of statistically significant differences in cytokine levels, total brain volume, and levels of APP and PS1. Bonferroni's post hoctest was used to identify groups contributing to the significance.
Statistical comparisons of cytokine, APP and PS1 levels between male and female young mice were not performed, due to the low number of young female Tg hsIL-1ra+/+ and WT mice (n = 2).
Correlations between levels of the transgene (hsIL-1ra), the proinflammatory cytokines, APP and PS1, were performed using simple linear regression analysis.
The statistica 6.01 software (StatSoft Scandinavia AB, Uppsala, Sweden) was used and a threshold of P < 0.05 was considered significant. The results are expressed as mean ± standard error of the mean (SEM).
Results
Total brain volume
The analysis of total brain volume was based on young (30–40 days) and adult (12 months) male mice of all genotypes. All groups contained at least 6 animals (for details see Materials and methods). The total brain volume was significantly influenced by the genotype (F(2, 39) = 12.05, P < 0.001). The post hoc analysis revealed that the brain volume of the Tg hsIL-1ra+/+ mice was lower than the brain volume in Tg hsIL-1ra+/− (P < 0.001) and WT (P < 0.001) mice, respectively (Fig. 1A). The age did not influence the total brain volume, and there was no significant interaction between genotype and age. Representative frontal sections showing the atrophic brain phenotype in Tg hsIL-1ra+/+ mice are shown in Figure 1B.
1.
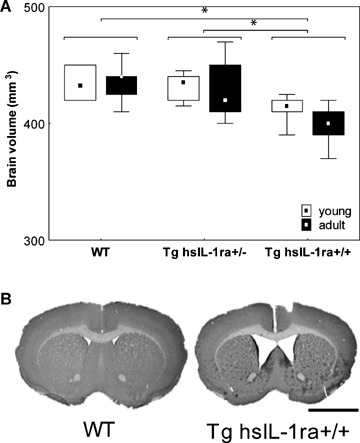
Total brain volume (A) in mice with transgenic expression of human soluble interleukin-1 receptor antagonist (hsIL-1ra) with homozygotic (Tg hsIL-1ra+/+) and heterozygotic (Tg hsIL-1ra+/−) genotype, and of wild type (WT) mice, of young (1month) and adult (13–14 months) age. Tg hsIL-1ra+/+ mice have a smaller brain volume than Tg IL-1ra+/− mice (*P < 0.001) and WT mice (*P < 0.001), respectively (A). B shows cresyl violet-stained sections of the brain at bregma +0.6 mm, from two adult mice, WT and homozygotic (Tg hsIL-1ra+/+), respectively, illustrating the reduced brain volume and enlarged ventricles. Scale bar = 2.5 mm.
Cytokine measurements
The measurements of hsIL-1ra, mIL-1β, mTNF-α and mIL-6, were performed in tissue extracts of the hippocampus, parietal cortex and cerebellum of Tg hsIL-1ra+/+, Tg hsIL-1ra+/− and their WT littermates, of 30–40 days and 13–14 months age. For details on the animal groups see Materials and methods.
hsIL-1ra
The genotype influenced the levels of hsIL-1ra in all brain regions analysed (Fig. 2A–F). The concentrations were approximately twice as high in the Tg hsIL-1ra+/+ mice as compared to the levels in the Tg hsIL-1ra+/− mice.
2.
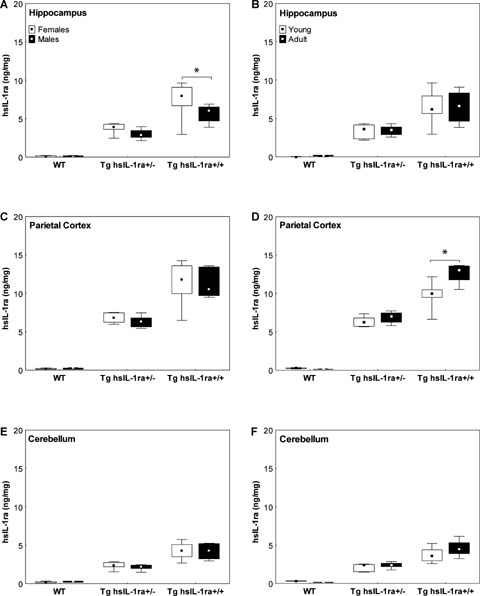
The levels of human interleukin-1 receptor antagonist (hIL-1ra) in hippocampus (A, B), parietal cortex (C, D) and cerebellum (E, F) of male and female mice with transgenic expression of human soluble IL-1ra (hsIL-1ra) with homozygotic (Tg hsIL-1ra+/+) and heterozygotic (Tg hsIL-1ra+/−) genotype, and of wild type (WT) mice. The levels are gene dosage-dependent in all the three brain regions (A–F; statistical data given in Results). In the hippocampus, female mice have higher levels than male mice (*P < 0.001) (A). An age-dependent increase is seen in the parietal cortex of homozygotic mice (*P < 0.001) (D).
In the hippocampus, there was a significant interaction effect between gender and genotype (F(2, 53) = 3.94, P < 0.05), i.e. the female Tg hsIL-1ra+/+ mice had higher levels of hsIL-1ra than the male Tg hsIL-1ra+/+ (P < 0.001) (Fig. 2A). A similar difference was observed when the data from adult mice were analysed separately (F(2, 24) = 9.44, P < 0.001) (data not shown). There was no influence of age on the levels of hsIL-1ra in the hippocampus (Fig. 2B).
In the parietal cortex, there was a significant interaction effect between age and genotype (F(2, 53) = 17.32, P < 0.001), i.e. the adult Tg hsIL-1ra+/+ mice had higher levels of hsIL-1ra than the young Tg hsIL-1ra+/+mice (P < 0.001) (Fig. 2D). A similar difference was observed when the data from male mice were analysed separately (F(2, 25) = 7.97, P < 0.01) (data not shown). Gender did not influence the concentrations of hsIL-1ra in the parietal cortex (Fig. 2C).
