Abstract
In June 2008, the world’s first whole tissue-engineered organ – the windpipe – was successfully transplanted into a 31-year-old lady, and about 18 months following surgery she is leading a near normal life without immunosuppression. This outcome has been achieved by employing three groundbreaking technologies of regenerative medicine: (i) a donor trachea first decellularized using a detergent (without denaturing the collagenous matrix), (ii) the two main autologous tracheal cells, namely mesenchymal stem cell derived cartilage-like cells and epithelial respiratory cells and (iii) a specifically designed bioreactor that reseed, before implantation, the in vitro pre-expanded and pre-differentiated autologous cells on the desired surfaces of the decellularized matrix. Given the long-term safety, efficacy and efforts using such a conventional approach and the potential advantages of regenerative implants to make them available for anyone, we have investigated a novel alternative concept how to fully avoid in vitro cell replication, expansion and differentiation, use the human native site as micro-niche, potentiate the human body’s site-specific response by adding boosting, permissive and recruitment impulses in full respect of sociological and regulatory prerequisites. This tissue-engineered approach and ongoing research in airway transplantation is reviewed and presented here.
Keywords: human trachea, decellularization, autologous epithelial cells, autologous mesenchymal stem cell derived chondrocytes, clinical transplantation, bionic tissue engineering
Introduction
In 2008, a stem-cell based tissue-engineered windpipe was successfully implanted in a young woman with end-stage post-tuberculosis left main bronchus collapse [1]. Her lung function was normalized after implant and approximately 2 years later she has a normal, active life and is in the workforce without immunosuppression, and with no immunological or clinical signs of rejection, to date. A small piece of history has been made by this achievement, being the first human transplant of a completely tissue-engineered organ.
In fact, tracheal replacement had been attempted in animals using a wide variety of autologous and synthetic tissues but none had been proved effective in human beings [2–4] until we experimentally demonstrated that an efficient reseeding of a donor trachea decellularized by using a detergent could be achieved using autologous respiratory epithelial cells and mesenchymal stem cell (MSC)-derived chondrocytes [5]. This paved the way for the first successful clinical transplantation of a fully tissue-engineered organ, where a donor trachea was first decellularized by using a detergent (without denaturing the collagenous matrix), recellularized in a bioreactor that reseeded the recipients’ MSC-derived chondrocytes cells on the outer and respiratory epithelial cells on the inner surfaces of the scaffold, and then implanted (Fig. 1).
Fig 1.
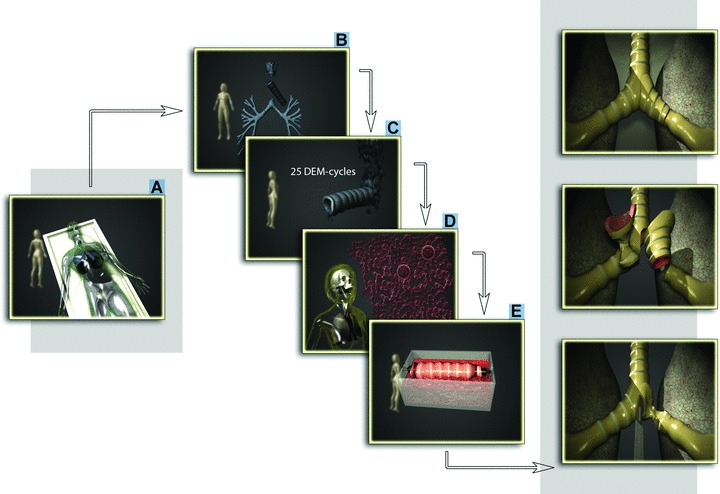
Steps towards completely tissue-engineered human trachea. A human donor trachea (A), (B) was decellularized (C), readily re-colonisated with epithelial cells and MSC-derived chondrocytes (cultured from cells taken from the recipient [D]) via a dynamic bioreactor with internal rotation of the graft (E) and used to successfully replace patient’s left main bronchus.
The purpose of this review is to provide an overview of the methods used to obtain bioengineered tracheal scaffolds and discuss future directions and potential synergies between recent scientific advances and regulations with the ultimate goal for safe, effective and worldwide regeneration of functional airway.
Rationales for tracheal transplantation
Today, the vast majority of benign or malignant tracheal lesions can be resected and primary reconstruction is safely carried out by end-to-end anastomosis. Unfortunately, when the disease length extends to more than 50% of the total (in adults) or one third (in small children) tracheal length, safe reconstruction is impossible and a tracheal replacement desirable. According to Belsey [6], a tracheal substitute must be laterally rigid, but longitudinally flexible, and possess a surface lining comprising ciliated respiratory epithelium. Furthermore, the conduit must be initially airtight and become integrated into adjacent tissues, so that chronic inflammation, granulation tissue, infection and erosion do not occur. In addition, materials for tracheal replacement must be biocompatible, non-toxic, non-immunogenic, non-carcinogenic and must not dislocate or erode over time. Ideally they should provide or facilitate epithelial resurfacing, avoid stenosis or late buckling, resist bacterial colonization and avoid accumulation of secretions. They must be permanent constructions. Finally, immunosuppressive therapy is undesirable for many reasons, especially because, unlike heart or lung transplantation, tracheal transplant is not a life-saving procedure.
Historical background
The apparent simplicity of the trachea encouraged investigations lasting a century, with tubular tracheal replacement using different potential substitutes like prosthetic materials, auto- and allografts [2]. Solid or nonporous prosthetic replacements have not been successful yet because these foreign materials cannot be incorporated by local tissues and there are problems of infection, dislodgement, migration, extrusion and stenosis. Re-epithelialization, was, of course, impossible on the inner lumen and led to the formation of granulation tissue or dehiscence at the interface between the prosthesis and the native trachea, usually within several months. Furthermore, solid tubes can never be removed, because the connective tissue tract formed around them proceeds to obstruct the new connective tissue formation and by contraction in the absence of a stent. For the above reasons, the non-porous tracheal prosthesis is now seldom used clinically. Even porous prosthesis was associated with an insufficient rate and completeness of epithelial migration and central granulations, cicatrization and stenosis could not be prevented.
Fresh or preserved allografts have been both experimentally and clinically used as tracheal replacements. Fresh tracheal allografts early demonstrated to stenose, necrose, liquefy [7–10] and, more important, to develop rejection without an immunosuppressive therapy. It has been demonstrated that tracheal transplants carry antigens [11] and that the human tracheal epithelium display HLA-DR antigens that activate T lymphocytes and thereby trigger graft rejection [12, 13]. Therefore, in order to reduce the antigenicity of the allograft itself and avoid its necrosis, several pre-treatment approaches of allogenic tracheal grafts, e.g. radiation therapy, chemical fixation (Tyrode’s solution, glutaraldeyhde, glycerol or formaldehyde), lyophilization and cryopreservation, have been reported both experimentally and clinically [2]. Allotransplantation of pre-irradiated tracheal grafts, without immunosuppressive drugs, is feasible only using high radiation doses and indirect omentum major vascularization [14]. Chemically treated tracheas, implanted as auto-, allo- or xenografts, do not induce any rejection, but no cartilage and epithelium development was reported [15]. Implanted cadaveric formalin fixed allografts showed reduced allogenicity and complete epithelization, but required frequent bronchoscopies to remove exuberant granulation tissue and ultimately became completely malacic [16, 17]. Cryopreservation has been demonstrated to have an immunomodulatory effect on several tissues resulting in the loss of class II HLA-antigen expression during freezing and thawing [18–20]. It has been suggested that a long period of cryopreservation, increasing the degree of degeneration in both epithelium and cartilage and reducing chondrocytes viability, may help to maintain a better patency of tracheal allografts by preventing an allogenic response [21, 22]. Transplantation of cryopreserved trachea allowed to preserve well the epithelium function and histological characteristics, but cartilaginous ischemic changes occurred [23–25], probably due to a slow and inadequate revascularization with insufficient graft blood supply consequently. It has been, indeed, demonstrated that only revascularized, cryopreserved allografts inhibited allogenicity and maintained structural functionality and integrity [26–28]. Results obtained clarified that it is biological impossible that a death cartilage, regardless the type of pre-treatment, could regenerate in a living tissue. Only a living substitute, therefore vascularized, can pretend to fulfil the anatomic mechanical and anti-infectious functions of the trachea [29]. More recently, the Leuven Tracheal Transplant Group has reported successful tracheal allotransplantation after withdrawal of immunosuppressive therapy [30]. Indirect revascularization of the graft was achieved by first placing the graft in a heterotopic position (the recipient’s forearm fascia). However, the membranous posterior wall of the allograft underwent avascular necrosis. Its ‘success’ must be questioned.
