Abstract
The existence of a new type of interstitial cells in the heart namely, interstitial Cajal-like cells (ICLC), has been described for the first time by Hinescu and Popescu in 2005. This study was then followed by an ascending trend of publications regarding the morphology, phenotype and distribution of myocardial ICLC in diverse species including human patients. Recently the new term ‘telocytes’ has been proposed for cells formerly known as ICLC, and the term ‘telopodes’ has been proposed for the prolongations of these cells. The identification of these cells is based on ultrastructural criteria. In addition, telocyters/telyopodes can be identified by several complementary approaches including methylene blue vital staining, silver impregnation and immunoreactivity against CD117/c-kit, vimentin, etc. This point of view presents critical data existing in literature, as well as own results, which unequivocally provide compelling evidence that telocytes are a new distinct cellular entity of myocardial interstitium. Several presumable functions of the myocardial telocytes are discussed: (i) intercellular signalling, (ii) cardiac repair/remodelling and (iii) stem cell nursing in cardiac renewal.
Keywords: telocytes, telopodes, interstitial Cajal-like cells (ICLC), myocardial remodelling, cardiac renewing, myocardial regeneration, interstitial cells
Introduction
For many decades, the existence of myocardial ICLCs interstitial Cajal-like cells (ICLCs) has been either ignored or overlooked. As recently stated, ICLCs ‘were mainly neglected due to the physical constraints of light and/or electron microscopy methodology’. [1]. This study was then followed by an ascending trend of publications regarding the morphology, phenotype and distribution of myocardial ICLC in diverse species including human patients. Recently the new term ‘telocytes’ has been proposed for cells formerly known as ICLC, and the term ‘telopodes’ has been proposed for the prolongations of these cells [1]. I share these arguments and for the sake of standardization; these terms will be used from this point of view.
Cellular compartments of telocytes
Transmission electron microscopy examination is fundamental in identifying the telocytes. The ultrastructural features of telocytes comprise distinct cellular compartments: (1) cell body (the proper telocytes), (2) cellular prolongations (telopodes) and (3) the labyrinthine system made of telopodes.
Figure 1 shows a typical telocyte (cell body) characterized by a thin perinuclear rim of cytoplasm with few cytoplasmic organelles and thin cytoplasmic veils with mitochondria.
Fig 1.
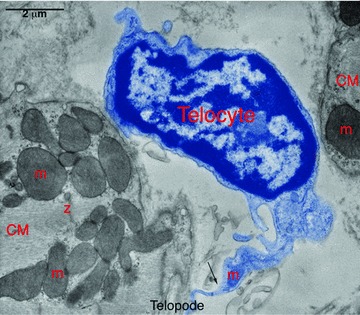
Digitally coloured electron micrograph of rat ventricular myocardium showing a typical cell body (telocyte, blue) in between two cardiomyocytes (CM). Note the sharp transition (Arrow) from cell body to long tortuous process (telocytes). m – mitochondria, Z – Z-band. Reproduced with permission from [3].
Telopodes, which represent cellular prolongations of the telocytes, are unique and probably the longest structures in the body (except some axons) [1, 2]. Figure 1 shows some of the major distinct ultrastructural features of the telopodes including:
Characteristic long (up to 100 μm), moniliform (with segments – about 100 nm thick) cell processes with a dichotomous branching pattern;
Caveolae and coated vesicles; mitochondria, relatively well-developed smooth and rough endoplasmic reticulum and
Intermediate and thin filaments, microtubules and undetectable thick filaments.
Another distinctive ultrastructural feature of telopodes is the formation of labyrinthine apparatuses by three-dimensional convolution and cytoplasmic overlapping. Figure 2 presents several examples of labyrinthine systems of telopodes which are interconnected via cell-to-cell contacts, thereby generating a real cellular network in the entire heart.
Fig 2.
