Abstract
In recent years, the use of anthraquinone laxatives, in particular senna, has been associated with damage to the intestinal epithelial layer and an increased risk of developing colorectal cancer. In this study, we evaluated the cytotoxicity of rhein, the active metabolite of senna, on human colon adenocarcinoma cells (Caco-2) and its effect on cell proliferation. Cytotoxicity studies were performed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), neutral red (NR) and trans-epithelial electrical resistance (TEER) assays whereas 3H-thymidine incorporation and Western blot analysis were used to evaluate the effect of rhein on cell proliferation. Moreover, for genoprotection studies Comet assay and oxidative biomarkers measurement (malondialdehyde and reactive oxygen species) were used. Rhein (0.1–10 μg/ml) had no significant cytotoxic effect on proliferating and differentiated Caco-2 cells. Rhein (0.1 and 1 μg/ml) significantly reduced cell proliferation as well as mitogen-activated protein (MAP) kinase activation; by contrast, at high concentration (10 μg/ml) rhein significantly increased cell proliferation and extracellular-signal-related kinase (ERK) phosphorylation. Moreover, rhein (0.1–10 μg/ml): (i) did not adversely affect the integrity of tight junctions and hence epithelial barrier function; (ii) did not induce DNA damage, rather it was able to reduce H2O2-induced DNA damage and (iii) significantly inhibited the increase in malondialdehyde and reactive oxygen species (ROS) levels induced by H2O2/Fe2+. Rhein was devoid of cytotoxic and genotoxic effects in colon adenocarcinoma cells. Moreover, at concentrations present in the colon after a human therapeutic dosage of senna, rhein inhibited cell proliferation via a mechanism that seems to involve directly the MAP kinase pathway. Finally, rhein prevents the DNA damage probably via an anti-oxidant mechanism.
Keywords: rhein, human colon adenocarcinoma cells, mitogen-activated protein kinase, genoprotection, antioxidant
Introduction
Constipation, a complaint conceptually regarded as disordered movement of stool through the large intestine, afflicts many people in the Western countries [1, 2]. The first approach for the treatment of constipation consists in lifestyle changes including increased fibre (about 30 g/day), and water (about 2 l/day) intake and physical exercise. If these measures are ineffective, laxative therapy has to be considered. Among laxatives, anthranoids are the oldest used drugs in clinical practice and as self-medication [3]. Senna is the most known anthranoid laxative; it is obtained from the dried leaves and pods of Cassia acutifolia Delile and Cassia angustifolia Vahl (Caesalpinaceae family). Senna contains sennosides that are not absorbed in the small intestinal tract; in the colon, sennosides are metabolized by the bacterial β-glucosidase and reductase to the pharmacologically active compound, rhein anthrone [4, 5]. Rhein anthrone is poorly absorbed and produces a laxative effect throughout two independent mechanisms: (i) changing of colonic motility leading to an accelerated large intestinal transit and (ii) alterations in colonic adsorption and secretion of water and electrolytes resulting in fluid accumulation [6].
In the last years, there has been the concern that therapeutic or chronic use of anthranoid laxatives can cause structural damage to colonic tissue and induce colonic neuropathy and colorectal cancer. However, human and animal data in this area are inconsistent. Three clinical studies have evaluated the potential side effects of long-term use of anthraquinone laxatives on colonic nerve tissue [7–9]. One study found a direct association between stimulant laxative use and anatomic changes in the colon [7] while two clinical trials did not support the hypothesis that anthraquinone containing laxatives are able to provoke relevant degenerative changes in the colonic nerve tissue [8, 9]. van Gorkom and colleagues reported three clinical studies showing increased cell proliferation in colonic tissue of patients treated with a single high dose of sennosides [10–12]. Most epidemiological studies failed to find any association between anthranoid laxative use and colorectal cancer [13–17], although a prospective study showed an increased cell proliferation (interpreted as higher risk for developing colorectal cancer) in patients using anthranoid laxatives [18]. Finally, two single case reports identified an increased cell proliferation in patients using anthranoid laxatives [19, 20]. Similar conflicting data have been reported on preclinical studies. As a consequence of these data in 1995, the German Federal Institute for Drugs and Medical Devices revised the product information of all medicinal products containing anthranoids and in 1998 the Food and Drug Administration (FDA) classified the stimulant laxatives (including senna) from category I (generally recognized as safe and effective) to category III (further testing is required). In 2002, the FDA re-classified senna, but not aloe and cascara, to category I. Although there is no conclusive clinical evidence that senna is dangerous, there are still doubts and unresolved questions on the safety of this drug and its components. On this basis, physicians often underuse anthraquinones laxatives and are more willing to use far more expensive drugs that have a much shorter track record concerning potential long-term consequences to the constipated patient.