The levels of hsIL-1ra in the cerebellum were influenced only by genotype (Fig. 2E–F). Neither the gender (Fig. 2E), nor age (Fig. 2F) influenced the levels of hsIL-1ra, when the data from all of the animals were pooled together.
IL-1β
There was no difference in the levels of IL-1β between the different genotypes, but a significant age-dependent increase in the levels of IL-1β was found in the hippocampus (F(1, 53) = 4.57, P < 0.05) (Fig. 3A), parietal cortex (F(1, 53) = 6.53, P < 0.05) (Fig. 3B) and the cerebellum (F(1, 53) = 6.53, P <0.001) (Fig. 3C) when data for both genders were pooled. In addition, age-dependent increase was observed in the cerebellum for male mice (F(1, 25) = 62.00, P < 0.001) (data not shown).
3.
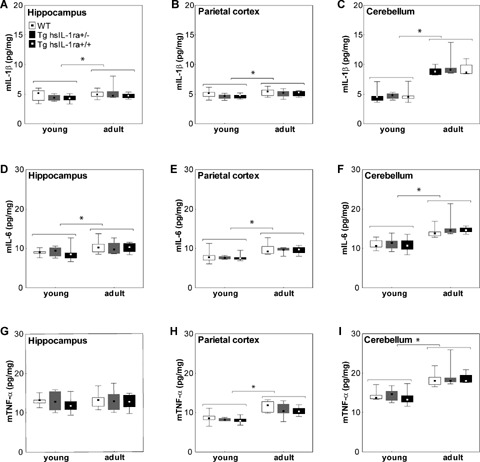
The levels of mouse interleukin-1β (mIL-1β) (A-C), IL-6 (D-F) and TNF-α (G-I) in the hippocampus (A, D and G), parietal cortex (B, E and H) and cerebellum (C, F and I) of mice with transgenic expression of human soluble interleukin- 1 receptor antagonist (hsIL-1ra) with homozygotic (Tg hsIL-1ra+/+) and heterozygotic (Tg hsIL-1ra+/−) genotype, and of wild type (WT) mice, of young (1 month) and adult (13–14 months) age. Adult mice have higher levels of mIL- 1β than the young mice in the hippocampus (*P < 0.05) (A), parietal cortex (*P < 0.05) (B) and cerebellum (*P < 0.001) (C). The levels of mIL-6 are higher in the adult than young mice in the hippocampus (*P < 0.001) (D), parietal cortex (*P < 0.001) (E) and cerebellum (*P < 0.001) (F). Adult mice have higher levels of mTNF-α levels than young mice in the parietal cortex (*P < 0.001) (H) and cerebellum (*P < 0.001) (I), but not in the hippocampus (G).
There were no gender differences with regard to IL-1β levels in any of the brain regions studied.
IL-6
There was no difference in the levels of IL-6 between the different genotypes, but there was an agedependent increase in the hippocampus, (F(1, 53) = 12.82, P < 0.001) (Fig. 3D), parietal cortex (F(1, 53) = 44.89, P < 0.001) (Fig. 3E) and cerebellum (F(1, 53) = 44.89, P < 0.001) (Fig. 3F) when the data from animals of both genders were included. In addition, when the data male mice were analysed separately, we found higher levels of IL-6 in the parietal cortex and the cerebellum (F(1, 25) = 19.47, P < 0.001 and (F(1, 25) = 21.93, P < 0.001) (data not shown).
The gender influenced the IL-6 levels only in the hippocampus where the levels of IL-6 were higher in female than in male mice, when data from both young and adult mice were pooled (F[1, 53]= 4.32, P < 0.05) (data not shown), as well as when comparing the data from the adult mice (F[1, 24]= 4.45, P < 0.05) (data not shown).
TNF-α
There were no significant differences in the hippocampal levels of TNF-α, neither as an effect of age (Fig. 3G), gender or genotype. Old mice had higher levels of TNF-α than young mice in the parietal cortex (F(1, 53) = 50.61, P < 0.001) (Fig. 3H), and cerebellum (F(1, 53) = 50.61, P < 0.001) when data from mice of both genders were pooled (Fig. 3I). A similar effect was found when data from male mice were analysed separately in the parietal cortex (F(1, 25) = 26.37, P < 0.001) and the cerebellum (F(1, 25) = 26.85, P < 0.001) (data not shown).
A significant effect of genotype in the parietal cortex was found in male mice when data from both young and adult were pooled (F(2, 25) = 4.42, P <0.05). The post hoc analysis showed that the concentration of TNF-α in the parietal cortex of the male Tg hsIL-1ra+/+ mice was significantly lower as compared to that in the male WT mice (P < 0.05).
The expression of TNF-α was not gender dependent in any of the brain regions analysed.
Analysis of APP and PS1
The levels of APP and PS1 in the hippocampus and cerebellum were measured by Western blotting (Fig. 4). The age influenced the levels of APP both in the hippocampus and cerebellum (Fig. 4B, C). Thus, higher levels were found in the hippocampus (F(1, 20) = 5.56, P < 0.05) (Fig. 4B) and cerebellum (F(1, 20) = 21.13, P < 0.001) (Fig. 4C), of adult male mice as compared to young male mice. The same effect was observed when mice of both genders were pooled, both for the hippocampus (F(1, 42) = 21.37, P < 0.001) (data not shown), and cerebellum (F(1, 42) = 41.07, P < 0.001) (data not shown).
4.