Regenerative approach to tracheal replacement
As mentioned above, prosthetic replacements, autologous tissue transfer and allografts have so far failed to offer functional solutions for the treatment of long circumferential tracheal defects. Interest has therefore shifted increasingly to the field of tissue engineering which applies the principles and methods of bioengineering, material science, cell transplantation and life sciences in an effort to develop in vitro biological substitutes able to restore, maintain or improve tissue and organ function.
A variety of regenerative medicine approaches have been proposed for airway replacement, ranging from collagen scaffolds supported by silicones stents to cartilaginous tubes created by in vitro culture methods [31–34]. Again, none of these strategies were adequate for tracheal replacement as a result of incomplete epithelialization with associated stricture, or a lack of mechanical integrity resulting in tracheomalacia [2]. To provide a biocompatible tracheal substitute with sufficient biological stability to facilitate its rapid epithelialization, several experiments have been conducted using the trachea itself as tracheal bioprosthesis. This approach relies on the fact that for tracheal cartilage reconstruction, complex anatomically shaped scaffolds better support tissue development than simple highly modelled designs [35].
Starting from the success of biological scaffolds derived from decellularized tissues and organs in both pre-clinical animal studies and in human clinical applications, attention has been driven to the possible use of decellularized tracheal matrix to realize functional tracheal replacement. The main goal of the decellularization approach is to remove most or all cellular and nuclear material from the tissue/organ (making substitutes non-immunogenic), without or minimally altering the composition, biological activity and mechanical integrity of the remaining extracellular matrix (ECM). The possibility of obtaining naturally biological devices that maintain the natural ECM composition, do not release toxic biodegradable products or induce inflammation, is of clinical relevance and has important advantages. In effect, a number of ECM-derived devices and related decellularization protocols, including human dermis, porcine SIS, porcine heart valves or porcine urinary bladder have already received regulatory approval for use in human beings [36].
To date, different decellularized matrices and decellularization protocols have been studied to obtain a suitable acellular tracheal graft. Small intestinal submucosa acellular matrices, used with or without stent, perichondrial flap or allogenic cartilages patch, have shown moderate success for small defects (less than half the circumference) with adequate structural support, but did not fully restore functional tracheal tissue [37–39]. Urinary bladder matrices, decellularized by peracetic acid, ethanol and deionized water, either not or cross-linked with carbodiimide (to provide additional mechanical support) were replaced with organized collagenous connective tissue and intact epithelial layer, facilitating closure of small-sized tracheal defects with no evidence of stenosis, tracheomalacia or inflammation. However, secretory, basal and ciliated cells and glandular structures were not fully restored and no evidence of cartilage formation was found [40]. A full thickness patch of porcine tracheal matrix, decellularized by trypsin/ethylenediaminetetraacetic acid (EDTA), deionized water and Triton X-100 and lyophilized, showed a spare epithelialization and no cartilage formation [40]. Negative results of these approaches were mainly related to the decellularization protocol used: acids, trypsin/EDTA, Triton X-100, and lyophilization treatments are highly efficient to remove cellular materials but cause disruption of glycosaminglycans and substantially reduced laminin and fibronectin ECM content, compromising the ability of the scaffold to provide mechanical support during the remodelling process [36, 40, 41]. Moreover, lyophilization causes the lack of viable cartilage with consequent absence of cartilage formation [40]. Using Meezan’s detergent-enzymatic method (DEM) [42], we [43] obtained a decellularized 10- to 15-cm-long porcine jejunal segment, which reseeded with autologous costal chondrocytes, smooth muscle cells, respiratory epithelium and endothelial progenitor cells, resulted in an in vitro directly vascularized bioartificial matrix with all cellular tracheal functioning elements [43]. Moreover, porcine tracheal matrices, decellularized by the same method, resulted to support in vitro adhesion of auricular chondrocytes and tracheal epithelial cells [44]. Mechanical and immunological properties studies of these tracheal constructs demonstrated that the DEM process is a simple and effective method to bioengineer tracheal matrices characterized by a preservation of their native structural integrity, lack of immunogenicity [5] and sufficient length for clinical interest.
Human tissue engineering tracheal replacement
The traditional method to generate a human tissue-engineered trachea bases on three key components: (i) a human donor trachea decellularized via a DEM, (ii) autologous cells (MSC-derived chondrocytes and epithelial cells) and (iii) a specific designed bioreactor that reseeds the autologous cells on the appropriate (external and internal) matrix decellularized surfaces. Each of these three components are revised and discussed herein.
Detergent enzymatic method
Our DEM procedure is based on several cycles of three washing steps (distilled water, sodium deoxycholate solution and DNAse-I solution) (Fig. 2). A key requirement is the determination of the minimum number of detergent cycles to remove donor cells and achieve implant immunotolerance, balancing this against the retention of biomechanical properties. In our previous experience with animal decellularized trachea, 17 cycles were able to preserve matrices’ biomechanical strength and induce loss of antigenicity (removal of all MHC class I and class II positive cells) [5]. In human beings, 17 cycles were insufficient and a diffuse immunoreactivity against both MHC class I and II antigens was still present, and this probably reflects the size discrepancy and evolutionary difference scale between pig and human tracheas. In effect, increasing the cycles from 17 to 25, epithelial cells and glands completely disappeared, the few detectable chondrocytes were distorted and mostly anuclear, and nearly all HLA+ cells were removed from the tracheal matrices (Fig. 3). It is of vital importance that MHC class I and II disappear from the matrix. MHC class II antigens, normally expressed by the dendritic cells of the trachea, function as antigen-presenting cells, and, hence, their loss minimizes allograft rejection [12]. Instead, the presence of some cellular elements (mainly in the cartilaginous area) could provide helpful signals, reduce the inflammatory response and preserve mechanical properties of the graft [5]. Moreover, it has been demonstrated that minor antigens expressed on cellular residues play a restricted part in clinical transplantation [1]. Another important feature of the DEM method, besides obtaining grafts with unaltered ECM ultrastructure and biomechanics and non-immunogenic, is the preservation of angiogenic factors, as β-fibroblast growth factor (FGF) or transforming growth factor (TGF)-β, within decellularized matrices [45, 46]. This is of vital clinical importance because a similar expression contributes to timely in vivo construct revascularization, solving, at least in part, the problem of the absence of a functional microvascular blood supply. The potential strength of our decellularization method lies in the similarity to normal anatomy and biomechanics that can be achieved. To this may be added observations of improved angiogenesis and cell migration, hypothetically via residual peptides left on the scaffold by the washing process, and by the presence of MSCs. In other words, the decellularized scaffold seemed to fulfil the properties of an ‘ideal matrix’, namely biocompatibility, bioabsorbability, non-immunogenity, support of cell attachment and growth, and an ability to induce angiogenesis.
Fig 2.