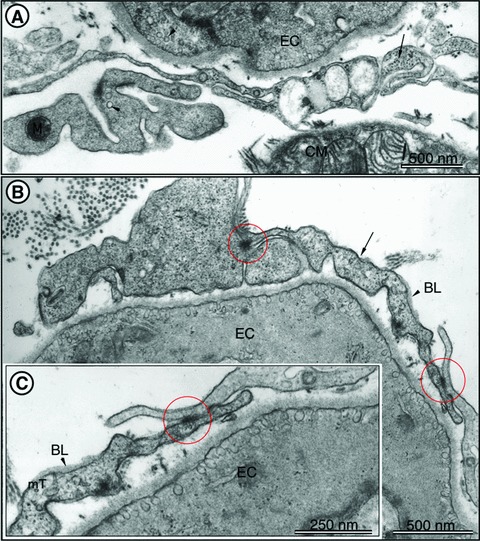
TEM images of rat left ventricular myocardium. (A) A typical example of telopodes (arrows) forming a labyrinthine system and located in vicinity of endothelial cells (EC) and cardiomyocytes. The presence of caveolae (arrowheads) is a typical feature of telopodes and ECs. Note the cross-section of intermediate filaments (arrow) inside the telopodes. (B) Telopodes (arrows) located in close vicinity of EC. Cell-to-cell contacts of telopodes are indicated with circles. Note the presence of the basal lamina (BL, arrowhead), which can occasionally be observed in telopodes. (C) Higher magnification of the right part of (B) showing junctional complexes (circle) between two telopodes. BL – basal lamina. Cross-sectioned microtubules (mT) can be seen in telopodes. Reproduced with permission from [3].
Taken together, all these characteristic ultrastructural features of telocytes/telopodes, which are already established as a diagnostic panel [1–4], underscore and make these cells completely different from other types of myocardial interstitial cells.
Distribution of telocytes in the heart
Growing evidence provide support to the concept that telocytes are not distributed uniformly in the heart. For example, the number of telocytes and telopodes has been found to be higher in atria than ventricles [2, 4]. The distribution of telocytes/telopodes within the left ventricle is also distinct in different myocardial layers: (i) subepicardially, as accumulations of these cells in the loose connective tissue (cardiac stem cell niche) and (ii) intramyocardially, as cellular networks.
Using laser scanning confocal microscopy, deconvolution and three-dimensional reconstruction software (Bitplane, Zürich, Switzerland) we have observed in the subepicardial loose connective tissue clusters of round c-kit+ cells (suggestive of telocytes) as they appear in the cardiac stem cell niches (Fig. 3). Such a distribution pattern of telocytes in the subepicardium has been also observed by electron microscopy [5–7]. Confocal microscopy of immunolabelled tissue sections for c-kit revealed that in the mid-myocardium interstitial c-kit+ cells with long processes (telopodes) intermingling with cardiomyocytes and forming a network-like distribution pattern could also be found. These results are also consistent with numerous electron microscopy findings.
Fig 3.
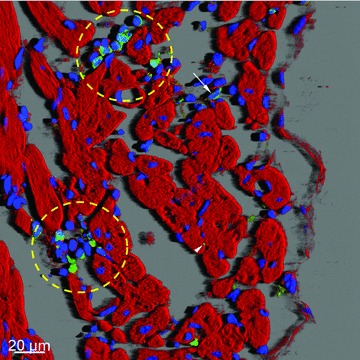
Confocal microscopy of c-kit+ cells in human left ventricular myocardium. Three-dimensional shadow projection image of transversally and obliquely oriented fibres image showing niche-like clusters (yellow round marks) or isolated round c-kit+ cells (arrow) in the subepicardial loose connective tissue. Note small green dots (arrowhead) which represent transversally sectioned telopodes of c-kit+ cells located in close proximity to cardiomyocytes. F-actin is red stained with phalloidin conjugated with TRITC and nuclei are blue stained with DAPI.
Possible functions of telocytes in the myocardium
The precise function(s) of telocytes are still obscure. However, based on several publications of myocardial telocytes, some relevant and potential roles were proposed [1–13].