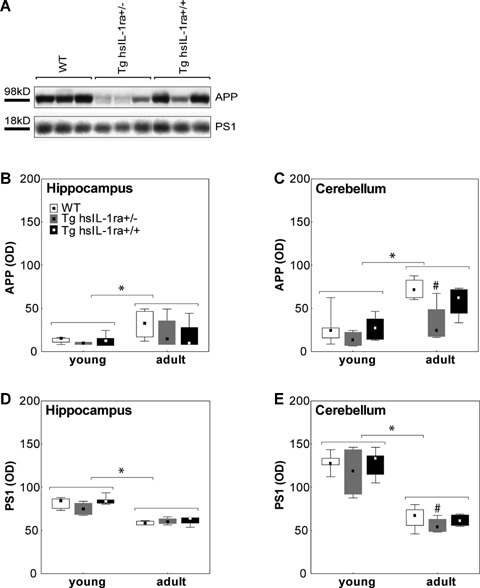
The levels of amyloid precursor protein (APP) (A–C) and presenilin-1 (PS1) (A, D and E) in the hippocampus (B and D) and cerebellum (A, C and E) of mice with transgenic expression of human soluble interleukin-1 receptor antagonist (hsIL-1ra) with homozygotic (Tg hsIL-1ra+/+) and heterozygotic (Tg hsIL-1ra+/−) genotype, and of wild type (WT) mice. Adult mice display higher levels of APP than young mice, both in the hippocampus (*P < 0.05) (B) and cerebellum (*P < 0.001) (C). In the male mice, APP levels are lower in Tg hsIL-1ra+/− mice than in WT mice (#P < 0.05) (A, C; adult mice). The above line in A shows a representative Western blot for APP, at the size of the full-length protein, in samples from the cerebellum of adult male mice. Regarding PS1 levels, young mice have higher levels than adult mice both in the hippocampus (*P < 0.001) (D) and cerebellum (*P < 0.001) (E). Tg hsIL-1ra+/− mice have lower levels of PS1 than adult WT in both males and females (#P < 0.05) (E; adult mice). The below line in A shows a representative Western blot for PS1, at the size of the full-length protein, in samples from the cerebellum of adult male mice.
The gender influenced the levels of APP in the cerebellum, but not hippocampus. Female mice had higher levels of APP in the cerebellum than the male mice, when data from adult animals were analysed separately (F(1, 18) = 6.86, P < 0.05) (data not shown), and when data from mice of both ages were pooled (F(1, 42) = 4.38, P < 0.05) (data not shown).
There was a significant effect on the levels of APP for genotype in the cerebellum, but not the hippocampus, of male mice, when both young and adult mice were pooled together (F(2, 20) = 5.18, P < 0.05) (data not shown). Comparison of the levels in the cerebellum of adult male mice showed that heterozygotic (Tg hsIL-1ra+/−) mice had lower levels than WT mice (P < 0.05) (Fig. 4A, C). The genotype effect did not reach statistical significance when the data from both genders were pooled (P = 0.06).
With some exceptions, the APP-levels were higher in the cerebellum than in the hippocampus when comparing the levels in each individual (see Fig. 4B, C). The levels of PS1 were influenced by age in male mice, i.e. higher levels were found in the hippocampus (F(1, 20) = 84.69, P < 0.001) (Fig. 4D) and cerebellum (F(1, 20) = 97.22, P < 0.001) (Fig. 4E), of young mice as compared to adult mice, regardless of genotype. The same effect was observed for the cerebellum (F(1, 42) = 155.85, P < 0.001), but not the hippocampus (F(1, 42) = 0.15), when mice of both genders were pooled (data not shown).
The levels of PS1 in the cerebellum were influenced by gender in adult mice, i.e. higher levels were found in female than in male mice (F(1, 18) = 19.71, P < 0.001) (data not shown), regardless of genotype. In the hippocampus, the levels were higher in adult female, than in adult male mice (F(1, 18) = 554.25, P < 0.001) (data not shown). When both young and adult animals were included, the levels of PS1 in the hippocampus were found to be higher in female than in male mice (F(1, 42) = 15.89, P < 0.001) (data not shown), whereas the levels in the cerebellum were not different.
There was a significant effect of genotype on the levels of PS1 in adult animals with regard to the cerebellum (F(2, 18) = 5.20, P < 0.05) (Fig. 4A, E), but not the hippocampus (F(2, 42) = 0.30) (data not shown). Thus, in the cerebellum of adult mice, male Tg hsIL-1ra+/− mice had lower levels of PS1 than the WT (P < 0.05) and Tg hsIL-1ra+/+ (P < 0.05) mice (Fig. 4A, E), and female Tg hsIL-1ra+/− mice had lower levels than the WT (P < 0.05) mice (data not shown).
Correlations between cytokines and AD-related proteins
Possible correlations between the levels of cytokines and AD-related proteins, as well as their relations to levels of the transgene, hsIL-1ra, were analysed in the hippocampus (Fig. 5) and cerebellum (Fig. 6)). Age affected the levels of most of the proteins analysed and therefore the correlation analyses were performed separately for young and adult animals. Only significant linear correlations are indicated. There was no significant correlation between the levels of the transgene and those of the proinflammatory cytokines IL-1β (Figs 5A, B and 6A, B), IL-6 (not shown) or TNF-α (not shown), neither in the hippocampus (Fig. 5A, B), nor in the parietal cortex (not shown) or cerebellum (Fig. 6A, B). The presence of the transgene product (hsIL-1ra) did not influence the levels of APP or PS1 neither in the hippocampus nor in the cerebellum (data not shown).
5.
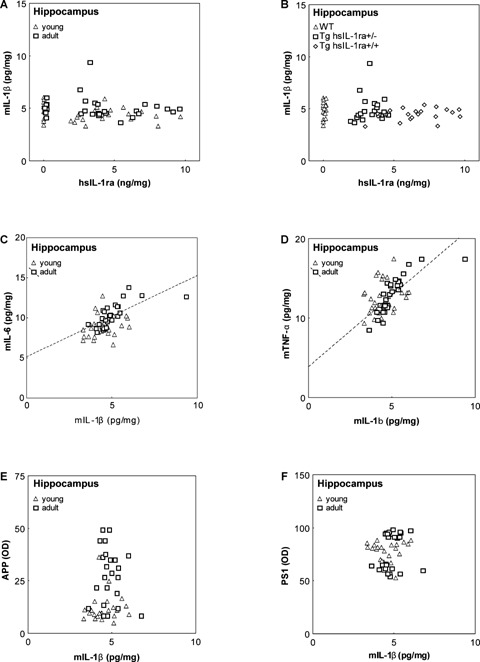
Correlations between the levels of mouse interleukin-1β (mIL-1β) and human soluble interleukin-1 receptor antagonist (hsIL-1ra) (A, B), mouse interleukin-6 (mIL-6) (C), mouse tumour necrosis factor-α (mTNF-β) (D), amyloid precursor protein (APP) (E) and presenilin 1 (PS1) (F), respectively, in the hippocampus of mice with transgenic expression of hsIL-1ra with homozygotic (Tg hsIL-1ra+/+) and heterozygotic (Tg hsIL-1ra+/−) genotype, and of wild type (WT) mice, of young and adult age. No correlation was found between the levels of mIL-1β and hIL-1ra (A). Labelling of the individual animals according to genotype illustrates the different levels of hsIL-1ra (B). A positive correlation was found between the levels of mIL-1β and those of mIL-6 in adult mice (r = 0.70, P < 0.001) (C), and between the levels of mIL-1β and those of mTNF-α in adult mice (r = 0.81, P < 0.001) (D). The levels of APP (E) and PS1 (F) do not correlate with the levels of mIL-1β.
