Abstract
We suggested that low-level laser irradiation (LLLI) precondition prior to cell transplantation might remodel the hostile milieu of infarcted myocardium and subsequently enhance early survival and therapeutic potential of implanted bone marrow mesenchymal stem cells (BMSCs). Therefore, in this study we wanted to address: (1) whether LLLI pre-treatment change the local cardiac micro-environment after myocardial infarction (MI) and (2) whether the LLLI preconditions enhance early cell survival and thus improve therapeutic angiogenesis and heart function. MI was induced by left anterior descending artery ligation in female rats. A 635 nm, 5 mW diode laser was performed with energy density of 0.96 J/cm2 for 150 sec. for the purpose of myocardial precondition. Three weeks later, qualified rats were randomly received with LLLI precondition (n= 26) or without LLLI precondition (n= 27) for LLLI precondition study. Rats that received thoracotomy without coronary ligation were served as sham group (n= 24). In the cell survival study, rats were randomly divided into 4 groups: serum-free culture media injection (n= 8), LLLI precondition and culture media injection (n= 8), 2 million male BMSCs transplantation without LLLI pre-treatment (n= 26) and 2 million male BMSCs transplantation with LLLI precondition (n= 25) group, respectively. Vascular endothelial growth factor (VEGF), glucose-regulated protein 78 (GRP78), superoxide dismutase (SOD) and malondialdehyde (MDA) in the infarcted myocardium were evaluated by Western blotting, real-time PCR and colorimetry, respectively, at 1 hr, 1 day and 1 week after laser irradiation. Cell survival was assayed with quantitative real-time PCR to identify Y chromosome gene and apoptosis was assayed with transferase-mediated dUTP end labelling staining. Capillary density, myogenic differentiation and left ventricular function were tested by immunohistochemistry and echocardiography, respectively, at 1 week. After LLLI precondition, increased VEGF and GRP78 expression, as well as the enhanced SOD activity and inhibited MDA production, was observed. Compared with BMSC transplantation and culture media injection group, although there was no difference in the improved heart function and myogenic differentiation, LLLI precondition significantly enhanced early cell survival rate by 2-fold, decreased the apoptotic percentage of implanted BMSCs in infarcted myocardium and thus increased the number of newly formed capillaries. Taken together, LLLI precondition could be a novel non-invasive approach for intraoperative cell transplantation to enhance cell early survival and therapeutic potential.
Keywords: precondition, low-level laser irradiation, bone marrow-derived mesenchymal stem cells, myocardial infarction, cell transplantation, cell survival
Introduction
Recent years have witnessed a growing interest and enthusiasm for the application of cell-based therapy to repair the damaged myocardium. Given their ease of harvesting, amenability to ex vivo expansion, multipotency and low immunogenicity, bone marrow derived mesenchymal stem cells (BMSCs) have drawn particular attention and have undergone intensive investigations [1, 2]. Both experimental studies and clinical trials have shown that BMSC transplantation leads to significant improvement of cardiac function via paracrine effects and possible myogenesis [3, 4]. However, the efficacy of cell therapy is limited by the poor survival of implanted cells. Massive cell death occurs in the first few days after transplantation owing to the ‘hostile’ milieu of infarcted myocardium [5, 6]. Although several strategies, such as heat shock [7, 8], gene modification [9–11] or pharmacological precondition [12, 13], have shown encouraging results in terms of donor cell survival, direct interventions to stem cells could generate safety considerations for their clinic application [14]. Thus, alternative precondition modalities to sustain cytoprotection in early stage after cell transplantation are desired.
Low-level laser irradiation (LLLI) has been applied for over 30 years in clinical medicine [15]. Unlike the higher powered lasers, such as transmyocardial revascularization (TMR), LLLI does not deliver power to damage tissue, but it does deliver adequate energy to activate distinct heat-independent biostimulation effects, such as anti-inflammation, microcirculation improvement and cardioprotection [16, 17]. It has been reported that LLLI could reduce the myocardial scar size as well as act as a favourable influence on myocardial remodelling after myocardial infarction (MI) [17–20]. Moreover, a preliminary observational study in patients with advanced multivessel coronary artery disease indicated that LLLI might improve functional capacity and clinical symptoms [21].
Considering the above facts, we suggested that LLLI precondition might be a feasible and effective approach to remodel the hostile milieu of infarcted myocardium and to subsequently enhance early survival and therapeutic potential of transplanted BMSCs. Therefore, in this study we wanted to address: (1) whether LLLI pre-treatment changes the local cardiac micro-environment after MI and (2) whether the LLLI preconditions enhance early cell survival and thus improve therapeutic angiogenesis and heart function.
Materials and methods
Animals
The study was performed in accordance with both the guidelines of the ‘Regulation to the Care and Use of Experimental Animals’ (1996) of the Beijing Council on Animal Care and the Guide for the Care and Use of Laboratory Animals (NIH Publication No.85–23, National Academy Press, Washington, DC, revised 1996). All procedures were approved by the Ethics Committee for Animal Study in Fu Wai hospital. Sprague-Dawley rats were obtained from Vital River Laboratory Animal, Inc. (Beijing, China). One hundred and ninety female rats weighing 260 to 280 g were enrolled in the study and 11 male rats weighing 60 to 80 g were served as cell donors.
Creation of myocardial infarction model and groups
MI was induced in female rats by permanent ligation of the left anterior descending (LAD) artery [22]. Briefly, the animals were anesthetized by intraperitoneal injection of 10% chlora hydrate (300 mg/kg body weight) and ventilated after oral intuba tion using Harvard Rodent Ventilator (Model 683, Harvard Apparatus, Holliston, MA, USA, http://www.harvardapparatus.com). Through a minimal left anterolateral thoracotomy, the heart was exposed and the LAD coronary artery was ligated 3 mm distally from where it branches off the aorta, using 6–0 polypropylene suture (Ethicon, Inc., Cincinnati, OH, USA, http://www.ethicon.com). The sham-operation rats received the same procedure of thoracotomy without coronary ligation. The incision was closed and penicillin G procaine (150,000 U/ml, 0.4 ml/rat) and morphine (0.3 mg/rat) were administered after operation. Three weeks after MI, the survived rats with left ventricular ejection fraction (LVEF) less than 60% and left ventricular fractional shortening (LVFS) less than 30% in ultrasonic assessment were randomly grouped according to the experimental protocol (Fig. 1A).
Fig 1.
