Abstract
Rapid apoptotic cell engulfment is crucial for prevention of inflammation and autoimmune diseases and is conducted by special immunocompetent cells like macrophages or immature dendritic cells. We recently demonstrated that endothelial cells (ECs) also participate in apoptotic cell clearance. However, in contrast to conventional phagocytes they respond with an inflammatory phenotype. To further confirm these pro-inflammatory responses human ECs were exposed to apoptotic murine ECs and changes in thrombospondin-1 (TSP-1) expression and in activation of intracellular signalling cascades were determined by real-time qPCR, immunoblotting and immunocytochemistry. Human primary macrophages or monocytic lymphoma cells (U937) were incubated with conditioned supernatant of human ECs exposed to apoptotic cells and changes in activation, migration and phagocytosis were monitored. Finally, plasma levels of TSP-1 in patients with anti-neutrophil cytoplasmic antibody(ANCA)-associated vasculitis (AAV) were determined by ELISA. We provided evidence that apoptotic cells induce enhanced expression of TSP-1 in human ECs and that this increase in TSP-1 is mediated by the mitogen-activated protein kinases (MAPK) ERK1 and 2 and their upstream regulators MEK and B-Raf. We also showed that plasma TSP-1 levels are increased in patients with AAV. Finally, we showed that conditioned supernatant of ECs exposed to apoptotic cells induces pro-inflammatory responses in monocytes or U937 cells and demonstrated that increased TSP-1 expression enhances migration and facilitates engulfment of apoptotic cells by monocyte-derived macrophages or U937 cells. These findings suggest that under pathological conditions with high numbers of uncleared dying cells in the circulation endothelial-derived elevated TSP-1 level may serve as an attraction signal for phagocytes promoting enhanced recognition and clearance of apoptotic cells.
Keywords: endothelial cells, phagocytosis, thrombospondin-1, autoimmune disease, phosphatidylserine
Introduction
Pauci-immune granulomatous anti-neutrophil cytoplasmic antibody(ANCA)-associated vasculitis (GAAV), microscopic polyangiitis (MPA) and immune complex associated systemic lupus erythematosis (SLE), all feature large numbers of circulating dead or dying cells [1, 2]. The host’s ability to clear these damaged cells by phagocytosis is presumably impaired in these diseases [3, 4]. Here, a defect in the macrophage phagocytosis rate and the appearance of membrane-bound or soluble factors that delay apoptotic cell uptake appear responsible [5–7]. For instance, the ANCA target in GAAV, proteinase 3, prevents clearance of apoptotic neutrophils without affecting macrophages directly [3]. On the other hand, macrophages from SLE patients display cellular defects resulting in delayed engulfment of apoptotic cells [8]. However, rapid apoptotic cell removal is important for tissue homeostasis. Once cells undergo apoptosis, they are rapidly engulfed by specialized immunocompetent phagocytes, including macrophages, immature dendritic cells and neutrophils. A key feature of apoptotic cell clearance is inhibition of pro-inflammatory signals and prevention of immune responses. Moreover, under certain conditions engulfment of apoptotic cells actively induces an anti-inflammatory and immunotolerant environment [9, 10]. On the other hand, defects in apoptotic cell removal lead to secondary necrosis and the release of noxious and immunogenic cytoplasmic contents, shifting the responses to inflammation and autoimmunity [11, 12]. In systemic AAV the endothelial layer is exposed to highly increased numbers of circulating late apoptotic or necrotic endothelial cells (ECs) and neutrophils [1, 13]. We recently found that ECs participate in apoptotic cell clearance, although their engulfment kinetics is inferior to macrophages. Unlike immunocompetent phagocytes, ECs become activated and respond with the release of inflammatory chemokines [14]. The aim of this study was to investigate the cellular pathways that are important to this process.
Materials and methods
Endothelial cell culture
Human umbilical cord endothelial cells (HUVEC) were isolated from umbilical cords as previously described [14]. HUVEC were cultured in EGM2 medium (Lonza, Vervier, France) and used up to passage 3. HMEC-1 cells were obtained from the CDC (Atlanta, GA, USA) and maintained in MCDB-131 medium supplemented with 10% foetal calf serum (FCS, PAA, Coelbe, Germany), 50 μg/ml gentamycin, 10 mM L-glutamine (Invitrogen, Karlsruhe, Germany) 10 ng/ml EGF and 1 μg/ml hydrocortisone (BD Biosciences, Heidelberg, Germany). Mouse embryonal microvascular ECs (eEND2) were a gift from Dr. Michael Leitges and cultured in D-MEM containing 4500 mg/l glucose, GlutaMaxI and pyruvate (Invitrogen) and supplemented with 10% FCS and gentamycin. U937 were maintained in RPMI 1640 supplemented with 2 mM L-glutamine, 10% FCS and gentamycin.
Isolation and culture of monocytic cells
Blood was obtained from healthy volunteers. Mononuclear cells were isolated by density-gradient centrifugation over isotonic Biocoll (Biochrom, Berlin, Germany) and cultivated in RPMI 1640 supplemented with 10% FCS and gentamycin at 37°C and 5% CO2. After 1 hr non-adherent lymphocytes were removed and monocytes were cultivated for further 6–8 days in RPMI 1640. For some experiments macrophages were labelled with 5-chloromethylfluorescein diacetate (CMFDA, Invitrogen) for 30 min. Approval for these studies was obtained from the institutional review board of the Medical School Hannover.
Induction of apoptosis in eEND2 cells
Apoptosis in eEND2 cells was induced by ultraviolet light (254 nm) as described previously for HMEC-1 cells [14]. Initiation of apoptosis was determined by annexinV/propidiumiodid staining (Roche Diagnostics, Penzberg, Germany), activity of caspase 3/7 and fragmentation of nuclear DNA (caspase-Glo 3/7 assay and Dead-End Fluorometric TUNEL system, Promega, Mannheim, Germany).
Phagocytosis assay
Human ECs were plated at 4 × 105 cells/ml in culture dishes or on μ-slides (IBIDI GmbH, Martinsried, Germany) and cultivated until reaching confluence. Cells were synchronized by cultivation for 16 hrs in MCDB-131 basal medium (HMEC-1 cells) or in EBM2 medium with reduced FCS content (0.5% FCS, HUVEC). After washing ECs were exposed to apoptotic eEND2 cells (1 × 106 cells/ml) for different time-points. Unbound or loosely adherent apoptotic eEND2 cells were removed by intensive washes with ice-cold PBS and cells were processed according to the subsequent assays.
Monocyte-derived macrophages or U937 cells were seeded onto μ-slides and synchronized over night in low-FCS medium. In some experiments cells were pre-incubated with RGD or RAD peptides (final concentration 50 μM, Enzo Life Sciences, Loerrach, Germany) for 30 min. Apoptotic eEND2 cells were pre-incubated for 30 min. with conditioned supernatant of HUVEC before they were added to the macrophages or U937 cells for further 60 min. To block thrombospondin-1 (TSP-1) the endothelial supernatant was pre-incubated with a neutralizing anti-TSP-1 antibody (clone A6.1 at a final concentration of 5 μg/ml, Invitrogen) or a corresponding mouse isotype control (5 μg/ml). Cells were washed intensively with ice-cold PBS, fixed with 4% paraformaldehyde (PFA) in PBS and counterstained with haematoxylin and eosin. The slides were analysed by bright field microscopy and phagocytosis was recorded as the phagocytic index, defined as the number of internalized apoptotic eEND2 cells per 100 macrophages or U937 cells based on assessment of at least 300 cells.