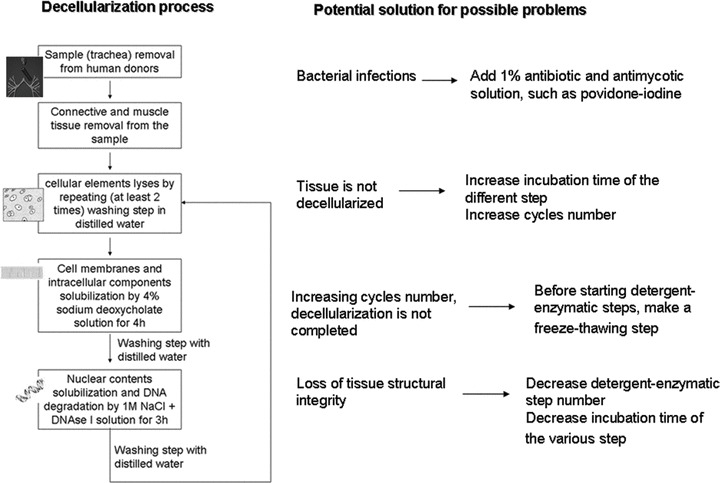
Tracheal decellularization process and potential solution to possible technical problems.
Fig 3.
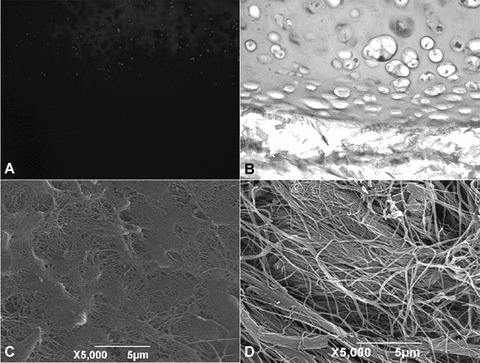
After 25 cycles of the detergent-enzymatic treatment only few chondrocytes (A, ×50) and a mild immunoreactivity against HLA-DR, HLA-DP, HLA-DQ antigens (B; ×200) were still present. SEM micrographs revealed that the basal lamina was partially maintained on the luminal surface (C), whereas an irregular network of collagen fibres was present on the external one (D).
Autologous cells
Mesenchymal stem cell derived chondrocytes
The main issue in cartilage tissue engineering is to determine which tissue-harvesting technique is the easiest, safest and minimally invasive, and which the more suitable in vitro culture conditions are to promote the retention of chondrocyte phenotype. The main sources of cartilage are auricular, nasal septum, tracheal and costal cartilage. Ear cartilage harvesting is the easiest, safest and minimally invasive but its use as tracheal hyaline cartilage is suboptimal because ear cartilage is elastic and may not provide ideal mechanical characteristics for making tracheal tissue. Moreover, auricular chondrocytes may cause the long-term development of intratracheal hair growth and sebum production with subsequent risk of tracheal infection and mucous stasis [47]. Nasal septum cartilage, in contrast, is very similar to the tracheal one and chondrocytes, and develops hyaline cartilage displaying excellent mechanical properties [48, 49]. Tracheal and costal porcine chondrocytes have been demonstrated to have similar vitality and metabolic activity, and the use of costal chondrocytes has been suggested, the clinical harvesting of these cell types being less invasive than others [50]. However, from a clinical point of view, cartilage harvested from these regions has several disadvantages including invasiveness of biopsy and eventually requiring loco-regional and/or general anaesthesia. Recently, it has been shown that bone marrow MSCs can be differentiated into chondrocytes [51]. The use of these cells as a cell line source has several theoretical and known advantages: bone marrow aspirates can be done under local anaesthesia averting the risks associated with general anaesthesia in critically injured patients; it is possible to obtain different cell types, sparing the patients from multiple procedures, and mesenchymal progenitors can be in vitro expanded to considerable numbers while retaining differentiation capacity [52, 53]. For these reasons autologous bone marrow MSCs have become an interesting new alternative source with which to tackle cartilage regeneration.
It is well known that once in vitro cultured, chondrocytes, regardless their source, change their gene expression and over several passages in culture go on to progressively lose their chondrogenic phenotype. It has been, indeed, demonstrated that maintaining differentiated chondrocytes in monolayer culture induces a shift of their biosynthetic profile to a fibroblast-like phenotype [54], with a probable consequent in vivo formation of fibrocartilage instead of hyaline cartilage in the implant [55]. On the contrary, chondrocytes cultured in 3D conditions [50] or in macroaggregates [56] remained vital, functional and with a stable phenotype. It has been demonstrated that the addition of growth factors, such as TGF-β, basic FGF (bFGF) and platelet derived growth factor-BB, to the culture medium of human chondrocytes greatly increases cellular proliferation rate, ECM production and the re-expression of a chondrogenic phenotype in 3D cultures [57–59]. Moreover, the use of growth factors in MSC expansion medium, prior differentiation, has been shown to have a significant effect on the ability of human MSCs to undergo chondrogenesis [52, 53, 60, 61]. TGF-β induction of a controlled differentiation of MSCs to chondrocytes was first described by Johnstone [62], using a high-density pellet culture of MSCs. The Hollander group has recently described methods for consistent growth of MSCs using bFGF to minimize variability between patients [63] and parathyroid hormone related protein to prevent terminal hypertrophic differentiation of MSC-derived chondrocytes [64] and reduce the risk of calcification of implanted cartilage. Based on these results, we cultured bone marrow MSCs in the presence of bFGF and induced their chondrogenesis with TGF-β3, dexamethasone and insulin in the presence of recombinant parathyroid hormone related protein. This in vitro culture approach permitted us to obtain vital, functional and phenotypically stable chondrocytes [1, 65].
Epithelial respiratory cells
Experimental and clinical studies in the development of tracheal prosthesis have demonstrated that the lack of an epithelial lining on the luminal surfaces is a critical factor involved in scar, and subsequent stenosis, formation [66, 67]. As a matter of fact, the airway epithelium regulates an array of airway functions, including control of lung fluid balance, attraction and activation of inflammatory cells, metabolism and clearance of inhaled agents, and regulation of airway smooth muscle function. To obtain an in vivo fully developed respiratory epithelium, a main role is played by the in vitro epithelial cell culture conditions. Indeed, primary epithelial cells are not always easy to culture and it has been demonstrated that tracheal epithelial cells dedifferentiated rapidly with increased passage number. Cells grown on rat tail collagen gels could be passaged four times without losing their ability to secrete mucins; however, increasing passages, the number of ciliated cell (native epithelium is approximately 90% ciliated) rapidly declined [68]. Yoon et al. [69] were unable to grow differentiated human nasal epithelial cells beyond the third passage. Yamaya et al. [70] suggested the importance of an air-interface system and of an appropriate growth substratum (vitrogen gel and Ultrosed G) in determining the differentiation level of primary human tracheal epithelium cultures. The same group demonstrated that culture media with high concentration of retinoic acid and Ca2+ levels induced a pseudo-stratified differentiation of primary epithelial cell cultures [71]. However in the study, no key factor in the composition of the partially defined media could be identified. Recently, Pfenninger et al. [72] suggested that (i) a basal lamina-equivalent of collagen fibres, (ii) extracellular factors secreted by fibroblasts and (iii) the creation of an air-liquid interface systems are the three key factors necessary to induce epithelial cells differentiation. However, these conditions are quite complex and the need for a fibroblast feeder layer persists. A simple method for reliably culturing and expanding primary human airway epithelial cells from normal individuals has been developed by Rees et al. [73]. The cells, grown in specific serum-free medium, supplemented with bovine pituitary factor and epidermal growth factor (removing the need for fibroblast feeder layer), were successfully expanded to passage 4, with epithelial morphology confirmation in each passage [73]. The application of this protocol allowed us to obtain stable and differentiated cultures of epithelial cells at the fourth passage [1]. However, some uncertainty about precisely which epithelial sources are best for particular tissue-engineering challenges still remains. Epithelial cells can be harvested from the nasal or bronchial mucosa. Although it has been described how nasal epithelial cells can be cultured for tissue-engineering purpose [74], in our clinical study, their proliferation rate was too fast and apoptosis occurred in earlier passages than bronchial cells. As consequence, only bronchial cells were used for graft development. More detailed studies will be necessary to say whether nasal or bronchial epithelial cells provide a more efficient and effective source with which re-epithelialize airway grafts.