(1) Telocytes and their telopodes are involved in intercellular signalling at distance because they are situated very close to cardiomyocytes, blood capillaries and nerve endings. In addition, telopodes form a three-dimensional network that would predict at least two mechanisms: (i) a paracrine and/or juxtacrine secretion of small signal molecules and (ii) shedding microvesicles, which play unique roles in the ‘horizontal’ transfer of important macromolecules (e.g., proteins, second messengers or RNAs) among neighbouring cells, necessary for the rapid physiological and phenotypic adjustments of the cells forming the myocardium.
(2) Telocytes, owing to their extremely long telopodes and their ability to form attachment plaques connecting them to the extracellular matrix (ECM), are predicted to function as mechanoreceptors/transducers (but not only!).
(3) Telocytes are key players in cardiac renewal and, likewise, in cardiac repair. This function is based on several studies demonstrating the presence of telocytes in subepicardial niches in adult rodent and human hearts. These results and the observations that telocytes and telopodes are in close contact with immature cardiomyoblasts provide support for the hypothesis that telocytes and their telopodes may ‘guide’ and ‘nurse’ the myocardial precursors, thereby significantly contributing to cardiac renewal and repair [5–7, 13, 14].
Strong arguments in favour of such functions of telocytes are provided by comparative biology of the mammalian heart with fishes and amphibians [15]. For many decades, it is known that these species can fully regenerate their organs, including the heart. In these species, the heart is highly trabeculated. Importantly, trabecula-supporting cells are in fact telocytes, and fulfil all (ultra)structural criteria for telocytes. Figures 4 and 5 show light microscopy and electron microscopy images of typical trabeculae in the newt and zebrafish hearts, respectively. In these species, after amputation of the apex of the ventricle, the heart regenerates and the telocytes and telopodes are the first cells to be involved in this process, which is characterized by proliferation and reorganization of telocytes and telopodes (Fig. 6) to form a three-dimensional network resulting in primitive trabeculae, which further direct and guide myocardial regeneration.
Fig 4.
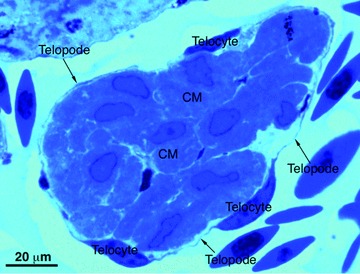
Light microscopy of toluidine-blue stained semithin sections of the normal heart of the newt ‘Notophthalmus viridescens’. A typical trabecula containing cardiomyocytes (CM) which are completely circumscribed by numerous telocytes and very thin telopodes (arrows).
Fig 5.
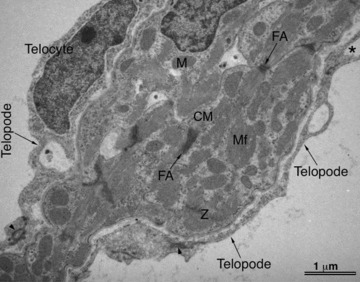
Transmission electron microscopy of the normal zebrafish heart. A typical trabecula containing cardiomyocytes (CM) which are in close contact with telocytes and telopodes (arrows). Asterisk indicates a dichotomous pattern of telocytes. M – mitochondria, Mf – myofilaments.
Fig 6.
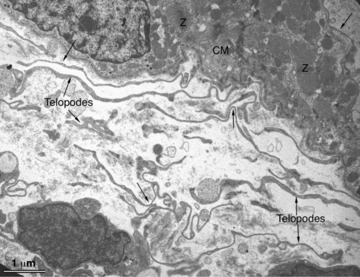
Transmission electron microscopy of the injured heart of the newt Notophthalmus viridescens. Numerous telopodes (arrows) are present in a healing myocardial region 4 days hours after amputation of the ventricular apex. Telopodes seem to originate from the intact myocardium and to extend to the injured site and show a strong tendency to form a cellular network.