6.
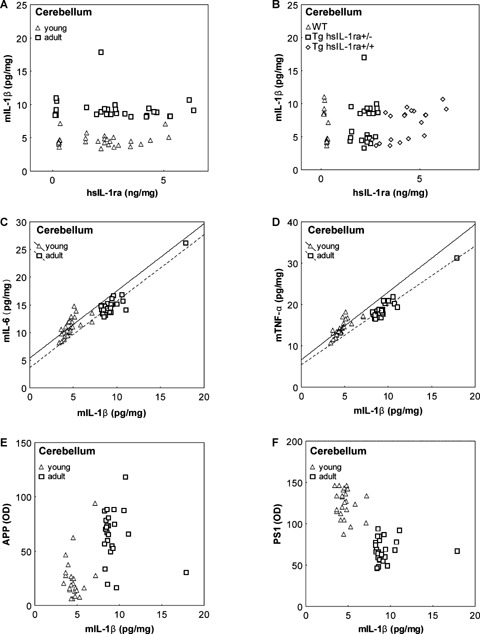
Correlations between the levels of mouse interleukin-1β (mIL-1β) and human soluble interleukin-1 receptor antagonist (hsIL-1ra) (A, B), mouse interleukin-6 (mIL-6) (C), mouse tumour necrosis factor-a (mTNF-α) (D), amyloid precursor protein (APP) (E) and presenilin 1 (PS1) (F), respectively, in the cerebellum of mice with transgenic expression of hsIL-1ra with homozygotic (Tg hsIL-1ra+/+) and heterozygotic (Tg hsIL-1ra+/−) genotype, and of wild type (WT) mice, of young and adult age. No correlation was found between the levels of mIL-1β and hsIL-1ra (A). Labelling of the individual animals according to genotype illustrates the different levels of hsIL-1ra (B). Adult mice have higher levels of mIL-1β than young mice of all genotypes (A, B). A positive correlation was found between the levels of mIL-1β and those of mIL-6 in both young (r = 0.66, P < 0.001), and adult (r = 0.92, P < 0.001) mice (C), and between the levels of mIL-1β and those of mTNF-α in both young (r = 0.78, P < 0.001), and adult (r = 0.95, P < 0.001) mice (D). APP (E) and PS1 (F) levels display a well-defined clustering pattern according to the age of the animals, but no correlation with mIL-1β levels.
Both the IL-6 and TNF-α levels were found to be positively correlated with the IL-1β levels in the hippocampus (Fig. 5C, D), parietal cortex (not shown) and cerebellum (Fig. 6C, D), in both young and adult animals, regardless of genotype. IL-6 correlates with IL-1β in young and the adult animals in the cerebellum (r = 0.66, P < 0.001, and r = 0.92, P < 0.001; Fig. 6C), the parietal cortex (r = 0.75, P < 0.001, and r = 0.72, P < 0.001; data not shown), and in the hippocampus of adult (r = 0.70, P < 0.001), but not young (r = 27, P = 0.16) animals (Fig. 5C). In the case of TNF-α, the correlations showed a similar pattern as for IL-6.Thus, TNF-α correlates with IL-1β in young and the adult animals in the cerebellum (r = 0.78, P < 0.001, and r = 0.95, P < 0.001; Fig. 6D), the parietal cortex (r = 0.62, P < 0.001, and r = 0.85, P < 0.001; data not shown), and in the hippocampus of adult (r = 0.81, P < 0.001), but not young (r = 25, P = 0.19) animals (Fig. 5D).
There was no correlation between the levels of APP and PS1, respectively, and those of IL-1β, neither in the hippocampus (Fig. 5E, F), nor in the cerebellum (Fig. 6E, F). In the cerebellum, the net effect of age on the levels of IL-1β, APP and PS1, determined a distinct clustering pattern regardless of genotype (Fig. 6E, F).
Discussion
This study presents some consequences of chronic, brain-directed overexpression of hsIL-1ra on the levels of the AD-related proteins APP and PS1, and of certain cytokines in different brain regions, as well as the effects on brain morphology. The transgenic mouse strain with overexpression of hsIL-1ra was developed previously using the promoter for GFAP to limit the expression to the CNS [31]. This transgenic mouse strain has been studied in different models for diseases in which neuroinflammation is believed to be a contributing factor, such as epilepsy [35], head trauma [36] and cerebral ischaemia [37]. Thus, seizures induced by bicuculline methiodide were inhibited [35], and the outcome of cerebral trauma was improved [36], in heterozygotic and homozygotic mice of this transgenic strain. However, in a mouse model of permanent focal cerebral ischaemia, the transgenic (heterozygotic) of hsIL-1ra did not provide neuroprotection [37]. The Tg hsIL-1ra mouse strain has also been used to study the involvement of the IL-1 system in activation of the hypothalamo-pituitary-adrenal (HPA) axis [38, 39], and the metabolism of brain monamines, i.e. dopamine and serotonin [39].