Bioreactor
Bioreactors can play a pivotal role in the field of tissue engineering and mass applicability because they assist and favour the interaction between biomaterials and the patients’ own cells. A bioreactor can act as a containment, a GMP compatible production unit and as a transport device. In the past, the development of hybrid tissues using stem cell types in a hierarchical and tissue-specific arrangement has been plagued by a shortage of suitable bioreactors. Following bionic principles, several bioreactors, with different operating parameters and environmental conditions, have been developed depending on tissue types and respective clinical applications [75–80].
Dynamic culture systems can offer several important advantages compared with the static ones: more uniform cell distribution in the scaffold, fluid flow can enhance mass transfer, nutrient supply and waste removal, while hydrodynamic shear stress, compression, pressure and stretch can enhance cell metabolic activity, proper differentiation, matrix secretion with consequent positive influences on tissue development. Regarding tracheal bioreactor-based tissue-engineering technology, it has been demonstrated that rotational bioreactors induced the development of a more functional cartilage tissue respect to static one [81]. Moreover, Tan et al. [82] shown that a continuous medium flow inside chondrocytes-poly (ethylene glycol)-terephthalate-poly (butylene terephthalate) scaffold cultures effectively combines chondrocyte seeding and culture systems for the reconstruction of tissue-engineered trachea. Lin et al. [83] demonstrated that chondrocytes, seeded on a poly(-caprolactone)-type II collagen scaffold and grown in a rotational bioreactor, were well organized, aligned along the flow direction and achieved morphology similar to that of native tracheal tissue, confirming that shear stress plays an important role in regulating cell function. Moreover, they suggest that an air-liquid bioreactor could provide essential oxygen and nutrient transport between chondrocytes and the culture media [83]. However, the developed bioreactor was suitable only for short tracheal segment grafts (1–2 cm long) and only for chondrocyte cultures and no epithelial cells seeding has been considered. For our stem cell-derived tissue-engineered trachea, two main key requirements for the bioreactor have been considered: the provision of different culture conditions on either side of the organ wall and the need for adequate mass transport of gases and nutrients within the construct that have to be more than 4 cm long to be clinically useful. Upon these criteria, the basic principles of the previously used bioreactor development have been: (i) the bioreactor should be constitute of two separate sterile compartments addressing the requirements of seeding and culturing of different cell types on either side of the tubular matrix; (ii) the device should rotate in order to move cells alternately between liquid (medium) and gaseous (air) phases, to enhance oxygenation, supply nutrient and remove waste, (iii) to rotate the construct, the system controlled DC motor should be isolated from the culture chamber, (iv) hydrodynamic stimuli should be present to promote metabolic activity and proper cells differentiation and (v) the different pieces constituting bioreactor should be autoclavable, ease to sterile handing, reliable and compatible with good laboratory practice. The mathematical studies on the base of bioreactor development, bioreactor characteristics and properties have been recently reported in detail by Asnaghi et al. [84]. The double-chamber bioreactor allows obtaining expansion and migration on the scaffold of two autologous cell types with different media requirement, to maintain construct oxygenation despite the thickness of the implant wall, to properly re-personalize a donor trachea and to successfully perform the first engineered airway transplantation without the need of any immunosuppressive therapy [1]. The bioreactor could eventually be further optimized to improve safety, standardization and traceability of the whole process (with a complete automation of the system), promote orientated ciliary function (with flow stimuli to the internal bioreactor compartment) and trigger angiogenesis (with radial-flow pressurized liquid perfusion inside the scaffold). Presently, we are evaluating a further bioreactor technology that previously has been successfully developed for automation and GMP processes in liver cells regeneration [75]– it allows simultaneous air and liquid perfusion and has been optimized for sterile handling in a clinical environment [76]. This bioreactor has been tested in a number of preclinical and clinical applications, especially in the cardiovascular field (heart valve and vessel), and recently also for a tracheal preparation process, providing GMP conditions for implant manufacture (Fig. 4).
Fig 4.
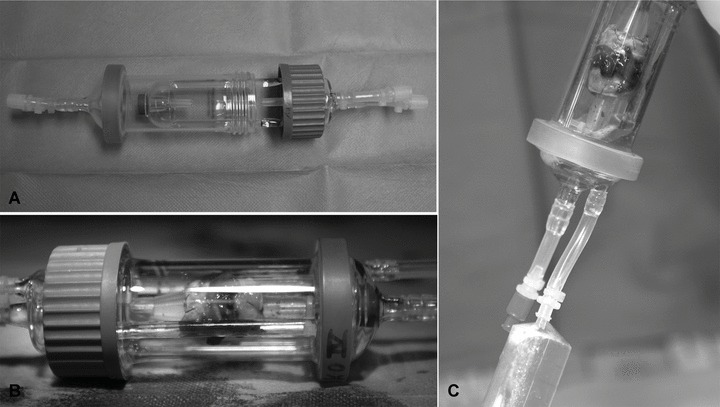
Rotational bioreactor developed for GMP processing and improved clinical handling and automation (A). Decellularized trachea, reseeded with MSCs, placed into the bioreactor (B), which allows simultaneous air and liquid perfusion and sterile handling (C).
Ongoing progress
Can tissue engineering ever translate into a social technology available for everyone? Presently, there are no satisfactory solutions available to treat the estimated 19,000 patients suffering from large airway diseases in the EU each year [85, 86]. Our recent clinical success is highly encouraging, but also serves to highlight the scientific, clinical and translational bottlenecks standing in the way of full integration of this regenerative medicine technology into routine clinical care.
Most of the biotechnological and surgical community are preparing themselves to move from non-viable implants to viable ones with great expectations. This will be paralleled to a clinical redefinition of the term ‘implant biocompatibility’ (which will include also the aspects of integration or assimilation into the host) and will require the development of interacting, intelligent implants with micro-environmental ‘sensory’ functions, capable of rapid and sustainable remodelling and differentiation inside the patient. As a consequence, for future implant therapies it will be essential to develop materials that could ‘contribute’ with physiological signalling to the future tissue components (i.e. the cells) and scaffolds that could be shaped directly by the recipient’s autologous cells. This is a formidable task to solve by the molecular and material sciences because nature has evolved not only the ECM but also molecular and cellular signalling, which is composed of a complex interplay essential to maintaining normal tissue architecture and function. This complexity has been widely neglected by conventional tissue-engineering approaches as it has not yet mimicked successfully. Moreover, bioreactors, as described above, can only assist in inducing a conditioning of the scaffolds with cells as a basis for remodelling.
The positive control of the implant’s fate in a therapeutic patient environment is what we want to describe as the holy grail of stem cell therapy and tissue engineering. Referring to our trachea technology this indispensable aspect relates to the persistence and formation of cartilage rings, lack of scar formation, quality and quantity of re-epithelialization or in short: the presence and performance of the right cells, the right matrix in the right place at the right time!
Bionic airway tissue-engineered replacement
Currently, engineering a 3D construct such as a trachea is complicated not only from the cell biology point of view but also because the organizational efforts of tissue engineering are indeed formidable. Typically, first a biopsy of tissue has to be taken, and then cells are expanded, reprogrammed and differentiated, often in 2D environments and almost for 2 weeks. Then cells are seeded on a scaffold using a bioreactor to accommodate the 3D graft. Thereafter the individualized ‘living graft’ is rapidly transported, with sterility precautions, to meet a scheduled operative intervention just in time [1]. This represents a generic limitation that is unique to cell culture. As we showed previously in cardiovascular systems and in our conventional approaches [87], in vitro cell culture can transform cells, cause oncogene activation, impose sterility risks and lead to qualitative and functional changes. Moreover, in vitro cell selections change multipotency in what may well be a too simplistic perception of what is really needed for a perfect graft. Multipotency and its differentiation control is a volatile good in conventional cell culture but achievable for a temporary phase. Tissue-derived progenitors and most likely many other mesenchymal cell types express unique characteristics known from bone marrow cells [88, 89], which indicates that we have stem cells as a first line of defence in most tissues and probably only one single underlying mechanism of regeneration induction.