Chemotaxis assay
HUVEC were seeded in the lower chamber of a transwell device (Corning/Fisher Scientific, Schwerte, Germany) and cultivated until reaching confluence. After synchronization cells were exposed to apoptotic eEND2 cells for 8 hrs. Subsequently, CMFDA-labelled human macrophages or PMA-stimulated U937 cells were added to the upper chamber and incubated for additional 60 min. In some experiments macrophages or PMA-stimulated U937 cells were pre-incubated for 30 min. with an RGD or RAD peptide. To block TSP-1, the neutralizing anti-TSP-1 antibody (5 μg/ml) or a corresponding isotype control was added to the supernatant in the lower chamber 30 min. prior to the experiment. After 60 min., transwell membranes were fixed with 4% PFA, stained with DAPI and mounted upside down onto microscope slides. The number of CMFDA-labelled macrophages that had migrated to the lower side of the transwell membrane was determined by fluorescence microscopy.
RNA extraction and real-time qPCR
Total RNA was extracted using the RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol and reverse transcribed using the total-script-OLS kit (Omni Life Science, Hamburg, Germany). qPCR was performed on a SDS 7700 system (Applied Biosystems, Darmstadt, Germany) with FastStart taq Polymerase (Roche Diagnostics) and gene-specific primers (Operon, Cologne, Germany) in combination with SYBR-Green chemistry (Invitrogen). Data were analysed using Q-gene software. Oligonucleotides for detection of TSP-1 (ordering number QT00028497)) and interleukin (IL)-8 (ordering number QT00000322) were purchased from Qiagen. The sequences for the remaining oligonucleotides were as follows: HPRT-1: TGACACTGGCAAAACAATGCA; GGTCCTTTTCACCAGCAAGCT, IL-10: CATCAAGGCGCATGTGAACT; ATTCTTCACCTGCTCCACGG, IL-1β: CTTTGAAGCTGATGGCCCTAAA; AGTGGTGGTCGGAGATTCGT, MCP-1: GCTCAGCCAGATGCAATCAA; CTCCTTGGCCACAATGGTCT, tumour necrosis factor (TNF)-α: GCCCATGTTGTAGCAAACCC; CCTCTGATGGCACCACCAG.
SDS-PAGE and immunoblot
Cells were lysed in ice-cold RIPA buffer containing protease and phosphatase inhibitor cocktail tablets (Roche Diagnostics) and protein content was determined using the BCA protein assay kit (Perbio Science, Bonn, Germany). Fifty micrograms of whole cell lysates were separated by SDS-PAGE electrophoresis and blotted on a PVDF nylon membrane. Filters were hybridized with the appropriate primary antibody followed by incubation with a HRP-conjugated secondary antibody. The bands were visualized by Western Lighting chemiluminiscence reagent (Perkin Elmer, Monza, Italy) and quantified by densitometry using a CCD camera and Quantity One software (Biorad, Muenchen, Germany). The individual primary antibodies used were anti-p-ERK1/2 and anti-pB-Raf (1:1000 dilutions, Cell Signaling, Danvers, MA, USA). Equal protein loading was verified by stripping off the original antibodies and reprobing with anti–ERK and anti-B-Raf antibody (1:1000, Cell Signaling).
Immunocytochemistry
Cells were grown onto collagen-coated glass cover slips or μ-slides (IBIDI GmbH) and fixed in ice-cold (–20°C) methanol. After aspirating the cells were blocked in normal donkey serum and incubated with the appropriate primary antibody for 1 hr. After washing the cover slips cells were incubated with a fluorochrome-conjugated secondary antibody for 1 hr, mounted on glass slides and processed for fluorescence or confocal microscopy. For counterstaining with phalloidin-Alexa 546 (Invitrogen) cells were fixed in 4% PFA and permeabilized with 0.1% Triton-X100. The following primary antibodies were used: rabbit anti-pB-Raf and rabbit anti-pErk1/2 (Cell Signaling) and mouse anti-TSP-1 (R&D Systems, Wiesbaden, Germany).
Measurement of TSP-1 concentrations in cell culture supernatant or plasma samples
HUVEC were grown to confluence in 24-well plates followed by a 16-hr incubation in synchronization medium. The next morning, HUVEC were cultivated in EBM basal medium supplemented with gentamycin. Apoptotic eEND2 cells were added to the endothelial monolayer for 3 and 8 hrs. The medium was removed, cleared of apoptotic cells and cellular debris by centrifugation for 10 min. at 14,000 ×g. For measurement of plasma TSP-1 concentrations citrated blood samples were centrifuged twice for 5 min. at 5000 ×g at room temperature to obtain platelet-poor plasma. Concentration of TSP-1 was determined using the Thrombospondin-1 Quantikine kit according to the manufacturers protocol (R&D Systems).
Patients
For measurement of plasma TSP-1 we collected blood from 19 patients with AAV. Fourteen patients had Wegener’s granulomatosis and five patients had MPA. Patients with vasculitis were classified in accordance with the Chapel Hill Consensus Conference. Patients with systemic infections were excluded. Characteristics of the patients are summarized in Table 1. Nine healthy age-matched volunteers without a history of hypertension, cardiovascular and inflammatory diseases served as controls. This study was conducted in accordance with the Declaration of Helsinki and the study protocol was approved by the local Institutional Ethical Committee. Informed consent was obtained from all patients included in this study prior to blood collections.
Table 1.
Characteristics of the patients
| Active AAV (n= 19) | |
|---|---|
| WG/MPA | 14/5 |
| Male/female | 15/4 |
| Age mean ± S.D., years | 59 ± 13 |
| BVAS (mean ± S.D.) | 6–23 (11.7 ± 6.9) |
| Creatinine mean ± S.D., μmol/l | 268 ± 212 |
| CRP mean ± S.D., mg/dl | 60 ± 59 |
AAV: active ANCA-associated vasculitis; WG: Wegener’s granulomatosis;
MPA: microscopic polyangiitis; BVAS: Birmingham Vasculitis Activity Score and CRP: C-reactive protein.
Statistical analysis
Results were expressed as mean ± S.D. or S.E.M. of at least three independent experiments. Where human donors were used, cells were from different donors. Results were analysed for statistical significance using two-tailed Mann-Whitney U test or Wilcoxon-signed ranks test.
Results
Binding of apoptotic cells enhances expression of TSP-1 in human ECs
Following our hypothesis that accumulation of apoptotic cells leads to the release of endothelial-derived chemokines and other soluble factors that facilitate phagocyte attraction as well as recognition and engulfment of apoptotic cells we examined changes in the expression of TSP-1 in human ECs exposed to apoptotic mouse eEnd2 cells. The assays used here to detect TSP-1 level, namely qPCR and ELISA or immune cytochemistry, were highly specific for the human TSP-1 ortholog and did not generate any signals for mouse TSP-1 (data not shown). Therefore, the use of a murine EC line for the generation of apoptotic cells allowed us to clearly distinguish between signals derived from the apoptotic mouse cell or from the engulfing human cell.