Therefore, the aim of this study was to investigate the potential damaging effects of the active anthranoid metabolite of senna, rhein, on a human colon adenocarcinoma cell line, Caco-2.
The cytotoxicity of rhein on proliferating and differentiating cells has been evaluated as well as its effects on cell proliferation, genotoxicity and epithelial integrity, all of which are thought to be associated with colorectal carcinogenesis.
Materials and methods
Chemicals
The 4,5-Dihydroxyanthraquinone-2-carboxylic acid (rhein), deoxycolic acid (DCA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and 1,1,3,3-tetramethoxypropane (malonaldehyde bis dimethyl acetal], standard MDA) were purchased from Sigma Aldrich S.r.l. (Milan, Italy). Monoclonal primary antibodies for phosphorylated extracellular-signal-related kinase ERK1/2 (pERK1/2) and ERK2 were obtained from Santa Cruz Biotechnology Inc. (CA, USA) while peroxidase-conjugated (HRP) antimouse IgG antibody was obtained from Amersham Biosciences Inc. (GE Healthcare, Milan, Italy). All reagents for Western blot analysis and cell culture were obtained from Sigma Aldrich S.r.l. (Milan, Italy), Bio-Rad Laboratories (Segrate, Italy) and Microglass Heim S.r.l. (Naples, Italy). Rhein was dissolved in dimethylsulphoxide (DMSO) to obtain a final concentration of 0.1% (v/v) in the culture medium. The concentration range for rhein (0.1–10 μg/ml) used in the study was selected on the basis that a human therapeutic dosage (20–40 mg of dianthrones derivatives) or an overdosage (80–100 mg) of senna generate rhein concentrations of approximately 0.1–1 μg/ml (0.35–3.5 μM) or 10 μg/ml (35 μM), respectively [3].
Cell culture
Caco-2, A-431 cells (American Type Culture Collection, LGC Promochen, Italy) and human fibroblasts were cultured in 75 cm2 polystyrene flasks (Falcon, Microglass Heim, Naples, Italy) as monolayers in modified Eagle medium (MEM) containing 10% foetal bovine serum (FBS), 100 Units/ml penicillin, 100 μg/ml streptomycin and 1% non-essential amino acids. Cells were cultured at 37°C in a humidified 5% CO2 and 95% filtered air and the culture medium was replaced every 2 days. After washing in phosphate buffered saline (PBS), cells were trypsinized with 0.25% trypsin-EDTA at 37°C for 5 min., centrifuged at 1000 ×g for 3 min. and then re-suspended in the appropriate medium. Cell viability was determined by trypan blue staining.
Cytotoxicity assay
MTT assay
The effect of rhein on cell survival was measured using the CellTiter 96® proliferation assay (MTT assay) (Promega, Madison, WI, USA) [21]. Caco-2 cells (passage between 26 and 29) were seeded in 96-wells plates at a concentration of 3 × 104 cells/well. After 48 hrs of culturing, the medium was removed and the cells were treated with rhein (0.1–10 μg/ml) at 37°C for 24 hrs. Following treatment, cells were washed and fresh medium was replaced. After 48 hrs of culturing at 37°C, 15 μl MTT dye solution were added to each well for 4 hrs. Finally, 100 μl of solubilization/stop solution were added to dissolve the purple crystals; absorbency of formazan was measured at a wavelength (λ) of 570 nm using a multiwell reader (Rainbow, SLT, Austria). Treatments were compared with a positive control, deoxycolic acid (250 μM). Experiments were repeated independently three times. The results are expressed as percentage of cell viability.