From a regulatory (Guidelines for ATMP and FDA) [90] and costwise point of view these procedures represent a hurdle that unlikely will allow availability of individualized implants in every region of the world. Realistic graft prices are beyond reach for a large part of mankind already. If it takes weeks to prepare these grafts, significant costs are justified, but often not enough to maintain the companies that manufacture them. Under the current regulatory requirements the grafts are treated like pharmaceutical drugs and a distribution license equivalent to a normal drug is required. These individualized methods result in ‘advanced therapy medicinal products (ATMP)’, which require a centralized approval by centralized agencies and include somatic cell therapy and tissue engineering [90]. On the other hand, the use of the patients’ own cells implies that these cells are the property of the patient to begin with and they cannot be standardized as well.
The reasons why tissue engineering does not evolve as a social technology available for everyone in any social setting are then clear: current products are too expensive and risky from a manufacturing point of view. A standard comparable to a normal drug production situation cannot even conceptually be ever achieved, because the basic components are individualized and cannot be standardized like a ‘normal drug’ having a defined chemical identity. What has been lacking is a technology that realizes the social potential of stem cell therapy and, at the same time, leads to the highest level of standardization and safety of use. In short, an industrialization technology is needed for something that cannot be industrialized. But individualization per se appears contrary to any strategy of mass availability. Just like the invention of the conveyer belt allowed Ford to go into mass production of cars with the Model T, the real shortage in tissue engineering has been the lack of a technological solution that allows ‘mass availability’ and would eliminate the current limitations in manufacturing.
Having put our fingers into the wounds of current teaching and practice of preparing bioartificial tissues we want to formulate an answer with respect to technological and clinical sustainability. The solution comes from full respect of sociological and regulatory prerequisites [90, 91] with the introduction of a technology that dramatically accelerates the speed of preparation of the implant. The manufacturing of the graft should not require 2–3 weeks, but should be done within minutes to make, as e.g. a trachea, a heart valve or any other tissue of choice. The whole process is ideally done intraoperatively and eliminates the need to send cells to laboratories. This solves the limitations of logistics, unnecessary anaesthesia and also cost. In the hands of the doctor treating the patient and not leaving the operating room, the process fully complies with current regulatory requirements (Fig. 5). The product is not the implant, but the process to make the implant as part of a therapy. This is the safest methodology existing with respect to avoiding transformation, infectious risks and increasing quality. The scaffold must be such to be able to react to remodelling stimuli and ideally all is prepared using fully closed GMP compliant one-way devices [79, 75, 92].
Fig 5.
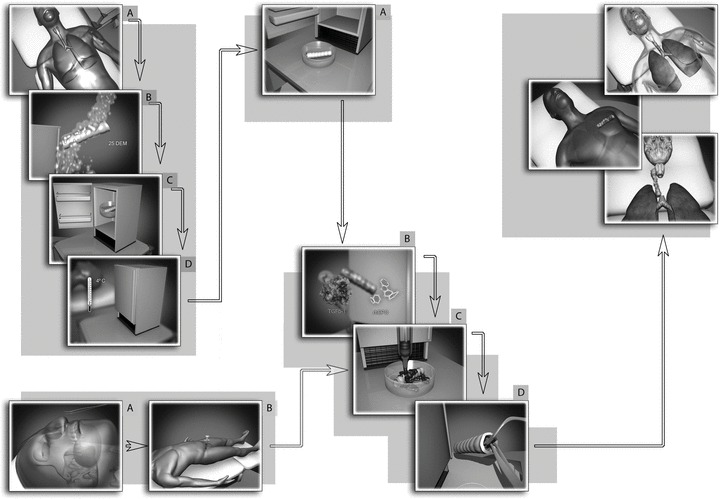
Bionic concept towards worldwide tissue-engineered tracheal replacement. The retrieved human tracheas are kept at –4°C and can be stored up to 11 months. Operations can be made as an elective procedure and purely intraoperatively (eliminating the need of human cell transportation, manipulation): a bone marrow aspiration of 30 to 60 ml needs to be centrifugated and then seeded on the tracheal matrix, without any further cell manipulation (thereby it will not be a cell therapy nor and advanced therapy). The own patient’s stem cells can be activated, controlled and induced to differentiation and proliferation by means local and systemic recruitment, boosting, permissive and commitment factors.
This technological solution is characterized by the fact that in vitro cell replication is fully avoided. We have recently discovered that many tissues express erythropoietin receptors and the tissue protective submit of the erythropoietin receptor (β-CR, Fig. 6). This molecule acts as a boosting accelerator in the regeneration process: erythropoietin-coated implants, such as trachea and valves have been implanted and a successful dramatic enhancement of tissue integration has been obtained in large pig models (Macchiarini P., unpublished results). We have also shown a significant advancement in wound healing in patients and reported similar effects in hepatic progenitors using the relating compound thrombopoietin [93]. We have used erythropoietin as a ‘boosting factor’ to enhance remodelling, reduce inflammation and activate stem cells to propagate and to protect against ischemia [94]. A site-specific activation process is provided according to our bionic concept by the local wound. During injury, permissive factors (i.e. trauma cytokines) are released and these factors represent a permissive situation of wound healing. Recruitment factors can be added to increase the number of stem cells both in situ and in the peripheral circulation (granulocyte macrophage-colony stimulating factor [GM-CSF], G-CSF) in addition or alternatively to in situ loading of the graft with intraoperativatively prepared stem cells. The control of multipotency is achieved by shifting cells towards a commitment. In our case, using TGF-β as a commitment factor, the in vivo differentiation towards cartilage rings with the goal of long-term sustainability could be achieved. These concepts extend the intrinsic micro-niche concept by adding triggering impulses to a receptive environment if needed. A result of such an approach is fast and efficient remodelling, that is 40–50% faster over a non-stimulated control and we have shown in ongoing large animal trials for trachea and heart valve engineering that this concept is fully in vivo applicable (Macchiarini P, personal communication).
Fig 6.
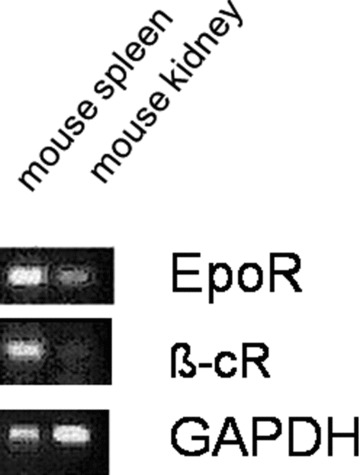
The mRNA expression profile of the erythropoietin receptor and of the tissue protective submit of the erythropoietin receptor (β-CR) in different mouse tissues. GAPDH was used as a housekeeping gene (PCR).
This bionic tissue-engineering concept benefits from the innate mechanisms of site-specific wound repair using them as co-triggering events and from the body’s capacity to formulate a site-specific response independent form the type and location of a tissue (Fig. 7). Human stem cells likely have receptors for those factors, e.g. erythropoietin and interleukin-6 and sense ischemia. If a co-stimulation occurs in the presence of an appropriate scaffold, the cells are triggered to achieve a strong and high-quality remodelling activity that is better, faster and more tightly controlled than in any in vitro activity. Thus, the evolutionary human limitation of being unable to regenerate large defects may be overcome in a very rapid manner without limits to a type of tissue or its location inside the human body.
Fig 7.
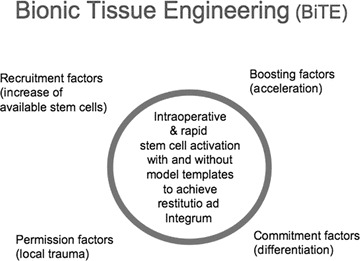
Bionic tissue-engineering concept.