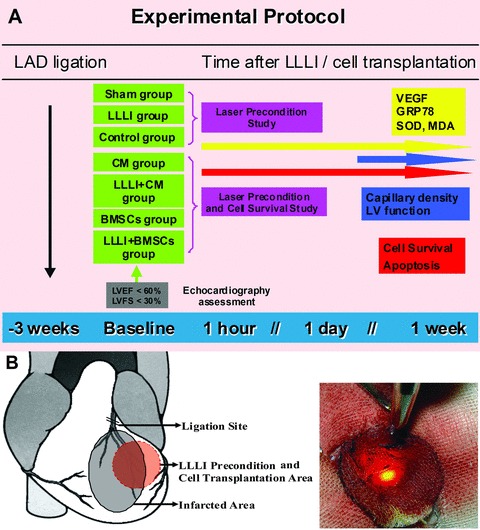
(A) Experimental protocol. LAD: left anterior descending artery; LLLI: Low-level laser irradiation; VEGF: vascular endothelial growth factor; GRP78: glucose-regulated protein 78; SOD: superoxide dismutase; MDA: malondialdehyde; LVEF: left ventricular ejection fraction; LVFS: left ventricular fractional shortening. (B) Illustration of LLLI precondition for the infarcted myocardium.
In laser precondition study, the qualified rats with chronic MI were divided into (1) LLLI group: chest-reopened with LLLI precondition (n= 26) and (2) Control group: chest-reopened without LLLI precondition (n= 27). And another 24 rats that received thoracotomy without coronary ligation were served as sham group.
In cell survival study, the qualified rats with chronic MI were divided into: (1) CM group: received no-cell culture media injection (n= 8); (2) LLLI + CM group: received LLLI precondition and culture media injection (n= 8); (3) BMSC group: received cell transplantation without LLLI pre-treatment (n= 26) and (4) LLLI + BMSC group: received cell transplantation with LLLI precondition (n= 25).
Laser precondition study
Laser irradiation for the infarcted myocardium
An indium-gallium-arsenate-phosphate (InGaAsP) diode laser with 600 quartz optical fibre (Model KDL-300, Beijing KeDian Microwave Electronics Co., Beijing, China) was used in this study. This system operated at a continuous wavelength of 635 nm and an adjustable power output from 0 to 200 mW. Power output was kept constantly at 5 mW in the continuous wave mode. After the chest opened, the optical fibre was fixed with a delivery arm and precisely positioned the fibre tip 26 mm above the myocardium, which allowed the laser beam width of 10 mm (Fig. 1B). An infrared viewer (Beijing KeDian Microwave Electronics Co.) was used to trace the infrared irradiation on the myocardium, and a LaserCheck energy meter (Model LM-91B, National Institute of Metrology, Beijing, China) was applied to measure the precise power density of the laser on the myocardium. The measured power density for the peri-infarcted area in left ventricular free wall, in which BMSCs would be transplanted, was kept at 6.37 mW/cm2. The duration of irradiation was 150 sec. and the energy density given to the tissue was 0.96 J/cm2. A round sterile absorbent gauze with a 10-mm-diameter hole served to shelter adjacent tissues from scattered light.
Western blot analysis for VEGF
Standard SDS-PAGE electrophoresis and Western blotting technique were used to quantify the protein expression of vascular endothelial growth factor (VEGF) in the infarcted hearts. One hour, 1 day and 1 week after LLLI, rat heart tissues were harvested from laser irradiated area of LV wall in LLLI group and from one part of LV free wall in the sham and control group, respectively (n= 4 at each time-point from each group). Samples were homogenized on ice in a homogenization buffer (1 mmol/l Tris-HCl, pH7.6, 1 mmol/l phenylmethane-sulfonylfluoride, 1 mmol/l ethylenediaminetetraacetic acid, 2 mg/ml aprotinin for 30 min.). The homogenates were centrifuged at 14,000 χg for 10 min. at 4°C. The amount of protein in each supernatant was determined by using a Bradford protein assay kit (Bio-Rad, Hercules, CA, USA, http://www.bio-rad.com). Equal amounts of protein were loaded, separated by 10% SDS-PAGE gel and transferred to nitrocellulose filter. The VEGF protein was probed with polyclonal VEGF antibody (1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA, http://www.scbt.com) with β-actin (1:500; Santa Cruz Biotechnology) as internal standard. Horseradish peroxidase-linked secondary antibodies (1:1000; Santa Cruz Biotechnology) were used and proteins were visualized by enhanced chemiluminescence. The bands were then exposed to radiography film, followed with density quantitation by TotalLab image analysis software (Nonlinear Dynamics, Newcastle upon Tyne, UK).
Real-time PCR for GRP 78
Real-time PCR technique was used to quantify the gene expression of glucose-regulated protein 78 (GRP78) in the infarcted hearts. One hour, 1 day and 1 week after LLLI, rat heart tissues were harvested from laser irradiated area of LV wall in LLLI group and from one part of LV free wall in the sham group and control group, respectively (n= 4) at each time-point from each group. The isolation of total RNA and cDNA synthesis were carried out as described earlier [23]. The forward primer was GCTGGCACTATTGCTGGACTGA and the reverse primer was AGACACATCGAAGGTTCCACCAC. The real-time PCR conditions were an initial denaturation step of 10 min. at 95°C, followed by 40 cycles of 95°C for 15 sec., and 60°C for 1 min. PCR products were run and imaged on 1.2% agarose gels stained with ethidium bromide. Expected fragment sizes are 141 bp for GRP78 and 195 bp for GAPDH. The expression of each target mRNA relative to GAPDH under experimental and control conditions was calculated based on the threshold cycle (CT) as r= 2-Δ (ΔCT), where ΔCT = CT target-CT GAPDH and Δ (ΔCT) =ΔCT experimental-ΔCT control. An ABI Prism 7300 sequence detection system (Applied Biosystems, Inc., Foster City, CA, USA, http://www.appliedbiosystems.com.), was used for PCR cycling reaction, real-time data collection and analysis.
Assay for antioxidant enzyme activities and lipid peroxidation
To detect the oxidative stress level in the post-infarct myocardium at 1 hr, 1 day and 1 week after LLLI, rat heart tissues were harvested from laser irradiated area of LV wall in the LLLI group and from one part of LV free wall in the control group and sham group, respectively (n= 4 at each time-point from each group). The enzymatic activities of superoxide dismutase (SOD) were measured according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The production of malondialdehyde (MDA), routinely used as an indicator of lipid peroxidation, was determined according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute).