Neutral red (NR) assay
The NR assay system, one of the most used and sensitive cytotoxicity test, is a mean of measuring living cells via the uptake of the vital dye neutral red. Viable cells will take up the dye by active transport and incorporate the dye into lysosomes, whereas non-viable cells will not take up the dye. An increase or decrease in the number of cells or their physiological state results in a concomitant change in the amount of dye incorporated by the cells in the culture [22]. Caco-2 cells (passage between 26 and 29) were seeded in 96-wells plates at a concentration of 1×104 cells/well. After 48 hrs of culturing, the medium was removed and the cells were treated with rhein (0.1–10 μg/ml) at 37°C for 24 hrs. Following treatment, cells were washed and 200 μl NR dye solution (50 μg/ml in DMEM) were added to each well for 3 hrs at 37°C. After washing in PBS, 100 μl of 1% acetic acid were added and the absorbency was measured at a wavelength (λ) of 532 nm using a multiwell reader (Perkin-Elmer Instruments Waltham, MA, USA). Treatments were compared with a positive control, deoxycolic acid (250 μM). The results are expressed as percentage of cell viability.
Trans-epithelial electrical resistance (TEER) assay
TEER was monitored as an indication of tight junction formation and epithelial monolayer integrity as previously described [23]. Falcon®-Transwell inserts (0.4 μm pore size; BD Bioscience, Buccinasco, Italy) were coated with 0.01% type I rat-tail collagen (Sigma) and left to dry overnight under ultraviolet (UV) lights in 6-well plates. Caco-2 cells (passage between 57 and 63) were seeded into these inserts in 2.5 ml aliquots at a concentration of 2.5 × 105 cells/ml. Culture medium (1.5 ml) was added to the basolateral compartment of each well. The cells were grown on these inserts and the medium was changed every 2 days. After 7 days of culturing TEER, readings (expressed as Ωcm2) were taken using an EVOM epithelial voltohmmeter with chopstick electrodes (World Precision Instruments Inc., Stevenage, UK). Readings were taken every 24 hrs until they stabilized (at days 14–16). At this point the cells, maintained at 37°C in a humidified 5% CO2 and 95% filtered air, were deemed fully differentiated. The culture medium was replaced every other day for 21 days. A final TEER reading was taken immediately before adding rhein (0.1–10 μg/ml) and after 24 and 48 hrs. Treatments were compared with a positive control, deoxycholic acid (250 μM). The results are expressed as percentage change in TEER compared with the TEER value at the start of the experiment (time 0). Three independent experiments were conducted.
Paracellular permeability assay
Caco-2 monolayers in 6-well plates were incubated with rhein (0.1–10 μg/ml) in presence of 10 μM fluorescein (Sigma). Samples (200 μl) were removed from the apical compartment at the beginning of the experiment (time 0) and from the basolateral compartment after 24 and 48 hrs. The samples fluorescein content was measured using a microplate reader (Tecan SPECTRA Rainbow, PAA Ltd, UK) (488 nm excitation and 510 nm emission). The paracellular permeability represents the amount of fluorescein in the basolateral compartment expressed as percentage of the amount added to the apical compartment at the start of the experiment, to give a measure of the relative amount of fluorescein travelling through the Caco-2 monolayer tight junctions (percentage of monolayer permeability). Three independent experiments were conducted.