Conclusions
The prospect of being able to build customised, non-immunogenic tracheas via tissue engineering is attractive. The potential strengths of the decellularized scaffold approach lie in the similarity to normal anatomy and biomechanics that can be achieved, its ability to permit autologous cells attachment, epithelial resurfacing, a vigorous angiogenesis and remodelling, and reduced non-immunogenicity without need of immunosuppresion. However, there are many questions that urgently need to be addressed to make this technology universally available, and the Moreover, it opens the door to the development of cell-based regenerative therapy in patients with early stage airway disease actually managed by far more invasive strategies by simply using the interactions between cell-based therapy and site-specific regenerative human response.
References
- 1.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–30. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 2.Grillo HC. Tracheal replacement: A critical review. Ann Thorac Surg. 2002;73:1995–2004. doi: 10.1016/s0003-4975(02)03564-6. [DOI] [PubMed] [Google Scholar]
- 3.Doss AE, Dunn SS, Kucera KA, et al. Tracheal replacements: part 2. ASAIO J. 2007;53:631–9. doi: 10.1097/MAT.0b013e318145ba13. [DOI] [PubMed] [Google Scholar]
- 4.Kucera KA, Doss AE, Dunn SS, et al. Tracheal replacements: part 1. ASAIO J. 2007;53:497–505. doi: 10.1097/MAT.0b013e3180616b5d. [DOI] [PubMed] [Google Scholar]
- 5.Jungebluth P, Go T, Asnaghi A, et al. Structural and morphologic evaluation of a novel detergent-enzymatic tissue-engineered tracheal tubular matrix. J Thorac Cardiovasc Surg. 2009;138:586–93. doi: 10.1016/j.jtcvs.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 6.Belsey R. Resection and reconstruction of the intrathoracic trachea. Br J Surg. 1950;38:200–5. doi: 10.1002/bjs.18003815008. [DOI] [PubMed] [Google Scholar]
- 7.Carter MG, Strieder JW. Resection of the trachea and bronchi; and experimental study. J Thorac Surg. 1950;20:613–27. [PubMed] [Google Scholar]
- 8.Ferguson DJ, Wild JJ, Wangensteen OH. Experimental resection of the trachea. Surgery. 1950;28:597–619. [PubMed] [Google Scholar]
- 9.Jackson TL, O’Brien EJ, Tuttle W, et al. The experimental use of homogenous tracheal transplants in the restoration of continuity of the tracheobronchial tree. J Thorac Surg. 1950;20:598–612. [PubMed] [Google Scholar]
- 10.Pacheco CR, Rivero O, Porter JK. Experimental reconstructive surgery of the trachea. J Thorac Surg. 1954;27:554–64. [PubMed] [Google Scholar]
- 11.Beigel A, Steffens-Knutzen R, Müller B, et al. Tracheal transplantation. III. Demonstration of transplantation antigens on the tracheal mucosa of inbred rat strains. Arch Otorhinolaryngol. 1984;241:1–8. doi: 10.1007/BF00457910. [DOI] [PubMed] [Google Scholar]
- 12.Bujia J, Wilmes E, Hammer C, et al. Tracheal transplantation: demonstration of HLA class II subregion gene products on human trachea. Acta Otolaryngol. 1990;110:149–54. doi: 10.3109/00016489009122530. [DOI] [PubMed] [Google Scholar]
- 13.Kalb TH, Chuang MT, Marom Z, et al. Evidence for accessory cell function by class II MHC antigen-expressing airway epithelial cells. Am J Respir Cell Mol Biol. 1991;4:320–9. doi: 10.1165/ajrcmb/4.4.320. [DOI] [PubMed] [Google Scholar]
- 14.Yokomise H, Inui K, Wada H, et al. High-dose irradiation prevents rejection of canine tracheal allografts. J Thorac Cardiovasc Surg. 1994;107:1391–7. [PubMed] [Google Scholar]
- 15.Scherer MA, Ascherl R, Geissdorfer K, et al. Experimental biosynthetic reconstruction of the trachea. Arch Otorhinolarynol. 1986;243:215–23. doi: 10.1007/BF00464433. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs JP, Elliott MJ, Haw MP, et al. Pediatric tracheal homograft reconstruction: a novel approach to complex tracheal stenosis in children. J Thorac Cardiovasc Surg. 1996;112:1549–60. doi: 10.1016/S0022-5223(96)70014-4. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs JP, Quintessenza JA, Andrews T, et al. Tracheal allograft reconstruction: the total North American and worldwide pediatric experiences. Ann Thorac Surg. 1999;68:1043–52. doi: 10.1016/s0003-4975(99)00878-4. [DOI] [PubMed] [Google Scholar]
- 18.Boren CH, Roon AJ, Moore WS. Maintenance of viable arterial allografts by cryopreservation. Surgery. 1978;83:382–91. [PubMed] [Google Scholar]
- 19.Kawabe N, Yoshinao M. Cryopreservation of cartilage. Int Orthop. 1990;14:231–5. doi: 10.1007/BF00178751. [DOI] [PubMed] [Google Scholar]
- 20.Niwaya K, Sakaguchi H, Kawachi K, et al. Effect of warm ischemia and cryopreservation on cell viability of human allograft valves. Ann Thorac Surg. 1995;60:S114–7. doi: 10.1016/0003-4975(95)00204-x. [DOI] [PubMed] [Google Scholar]
- 21.Nakanishi R, Hashimoto M, Muranaka H, et al. Effect of cryopreservation period on rat tracheal allografts. J Heart Lung Transplant. 2001;20:1010–5. doi: 10.1016/s1053-2498(01)00288-1. [DOI] [PubMed] [Google Scholar]
- 22.Shi H, Xu H, Lu D, Wu J. Animal models of tracheal allotransplantation using vitrified cryopreservation. J Thorac Cardiovasc Surg. 2009;138:1222–6. doi: 10.1016/j.jtcvs.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 23.Deschamps C, Trastek VF, Ferguson JL, et al. Cryopreservation of canine trachea: functional and histological changes. Ann Thorac Surg. 1989;47:208–12. doi: 10.1016/0003-4975(89)90270-1. [DOI] [PubMed] [Google Scholar]
- 24.Messineo A, Filler RM, Bahoric A, et al. Repair of long tracheal defects with cryopreserved cartilaginous allografts. J Pediatr Surg. 1992;27:1131–4. doi: 10.1016/0022-3468(92)90574-q. [DOI] [PubMed] [Google Scholar]
- 25.Lenot B, Macchiarini P, Duhnet E, et al. Tracheal allograft replacement. An unsuccessful method. Eur J Cardio-thorac Surg. 1993;7:648–52. doi: 10.1016/1010-7940(93)90261-9. [DOI] [PubMed] [Google Scholar]
- 26.Yokomise H, Inui K, Wada H, et al. Long-term cryopreservation can prevent rejection of canine tracheal allografts with preservation of graft viability. J Thorac Cardiovasc Surg. 1996;111:930–4. doi: 10.1016/s0022-5223(96)70366-5. [DOI] [PubMed] [Google Scholar]
- 27.Mukaida T, Shimizu N, Aoe M, et al. Experimental study of tracheal allotransplantation with cryopreserved grafts. J Thorac and Cardiovasc Surg. 1998;116:262–6. doi: 10.1016/s0022-5223(98)70125-4. [DOI] [PubMed] [Google Scholar]
- 28.Tojo T, Niwaya K, Sawabata N, et al. Tracheal replacement with cryopreserved tracheal allograft: experiments in dogs. Ann Thorac Surg. 1998;66:209–13. doi: 10.1016/s0003-4975(98)00270-7. [DOI] [PubMed] [Google Scholar]
- 29.Macchiarini P. Berançon. 1997. La transplantation de trachée et trachéo-oesophagienne. France: Université Franche-Comte; [Google Scholar]
- 30.Delaere P, Vranckx J, Verleden G, et al. Leuven Tracheal Transplant Group. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med. 2010;362:138–45. doi: 10.1056/NEJMoa0810653. [DOI] [PubMed] [Google Scholar]
- 31.Teramachi M, Nakamura T, Yamamoto Y, et al. Porous-type tracheal prosthesis sealed with collagen sponge. Ann Thorac Surg. 1997;64:965–9. doi: 10.1016/s0003-4975(97)00755-8. [DOI] [PubMed] [Google Scholar]