Cell survival with laser precondition study
BMSC preparation and labelling
BMSCs were isolated from male donor rats and cultured as previously reported [24]. In brief, bone marrow cells were obtained from the tibias and cultured in DMEM (Gibco, Grand Island, NY, USA, http://www.invitrogen.com) supplemented with 10% (v/v) foetal bovine serum (FBS, Gibco). The non-adherent cells were removed at 24 hrs and adherent cells were incubated in a humidified atmosphere of 95% air and 5% CO2 at 37°C. Passage 2–3 BMSCs were used in the study. The culture medium was changed by fresh medium every 4 days.
To track the injected cells, BMSCs at 80–90% confluence were labelled with 4′, 6-diamidino-2-phenylindole (DAPI; Sigma, St. Louis, MO, USA, http://www.sigmaaldrich.com) before transplantation. Briefly, sterile DAPI stock solution was added to culture medium at a final concentration of 50 μg/ml for 30 min. After labelling, cells were rinsed six times with phosphate buffered saline (PBS) to remove unbound DAPI. This procedure made 100% cell nuclei labelled.
Cell transplantation
The infarcted rat heart was exposed through the original thoracotomy incision under anaesthesia, and the intramyocardial injections were performed at a single site in the border zone of MI [22]. Immediately after LLLI precondition, 50 μl of culture media or the same volume DMEM containing 2 χ 106 DAPI labelled BMSCs was injected in the LLLI + CM or LLLI + BMSC group, respectively. Rats in the Control group and BMSC group were injected with the same volume of culture media or labelled cells without LLLI pre-treatment.
DNA preparation and quantitation of Y chromosomal DNA by real-time PCR
Genomic DNA was isolated by using a Wizard® genomic DNA purification kit (Promega, Madison, WI, USA, http://www.promega.com). The number of implanted donor male BMSCs in the female recipient hearts were estimated by the real-time PCR technique for the Y chromosome-specific Sry gene, as described [25]. Standard curves were generated by serially diluting male rat genomic DNA prepared from male BMSCs (n= 3). At 1 hr, 1 day and 1 week after cell injection, host female hearts were collected (n= 4 at each time-point from BMSCs and LLLI + BMSC groups) under terminal anaesthesia. The LV wall was homogenized and diluted to produce samples to be assayed for the amount of Y chromosome. Primers used for detection of rat male specific sry gene were as follows: (sense 5-CATCGAAGGGTTAAAGTGCCA-3; antisense 5-ATAGTGTGTAGGTTGTTGTCC-3). The real-time PCR conditions were an initial denaturation step of 10 min. at 95°C, followed by 40 cycles of 95°C for 15 sec., and 60°C for 1 min. on the ABI PRISM 7300 Sequence Detection System (Applied Biosystems) using the Master Mix SYBR Green Kit (Applied Biosystems).
Evaluation of left ventricular function
Transthoracic echocardiography was performed at 3 weeks after MI (baseline) and 1 week after cell transplantation (n= 6 in each group). Each animal was anesthetized and fixed in supine position. Images were recorded using a 12-MHz high-frequency liner phased-array transducer (Philips SONOS 5500, Philip Corp., Beijing, China, http://www.philips.com.cn). Parasternal long- and short-axis views were obtained with both M-mode and two-dimensional echocardiography images. As a measure of LV function, LV end-systolic (LVESd) and end-diastolic (LVEDd) diameters were measured and LVFS and LV ejection fraction (LVEF) were calculated as follows: LVFS (%) = (LVEDd – LVESd)/LVEDd χ 100 and LVEF (%) = ([LVEDd3– LVESd3]/LVEDd3) χ 100. Dimensions were measured just below the level of the papillary muscle. All measurements were averaged on three consecutive cardiac cycles and were analysed by two independent experienced investigators who were blinded to the treatment status of the animals [22].
Vascular densities, cell apoptosis and myogenic differentiation
Rats from LLLI + CM, BMSCs and LLLI + BMSC groups (n= 3 at each time-point) were killed under deep anaesthesia. Hearts were harvested, embedded in paraffin or cryopreserved in TissueTek® OCT compound (Sakura Finetechnical Co, Ltd., Tokyo, Japan), sectioned into 6-μm-thick sections (n= 8 from each sample) and sent to examine blindly. The survival of implanted BMSCs was demonstrated by the presence of DAPI-labelled cells. Image acquisition was performed under light or phase contrast at 400χ magnification in five fields per slide on a Olympus BX61 microscope (Olympus, Tokyo, Japan) by two double-blinded investigators.
For in situ detection of apoptotic BMSCs at 1 hr, 1 day and 1 week after cell transplantation, we performed the terminal deoxynucleotidyl transferase-mediated dUTP end labelling (TUNEL) assay by using a TdT FragEL-DNA fragmentation detection kit (EMD Biosciences, Inc., Darmstadt, Germany, http//www.calbiochem.com) in frozen tissue sections according to the manufacturer’s protocol. The percentage of apoptotic BMSCs is calculated as the number of TUNEL+ cell number/DAPI-labelled nuclear numbers of BMSCs χ 100.
For determination of capillary density in the infarct and peri-infarct regions at 1 week after cell transplantation, paraffin sections were immunohistochemically stained with primary antibody against CD31 (platelet endothelial cell adhesion molecule-1, PECAM-1) antibodies (Santa Cruz Biotechnology) followed by incubation with peroxidase conjugated secondary antibody (Zhongshan Goldenbridge Biotechnology Co. Ltd., Beijing, China, http://www.zsbio.com). The number of capillaries was measured and the results were expressed as capillaries per high power field (0.2 mm2).
For in situ detection of myogenic differentiation of BMSCs at 1 week after cell transplantation, frozen tissue sections were stained using the following primary antibodies: α-sarcomeric actin (1:50, GeneTech Co. Ltd, Shanghai, China) and desmin (1:50, GeneTech) followed by incubation with a goat antimouse conjugated rhodamine IgG (Zhongshan Goldenbridge Biotechnology).
Statistics
Data were presented as means ± standard deviation (S.D.). After having evaluated the homogeneity of variance and normal distribution of data, differences among groups were tested by ANOVA followed by the least significant difference (L.S.D.) test for multiple comparisons among groups. Comparisons between two groups were evaluated using Student’s t-test. Values were analysed using the statistical package SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). A value of P < 0.05 was accepted as statistical significance.
Results
Effect of LLLI precondition on expression of VEGF and GRP78, the activity of SOD and the production of MDA in the infarcted myocardium
As shown in Fig. 2, effects of LLLI precondition on the infarcted myocardium were summarized according to the time course. At 1 hr and 1 day after LLLI precondition, the expression of VEGF (A) and GRP78 (B) and the activity of SOD (C) in the LLLI group were significantly higher than that in the control group, whereas the production of MDA (D) was significantly lower than that in control group. At 1 week after LLLI precondition, there were no significant differences between LLLI group and control group.