In both, HUVEC and HMEC-1 cells, we observed a significant increase in TSP-1 transcripts 4 hrs after addition of apoptotic eEND2 cells (Fig. 1A). Interestingly, the basal levels of TSP-1 transcript in unstimulated EC did not differ markedly between the two cell types (the ratio of TSP-1/HPRT-1 mRNA in untreated HUVEC was 0.59 ± 0.3 compared to a ratio of 0.41 ± 0.1 in untreated HMEC-1 cells). In addition, the increase in TSP-1 expression after incubation with apoptotic eEND2 cells followed a similar pattern in both cell types (3.6-fold increase in HUVEC (P < 0.001; n= 8) compared to 3.9-fold increase in HMEC-1 (P < 0.05; n= 8). To verify that the observed up-regulation of TSP-1 expression was specific for the binding of apoptotic cells we incubated human ECs with necrotic (obtained by several freeze-thaw cycles) or with untreated eEND2 cells. Under both conditions we did not record any changes in TSP-1 expression (data not shown).
Fig 1.
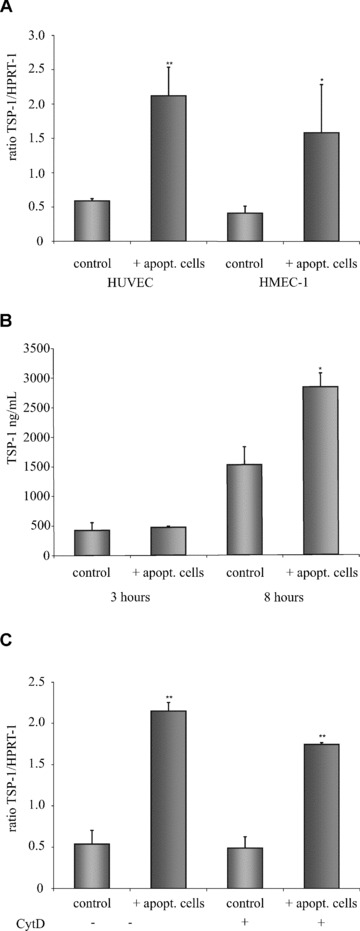
Apoptotic cells induce increased TSP-1 expression in ECs. (A) HUVEC or HMEC-1 cells were exposed to apoptotic eEND2 cells for 4 hrs and differences in the expression of TSP-1 transcript was measured by real-time qPCR (n= 8). (B) HUVEC were exposed to apoptotic eEND2 cells for three and for 8 hrs and TSP-1 protein in the supernatant was measured by ELISA (n= 4). (C) Pre-incubation with cytochalasin D did not prevent apoptotic-cell-induced increase in TSP-1 mRNA expression in HUVEC (n= 3). Data are given as mean ± S.D. (*P < 0.05 **P < 0.001).
We also measured a significant increase in the release of TSP-1 protein from HUVEC following 8-hr incubation with apoptotic eEND2 cells. The extended time period for protein measurement was chosen to account for the different kinetics in transcript and protein synthesis. Exposure to apoptotic cells for 3 hrs did not result in any significant changes in TSP-1 production whereas exposure to apoptotic cells for 8 hrs increased the TSP-1 protein content in the cell culture supernatant from 1523.4 ng/ml ± 288 to 2833.3 ng/ml ± 219 (n= 4, P < 0.005) (Fig. 1B).
Next, we pre-incubated HUVEC with cytochalasin D (CytD) to prevent engulfment but not binding of apoptotic eEND2 cells. CytD is a toxin that rapidly inhibits the polymerization of microtubules. Pre-treatment with CytD for 30 min. did not result in significant changes in apoptotic cell-induced TSP-1 expression in HUVEC (Fig. 1C). These findings implicate that binding but not necessarily engulfment of apoptotic cells is sufficient to initiate the intracellular signals required for enhanced TSP-1 expression in ECs.
We also wondered whether the increase in TSP-1 transcripts was due to paracrinic signalling of HUVEC that have bound and phagocytosed apoptotic eEND2 cells. Therefore, we incubated HUVEC with either Golgi-plug or Golgi-stop for 30 min. prior to the exposure to apoptotic eEND2 cells. Treatment with Golgi-plug or Golgi-stop inhibits the release of secretory proteins from the cell. Single or combined treatment with both drugs did not interfere with the observed increase in TSP-1 transcript expression in HUVEC after incubation with apoptotic cells (data not shown). Therefore, we concluded that the increase in TSP-1 generation represents an event that is directly linked to the binding of the apoptotic cell to the surface of the EC and is not induced by paracrine or autocrine signals.
Apoptotic cell-induced TSP-1 expression is mediated by the B-Raf/MEK/ERK pathway
Next, we examined the intracellular signalling pathways that are responsible for apoptotic cell-induced increase in TSP-1 generation. Expression of TSP-1 is known to be modulated by members of the MAPK pathway in several cell types. Therefore, we further assessed whether members of the MAPK pathway are involved in apoptotic cell-induced TSP-1 expression. We first measured changes in the phosphorylation status of ERK1/2 by immunoblot analysis. Exposure of HUVEC to apoptotic eEND2 cells resulted in a rapid activation of both ERK isoforms with a peak after 10 min. and a subsequent decline to basal phosphorylation level in the next 30 min. whereas non-apoptotic cells did not have any impact on ERK activation (data not shown). B-Raf, one of the main effector kinases regulating activation of the MEK/ERK pathway, showed similar phosphorylation pattern in HUVEC incubated with apoptotic eEND2 cells (Fig. 2A). In contrast, we could not detect any short-term changes in the activation of c-Jun N-terminal kinase (JNK) or p38 MAPK, respectively (data not shown). Immunostaining of HUVEC with an anti-pERK1/2 or an anti-pB-Raf antibody demonstrated increased ERK1/2 and B-Raf phosphorylation shortly after exposure to apoptotic eEND2 cells (Fig. 2B). We also measured phosphorylation of the B-Raf homologues A-Raf and C-Raf but did not detect any changes in their phosphorylation status (data not shown).
Fig 2.