- 32.Kojima K, Bonassar LJ, Roy AK, et al. Autologous tissue-engineered trachea with sheep nasal chondrocytes. J Thorac Cardiovasc Surg. 2002;123:1177–84. doi: 10.1067/mtc.2002.121161. [DOI] [PubMed] [Google Scholar]
- 33.Kanzaki M, Yamato M, Hatakeyama H, et al. Tissue engineered epithelial cell sheets for the creation of a bioartificial trachea. Tissue Eng. 2006;12:1275–83. doi: 10.1089/ten.2006.12.1275. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita M, Kanemaru S, Hirano S, et al. Tracheal regeneration after partial resection: a tissue engineering approach. Laryngoscope. 2007;117:497–502. doi: 10.1097/MLG.0b013e31802e223d. [DOI] [PubMed] [Google Scholar]
- 35.Moroni L, Curti M, Welti M, et al. Anatomical 3D fiber-deposited scaffolds for tissue engineering: designing a neotrachea. Tissue Eng. 2007;13:2483–93. doi: 10.1089/ten.2006.0385. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Park JW, Pavcnik D, Uchida BT, et al. Small intestinal submucosa covered expandable Z stents for treatment of tracheal injury: an experimental pilot study in swine. J Vasc Interv Radiol. 2000;11:1325–30. doi: 10.1016/s1051-0443(07)61310-4. [DOI] [PubMed] [Google Scholar]
- 38.Gubbels SP, Richardson M, Trune D, et al. Tracheal reconstruction with porcine small intestine submucosa in a rabbit model. Otolaryngol Head Neck Surg. 2006;134:1028–35. doi: 10.1016/j.otohns.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Liu Z, Cui P, et al. SIS with tissue-cultured allogenic cartilages patch tracheoplasty in a rabbit model for tracheal defect. Acta Otolaryngol. 2007;127:631–6. doi: 10.1080/00016480600987750. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert TW, Gilbert S, Madden M, et al. Morphologic assessment of extracellular matrix scaffolds for patch tracheoplasty in a canine model. Ann Thorac Surg. 2008;86:967–74. doi: 10.1016/j.athoracsur.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 41.Behrend M, Kluge E, Von Wasielewski R, et al. The mechanical influence of tissue engineering techniques on tracheal strength: an experimental study on sheep trachea. J Invest Surg. 2002;15:227–36. doi: 10.1080/08941930290086010. [DOI] [PubMed] [Google Scholar]
- 42.Meezan E, Hjelle JT, Brendel K. A simple, versatile, non disruptive method for the isolation of morphologically and chemically pure basement membranes from several tissues. Life Sci. 1975;17:1721–32. doi: 10.1016/0024-3205(75)90119-8. [DOI] [PubMed] [Google Scholar]
- 43.Walles T, Giere B, Hofmann M, et al. Experimental generation of a tissue-engineered functional and vascularized trachea. J Thorac Cardiovasc Surg. 2004;128:900–6. doi: 10.1016/j.jtcvs.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 44.Conconi MT, De Coppip, Di Liddo R, et al. Tracheal matrices, obtained by a detergent-enzymatic method, support in vitro the adhesion of chondrocytes and tracheal epithelial cells. Transpl Int. 2005;18:727–34. doi: 10.1111/j.1432-2277.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 45.Ribatti D, Conconi MT, Nico B, et al. Angiogenic response induced by acellular brain scaffolds grafted onto the chick embryo chorioallantoic membrane. Brain Res. 2003;989:9–15. doi: 10.1016/s0006-8993(03)03225-6. [DOI] [PubMed] [Google Scholar]
- 46.Conconi MT, De Coppi P, Bellini S, et al. Homologous muscle acellular matrix seeded with autologous myoblasts as a tissue-engineering approach to abdominal wall-defect repair. Biomaterials. 2005;26:267–74. doi: 10.1016/j.biomaterials.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 47.Weidenbecher M, Tucker HM, Awadallah A, et al. Fabrication of a neotrachea using engineered cartilage. Laryngoscope. 2008;118:593–8. doi: 10.1097/MLG.0b013e318161f9f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kojima K, Bonassar LJ, Ignotz RA, et al. Comparison of tracheal and nasal chondrocytes for tissue engineering of the trachea. Ann Thorac Surg. 2003;76:1884–8. doi: 10.1016/s0003-4975(03)01193-7. [DOI] [PubMed] [Google Scholar]
- 49.Kojima K, Bonassar LJ, Roy AK, et al. A composite tissue-engineered trachea using sheep nasal chondrocyte and epithelial cells. FASEB J. 2003;17:823–8. doi: 10.1096/fj.02-0462com. [DOI] [PubMed] [Google Scholar]
- 50.Walles T, Giere B, Macchiarini P, et al. Expansion of chondrocytes in a three-dimensional matrix for tracheal tissue engineering. Ann Thorac Surg. 2004;78:444–8. doi: 10.1016/j.athoracsur.2004.02.122. [DOI] [PubMed] [Google Scholar]
- 51.Noth U, Tuli R, Osyczka AM, et al. In vitro engineered cartilage constructs produced by press-coating biodegradable polymer with human mesenchymal stem cells. Tissue Eng. 2002;8:131–44. doi: 10.1089/107632702753503126. [DOI] [PubMed] [Google Scholar]
- 52.Tsutsumi S, Shimazu A, Miyazaki K, et al. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001;288:413–9. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- 53.Bianchi G, Banfi A, Mastrogiacomo M, et al. Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp Cell Res. 2003;287:98–105. doi: 10.1016/s0014-4827(03)00138-1. [DOI] [PubMed] [Google Scholar]
- 54.Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;365:149–16. doi: 10.1097/00003086-199908000-00020. [DOI] [PubMed] [Google Scholar]
- 55.Hedbom E, Antonsson P, Hjerpe A, et al. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992;267:6132–6. [PubMed] [Google Scholar]
- 56.Wu W, Cheng X, Zhao Y, et al. Tissue engineering of trachea-like cartilage grafts by using chondrocyte macroaggregate: experimental study in rabbits. Artif Organs. 2007;31:826–34. doi: 10.1111/j.1525-1594.2007.00474.x. [DOI] [PubMed] [Google Scholar]
- 57.Kato Y, Gospodarowicz D. Sulfated proteoglycan synthesis by confluent cultures of rabbit costal chondrocytes grown in the presence of fibroblast growth factor. J Cell Biol. 1985;100:477–85. doi: 10.1083/jcb.100.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin I, Vunjak-Novakovic G, Yang J, et al. Mammalian chondrocytes expanded in the presence of fibroblast growth factor 2 maintain the ability to differentiate and regenerate three-dimensional cartilaginous tissue. Exp Cell Res. 1999;253:681–8. doi: 10.1006/excr.1999.4708. [DOI] [PubMed] [Google Scholar]
- 59.Barbero A, Ploegert S, Heberer M, et al. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003;48:1315–25. doi: 10.1002/art.10950. [DOI] [PubMed] [Google Scholar]
- 60.Mastrogiacomo M, Cancedda R, Quarto R. Effect of different growth factors on the chondrogenic potential of human bone marrow stromal cells. Osteoarthritis Cartilage. 2001;9:S36–40. doi: 10.1053/joca.2001.0442. [DOI] [PubMed] [Google Scholar]
- 61.Solchaga LA, Penick K, Porter JD, et al. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203:398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 62.Johnstone B, Hering TM, Caplan AI, et al. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–72. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 63.Kafienah W, Mistry S, Williams C, et al. Nucleostemin is a marker of proliferating stromal stem cells in adult human bone marrow. Stem Cells. 2006;24:1113–20. doi: 10.1634/stemcells.2005-0416. [DOI] [PubMed] [Google Scholar]