The present study demonstrates a marked reduction in brain volume in the Tg hsIL-1ra mice, with a correlation to the amount of transgenic expression, suggesting a dose-dependent effect due to the degree of central blockade of IL-1 transmission. Furthermore, the reduction in brain volume was seen in both young and adult mice, suggesting that the transgenic expression of hsIL-1ra interferes with brain development. These conclusions are supported by the findings that IL-1 promotes astroglial proliferation during embryogenesis, and that IL-1 released from amoeboid microglia during development is involved in regulating growth of the CNS [5]. Interestingly, IL-1 has been shown to stimulate the differentiation of mesencephalic progenitor cells to dopaminergic neurons [40], and contribute to neuronal survival in dissociated spinal cord cultures derived from foetal mice [41]. Studies on other species, such as the demonstration of IL-1β and IL-1RI in developing neural circuits in the frog [42] and of IL-1β in the developing sheep neocortex [43], support the idea that IL-1β is a highly conserved protein, with a potentially important role in CNS development.
Interpretation of the results obtained in studies on the Tg hsIL-1ra mice is dependent upon the potential compensatory mechanisms that may develop secondary to central blockade of IL-1RI-mediated transmission. Thus, it was of interest to analyse the levels of cytokines that are known to be related to IL- 1R-mediated activities, including IL-1β, but also TNF-α, a proinflammatory cytokine that has similar actions as IL-1β, as well as IL-6, a cytokine that is induced downstream of IL-1 signalling. However, the transgenic expression of hsIL-1ra did not affect the levels of IL-1β, IL-6 or TNF-α, with one exception, regarding the TNF-α levels in one brain region. The results, also illustrated by the correlation analysis, do not favour a major compensatory up-regulation of the presently analysed cytokines, even upon homozygotic expression of the transgene, suggesting other mechanisms for the differences observed between the transgenic and WT mice.
Earlier studies showed higher levels of TNF-α in the cerebral cortex of heterozygotic (Tg hsIL-1ra+/−) mice as compared to WT mice [36]. The discrepancy with the present data showing lower levels of TNF-α in parietal cortex of Tg hsIL-1ra+/− mice may be due to the different procedures for tissue processing.
Thus, the present data were obtained from tissues homogenised in the presence of detergents, in order to dissolve cellular membranes, conceivably resulting in measurements of both the 17 kDa soluble form and a 26 kDa membrane-bound form [44], whereas Tehranian et al.[36] performed the analysis on tissues prepared without detergents. Furthermore, the different results may be due to analysis of different anatomical parts of the cerebral cortex.
The involvement of IL-1 in the pathogenesis of AD has emerged from analyses of clinical material, genetic studies as well as studies on experimental models of AD (for review see ref. [22]). Several studies indicate reciprocal interactions between IL-1 and APP/Aβ-peptide, including the stimulatory effects of IL-1 on the synthesis and processing of APP [23–25], and the stimulatory effect of Aβ-peptide on the secretion of IL-1 and IL-6 from microglia [45–47]. The present finding that the levels of APP in the cerebellum were lower in Tg hsIL-1ra+/− than in WT mice, is consistent with the stimulatory effects of IL-1 on the APP gene promoter and synthesis [46, 48], i.e. blocking IL-1R-mediated activity in the brain results in a reduced stimulation of APP synthesis. However, the homozygotic (Tg hsIL-1ra+/+) mice had similar levels of APP as the WT mice and higher levels than the heterozygotic (Tg hsIL-1ra+/−) mice, suggesting that effects of IL-1 on APP have some degree of redundancy. Furthermore, these findings imply that the nature of central blockade of IL-1 signalling in the brain is different in the heterozygotic and homozygotic Tg hsIL-1ra mice, as suggested also by the brain morphology (see above). However, further studies are needed to elucidate the interrelations between IL-1 signalling in the brain and APP expression.
A variety of missense mutations in the gene for PS1 have been found in early-onset autosomal dominant familial forms of AD (FAD) [28–30]. Studies on the effects of various cytokines in different human neuronal cell lines showed unchanged levels of PS1 mRNA [49], whereas the combination of IL-1β and Aβ1–42 synergistically activated PS1 and cyclooxygenase (COX) II synthesis in primary cultures of human neural progenitor cells [50]. The present studies demonstrate an influence of the transgenic overexpression of hsIL-1ra on the PS1 levels in the cerebellum, i.e. lower levels were encountered in transgenic as compared to WT mice. However, this effect was only seen in animals with heterozygotic expression of hsIL-1ra. Interestingly, mice with transient expression of mutant PS1 displayed an enhanced response to immune activation by bacterial lipopolysaccharides (LPS) in terms of increased expression of IL-1α, IL-1β, IL-1ra, IL-6 and TNF-α mRNA in the hippocampus and cerebral cortex [51]. Taken together, these findings support a role of PS1 function in inflammation, and vice versa. Further support for this notion is provided by the findings that IL-1β and TNF-α stimulate amyloidogenic γ-secretase cleavage of APP [26].
The expression of the transgene product, hsIL-1ra, in homozygotic (Tg hsIL-1ra+/+) mice was, as would be expected, about double the concentration in the heterozygotic (Tg hsIL-1ra+/−) mice. Furthermore, the homozygotic animals displayed an age-dependent increase in hsIL-1ra levels in the parietal cortex. The levels of pro-inflammatory cytokines also increased with age, regardless of genotype and gender, with the exception of TNF-α in the hippocampus. The results agree with previous findings showing higher levels of proinflammatory cytokines in the brain of aging rodents as compared to younger animals ([52], for review see ref. [53]). In addition, both APP- and PS1-levels changed with age, although in different directions. Thus, the levels of APP increased with age, both in the hippocampus and cerebellum, regardless of genotype. An age-dependent increase in APP-levels was also described in the rat [54] and human brain [55]. In contrast to APP, the levels of PS1 were found to be higher in young than in adult animals, with the exception of the hippocampus in adult female mice, which had higher levels than young females. Altogether, the analysis of age-related changes indicates a positive co-regulation between the levels of cytokines and APP, and a negative co-regulation between the cytokine levels and PS1. Consequently, there is an inverse relation between the levels of APP and PS1. However, further studies will be required to understand to what extent this means higher levels of full-length APP and/or Aβ, i.e. to determine which forms of APP immunoreactive material that are increased with age.