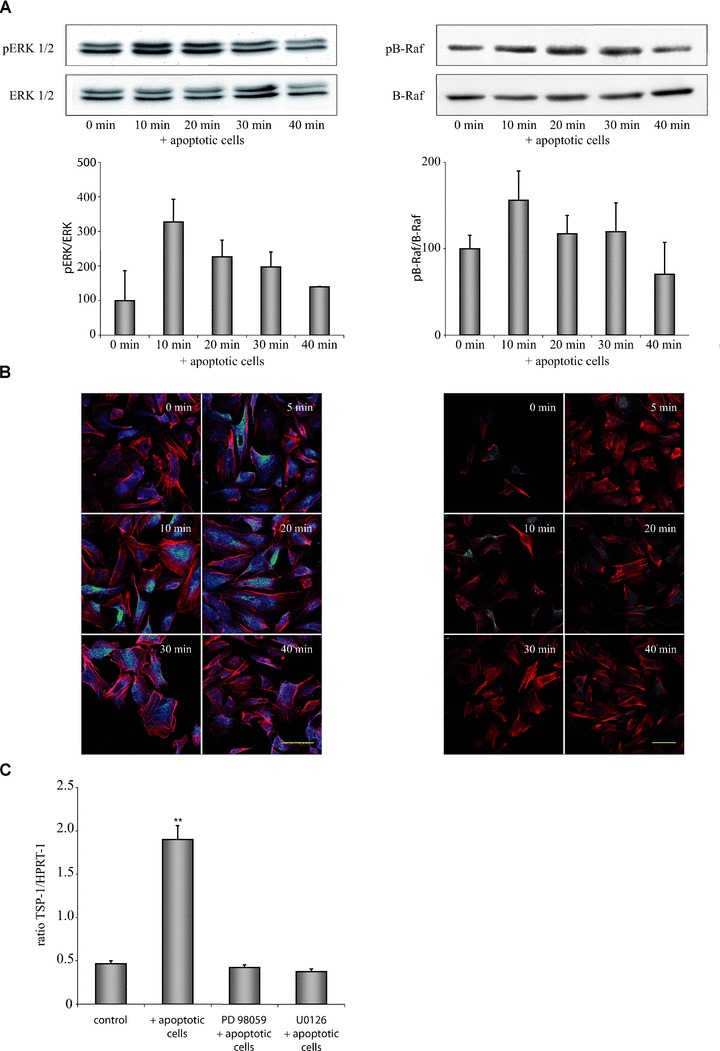
Apoptotic cell-induced expression of TSP-1 in HUVEC is mediated by the B-Raf/MEK/ERK pathway. (A) Exposure of HUVEC to apoptotic eEND2 cells led to a rapid activation of ERK1/2 (left) and B-Raf (right). Changes in phosphorylation were determined by calculation of the ratio of phosphorylated to unphosphorylated ERK1/2 or B-Raf protein, respectively (n= 6). (B) Immunocytochemical staining of pERK1/2 and pB-Raf in HUVEC exposed to apoptotic eEND2 cells. Cells were counterstained with phalloidin-Alexa546 and analysed by confocal microscopy. Shown are representative micrographs of three independent experiments. Scale bars represent 50 μm. (C) Inhibition of ERK phosphorylation by U0126 or PD98059 circumvented apoptotic cell-induced enhanced TSP-1 expression in HUVEC as assessed by real-time qPCR measurement (n= 4; **P < 0.001).
In additional experiments, we demonstrated that activation of ERK1/2 is necessary for apoptotic cell-induced TSP-1 expression in HUVEC. Blocking ERK phosphorylation by pre-incubating HUVEC for 30 min. with the MEK-specific inhibitors U0126 or PD98059 completely prevents the observed increase in TSP-1 expression following incubation with apoptotic eEND2 cells (Fig. 2C). Interestingly, blocking of ERK1/2 phosphorylation by U0126 or PD98059 did not have any impact on the basal TSP-1 expression suggesting that apoptotic cell-induced TSP-1 synthesis and basal TSP-1 generation in HUVEC are controlled by different pathways.
HUVEC-derived TSP-1 is bound by apoptotic mouse eEND2 cells
Although it is well known that TSP-1 serves as a bridging molecule that facilitates recognition and binding of apoptotic cells by phagocytes little is known about the role of endothelial-derived TSP-1 in the course of apoptotic cell clearance. Therefore, apoptotic mouse eEND2 cells were incubated for 1 hr with conditioned supernatant of HUVECs that were exposed to apoptotic cells for 8 hrs (cS/N). Subsequently, the apoptotic eEND2 cells were fixed and stained with a monoclonal antibody against human TSP-1. As shown in Fig. 3A and B the antibody recognizes human TSP-1 bound to the outer plasma membrane of the apoptotic mouse eEND2 cell. Control staining of apoptotic eEND2 cells incubated with endothelial basal medium or native eEND2 cells as well as with an unspecific mouse IgG control did not reveal any positive TSP-1 staining (data not shown). Next, we incubated HUVEC with apoptotic eEND2 cells for 30 min. and again stained against human TSP-1. TSP-1 staining could be seen predominantly at perinuclear regions and to a lesser extent at the plasma membrane of HUVEC. Apoptotic eEND2 cells displayed TSP-1 staining at the whole surface of the cell (Fig. 3C, D). Using confocal microscopy we could confirm the TSP-1 staining pattern around the nucleus and at the plasma membrane. Moreover, the surface of the apoptotic cell that is attached to the plasma membrane of the EC displays a strong TSP-1 signal (Fig. 3E, F).
Fig 3.
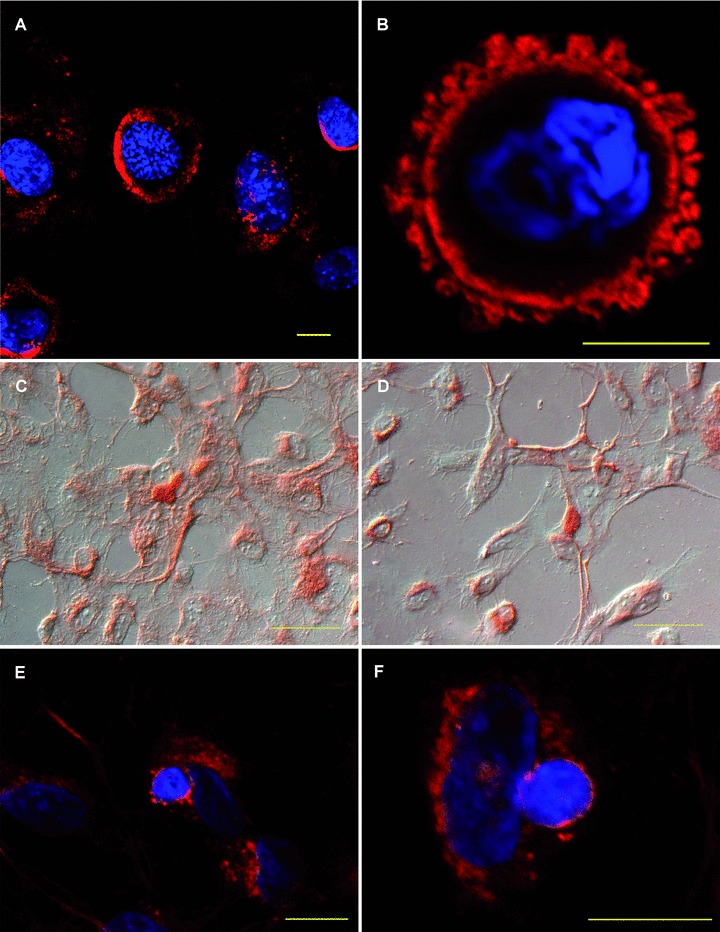
HUVEC-derived TSP-1 is bound by apoptotic murine eEND2 cells. (A, B) Apoptotic murine eEND2 cells were incubated with conditioned supernatant of HUVEC exposed to apoptotic cells and stained for human TSP-1. Shown are representative confocal images of four independent experiments. The nucleus was counterstained with DAPI. Scale bar represents 10 μm. (C, D) HUVEC were exposed to apoptotic eEND2 cells for 30 min. Cells were counterstained with haematoxylin and eosin and analysed by conventional microscopy. (E, F) HUVEC were seeded onto μ-slides and exposed to apoptotic eEND2 cells for 30 min. Cells were stained for human TSP-1 and applied to confocal microscopy. The nucleus was counterstained with DAPI. Scale bars represent 50 μm.