Proliferation assay
3H-Thymidine incorporation
Caco-2 cells (passage between 28 and 34) were seeded in 24-well plates at a density of 1.0 ×104 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS. After 48 hrs of incubation, cells were treated with rhein (0.1–10 μg/ml) in the presence of 3H-thymidine (1 μCi/well) for 24 hrs. Medium was removed by aspiration, and after washing in PBS, cells were scraped in 1 M NaOH (100 μl/well) and collected on glass fibre filter mats using an LKB (Bromma, Sweden) automatic cell harvester prior to liquid scintillation counting. The effect of rhein on cell proliferation was expressed as count per minute/mg of protein (CPM/mg protein) of incorporating 3H-Thymidine cells. The treatments were carried out in triplicate and three independent experiments were performed.
Preparation of cytosolic fractions and Western blot analysis
Caco-2 cytosolic extracts were prepared as previously described [24]. Briefly, after rhein (0.1–10 μg/ml) incubation for 24 hrs, the medium was removed and cells were washed with ice-cold PBS. The cells were collected by scraping for 10 min. at 4°C with lysis buffer (50 mM Tris-HCl pH = 7.4, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA, 1 mM NaF, 1% NP-40, 1mM PMSF, 1 mM Na3VO4 containing complete protease inhibitor cocktail [Roche Diagnostics, Mannheim, Germany]). After centrifugation at 16,200 g for 15 min. at 4°C, the supernatants were collected and protein concentration was determined by Bio-Rad Protein Assay (Bio-Rad, Milan, Italy). For Western blot analysis, lysate aliquots containing 70 μg of proteins were separated on a 12% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane (Protran®, Protran Nitrocellulose Transfer Membrane Schleicher & Schuell Bioscience, Dassel, Germany) using a Bio-Rad Transblot (350 mA, 3 hrs). Proteins were visualized on the filters by reversible staining with Ponceau-S solution (Sigma) and de-stained in PBS containing 0.1% Tween 20. Membranes were blocked at 4°C in milk buffer (5% non-fat dry milk in PBS/Tween 0.1%) and then incubated overnight at 4°C with mouse monoclonal antibodies for pERK1/2 and ERK2 (Santa Cruz, DBA S.r.l, Italy). The mouse monoclonal anti-pERK1/2 and anti-ERK2 were used at 1:1000 dilution in milk buffer (5% non-fat dry milk in PBS/Tween 0.1%). Subsequently, the membranes were incubated for 1 hr at room temperature with 1:2000-dilution of antimouse IgG-HRP-conjugated secondary antibody (Amersham Biosciences, UK). After washing in PBS/Tween 0.1%, the membranes were analysed by enhanced chemiluminescence’s (ECL; Amersham Biosciences). The optical density of the bands on autoradiographic films was determined by an image analysis system (GS 700 Imaging Densitometer, Bio-Rad) equipped with a software Molecular Analyst (IBM, Milan, Italy). The effect of rhein on the mitogen-activated protein (MAP) kinase activation was expressed as ratio of densitometric analysis of pERK1/2/total ERK bands.
Genotoxicity assay
DNA damage was examined by single-cell gel electrophoresis (Comet assay) [25]. Caco-2 cells (passage between 33 and 37) were seeded in 25 cm2 polystyrene flasks (Falcon) and grown to ∼70% confluence. Then, cells were incubated with rhein (0.1–10 μg/ml) for 24 hrs and subsequently they were trypsinized to obtain a suspension of 1.5 ×105 cells/ml. Aliquots of cell suspension were incubated with and without H2O2 challenge (75 μM for 5 min. on ice) and then centrifuged at 1000 ×g for 5 min. at 4°C. The supernatants were discarded and the pellets were mixed with 85 μl of 0.85% low melting point agarose in PBS. Cells were added to previously prepared gels of 1% normal melting point agarose in PBS. The gels on frosted slides were suspended in lysis solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris and 1% Triton X-100, pH 10) at 4°C for 1 hr, and then electrophoresed in buffer (300 mM NaOH, 1 mM Na2EDTA, pH>12) at 26 V, 300 mA for 20 min. After electrophoresis, gels were immersed in 0.4 M Tris–HCl, pH 7.5 (3 × 5 min. washes) to neutralize the alkaline pH, and stained with 20 μl of ethidium bromide (2 μg/ml) before scoring. Images were analysed using a fluorescence microscope (Nikon, Florence, Italy) interfaced with a computer. DNA damage (percentage of tail DNA) was quantified using Komet 5.0 image analysis software (Kinetic Imaging, Liverpool, UK). Positive (H2O2; 75 μmol/L) and negative (PBS) controls were included for all experiments. The mean percentage of tail DNA was calculated from 100 cells per gel (each sample in triplicate) and the mean of each independent experiment (n = 3) was used in the statistical analysis.