The age-dependent increase in the levels of IL-1β, IL-6 and TNF-α, were not influenced by the Tg hsIL-1ra genotype in the age/groups examined. Instead, a marked positive correlation was found between the three cytokines in all brain regions analysed, regardless of genotype. Interestingly, IL-6 and TNF-α correlated with IL-1β levels in the hippocampus of adult, but not young mice, suggesting that the influence of IL-1β on the expression of IL-6 and TNF-α increases with age.
Some gender differences were observed, regardless of genotype, i.e. the hippocampal levels of IL-6, hsIL-1ra and PS1, were higher in female than in male mice. Indeed, higher numbers of astrocytes and microglia have been found in the hippocampus of female mice as compared to male mice [56]. In addition, we show that APP and PS1 levels in the cerebellum were higher in female mice.
In conclusion, the Tg hsIL-1ra mouse strain may provide a useful tool in further studies on the effects of IL-1R-mediated activity on different isoforms and cleavage products of APP, as well as on the role of PS1. The most conspicuous finding in these mice was the marked changes in brain volume, conceivably due to a developmental effect of the chronic, brain-directed overexpression of hsIL-1ra. Our data indicate that compensatory mechanisms due to the chronic blocking of IL-1 receptors in the brain have not occurred with regard to the brain cytokine levels. Thus, IL-1β, IL-6 and TNF-α levels in the brain were essentially unaffected, suggesting that the phenotypic characteristics are probably not due to these proinflammatory cytokines. The measurements of cytokines were performed in physiological conditions, but genotype-dependent differences may appear under pathological conditions. A marked agedependent increase in cytokine levels was found in both transgenic and WT mice, in parallel with increased levels of APP, and decreased levels of PS1.
Acknowledgments
This work was supported by grants from the Swedish Research Council (072194), Karolinska Institutet Research Funds, Stiftelsen för Gamla Tjänarinnor, Gun och Bertil Stohnes stiftelse and Insamlingsstiftelsen för Alzheimeroch Demensforskning.
References
- 1.Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–25. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- 2.Minami M, Kuraishi Y, Yabuuchi K, Yamazaki A, Satoh M. Induction of interleukin-1β mRNA in rat brain after transient forebrain ischemia. J Neurochem. 1992;58:390–2. doi: 10.1111/j.1471-4159.1992.tb09324.x. [DOI] [PubMed] [Google Scholar]
- 3.Stroemer RP, Rothwell NJ. Exacerbation of ischemic brain damage by localized striatal injection of interleukin- 1β in the rat. J Cereb Blood Flow Metab. 1998;18:833–9. doi: 10.1097/00004647-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Blume AJ, Vitek MP. Focusing on IL-1-promotion of β-amyloid precursor protein synthesis as an early event in Alzheimer's disease. Neurobiol Aging. 1989;10:406–8. doi: 10.1016/0197-4580(89)90077-8. [DOI] [PubMed] [Google Scholar]
- 5.Giulian D, Young DG, Woodward J, Brown DC, Lachman LB. Interleukin-1 is an astroglial growth factor in the developing brain. J Neurosci. 1988;8:709–14. doi: 10.1523/JNEUROSCI.08-02-00709.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- 7.Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, Yirmiya R. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–34. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- 8.Schneider H, Pitossi F, Balschun D, Wagner A, Del Rey A, Besedovsky HO. A neuromodulatory role of interleukin-1β in the hippocampus. Proc Natl Acad Sci USA. 1998;95:7778–83. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–52. [PubMed] [Google Scholar]
- 10.Eisenberg SP, Evans RJ, Arend WP, Verderber 0E, Brewer MT, Hannum CH, Thompson RC. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990;343:341–6. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- 11.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–40. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson C, Winblad B, Schultzberg M. Kainic acid induced expression of interleukin-1 receptor antagonist mRNA in the rat brain. Mol Brain Res. 1998;58:195–208. doi: 10.1016/s0169-328x(98)00125-9. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson C, Van Dam AM, Lucassen PJ, Bol JG, Winblad B, Schultzberg M. Immunohistochemical localization of interleukin-1β, interleukin-1 receptor antagonist and interleukin-1β-converting enzyme/caspase-1 in the rat brain after peripheral administration of kainic acid. Neuroscience. 1999;93:915–30. doi: 10.1016/s0306-4522(99)00178-5. [DOI] [PubMed] [Google Scholar]
- 14.Vezzani A, Moneta D, Richichi C, Aliprandi M, Burrows SJ, Ravizza T, Perego C, De Simoni MG. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia. 2002;43:30–5. doi: 10.1046/j.1528-1157.43.s.5.14.x. [DOI] [PubMed] [Google Scholar]
- 15.Oprica M, Eriksson C, Schultzberg M. Inflammatory mechanisms associated with brain damage induced by kainic acid with special reference to the interleukin-1 system. J Cell Mol Med. 2003;7:127–40. doi: 10.1111/j.1582-4934.2003.tb00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Barone FC, Aiyar NV, Feuerstein GZ. Interleukin-1 receptor and receptor antagonist gene expression after focal stroke in rats. Stroke. 1997;28:155–61. doi: 10.1161/01.str.28.1.155. [DOI] [PubMed] [Google Scholar]
- 17.Hill JK, Gunion-Rinker L, Kulhanek D, Lessov N, Kim S, Clark WM, Dixon MP, Nishi R, Stenzel-Poore MP, Eckenstein FP. Temporal modulation of cytokine expression following focal cerebral ischemia in mice. Brain Res. 1999;820:45–54. doi: 10.1016/s0006-8993(98)01140-8. [DOI] [PubMed] [Google Scholar]