Conditioned supernatant of ECs induced macrophage activation
We recently showed that apoptotic cells have the potential to induce an inflammatory phenotype in human ECs [14]. To answer the question whether this effect has any impact on macrophage activation we measured different cytokine and chemokine transcript profiles in human primary monocyte-derived macrophages and U937 cells. In primary macrophages cS/N of HUVEC induced a 4- to 10-fold increase in transcript amount of IL-1β, TNF-α and IL-8 after 3 hrs compared to supernatant of unstimulated cells whereas expression of MCP-1 and IL-10 was reduced after stimulation with cS/N. M-CSF obviously had no effect on expression of the pro-inflammatory markers and IL-10 whereas MCP-1 transcript amount was slightly increased (Fig. 4A). U937 cells exposed to conditioned supernatant respond with slight increases in IL-8 and TNF-α expression and a reduction in IL-10 transcript amount (Fig. 4B). Taken together, these results suggest that cS/N of HUVEC exposed to apoptotic ECs led to an activation of primary human monocytes and, to a lesser extent, of U937 cells.
Fig 4.
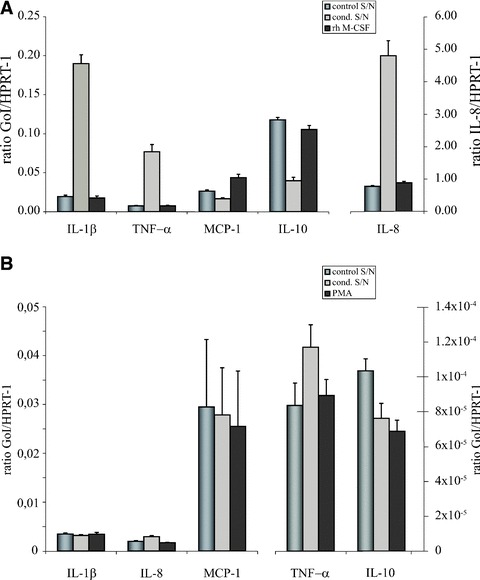
Supernatant of HUVEC exposed to apoptotic eEND2 cells activates monocyte-derived macrophages and U937 cells. (A) Human monocyte-derived macrophages were exposed to supernatant of HUVEC exposed to apoptotic eEND2 cells, to supernatant of untreated HUVEC or to rh M-CSF for 3 hrs and expression level of different cytokines and chemokines were determined by real-time qPCR. Plotted are the average expression levels of three experiments with macrophages derived from different donors. Note the different y-scale for expression of IL-8. (B) U937 mRNA expression profiles of the same set of cytokines/chemokines after stimulation for 3 hrs with supernatant of untreated HUVEC, HUVEC exposed to apoptotic cells and PMA (n= 3). Note the different y-scale for expression level of MCP-1, TNF-α and IL-10. GoI: Gene of interest.
Endothelial TSP-1 enhances recognition and binding of apoptotic cells by macrophages
We next examined whether HUVEC-derived TSP-1 facilitates clearance of apoptotic mouse eEND2 cells. In a first set of experiments we neutralized secreted TSP-1 with an inhibitory antibody or blocked αVβ3 integrin or CD36 on the surface of HUVEC and measured changes in the binding or uptake of apoptotic eEND2 cells after 1 and 6 hrs. Surprisingly, pre-incubation of HUVEC with the RGD peptide or an anti-CD36 antibody as well as neutralizing TSP-1 in the cS/N did not result in any changes in apoptotic cell engulfment or even cell binding. We repeated these experiments with both αVβ3 integrin and CD36 being blocked by a mix of antibodies and RGD peptides before apoptotic cells were added. Additionally, TSP-1 in the cS/N was neutralized by an inhibitory anti-TSP-1 antibody. Even under these conditions binding of apoptotic eEND2 cells to HUVEC was not significantly altered (Fig. 5A).
Fig 5.
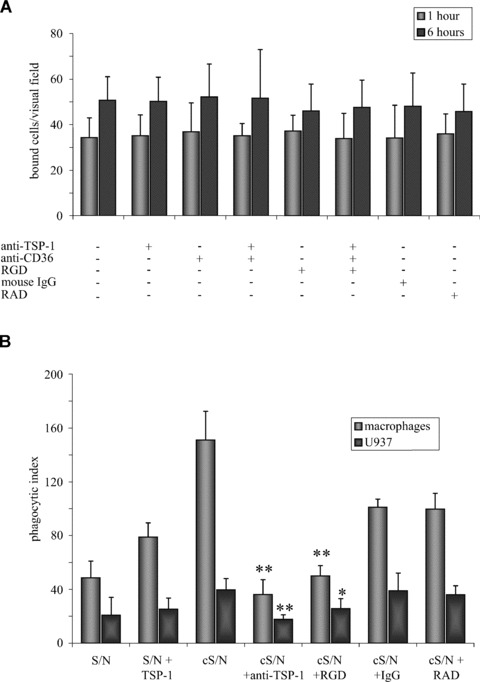
TSP-1 mediates engulfment of apoptotic cells by macrophages but not by ECs. (A) Blocking TSP-1 in the supernatant with a neutralizing anti-TSP-1 antibody (5 μg/ml) or pre-treatment of HUVEC with RGD-containing peptides (50 μM) or anti-CD36 antibodies (10 μg/ml) did not influence binding and engulfment of apoptotic eEND2 cells. (n= 7). For each experiment at least 10 visual fields were counted and averaged. (B) Blocking RGD-sensitive receptors of monocyte-derived macrophages and PMA-stimulated U937 cells or neutralizing TSP-1 in the conditioned supernatant of apoptotic cell-activated HUVEC reduced the number of engulfed apoptotic cells whereas exogenous TSP-1, added to supernatant of untreated HUVEC, slightly increased the engulfment of apoptotic eEND2. Shown are the results of four to five independent experiments (*P < 0.05; **P < 0.001 compared to pre-incubation with conditioned supernatant alone).
We then tested whether HUVEC-derived TSP-1 augmented engulfment of apoptotic cells by monocyte-derived macrophages or by PMA-treated U937 cells (Fig. 5B). Pre-incubation of apoptotic eEND2 cells with cS/N of HUVEC increased the phagocytic index of monocyte-derived macrophages from 48.6 ± 12.4 (supernatant of untreated HUVEC) to 151 ± 21.32 (cS/N). This increase in engulfment could be prevented by either blocking RGD peptide-sensitive receptors (50 ± 7.6 compared to 99.6 ± 11.8 for the control RAD peptide) or by neutralizing TSP-1 in the cS/N (36.17 ± 11.1 compared to 101 ± 6.2 for a mouse IgG isotype control). On the other hand, supplementation of supernatant of untreated HUVEC with 3 μg/ml of recombinant human TSP-1 slightly increased the numbers of ingested apoptotic eEND2 cells (78.83 ± 10.63) compared to S/N of untreated HUVEC alone.
Monocytic U937 cells showed similar results although the overall phagocytosis index was much lower compared to monocyte-derived macrophages. Pre-incubation of apoptotic eEND2 cells with cS/N raised the phagocytic index from 20.8 ± 13.2 to 39.7 ± 8.4 whereas neutralizing TSP-1 or blocking RGD sensitive receptors led to a decline of the phagocytosis rate (17.7 ± 3.4 for the anti-TSP-1 antibody and 25.8 ± 7.3 for the RGD peptide). Pre-incubation with the control RAD peptide or with the mouse IgG isotype control did not reveal any differences to supernatant from untreated HUVEC (39.1 ± 13.5 for the RAD peptide and 36 ± 6.7 for the isotype control). Addition of recombinant TSP-1 led to only a slight increase in the phagocytosis rate (25.33 ± 8.19 compared to 20.8 ± 13.2 for supernatant of untreated HUVEC).