Fig 2.
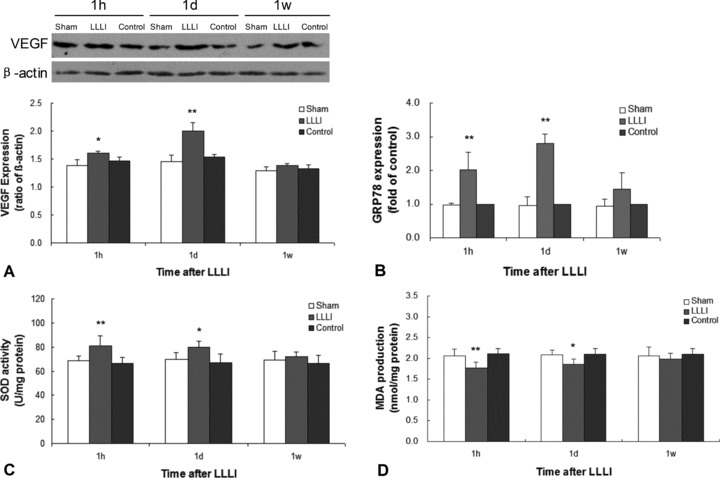
Effects of LLLI precondition on the infarcted myocardium at 1 hr, 1 day and 1 week after LLLI. (A) Western blotting demonstrated that the expression of VEGF protein were significantly higher in the LLLI group in comparison with the control group at 1 hr and 1 day after LLLI. (B) Real-time PCR demonstrated that the expression of GRP78 mRNA were significantly higher in the LLLI group in comparison with the control group at 1 hr and 1 day after LLLI. (C) The activity of SOD in the LLLI group was significantly higher than that in control group at 1 hr and 1 day after LLLI. (D) The production of MDA in the LLLI group was remarkably less than that in the control group at 1 hr (**P < 0.01 versus control group) and 1 day (*P < 0.05 versus control group) after LLLI. However, all significant differences were observed within 1 day after LLLI and then were vanished at 1 week after LLLI. Values were expressed as mean ± S.D. *P < 0.05, **P < 0.01 versus control group.
Effect of LLLI precondition on the survival of implanted BMSCs
We generated the standard curve by serially diluting rat male genomic DNA prepared from male BMSCs and quantified SRY gene by using real-time PCR. Threshold cycles of SRY gene in male BMSCs have a good relation with gradually diluted cells (Fig. 3A and 3B), R2= 0.9994. As shown in Fig. 3D, at 1 hr, 1 day and 1 week after BMSC transplantation, survival rate of implanted BMSCs was decreased gradually from 1 hr to 1 week in both BMSC-transplanted groups. The survival rate of implanted BMSCs was significantly higher in the LLLI + BMSC group than that in BMSC group at each detected time-point. At 1 hr after cell transplantation, 48.40 ± 17.75% of the initially implanted BMSCs in the LLLI + BMSC group was detected, which was significantly higher compared with 25.50 ± 4.62% in the BMSC group (P= 0.001). Meanwhile, the survival rates at 1 day and 1 week in the LLLI + BMSC group (31.82 ± 12.65% and 1.07 ± 0.28%, respectively) were significantly higher than that in the BMSC group (17.22 ± 3.92% and 0.68 ± 0.22%, respectively) (P= 0.025 and P= 0.03, respectively).
Fig 3.
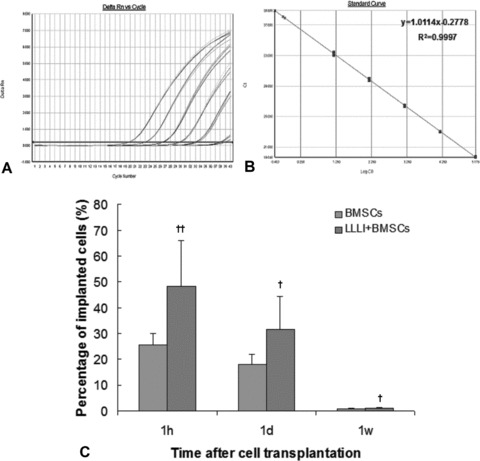
Quantifying implanted male BMSCs in the female infarcted myocardium by real-time PCR. (A) Real-time amplification plot showing change in normalized reporter dye fluorescence (Rn) versus number of amplification cycles in sample containing serially diluted male BMSC genomic DNA. (B) Standard curve generated from data in (A) showing relationship between threshold cycle (Ct) and number of male BMSCs. (C) Time course of implanted BMSC survival (%). The percentage of cell survival in the infarcted myocardium was significantly higher in the LLLI + BMSC group than that in the BMSC group. Values were expressed as mean ± S.D. †P < 0.05, ††P < 0.01 versus BMSC group.
In a preliminary study we did not detect any male cells in CM or LLLI + CM group following the same protocol.
Effect of LLLI precondition on implanted BMSC apoptosis and myogenic differentiation
As shown in Fig. 4, at 1 hr, 1 day and 1 week after cell transplantation, DAPI-labelled implanted cells could be detected and cell nuclei became smaller in shape and were frequently found to distribute along the host cardiomyocytes. The percentage of apoptotic BMSCs was significantly lower in the LLLI + BMSC group than that in the BMSC group at each time-point (1 hr: 1.17 ± 0.42%versus 2.15 ± 0.8%, P= 0.026; 1 day: 7.02 ± 2.07%versus 18.33 ± 6.07%, P= 0.016; 1 week: 1.81 ± 0.63%versus 3.55 ± 0.89%, P= 0.022). At 1 week after cell transplantation, neither α-sarcomeric actin- nor desmin-positive immunostaining cells colocalized with DAPI-labelled nuclei were identified in the myocardium (Fig. 5).
Fig 4.