Telocytes and myocardial remodelling
The classical definition of myocardial remodelling, ‘genome expression resulting in molecular, cellular and interstitial changes’, has been suggested by Cohn and coauthors in 2000 [16]. According to this definition, ECM remodelling is an important component of myocardial remodelling. In the last years we have intensively studied ECM in diverse cardiac diseases in human patients with pressure-overloaded hearts, cardiomyopathies and atrial fibrilation [17–19]. These studies have demonstrated that ECM remodelling of diseased human hearts is associated with a specific matrix metalloproteinase/tissue inhibitor of metalloproteinase (MMP)/(TIMP) balance, and that each aetiology of cardiac disease possesses a specific MMP and TIMP portfolio. However, in addition to the different MMP/TIMP profiles and different changes of regulatory factors, the failing myocardium is characterized by discordant changes in collagen type I and III metabolism. This observation implies that multiple mechanisms acting alone or in concert are active in fibrosis development. Recently we embarked on several studies aiming at clarifying the role of telocytes in ECM remodelling. Our preliminary data indicate that the number of telocytes/telopodes correlates positively with the amount of denaturated collagens, deposition of non-fibrillar collagens and with the grade of acute or chronic myocardial inflammation. On the other hand, severe interstitial fibrosis and increased amounts of fibrillar collagens, upon achieving a threshold, lead to telocyte cell death via apoptosis and shrinkage and shortening of telopodes (unpublished data). Taken together, our studies suggest that telocytes/telopodes are very flexible cell/structures and respond promptly to any quantitative and qualitative changes in the ECM composition.
Unresolved issues regarding myocardial telocytes
Although in the last 5 years we have witnessed an increased interest and knowledge about myocardial telocytes, several unresolved issues regarding these cells should be emphasized. First, very little is known about telocytes in cardiac pathologies. Apart from the above mentioned preliminary results and one study reporting the presence of telopodes in myocardial sleeves of pulmonary veins [10], which is the source of atrial fibrillation, there are virtually no studies to address the role of telocytes in cardiac diseases. Second, as mentioned in this review, the ultrastructural identity of telocytes is well established and unequivocal. However, the exact immunophenotype and gene expression profile of these cells is far from being resolved. This is an important aspect in this field, given that such knowledge may lead to better understanding the function(s) of telocytes. Another attractive aspect is whether there are organ- or tissue-specific telocytes. As an example, it has been shown that telocytes in human myometrium and Fallopian tube express oestrogen and progesterone receptors [20–22], which raises the possibility that myocardial telocytes might also express tissue-specific signalling molecules, growth factors, receptors, etc.
References
- 1.Popescu LM, Faussone-Pellegrini MS. TELOCYTES – a case of serendipity: the winding way from interstitial cells of Cajal (ICC), via interstitial Cajal-like cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popescu LM, Gherghiceanu M, Hinescu ME, et al. Insights into the interstitium of ventricular myocardium: interstitial Cajal-like cells (ICLC) J Cell Mol Med. 2006;10:429–58. doi: 10.1111/j.1582-4934.2006.tb00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kostin S, Popescu LM. A distinct type of cell in myocardium: interstitial Cajal-like cells (ICLCs) J Cell Mol Med. 2009;13:295–308. doi: 10.1111/j.1582-4934.2008.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinescu ME, Gherghiceanu M, Mandache E, et al. Interstitial Cajal-like cells (ICLC) in atrial myocardium: ultrastructural and immunohistochemical characterization. J Cell Mol Med. 2006;10:243–57. doi: 10.1111/j.1582-4934.2006.