Endothelial TSP-1 has chemotactic properties
Having demonstrated that ECs respond with enhanced TSP-1 synthesis upon exposure to apoptotic cells we tested whether increased TSP-1 secretion plays a role in attraction of macrophages or PMA-stimulated U937 cells. We used a transwell chamber system with HUVEC seeded into the lower compartment. After exposure to apoptotic eEND2 cells for 8 hrs monocyte-derived macrophages or PMA-treated U937 cells were placed in the upper chamber and allowed to transmigrate for 60 min. As shown in Fig. 6 conditioned medium of HUVEC exposed to apoptotic eEND2 cells significantly increased migration of macrophages and U937 cells more than two-fold compared to supernatant of untreated HUVEC. To further ascertain whether TSP-1 directly influences migration of macrophages or U937 cells RGD peptide-sensitive receptors of the cells were blocked by adding a RGD peptide before they were added to the upper compartment. Pre-treatment with the RGD peptide circumvented the increased transmigration of both monocyte-derived macrophages and PMA-stimulated U937 cells in response to HUVEC-derived TSP-1 whereas the control RAD peptide had no significant impact on macrophage migration. Also neutralizing TSP-1 in the conditioned supernatant led to a decrease in migration of both macrophages and U937 cells. These results suggest that endothelial TSP-1 acts, at least partly, as a chemoattractant for macrophages.
Fig 6.
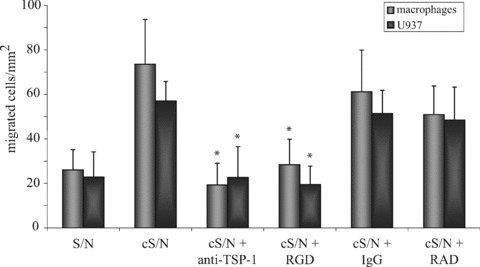
Migration of macrophages or U937 cells is influenced by TSP-1. HUVEC were seeded into the lower compartment of transwell devices and exposed to apoptotic eEND2 cells for 8 hrs or left untreated. Fluorescence-labelled macrophages or U937 cells were added to the upper compartment. In some experiments macrophages or U937 cells were pre-incubated with RGD peptides (50 μM) or TSP-1 in the supernatant was blocked by neutralizing antibodies (5 μg/ml). After 60 min. transmigration of macrophages or U937 cells through the transwell membrane was counted using fluorescence microscopy. Results are given as mean ± S.D. of at least three independent experiments (*P < 0.05).
Plasma TSP-1 level are increased in patients with AAV
In our in vitro model apoptotic cells are capable of inducing TSP-1 production in ECs. To investigate, whether these findings have any equivalence in vivo we measured TSP-1 protein concentrations in platelet-poor plasma of patients with AAV and in healthy controls. As shown in Fig. 7, TSP-1 plasma concentrations are significantly increased in patients with AAV (657.47 ± 64.17 pg/ml) compared to healthy controls (253.78 ± 37.10 pg/ml, P < 0.005).
Fig 7.
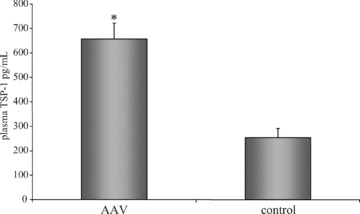
Plasma TSP-1 concentrations are increased in patients with AAV. Platelet-poor plasma from 19 patients with AAV and from nine healthy volunteers were collected and assayed for TSP-1 concentration using the TSP-1 Quantikine ELISA kit. Results are given as mean ± S.E.M. (*P < 0.005).
Discussion
Autoimmune diseases like AAV or SLE as well as other diseases with vascular involvement are characterized by high numbers of detached inflammatory ECs in the circulation [1, 15–17]. To delineate the impact of these damaged cells on healthy endothelium we established an in vitro cell culture model and showed recently that ECs exhibit a pro-inflammatory phenotype in response to damaged ECs [14]. In the present study we demonstrated that exposure of apoptotic murine ECs to human endothelium enhances synthesis and release of the homotrimeric glycoprotein TSP-1. We provided evidence that binding but not uptake of the apoptotic cell is sufficient for increased TSP-1 synthesis and that apoptotic cell-induced TSP-1 expression in ECs is mediated by the B-Raf/MEK/ERK pathway. We further demonstrated that human TSP-1 is recognized by surface proteins of apoptotic mouse cells and acts as chemoattractant for monocyte-derived macrophages or monocytic U937 cells. To note, besides its chemoattractant properties cS/N of HUVEC exposed to apoptotic ECs induces pro-inflammatory responses in macrophages. Finally, we showed that TSP-1 plasma levels are significantly elevated in patients with AAV. These in vitro and in vivo data led us to the conclusion that increased TSP-1 expression, in response to elevated numbers of circulating apoptotic cells, serves as an endothelial emergency signal recruiting and activating phagocytes and thus facilitating clearance of apoptotic cells.
Although there is no evidence that engulfment of apoptotic cells is defective in acute AAV there are many hints suggesting that clearance of apoptotic cells is at least delayed. Harper and colleagues showed that opsonizing neutrophils with ANCA IgG resulted in accelerated apoptosis and delayed clearance rates compared to normal IgG [7]. Also proteinase-3, one of the major ANCA antigens, negatively affects the clearance rate of macrophages [3]. We do not have a proof that elevated TSP-1 amounts in active AAV are derived from activated endothelium and not from other cell types like apoptotic fibroblasts as reported by Moodley and colleagues [18]. Nevertheless, ECs are known constitutive producers of TSP-1. Moreover, apoptotic fibroblasts play a minor role in acute AAV whereas activated or detached ECs as well as primed neutrophils are believed to be the most important cell types involved in AAV. Therefore, it is conceivable that elevated TSP-1 level in AAV may derive, at least in parts, from the endothelium. But certainly, further in vivo studies are necessary to verify the hypothesis that enhanced TSP-1 amounts modulate apoptotic cell clearance in active AAV.
Our findings provide new insights into the as yet poorly understood interactions between circulating inflammatory ECs and the vascular endothelium. Apoptosis and the subsequent removal of dying cells are natural occurring processes and of vital importance for maintaining homeostasis and integrity of the organism. Under normal circumstances one of the hallmarks of apoptotic cell clearance is the prevention of pro-inflammatory signalling and the absence of immune responses [19, 20]. Whereas mostly professional phagocytes like macrophages, immature dendritic cells or neutrophils are involved in apoptotic cell clearance also neighbouring cells like epithelial or ECs could actively participate in the rapid removal of their dying fellows [21]. We recently demonstrated that in vitro both HUVEC and immortalized microvascular ECs recognized, bound and phagocytosed their apoptotic counterparts. In contrast to the anti-inflammatory responses that are normally associated with apoptotic cell clearance ECs became activated and released a number of chemotactic and inflammatory chemokines [14]. As a consequence, monocytes incubated with the supernatant of activated ECs respond with an inflammatory phenotype in vitro. Interestingly, elevated serum level of these cytokines have also been found in patients with acute AAV [22, 23]. The increase in endothelial TSP-1 production and release in response to apoptotic cells, as reported in this study, further strengthen the hypothesis of apoptotic cell-induced EC activation.