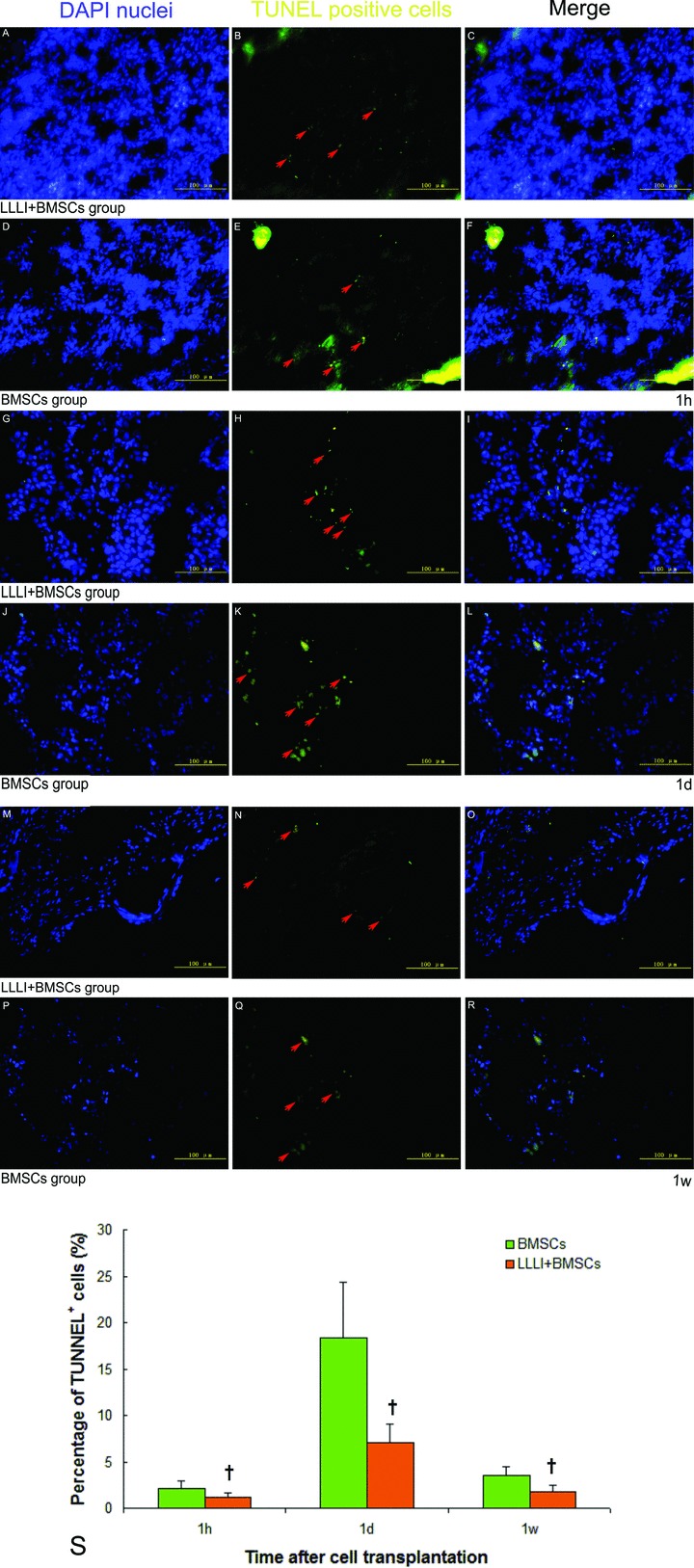
Apoptosis of implanted BMSCs in the infarcted myocardium at 1 hr, 1 day and 1 week after cell transplantation by TUNEL assay. (A, D, G, J, M and P) DAPI-labelled nuclei were shown in blue; (B, E–H, K, N and Q) TUNEL+ nuclei was in yellow (arrows); (C, F, I, L, O and R) Merged images; (S) Significantly lower percentage of TUNEL+ implanted cell nuclei in the infarcted myocardium was observed in the LLLI + BMSC group. Values were expressed as mean ± S.D. †P < 0.05 versus BMSC group.
Fig 5.
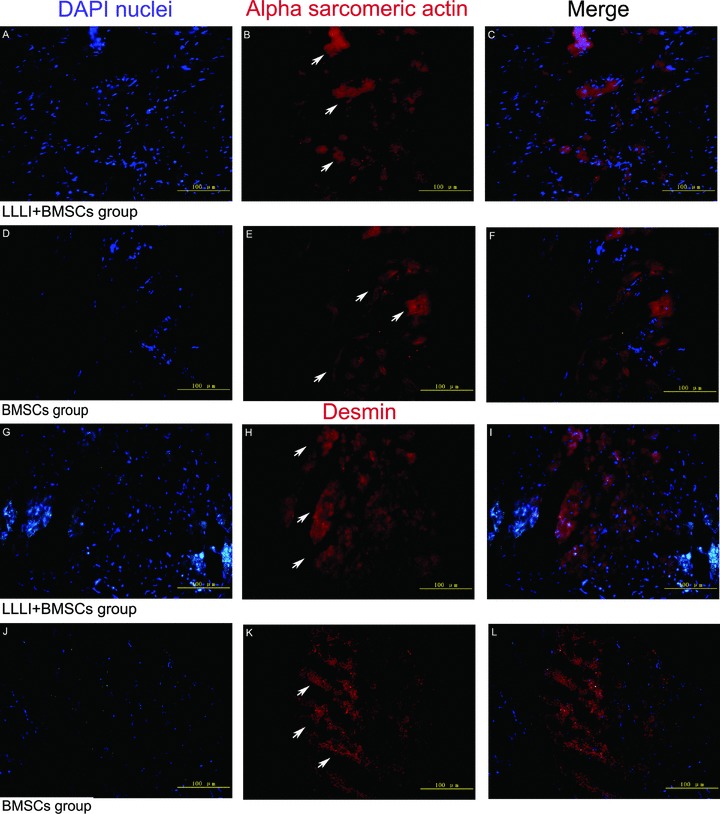
Myogenic differentiation of BMSCs in the infarcted myocardium at 1 week after cell transplantation by immunofluorescence staining for α-sarcomeric actin and desmin, respectively. (A, D, G and J) DAPI-labelled nuclei were shown in blue; (B and E) α-sarcomeric actin in red (arrows); (H and K) desmin in red (arrows); (C, F, I and L) Merged images. There was no significant overlap between 2 fluorophores at 1 week, indicating that implanted BMSCs did not express α-sarcomeric actin or desmin at this time.
Quantitative study for the newly formed capillaries
CD31, a glycoprotein expressed on endothelial cells, was used as a marker for quantifying newly formed capillaries (Fig. 6A–H). At 1 week after cell transplantation, quantitative vascular densities in the infarct and peri-infarct regions showed that the number of capillaries in the BMSC group (19.0 ± 2.28 and 35.17 ± 5.78 vessels/0.2 mm2, respectively) was significantly higher than that in the control group (12.83 ± 3.60 and 24.33 ± 3.93 vessels/0.2 mm2, respectively) (P= 0.004 and P < 0.01, respectively) and LLLI + CM group (13.17 ± 3.66 and 24.67 ± 4.13 vessels/0.2 mm2, respectively) (P= 0.005 and P= 0.001, respectively). Notably, the number of capillaries in the LLLI + BMSC group (24.0 ± 3.22 and 42.33 ± 3.44 vessels/0.2 mm2, respectively) was significantly higher than that in BMSC group (P= 0.015 and P= 0.011, respectively) (Fig. 6I).