- 18.Relton JK, Martin D, Thompson RC, Russel DA. Peripheral administration of interleukin-1 receptor antagonist inhibits brain damage after focal cerebral ischemia in the rat. Exp Neurol. 1996;138:206–13. doi: 10.1006/exnr.1996.0059. [DOI] [PubMed] [Google Scholar]
- 19.Betz AL, Yang GY, Davidson BL. Attenuation of stroke size in rats using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist in brain. J Cereb Blood Flow Metab. 1995;15:547–51. doi: 10.1038/jcbfm.1995.68. [DOI] [PubMed] [Google Scholar]
- 20.Yang GY, Liu XH, Kadoya C, Zhao YJ, Mao Y, Davidson BL, Betz AL. Attenuation of ischemic inflammatory response in mouse brain using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist. J Cereb Blood Flow Metab. 1998;18:840–7. doi: 10.1097/00004647-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Loscher CE, Mills KH, Lynch MA. Interleukin-1 receptor antagonist exerts agonist activity in the hippocampus independent of the interleukin-1 type I receptor. J Neuroimmunol. 2003;137:117–24. doi: 10.1016/s0165-5728(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 22.Griffin WS, Mrak RE. Interleukin-1 in the genesis and progression of and risk for development of neuronal degeneration in Alzheimer's disease. J Leukoc Biol. 2002;72:233–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Brugg B, Dubreul YL, Huber G, Wollman EE, Delhaye-Bouchaud N, Mariani J. Inflammatory processes induce β-amyloid precursor protein changes in mouse brain. Proc Natl Acad Sci USA. 1995;92:3032–5. doi: 10.1073/pnas.92.7.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buxbaum JD, Oishi M, Chen HI, Pinkas-Kramarski R, Jaffe EA, Gandy SE, Greengard P. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer β/A4 amyloid protein precursor. Proc Natl Acad Sci. 1992;89:10075–8. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forloni G, Demicheli F, Giorgi S, Bendotti C, Angeretti N. Expression of amyloid precursor protein mRNAs in endothelial, neuronal and glial cells: modulation by interleukin-1. Mol Brain Res. 1992;16:128–34. doi: 10.1016/0169-328x(92)90202-m. [DOI] [PubMed] [Google Scholar]
- 26.Liao YF, Wang BJ, Cheng HT, Kuo LH, Wolfe MS. Tumor necrosis factor-α, interleukin-1β, and interferon- γ stimulate γ-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J Biol Chem. 2004;279:49523–32. doi: 10.1074/jbc.M402034200. [DOI] [PubMed] [Google Scholar]
- 27.Herreman A, Serneels L, Annaert W, Collen D, Schoonjans L, De Strooper B. Total inactivation of γ-secretase activity in presenilin-deficient embryonic stem cells. Nature Cell Biol. 2000;2:461–2. doi: 10.1038/35017105. [DOI] [PubMed] [Google Scholar]
- 28.Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, Crowley AC, Fu Y-H, Guenette SY, Galas D, Nemens E, Wijsman EM, Bird TD, Schellenberg GD, Tanzi RE. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995;269:973–7. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 29.Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, Mar L, Sorbi S, Nacmias B, Piacentini S, Amaducci L, Chumakov I, Coben D, Lannfelt L, Freaser PE, Rommens JM, St George-Hyslop PH. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature. 1995;376:775–8. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 30.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Holman K, Tsuda T, Mar L, Foncin J-F, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HAR, Haines JL, Pericak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH. Cloning of a gene gearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–60. [Google Scholar]
- 31.Lundkvist J, Sundgren-Andersson AK, Tingsborg S, Ostlund P, Engfors C, Alheim K, Bartfai T, Iverfeldt K, Schultzberg M. Acute-phase responses in transgenic mice with CNS overexpression of IL-1 receptor antagonist. Am J Physiol. 1999;276:R644–51. doi: 10.1152/ajpregu.1999.276.3.R644. [DOI] [PubMed] [Google Scholar]
- 32.Zhang YH, Lin JX, Vilcek J. Interleukin-6 induction by tumor necrosis factor and interleukin-1 in human fibroblasts involves activation of a nuclear factor binding to a kappa B-like sequence. Mol Cell Biol. 1990;10:3818–23. doi: 10.1128/mcb.10.7.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bargetzi MJ, Lantz M, Smith CG, Torti FM, Olsson I, Eisenberg SP, Starnes HF., Jr Interleukin-1β induces interleukin-1 receptor antagonist and tumor necrosis factor binding protein in humans. Cancer Res. 1993;53:4010–3. [PubMed] [Google Scholar]
- 34.Ledesma E, Martinez I, Cordova Y, Rodriguez-Sosa M, Monroy A, Mora L, Soto I, Ramos G, Weiss B, Santiago Osorio E. Interleukin-1β (IL-1β) induces tumor necrosis factor α (TNF-α) expression on mouse myeloid multipotent cell line 32D cl3 and inhibits their proliferation. Cytokine. 2004;26:66–72. doi: 10.1016/j.cyto.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Vezzani A, Moneta D, Conti M, Richichi C, Ravizza T, De Luigi A, De Simoni MG, Sperk G, Andell-Jonsson S, Lundkvist J, Iverfeldt K, Bartfai T. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci USA. 2000;97:11534–9. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tehranian R, Andell-Jonsson S, Beni SM, Yatsiv I, Shohami E, Bartfai T, Lundkvist J, Iverfeldt K. Improved recovery and delayed cytokine induction after closed head injury in mice with central overexpression of the secreted isoform of the interleukin-1 receptor antagonist. J Neurotrauma. 2002;19:939–51. doi: 10.1089/089771502320317096. [DOI] [PubMed] [Google Scholar]