We also demonstrated that apoptotic cell-induced TSP-1 synthesis in HUVEC is mediated via activation of the B-Raf/MEK/ERK pathway and that blocking ERK1/2 phosphorylation abolishes this increase in TSP-1 expression. Currently, ERK1/2 activation in the context of cell clearance is mostly ascribed to engulfment of necrotic cell corpses or apoptotic cells entering secondary necrosis. These processes are mostly accompanied by pro-inflammatory responses [24]. Recently, Patel and colleagues demonstrated very elegantly that incubation of bone marrow-derived macrophages with apoptotic cells not only failed to activate ERK1/2 but even restored necrotic cell-induced activation of ERK [25]. On the other hand, p38 and JNK, the main MAPK in macrophage-mediated apoptotic cell engulfment [25], seemed not to be activated during EC-mediated cell clearance. Therefore, it is assumable that ECs utilize different signalling pathways compared to macrophages or other professional phagocytes. A possible explanation for these altered molecular responses could be the fact that ECs do not belong to the typical group of phagocytes. Moreover, due to their proximity to blood-derived leucocytes or dendritic cells it seems conceivable that the ability of the endothelium to engulf apoptotic cells is only rudimentarily developed and that ECs have to take part in apoptotic cell clearance only under pathological conditions like AAV or other vascular diseases where large amounts of damaged circulating cells are present in the circulation [26, 27].
There is evidence that the responses of the phagocyte are dependent on the nature of the apoptotic cell and the physiological conditions found at the site of cell clearance. For instance, although macrophages normally exhibit an anti-inflammatory phenotype during apoptotic cell clearance exposure to late apoptotic cells led to ERK-mediated synthesis of inflammatory chemokines [28]. Moreover, treatment of macrophages with oxidized lipoprotein as an in vitro model to mimic the conditions found in chronically inflamed tissues shifts the cellular response towards inflammation [29]. This idea of an interdependency between the phenotype of the apoptotic cell and the microenvironment on the one hand and the responses of the engulfing cell on the other hand certainly raises the question whether ECs would also become activated if they would be exposed to apoptotic cells under physiological conditions.
ECs, as well as platelets, are known as constituent producers of TSP-1. Therefore, it was quite surprising that exposure of HUVEC to apoptotic eEND2 cells even further enhanced TSP-1 synthesis. Moreover, our finding that basal endothelial TSP-1 expression is not affected by ERK inhibition suggests that the apoptotic cell-induced increase in TSP-1 relies on alternative pathways compared to TSP-1 synthesis in HUVEC under physiological conditions. TSP-1 is a secreted multidomain glycoprotein that possesses many different functions. Besides its role in cell proliferation, adhesion and migration TSP-1 facilitates recognition and binding of apoptotic cells by phagocytes and exhibits anti-inflammatory properties [30, 31]. In fact, TSP-1 expression is rapidly increased at sites of tissue inflammation or injury and TSP-1 null mice display persistent multi-organ inflammation [32–34]. The functional significance of enhanced TSP-1 generation in ECs in response to apoptotic cells is yet not clear. TSP-1 serves as bridging molecule linking externalized phosphatidylserine of the apoptotic cell and αVβ3 integrin or CD36 on the membrane of the phagocyte facilitating recognition and binding of the apoptotic cell [35]. Furthermore, TSP-1 has been shown to play a role in attracting macrophages to the site of inflammation or injury [18, 36]. In the present study, we demonstrated that apoptotic murine eEND2 cells bound human TSP-1. We also showed that conditioned supernatant of HUVEC exposed to apoptotic eEND2 cells significantly enhanced migration of macrophages in vitro and that this effect was partly reversed by blocking macrophage αVβ3 integrin or by neutralizing TSP-1. Moreover, phagocytosis rate of macrophages, but not of ECs, was reduced by blocking αVβ3 integrin or by inhibiting TSP-1 in the supernatant. Therefore, we concluded that enhanced endothelial TSP-1 release preferably influences immune cells but not the ECs themselves. Thus, the role of the endothelium in apoptotic cell clearance is rather that of a fine-tuned sensor reporting errors in the clearance program to the immune system.
Besides, these findings indicate that ECs utilize their own engulfment system since inhibition of endothelial CD36 or αVβ3 integrin did not significantly reduce binding and uptake of apoptotic eEND2 cells. Indeed, evidence exists that different cell types utilize different receptors for recognition of apoptotic cells. For example, Seitz and colleagues demonstrated cell and organ specificity for members of the Axl/Tyro3/Mertk family of tyrosine receptors [37]. Likewise, phagocytosis of apoptotic fibroblasts by macrophages is reported to be critically depended on macrophage CD36 and αVβ3 integrin [18]. Therefore, it is conceivable that endothelial-derived TSP-1 forces engulfment of apoptotic cells by macrophages but do not necessarily influence the vascular ECs.
In summary, our data demonstrated that binding of apoptotic ECs to native endothelium induces, via activation of the ERK/MEK/B-Raf pathway, a significant increase in TSP-1 secretion in vitro and that elevated TSP-1 levels act as chemoattractants directing macrophages to the site of injury and facilitate clearance of apoptotic cells. These results, combined with the finding that TSP-1 plasma concentrations are elevated in patients with active AAV, suggest that TSP-1 may play a certain role in conditions where apoptotic cell clearance is disturbed.