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popescu L, Gherghiceanu M, Manole C, Faussone-Pellegrini MS. Cardiac renewing: interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches. J Cell Mol Med. 2009;13:866–86. doi: 10.1111/j.1582-4934.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gherghiceanu M, Popescu L. Human epicardium: ultrastructural ancestry of mesothelium and mesenchymal cells. J Cell Mol Med. 2009;13:2949–51. doi: 10.1111/j.1582-4934.2009.00869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gherghiceanu M, Popescu LM. Cardiomyocyte precursors and telocytes in epicardial stem cell niche. J Cell Mol Med. 2010;14:871–7. doi: 10.1111/j.1582-4934.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinescu ME, Popescu LM. Interstitial Cajal-like cells (ICLC) in human atrial myocardium. J Cell Mol Med. 2005;9:972–5. doi: 10.1111/j.1582-4934.2005.tb00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandache E, Popescu LM, Gherghiceanu M. Myocardial interstitial Cajal-like cells (ICLC) and their nanostructural relationships with intercalated discs: shed vesicles as intermediates. J Cell Mol Med. 2007;11:1175–84. doi: 10.1111/j.1582-4934.2007.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gherghiceanu M, Hinescu ME, Andrei F, et al. Interstitial Cajal-like cells (ICLC) in myocardial sleeves of human pulmonary veins. J Cell Mol Med. 2008;12:1777–81. doi: 10.1111/j.1582-4934.2008.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suciu L, Popescu LM, Regalia T, et al. Epicardium: interstitial Cajal-like cells (ICLC) highlighted by immunofluorescence. J Cell Mol Med. 2009;13:771–7. doi: 10.1111/j.1582-4934.2009.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gherghiceanu M, Hinescu ME, Popescu LM. Myocardial interstitial Cajal-like cells (ICLC) in caveolin-1 KO mice. J Cell Mol Med. 2009;13:202–6. doi: 10.1111/j.1582-4934.2008.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faussone-Pellegrini MS, Bani D. Relationships between telocytes and cardiomyocytes during pre- and post-natal life. J Cell Mol Med. 2010;14:1061–3. doi: 10.1111/j.1582-4934.2010.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Limana F, Bertolami C, Mangoni A, et al. Myocardial infarction induces embryonic reprogramming of epicardial c-kit(+) cells: role of the pericardial fluid. J Mol Cell Cardiol. 2010;48:609–18. doi: 10.1016/j.yjmcc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Borchard T, Kostin S. Plastizitat von Herzmusklezellen des Molches. Max-Plack-Gesellschaft Entwicklungs &Evolutionsbiologie. 2007:237–43. [Google Scholar]
- 16.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling-concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–82. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 17.Polyakova V, Hein S, Kostin S, et al. Matrix metalloproteinases and their tissue inhibitors in pressure-overloaded human myocardium during heart failure progression. J Am Coll Cardiol. 2004;44:1609–18. doi: 10.1016/j.jacc.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Polyakova V, Loeffler I, Hein S, et al. Fibrosis in endstage human heart failure: Disease-specific changes in collagen metabolism and MMP/TIMP profiles. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.04.053. DOI: 10.1016/j.ijcard.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 19.Polyakova V, Miyagawa S, Szalay Z, et al. Atrial extracellular matrix remodelling in patients with atrial fibrillation. J Cell Mol Med. 2008;12:189–208. doi: 10.1111/j.1582-4934.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cretoiu D, Ciontea SM, Popescu LM, et al. Interstitial Cajal-like cells (ICLC) as steroid hormone sensors in human myometrium: immunocytochemical approach. J Cell Mol Med. 2006;10:789–95. doi: 10.1111/j.1582-4934.2006.tb00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popescu LM, Ciontea SM, Cretoiu D. Interstitial Cajal-like cells in human uterus and fallopian tube. Ann NY Acad Sci. 2007;1101:139–65. doi: 10.1196/annals.1389.022. [DOI] [PubMed] [Google Scholar]
- 22.Cretoiu SM, Cretoiu D, Suciu L, et al. Interstitial Cajal-like cells of human Fallopian tube express estrogen and progesterone receptors. J Mol Histol. 2009;40:387–94. doi: 10.1007/s10735-009-9252-z. [DOI] [PubMed] [Google Scholar]