Fig 6.
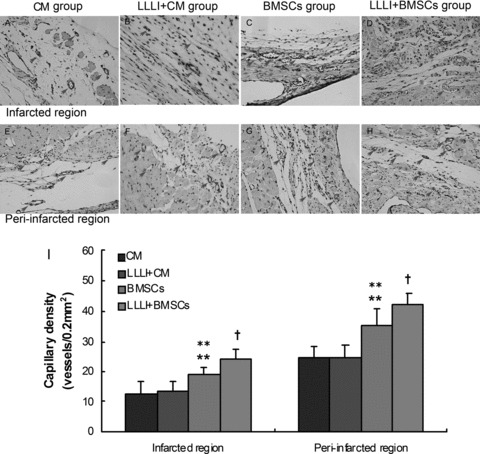
Capillary density of the infarct and peri-infarct regions in various groups by immunohistochemical staining for CD31 at 1 week after cell transplantation. (A, E) CM group; (B, F) LLLI + CM group; (C, G) BMSC group; (D, H) the LLLI + BMSC group; (I) Significantly increased number of blood vessels was observed in both infarcted and peri-infarcted region in the BMSC group. Further enhanced angiogenesis was observed in the LLLI + BMSC group. Values were expressed as mean ± S.D. **P < 0.01 versus CM group; **P < 0.01 versus LLLI + CM group; †P < 0.05 versus BMSC group. Arrows indicated the CD31+ cells.
Assessment of the left ventricular function
Table 1 summarized the results of echocardiographic assessment at 1 week after cell transplantation. EF and FS in the LLLI + BMSC group tended to be higher than that in the control group (P= 0.060 and P= 0.052, respectively) and LLLI + DMEM group (P= 0.129 and P= 0.114, respectively). In contrast, LVEDD and LVESD in the LLLI + BMSC group tended to be decreased compared with the control group (P= 0.123 and P= 0.082, respectively) and LLLI + DMEM group (P= 0.140 and P= 0.114, respectively). However, there were no significant differences among the control group, LLLI + DMEM group, BMSC group and LLLI + BMSC group.
Table 1.
Evaluation of left ventricular function by echocardiography at 1 week
| Group | CM | LLLI + CM | BMSCs | LLLI + BMSCs |
|---|---|---|---|---|
| LVEDd (mm) | 7.49 ± 0.61 | 7.45 ± 0.52 | 7.10 ± 0.70 | 6.89 ± 0.73 |
| LVESd (mm) | 6.24 ± 0.50 | 6.17 ± 0.52 | 5.77 ± 0.81 | 5.53 ± 0.82 |
| LVEF (%) | 41.86 ± 3.46 | 43.26 ± 5.02 | 46.49 ± 6.62 | 48.64 ± 7.57 |
| LVFS (%) | 16.56 ± 1.67 | 17.27 ± 2.44 | 18.92 ± 3.21 | 20.08 ± 3.95 |
LVEDd: left ventricular end-diastolic diameter; LVESd: left ventricular end-systolic diameter; LVEF: left ventricular ejection fraction; LVFS: left ventricular fractional shortening. No significant differences among groups.
Discussion
Cell transplantation has emerged as a promising approach to regenerate the injured myocardium, and research in this area has progressed rapidly. Despite encouraging results emanating from experimental studies as well as clinical trials, low survival rate after transplantation has major negative impact on cell therapy for the treatment of MI. It has been reported that as low as 1% of the donor cells survived during first 24 hrs after transplantation [26]. Similarly, the percentage of donor cells in the heart decreased rapidly from 34–80% of injected cells at 0 hr to 0.3–3.5% at 6 weeks [5]. Besides cell escape from the injection site, the infarcted myocardial milieu in settings of matrix detachment, ischemia and inflammation is even more ‘hostile’ to implanted cells [27, 28]. After cell transplantation, the grafted cells will face the great challenge of such a hostile environment which they may not survive. Therefore, it is imperative to create a more ‘friendly’ myocardial milieu prior to cell transplantation to support the cell early survival and thus improve the efficacy of cell therapy.
Several cell preconditioning strategies such as ex vivo genetic modulation of stem cells prior to transplantation have been extensively studied for enhancing the implanted cell survival. However, complicated techniques are always needed and such interventions may lead to undesired effects on the donor stem cells [14]. Besides direct cytoprotective strategies, cardioprotective interventions through improving the myocardial milieu, such as mechanical pre-treatment of infarcted myocardium by TMR [29], may provide possibilities to reduce implanted cell death, but these approaches have been studied to a limited extent and were associated with potential myocardial injury. LLLI is a U.S. Food and Drug Administration approved non-significant risk device and it is widely accepted because of its safety, lower cost and technically easier handling. The wavelength of 635 nm is similar to the widely studied wavelength of 632.8 nm [30] and also ranges to the visible red wavelengths, at which, LLLI could be easily manipulated in surgical procedures and focused on targeted positions. An energy density of 1–4 J/cm2 has been claimed to be an ‘optimal’ energy density to achieve beneficial effects by LLLI in various tissues [16]. Furthermore, a previous study has demonstrated that LLLI at the energy density of 0.96 J/cm2 showed beneficial effects for the ischemic heart tissue through up-regulating VEGF and iNOS expression in the infarcted rat heart [20]. Therefore, as a preconditioning approach prior to cell transplantation, the duration of LLLI was set to 150 sec. in order to give energy densities at 0.96 J/cm2 (when the corresponding laser power densities are kept at 6.37mW/ cm2) in present study.
In the LLLI precondition study, LLLI significantly up-regulated the expression of VEGF and GRP78 in the infarcted myocardium. It has been reported that VEGF and GRP78 were two key factors that played a major role in cytoprotection against myocardial ischemic injury [31, 32]. Particularly, constitutive expression of VEGF gene or co-injection of VEGF peptide could protect BMSCs from culture-induced cellular stress and improve their viability in ischemic myocardium [33, 34]. Meanwhile, LLLI significantly reserved the activity of SOD and decreased the production of MDA in the infarcted myocardium. Levels of oxidative stress are elevated in chronic failing hearts, which resulted in the development of heart failure and antioxidant therapies could improve cardiac function against heart failure [35, 36]. A previous study has demonstrated that superoxide played a causative role in the initial graft death and co-injecting the free radical scavenger SOD, CuZn-SOD, with skeletal myoblasts yielded a 2-fold increase in graft survival 3 days after engraftment [37]. Therefore, LLLI precondition has a mitigating influence on the deleterious effects of the free radicals in the infarcted myocardium. Our findings are mainly in agreement with recent study by Zhu et al.[38], who observed that LLLI at 660 nm wavelength improved functional recovery of the cold-stored rat heart possibly via conservation of ATP and antioxidant enzyme activity. Although there is strong evidence of cardioprotective effects of LLLI, the underlying mechanisms remain unclear. These beneficial effects are most likely mediated through increasing ATP synthesis [17] and antioxidative enzyme activity [18], elevating shock proteins content [19] and enhancing expression of VEGF and inducible nitric oxide (iNOS) [20]. Further studies are required to understand the mechanisms leading to the cardioprotective effects of LLLI.