References
- 1.Woywodt A, Streiber F, De Groot K, et al. Circulating endothelial cells as markers for ANCA-associated small-vessel vasculitis. Lancet. 2003;361:206–10. doi: 10.1016/S0140-6736(03)12269-6. [DOI] [PubMed] [Google Scholar]
- 2.Munoz LE, Gaipl US, Franz S, et al. SLE–a disease of clearance deficiency? Rheumatology. 2005;44:1101–7. doi: 10.1093/rheumatology/keh693. [DOI] [PubMed] [Google Scholar]
- 3.Kantari C, Pederzoli-Ribeil M, Amir-Moazami O, et al. Proteinase 3, the Wegener autoantigen, is externalized during neutrophil apoptosis: evidence for a functional association with phospholipid scramblase 1 and interference with macrophage phagocytosis. Blood. 2007;110:4086–95. doi: 10.1182/blood-2007-03-080457. [DOI] [PubMed] [Google Scholar]
- 4.Gaipl US, Munoz LE, Grossmayer G, et al. Clearance deficiency and systemic lupus erythematosus (SLE) J Autoimmun. 2007;28:114–21. doi: 10.1016/j.jaut.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Gaipl US, Kuhn A, Sheriff A, et al. Clearance of apoptotic cells in human SLE. Curr Dir Autoimmun. 2006;9:173–87. doi: 10.1159/000090781. [DOI] [PubMed] [Google Scholar]
- 6.Licht R, Dieker JW, Jacobs CW, et al. Decreased phagocytosis of apoptotic cells in diseased SLE mice. J Autoimmun. 2004;22:139–45. doi: 10.1016/j.jaut.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Harper L, Ren Y, Savill J, et al. Antineutrophil cytoplasmic antibodies induce reactive oxygen-dependent dysregulation of primed neutrophil apoptosis and clearance by macrophages. Am J Pathol. 2000;157:211–20. doi: 10.1016/S0002-9440(10)64532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tas SW, Quartier P, Botto M, et al. Macrophages from patients with SLE and rheumatoid arthritis have defective adhesion in vitro, while only SLE macrophages have impaired uptake of apoptotic cells. Ann Rheum Dis. 2006;65:216–21. doi: 10.1136/ard.2005.037143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Distler JH, Huber LC, Hueber AJ, et al. The release of microparticles by apoptotic cells and their effects on macrophages. Apoptosis. 2005;10:731–41. doi: 10.1007/s10495-005-2941-5. [DOI] [PubMed] [Google Scholar]
- 10.Skoberne M, Beignon AS, Larsson M, et al. Apoptotic cells at the crossroads of tolerance and immunity. Curr Top Microbiol Immunol. 2005;289:259–92. doi: 10.1007/3-540-27320-4_12. [DOI] [PubMed] [Google Scholar]
- 11.Schrijvers DM, De Meyer GR, Kockx MM, et al. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–61. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 12.Liu G, Wu C, Wu Y, et al. Phagocytosis of apoptotic cells and immune regulation. Scand J Immunol. 2006;64:1–9. doi: 10.1111/j.1365-3083.2006.01771.x. [DOI] [PubMed] [Google Scholar]
- 13.Van Rossum AP, Limburg PC, Kallenberg CG. Activation, apoptosis, and clearance of neutrophils in Wegener’s granulomatosis. Ann N Y Acad Sci. 2005;1051:1–11. doi: 10.1196/annals.1361.041. [DOI] [PubMed] [Google Scholar]
- 14.Kirsch T, Woywodt A, Beese M, et al. Engulfment of apoptotic cells by microvascular endothelial cells induces proinflammatory responses. Blood. 2007;109:2854–62. doi: 10.1182/blood-2006-06-026187. [DOI] [PubMed] [Google Scholar]
- 15.Woywodt A, Schroeder M, Mengel M, et al. Circulating endothelial cells are a novel marker of cyclosporine-induced endothelial damage. Hypertension. 2003;41:720–3. doi: 10.1161/01.HYP.0000052948.64125.AB. [DOI] [PubMed] [Google Scholar]
- 16.Sesin CA, Yin X, Esmon CT, et al. Shedding of endothelial protein C receptor contributes to vasculopathy and renal injury in lupus: in vivo and in vitro evidence. Kidney Int. 2005;68:110–20. doi: 10.1111/j.1523-1755.2005.00385.x. [DOI] [PubMed] [Google Scholar]
- 17.Boos CJ, Lip GY, Blann AD. Circulating endothelial cells in cardiovascular disease. J Am Coll Cardiol. 2006;48:1538–47. doi: 10.1016/j.jacc.2006.02.078. [DOI] [PubMed] [Google Scholar]
- 18.Moodley Y, Rigby P, Bundell C, et al. Macrophage recognition and phagocytosis of apoptotic fibroblasts is critically dependent on fibroblast-derived thrombospondin 1 and CD36. Am J Pathol. 2003;162:771–9. doi: 10.1016/S0002-9440(10)63874-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fadok VA, Bratton DL, Konowal A, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/ paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krispin A, Bledi Y, Atallah M, et al. Apoptotic cell thrombospondin-1 and heparin-binding domain lead to dendritic-cell phagocytic and tolerizing states. Blood. 2006;108:3580–9. doi: 10.1182/blood-2006-03-013334. [DOI] [PubMed] [Google Scholar]
- 21.Golpon HA, Fadok VA, Taraseviciene-Stewart L, et al. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J. 2004;18:1716–8. doi: 10.1096/fj.04-1853fje. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh SC, Yu HS, Cheng SH, et al. Anti-myeloperoxidase antibodies enhance phagocytosis, IL-8 production, and glucose uptake of polymorphonuclear neutrophils rather than anti-proteinase 3 antibodies leading to activation-induced cell death of the neutrophils. Clin Rheumatol. 2007;26:216–24. doi: 10.1007/s10067-006-0285-3. [DOI] [PubMed] [Google Scholar]
- 23.Yang JJ, Preston GA, Alcorta DA, et al. Expression profile of leukocyte genes activated by anti-neutrophil cytoplasmic autoantibodies (ANCA) Kidney Int. 2002;62:1638–49. doi: 10.1046/j.1523-1755.2002.00619.x. [DOI] [PubMed] [Google Scholar]
- 24.Reddy SM, Hsiao KH, Abernethy VE, et al. Phagocytosis of apoptotic cells by macrophages induces novel signaling events leading to cytokine-independent survival and inhibition of proliferation: activation of Akt and inhibition of extracellular signal-regulated kinases 1 and 2. J Immunol. 2002;169:702–13. doi: 10.4049/jimmunol.169.2.702. [DOI] [PubMed] [Google Scholar]
- 25.Patel VA, Longacre A, Hsiao K, et al. Apoptotic cells, at all stages of the death process, trigger characteristic signaling events that are divergent from and dominant over those triggered by necrotic cells: implications for the delayed clearance model of autoimmunity. J Biol Chem. 2006;281:4663–70. doi: 10.1074/jbc.M508342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boos CJ, Lip GYH, Blann AD. Circulating endothelial cells in cardiovascular disease. J Am Coll Cardiol. 2006;48:1538–47. doi: 10.1016/j.jacc.2006.02.078. [DOI] [PubMed] [Google Scholar]
- 27.Grundmann M, Woywodt A, Kirsch T, et al. Circulating endothelial cells: a marker of vascular damage in patients with preeclampsia. Am J Obstet Gynecol. 2008;198:317e1–5. doi: 10.1016/j.ajog.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 28.Kurosaka K, Takahashi M, Kobayashi Y. Activation of extracellular signal-regulated kinase 1/2 is involved in production of CXC-chemokine by macrophages during phagocytosis of late apoptotic cells. Biochem Biophys Res Commun. 2003;306:1070–4. doi: 10.1016/s0006-291x(03)01105-7. [DOI] [PubMed] [Google Scholar]
- 29.Khan M, Pelengaris S, Cooper M, et al. Oxidised lipoproteins may promote inflammation through the selective delay of engulfment but not binding of apoptotic cells by macrophages. Atherosclerosis. 2003;171:21–9. doi: 10.1016/j.atherosclerosis.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Herndon ME, Lawler J. The cell biology of thrombospondin-1. Matrix Biol. 2000;19:597–614. doi: 10.1016/s0945-053x(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 31.Lawler J. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol. 2000;12:634–40. doi: 10.1016/s0955-0674(00)00143-5. [DOI] [PubMed] [Google Scholar]
- 32.Raugi GJ, Olerud JE, Gown AM. Thrombospondin in early human wound tissue. J Invest Dermatol. 1987;89:551–4. doi: 10.1111/1523-1747.ep12461198. [DOI] [PubMed] [Google Scholar]
- 33.Lawler J, Sunday M, Thibert V, et al. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest. 1998;101:982–92. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crawford SE, Stellmach V, Murphy-Ullrich JE, et al. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–70. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 35.Savill J, Hogg N, Ren Y, et al. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–22. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mansfield P, Suchard S. Thrombospondin promotes chemotaxis and haptotaxis of human peripheral blood monocytes. J Immunol. 1994;153:4219–29. [PubMed] [Google Scholar]
- 37.Seitz HM, Camenisch TD, Lemke G, et al. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol. 2007;178:5635–42. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]