- 37.Oprica M, Van Dam AM, Lundkvist J, Iverfeldt K, Winblad B, Bartfai T, Schultzberg M. Effects of chronic overexpression of interleukin-1 receptor antagonist in a model of permanent focal cerebral ischemia in mouse. Acta Neuropathol. 2004;108:69–80. doi: 10.1007/s00401-004-0868-5. [DOI] [PubMed] [Google Scholar]
- 38.Goshen I, Yirmiya R, Iverfeldt K, Weidenfeld J. The role of endogenous interleukin-1 in stress-induced adrenal activation and adrenalectomy-induced adrenocorticotropic hormone hypersecretion. Endocrinology. 2003;144:4453–8. doi: 10.1210/en.2003-0338. [DOI] [PubMed] [Google Scholar]
- 39.Oprica M, Zhu S, Goiny M, Pham TM, Mohammed AH, Winblad B, Bartfai T, Schultzberg M. Transgenic overexpression of interleukin-1 receptor antagonist in the CNS influences behaviour, serum corticosterone and brain monoamines. Brain Behav Immun. 2005;19:223–34. doi: 10.1016/j.bbi.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Jelaso AM, Acevedo S, Dang T, Lepere A, Ide CF. Interleukin-1β and its type 1 receptor are expressed in developing neural circuits in the frog, Xenopus laevis. J Comp Neurol. 1998;394:242–51. doi: 10.1002/(sici)1096-9861(19980504)394:2<242::aid-cne8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 41.Brenneman DE, Schultzberg M, Bartfai T, Gozes I. Cytokine regulation of neuronal survival. J Neurochem. 1992;58:454–60. doi: 10.1111/j.1471-4159.1992.tb09743.x. [DOI] [PubMed] [Google Scholar]
- 42.Jelaso AM, Acevedo S, Dang T, Lepere A, Ide CF. Interleukin-1beta and its type 1 receptor are expressed in developing neural circuits in the frog, Xenopus laevis. J Comp Neurol. 1998;394:242–51. doi: 10.1002/(sici)1096-9861(19980504)394:2<242::aid-cne8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 43.Dziegielewska KM, Moller JE, Potter AM, Ek J, Lane MA, Saunders NR. Acute-phase cytokines IL-1β and TNF-α in brain development. Cell Tissue Res. 2000;299:335–45. doi: 10.1007/s004419900157. [DOI] [PubMed] [Google Scholar]
- 44.Schottelius AJ, Moldawer LL, Dinarello CA, Asadullah K, Sterry W, Edwards CK., 3rd Biology of tumor necrosis factor-_α- implications for psoriasis. Exp Dermatol. 2004;13:193–222. doi: 10.1111/j.0906-6705.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- 45.Barger SW, Harmon AD. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature. 1997;388:878–81. doi: 10.1038/42257. [DOI] [PubMed] [Google Scholar]
- 46.Del Bo R, Angeretti N, Lucca E, De Simoni MG, Forloni G. Reciprocal control of inflammatory cytokines, IL-1 and IL-6, and β-amyloid production in cultures. Neurosci Lett. 1995;188:70–4. doi: 10.1016/0304-3940(95)11384-9. [DOI] [PubMed] [Google Scholar]
- 47.Lindberg C, Hjorth E, Post C, Winblad B, Schultzberg M. Cytokine production by a human microglial cell line: effects of β-amyloid and α-melanocyte-stimulating hormone. Neurotoxicity Res. 2005;8:267–76. doi: 10.1007/BF03033980. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Quitschke WW, Brewer GJ. Upregulation of amyloid precursor protein gene promoter in rat primary hippocampal neurons by phorbol ester, IL-1 and retinoic acid, but not by reactive oxygen species. Brain Res Mol Brain Res. 1998;60:40–9. doi: 10.1016/s0169-328x(98)00164-8. [DOI] [PubMed] [Google Scholar]
- 49.Satoh J-I, Kuroda Y. Constitutive and cytokine-regulated expression of presenilin-1 and presenilin-2 genes in human neural cell lines. Neuropathol Appl Neurobiol. 1999;25:492–503. doi: 10.1046/j.1365-2990.1999.00209.x. [DOI] [PubMed] [Google Scholar]
- 50.Bazan NG, Lukiw WJ. Cyclooxygenase-2 and presenilin-1 gene expression induced by interleukin-1β and amyloid β42 peptide is potentiated by hypoxia in primary human neural cells. J Biol Chem. 2002;277:30359–67. doi: 10.1074/jbc.M203201200. [DOI] [PubMed] [Google Scholar]
- 51.Lee J, Chan SL, Mattsson MP. Adverse effects of a presenilin-1 mutation in microglia results in enhanced nitric oxide and inflammatory cytokine responses to immune challenge in the brain. Neuromolecular Med. 2002;2:29–45. doi: 10.1385/NMM:2:1:29. [DOI] [PubMed] [Google Scholar]
- 52.Sharman KG, Sharman EH, Yang E, Bondy SC. Dietary melatonin selectively reverses age-related changes in cortical cytokine mRNA levels, and their responses to an inflammatory stimulus. Neurobiol Aging. 2002;23:633–8. doi: 10.1016/s0197-4580(01)00329-3. [DOI] [PubMed] [Google Scholar]
- 53.Nordstedt C, Gandy SE, Alafuzoff I, Caporaso GL, Iverfeldt K, Grebb J, Winblad B, Greengard P. Alzheimer β/A4 amyloid precursor protein in human brain: aging-associated increases in holoprotein and in a proteolytic fragment. Proc Natl Acad Sci. 1991;88:8910–4. doi: 10.1073/pnas.88.20.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawarabayashi T, Shoji M, Yamaguchi H, Tanaka M, Harigaya Y, Ishiguro K, Hirai S. Amyloid beta protein precursor accumulates in swollen neurites throughout rat brain with aging. Neurosci Lett. 1993;153:73–6. doi: 10.1016/0304-3940(93)90080-5. [DOI] [PubMed] [Google Scholar]
- 55.Nordstedt C, Gandy SE, Alafuzoff I, Caporaso GL, Iverfeldt K, Grebb JA, Winblad B, Greengard P. Alzheimer beta/A4 amyloid precursor protein in human brain: aging-associated increases in holoprotein and in a proteolytic fragment. Proc Natl Acad Sci USA. 1991;88:8910–4. doi: 10.1073/pnas.88.20.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mouton PR, Long JM, Lei DL, Howard V, Jucker M, Calhoun ME, Ingram DK. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 2002;956:30–5. doi: 10.1016/s0006-8993(02)03475-3. [DOI] [PubMed] [Google Scholar]