To further study the cardioprotective effects of LLLI precondition on implanted BMSC survival, the time-course cell survival after transplantation was quantified by real-time PCR analysis from male donor cells in female recipient hearts. Real-time PCR assay in gender-mismatched transplant represents a sensitive and specific method in quantifying implanted BMSC engraftment in vivo. Using this technique, we acquired a 25% cell survival at 1 hr, 17% at 1 day and 0.68% at 1 week in the BMSC group without laser precondition. Although the number of implanted cells decreased dramatically within 1 week after cell transplantation, LLLI precondition enhanced cell survival by approximate 2-fold. In contrast, data from TUNEL apoptosis assay showed that there was the lower percentage of apoptotic BMSCs at each time-point in LLLI + BMSC group compared with the BMSC group. These results suggested that LLLI precondition support more BMSC survival, which might result from the remodelling myocardial milieu. Interestingly, the time-course studies in previous LLLI precondition study showed that remarkable changes with respect to VEGF, GRP78, SOD and MDA only occurred on the first day after precondition and the differences vanished at 1 week. Although the cell number in the damaged myocardium continuously decreased with time even after LLLI precondition, the higher survival percentage of total implanted BMSCs still was observed at 1 week. It confirmed the generally admitted fact that maximizing early cell survival will ultimately result in greater late survival.
A number of studies have demonstrated that BMSC transplantation can restore the damaged heart function [3, 39]. Because the present study focused on the cell early survival, we limited the time course within 1 week. The myogenic differentiation of grafted cells and restored heart function were usually identified several weeks after cell injection [26]. Therefore, it would be very difficult to find the evidence of differentiation and improved heart function in the present study. However, we observed the improvement tendency in LV function after BMSC transplantation with or without laser precondition. Although LLLI precondition did not improve the heart function and the expression of muscle-specific marker of implanted BMSCs within 1 week, it enhanced the angiogenesis both in the infarcted and peri-infarcted region. The therapeutic potential of BMSCs has been partly attributed to their ability to participate in angiogenesis through paracrine mechanisms [40]. It has been demonstrated that implanted BMSCs can enhance vascular density as early as day 5 after implantation in a rat MI model [41]. In present study, we found that BMSC transplantation alone significantly enhanced vascular density after MI at 1 week. Notably, the enhanced angiogenesis was further intensified in the LLLI + BMSC group. Moreover, data from the LLLI precondition study showed that LLLI precondition could enhance the expression of VEGF in the infarcted myocardium; however, the capillary density in the LLLI + CM group was not significantly increased at 1 week. These suggested that the intensified angiogenesis in LLLI + BMSC group was mainly attributed to the enhanced cell survival by LLLI precondition, which directly reinforced paracrine mechanisms.
In present study, the effects of LLLI precondition on the infarcted myocardium vanished rapidly within 1 week, although temporary cardioprotective effects of LLLI precondition may be correlated to the enhanced survival rate of implanted cell (0.68%) at the end time-point. These suggested that repeated irradiation may be necessary to amplify the beneficial effects of LLLI precondition. Considering that 635 nm low level laser provided poor tissue penetration capacity [42], post-operative percutaneous irradiation might not lead to stable laser-derived effects. Therefore, it is necessary to improve the irradiation technique and equipment of LLLI. In the future clinical application, the cardiac surgeons could loosen the suture of the optical fibres on the surface of the heart and then place the fibres percutaneously. Hence it would allow easy access to the laser device and therefore offer a novel approach for percutaneous laser precondition to perform multiple irradiations after cell transplantation. Another limitation of our study is that only 635 nm LLLI with one energy density was tested. Indeed, LLLI-derived effects on tissues are wavelength and dose dependent. Our ongoing studies will explore the beneficial effects with various wavelength and energy densities and search for the optimal wavelength and dose for myocardial LLLI precondition.
Conclusions
Our study, for the first time, showed that precondition the infarcted myocardium with LLLI prior to cell transplantation could attenuate the hostile milieu of infarcted myocardium, inhibit implanted BMSC apoptosis and thus improve early survival of implanted BMSCs, which resulted in enhanced angiogenesis and potential improvement of LV function. Therefore LLLI precondition may offer a simple and novel non-invasive approach for intraoperative cell therapy to enhance cell early survival and therapeutic potential.
Acknowledgments
The authors wish to thank Ms. Jun Li, and Drs. Shao-peng Fu, Sheng-wen Liu and Yuan Xin for the technical assistance. This study was supported by National Science Foundation of China (30672083), National 863 Project (2006AA02A106) and Beijing Nova Project (2006A85).
Disclosures
The authors have no conflict of interest to report.