- 64.Kafienah W, Mistry S, Dickinson SC, et al. Three-dimensional cartilage tissue engineering using adult stem cells from osteoarthritis patients. Arthritis Rheum. 2007;56:177–87. doi: 10.1002/art.22285. [DOI] [PubMed] [Google Scholar]
- 65.Baiguera S, Damasceno KL, Macchiarini P. Detergent-enzymatic method for bioengineering human airways. In: Yarmush M, Langer R, editors. Organ perfusion and culture methodology. Methods in bioengineering. Boston: Artech House; ; in press. [Google Scholar]
- 66.Shi H, Xu Z, Qin X, et al. Experimental study of replacing circumferential tracheal defects with new prosthesis. Ann Thorac Surg. 2005;79:672–6. doi: 10.1016/j.athoracsur.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 67.Martinod E, Seguin A, Pfeuty K, et al. Long-term evaluation of the replacement of the trachea with an autologous aortic graft. Ann Thorac Surg. 2003;75:1572–8. doi: 10.1016/s0003-4975(03)00120-6. [DOI] [PubMed] [Google Scholar]
- 68.Gray TE, Guzman K, Davis CW, et al. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1996;14:104–12. doi: 10.1165/ajrcmb.14.1.8534481. [DOI] [PubMed] [Google Scholar]
- 69.Yoon JH, Kim KS, Kim SS, et al. Secretory differentiation of serially passaged normal human nasal epithelial cells by retinoic acid: expression of mucin and lysozyme. Ann Otol Rhinol Laryngol. 2000;109:594–601. doi: 10.1177/000348940010900612. [DOI] [PubMed] [Google Scholar]
- 70.Yamaya M, Finkbeiner WE, Chun SY, et al. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol. 1992;262:L713–24. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 71.Sachs LA, Finkbeiner WE, Widdicombe JH. Effects of media on differentiation of cultured human tracheal epithelium. In Vitro Cell Dev Biol Anim. 2003;39:56–62. doi: 10.1290/1543-706X(2003)039<0056:EOMODO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 72.Pfenninger C, Leinhase I, Endres M, et al. Tracheal remodeling: comparison of different composite cultures consisting of human respiratory epithelial cells and human chondrocytes. In Vitro Cell Dev Biol Anim. 2007;43:28–36. doi: 10.1007/s11626-006-9000-6. [DOI] [PubMed] [Google Scholar]
- 73.Rees LE, Gunasekaran S, Sipaul F, et al. The isolation and characterisation of primary human laryngeal epithelial cells. Mol Immunol. 2006;43:725–30. doi: 10.1016/j.molimm.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 74.Candrian C, Vonwil D, Barbero A, et al. Engineered cartilage generated by nasal chondrocytes is responsive to physical forces resembling joint loading. Arthritis Rheum. 2008;58:197–208. doi: 10.1002/art.23155. [DOI] [PubMed] [Google Scholar]
- 75.Bader A, Knop E, Böker K, et al. A novel bioreactor design for in vitro reconstruction of in vivo liver characteristics. Artif Organs. 1995;19:368–74. doi: 10.1111/j.1525-1594.1995.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 76.Karim N, Golz K, Bader A. The cardiovascular tissue-reactor: a novel device for the engineering of heart valves. Artif Organs. 2006;30:809–14. doi: 10.1111/j.1525-1594.2006.00304.x. [DOI] [PubMed] [Google Scholar]
- 77.Schmitmeier S, Langsch A, Jasmund I, et al. Development and characterization of a small-scale bioreactor based on a bioartificial hepatic culture model for predictive pharmacological in vitro screenings. Biotechnol Bioeng. 2006;95:1198–206. doi: 10.1002/bit.21089. [DOI] [PubMed] [Google Scholar]
- 78.Bilodeau K, Mantovani D. Bioreactors for tissue engineering: focus on mechanical constraints. A comparative review Tissue Eng. 2006;12:2367–83. doi: 10.1089/ten.2006.12.2367. [DOI] [PubMed] [Google Scholar]
- 79.Schulz RM, Wüstneck N, Van Donkelaar C, et al. Development and validation of a novel bioreactor system for load- and perfusion-controlled tissue engineering of chondrocyte-constructs. Biotechnol Bioeng. 2008;101:714–28. doi: 10.1002/bit.21955. [DOI] [PubMed] [Google Scholar]
- 80.Chen HC, Hu YC. Bioreactors for tissue engineering. Biotechnol Lett. 2006;28:1415–23. doi: 10.1007/s10529-006-9111-x. [DOI] [PubMed] [Google Scholar]
- 81.Vunjak-Novakovic G, Martin I, Obradovic B, et al. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;7:130–8. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 82.Tan Q, Hillinger S, Van Blitterswijk CA, et al. Intra-scaffold continuous medium flow combines chondrocyte seeding and culture systems for tissue engineered trachea construction. Interact Cardiovasc Thorac Surg. 2009;8:27–30. doi: 10.1510/icvts.2008.179804. [DOI] [PubMed] [Google Scholar]
- 83.Lin CH, Hsu SH, Huang CE, et al. A scaffold-bioreactor system for a tissue-engineered trachea. Biomaterials. 2009;30:4117–26. doi: 10.1016/j.biomaterials.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 84.Asnaghi MA, Jungebluth P, Raimondi MT, et al. A double-chamber rotating bioreactor for the development of tissue-engineered hollow organs: from concept to clinical trial. Biomaterials. 2009;30:5260–9. doi: 10.1016/j.biomaterials.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 85.Ferlay J, Autier P, Boniol M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–92. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 86.Nouraei SA, Ma E, Patel A, et al. Estimating the population incidence of adult post-intubation laryngotracheal stenosis. Clin Otolaryngol. 2007;32:411–2. doi: 10.1111/j.1749-4486.2007.01484.x. [DOI] [PubMed] [Google Scholar]
- 87.Steinhoff G, Stock U, Karim N, et al. Tissue Engineering of pulmonary heart valves on allogeneic acellular matrix conduits in vivo restoration of valve tissue. Circulation. 2000;102:III50–55. doi: 10.1161/01.cir.102.suppl_3.iii-50. [DOI] [PubMed] [Google Scholar]
- 88.Lorenz K, Rupf T, Salvetter J, et al. Enrichment of human β1bri/α6bri/CD71dim keratinocytes after culture in defined media. Cells Tissues Organs. 2009;189:382–90. doi: 10.1159/000151291. [DOI] [PubMed] [Google Scholar]
- 89.Lorenz K, Sicker M, Schmelzer E, et al. Multilineage differentiation potential of human dermal skin derived fibroblasts. ExpDerm. 2008;17:925–32. doi: 10.1111/j.1600-0625.2008.00724.x. [DOI] [PubMed] [Google Scholar]
- 90.Guidelines for ATMP by EMEA. http://www.emea.europa.eu/htms/human/mes/advancedtherapies.htm.
- 91.Transatlantic collaboration. http://www.fda.gov/oia/FDA-EU_action_plan_062008.htm. . Accessed 5 May 2010.
- 92.Schulz R, Zscharnack M, Hanisch I, et al. Cartilage tissue engineering by collagen matrix associated bone marrow derived mesenchymal stem cells. Biomed Mater Eng. 2008;18:55–70. [PubMed] [Google Scholar]
- 93.Schmelzer E, Deiwick A, Bader A. Thrombopoietin is a growth factor for hepatic progenitors. Eur J Gastroenterol Hepatol. 2008;20:209–16. doi: 10.1097/MEG.0b013e3282f246e6. [DOI] [PubMed] [Google Scholar]
- 94.Zscharnack M, Poesel C, Galle J, et al. Low oxygen expansion improves subsequent chondrogenesis of ovine bone-marrow-derived mesenchymal stem cells in collagen type I hydrogel. Cells Tissues Organs. 2009;190:81–93. doi: 10.1159/000178024. [DOI] [PubMed] [Google Scholar]