References
- 1.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 4.Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47:1777–85. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Müller-Ehmsen J, Krausgrill B, Burst V, et al. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol. 2006;41:876–84. doi: 10.1016/j.yjmcc.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Robey TE, Saiget MK, Reinecke H, et al. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–81. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang M, Methot D, Poppa V, et al. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–21. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Smolenski RT, Jayakumar J, et al. Heat shock treatment enhances graft cell survival in skeletal myoblast transplantation to the heart. Circulation. 2000;102:III216–21. doi: 10.1161/01.cir.102.suppl_3.iii-216. [DOI] [PubMed] [Google Scholar]
- 9.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 10.Yau TM, Kim C, Li G, et al. Maximizing ventricular function with multimodal cell-based gene therapy. Circulation. 2005;112:I123–8. doi: 10.1161/CIRCULATIONAHA.104.525147. [DOI] [PubMed] [Google Scholar]
- 11.Dai Y, Xu M, Wang Y, et al. HIF-1alpha induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol. 2007;42:1036–44. doi: 10.1016/j.yjmcc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasha Z, Wang Y, Sheikh R, et al. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77:134–42. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Hou J, Shi L, et al. Lysophosphatidic acid protects mesenchymal stem cells against ischemia induced apoptosis in vivo. Stem Cells Dev. 2009;18:947–54. doi: 10.1089/scd.2008.0352. [DOI] [PubMed] [Google Scholar]
- 14.Fazel SS, Angoulvant D, Butany J, et al. Mesenchymal stem cells engineered to overexpress stem cell factor improve cardiac function but have malignant potential. J Thorac Cardiovasc Surg. 2008;136:1388–9. doi: 10.1016/j.jtcvs.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 15.Moshkovska T, Mayberry J. It is time to test low level laser therapy in Great Britain. Postgrad Med J. 2005;81:436–41. doi: 10.1136/pgmj.2004.027755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conlan MJ, Rapley JW, Cobb CM. Biostimulation of wound healing by low-energy laser irradiation A review. J Clin Periodontol. 1996;23:492–6. doi: 10.1111/j.1600-051x.1996.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 17.Oron U, Yaakobi T, Oron A, et al. Low-energy laser irradiation reduces formation of scar tissue after myocardial infarction in rats and dogs. Circulation. 2001;103:296–301. doi: 10.1161/01.cir.103.2.296. [DOI] [PubMed] [Google Scholar]
- 18.Oron U, Yaakobi T, Oron A, et al. Attenuation of infarct size in rats and dogs after myocardial infarction by low-energy laser irradiation. Lasers Surg Med. 2001;28:204–11. doi: 10.1002/lsm.1039. [DOI] [PubMed] [Google Scholar]
- 19.Yaakobi T, Shoshany Y, Levkovitz S, et al. Long-term effect of low energy laser irradiation on infarction and reperfusion injury in the rat heart. J Appl Physiol. 2001;90:2411–9. doi: 10.1152/jappl.2001.90.6.2411. [DOI] [PubMed] [Google Scholar]
- 20.Tuby H, Maltz L, Oron U. Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis. Lasers Surg Med. 2006;38:682–8. doi: 10.1002/lsm.20377. [DOI] [PubMed] [Google Scholar]
- 21.Zyciński P, Krzemińnska-Pakuła M, et al. Laser biostimulation in end-stage multivessel coronary artery disease–a preliminary observational study. Kardiol Pol. 2007;65:13–21. [PubMed] [Google Scholar]
- 22.Su W, Zhang H, Jia Z, et al. Cartilage-derived stromal cells: is it a novel cell resource for cell therapy to regenerate infarcted myocardium. Stem Cells. 2006;24:349–56. doi: 10.1634/stemcells.2005-0168. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Baydoun AR, Xu R, et al. Lysophosphatidic acid protects mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis. Stem Cells. 2008;26:135–45. doi: 10.1634/stemcells.2007-0098. [DOI] [PubMed] [Google Scholar]
- 24.Zhu W, Chen J, Cong X, et al. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells. 2006;24:416–25. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Song P, Tang Y, et al. Injection of bone marrow mesenchymal stem cells in the borderline area of infarcted myocardium: heart status and cell distribution. J Thorac Cardiovasc Surg. 2007;134:1234–40. doi: 10.1016/j.jtcvs.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–8. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 27.Du XJ. Re-modelling ‘hostile’ milieu of diseased myocardium via paracrine function of transplanted cells or relaxin. J Cell Mol Med. 2007;11:1101–4. doi: 10.1111/j.1582-4934.2007.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penna C, Raimondo S, Ronchi G, et al. Early homing of adult mesenchymal stem cells in normal and infarcted isolated beating hearts. J Cell Mol Med. 2008;12:507–21. doi: 10.1111/j.1582-4934.2007.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiegelstein D, Kim C, Zhang Y, et al. Combined transmyocardial revascularization and cell-based angiogenic gene therapy increases transplanted cell survival. Am J Physiol Heart Circ Physiol. 2007;293:H3311–6. doi: 10.1152/ajpheart.00178.2007. [DOI] [PubMed] [Google Scholar]
- 30.Posten W, Wrone DA, Dover JS, et al. Low-level laser therapy for wound healing: mechanism and efficacy. Dermatol Surg. 2005;31:334–40. doi: 10.1111/j.1524-4725.2005.31086. [DOI] [PubMed] [Google Scholar]
- 31.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–8. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 32.Shintani-Ishida K, Nakajima M, Uemura K, et al. Ischemic preconditioning protects cardiomyocytes against ischemic injury by inducing GRP78. Biochem Biophys Res Commun. 2006;345:1600–5. doi: 10.1016/j.bbrc.2006.05.077. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki K, Murtuza B, Smolenski RT, et al. Cell transplantation for the treatment of acute myocardial infarction using vascular endothelial growth factor-expressing skeletal myoblasts. Circulation. 2001;104:I207–12. doi: 10.1161/hc37t1.094524. [DOI] [PubMed] [Google Scholar]
- 34.Pons J, Huang Y, Arakawa-Hoyt J, et al. VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem Biophys Res Commun. 2008;376:419–22. doi: 10.1016/j.bbrc.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Hill MF, Singal PK. Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am J Pathol. 1996;148:291–300. [PMC free article] [PubMed] [Google Scholar]
- 36.Yano M, Okuda S, Oda T, et al. Correction of defective inter-domain interaction within ryanodine receptor by antioxidant is a new therapeutic strategy against heart failure. Circulation. 2005;112:3633–43. doi: 10.1161/CIRCULATIONAHA.105.555623. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki K, Murtuza B, Beauchamp JR, et al. Dynamics and mediators of acute graft attrition after myoblast transplantation to the heart. FASEB J. 2004;18:1153–5. doi: 10.1096/fj.03-1308fje. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Q, Yu W, Yang X, et al. Photo-irradiation improved functional preservation of the isolated rat heart. Lasers Surg Med. 1997;20:332–9. doi: 10.1002/(sici)1096-9101(1997)20:3<332::aid-lsm12>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 39.Hou M, Yang KM, Zhang H, et al. Transplantation of mesenchymal stem cells from human bone marrow improves damaged heart function in rats. Int J Cardiol. 2007;115:220–8. doi: 10.1016/j.ijcard.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 40.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–9. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 41.Davani S, Marandin A, Mersin N, et al. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003;108:II253–8. doi: 10.1161/01.cir.0000089186.09692.fa. [DOI] [PubMed] [Google Scholar]
- 42.Kolari PJ, Airaksinen O. Poor penetration of infra-red and helium neon low power laser light into the dermal tissue. Acupunct Electrother Res. 1993;18:17–21. doi: 10.3727/036012993816357566. [DOI] [PubMed] [Google Scholar]