Antioxidant assay
TBARs assay
Lipid peroxidation products from Caco-2 cells were measured by the thiobarbituric acid colorimetric assay [26]. The reaction of lipid peroxides (formed after the oxidative stress) and the thiobarbituric acid (TBA) leads to the formation of malondialdehyde that can be measured by colorimetric detection of the chromogen (pink colour complex). Briefly, Caco-2 cells were seeded in 6-well plates at a density of 3.0 × 106 in DMEM supplemented with 10% FBS. After treatment with rhein (0.1–10 μg/ml) for 24 hrs, cells were washed with PBS and incubated with the Fenton’s reagent (Fe2+/H2O2 1 mM) for 3 hrs at 37 °C. After incubation, cells were washed and scraped in PBS. Cells were lysed by six cycles of freezing and thawing in PBS and then centrifuged at 12,600 ×g for 10 min. at 4°C. To 150 μl of cellular lysate were added 300 μl of 10% (w/v) trichloroacetic acid (TCA) and, after centrifugation at 16,200 ×g for 10 min., 0.67% (w/v) thiobarbituric acid was added and the mixture was heated at 80°C for 30 min. After cooling, malondialdehyde (MDA) production was recorded (A490nm) in a Beckman DU-62 spectrophotometer (Beckman, Milan, Italy) and the results are presented as μmol of MDA/mg of cell proteins determined by the Bio-Rad protein assay. A standard curve of MDA was used to quantify the MDA levels formed during the experiments.
Intracellular reactive oxygen species (ROS) measurement
The generation of intracellular ROS was estimated using the fluorescence probe 2′,7′-dichlorfluorescein-diacetate (H2DCF-DA) [27]. The H2DCF-DA is able to diffuse passively into cells, where the acetate is cleaved by intracellular esterases to the non-fluorescent H2DCF that thereby traps it within the cell. In the presence of intracellular ROS, H2DCF is rapidly oxidized to the highly fluorescent 2′,7′- dichlorofluorescein (DCF). The DCF fluorescence intensity is paralleled to the amount of ROS formed intracellularly. For the experiments, cells were plated in 96 multi-well black plates (Corning, USA) at the density of 1×104 cells per well and led to the differentiation. Confluent Caco-2 cell monolayers were incubated for 24 hrs at 37°C with rhein (0.1–10 μg/ml). After washing in PBS, cells were incubated for 30 min. with 200 μl of 100 μM DCFH-DA in Hank’s balanced salt solution (HBSS) containing 1% FBS. Finally, cells were rinsed and incubated with the Fenton reagent (H2O2/Fe2+ 2 mM) for 3 hrs at 37°C. The DCF fluorescence intensity was detected using a fluorescent microplate reader (Perkin-Elmer Instruments) at the excitation wavelength (λ) of 485 nm and the emission wavelength (λ) of 538 nm. The ROS levels were expressed as fluorescence intensity (picogreen).
Statistical analysis
Results are expressed as mean ± S.E.M of n experiments. The analyses of results were carried out using GraphPad Prism Software (GraphPad Software, Inc. San Diego, CA, USA). Comparisons between two sets of data were made by Student’s t-test for paired data. When multiple comparisons against a single control were made, one-way ANOVA was used, followed by Turkey-Kramer multiple comparisons test. A P-value less than 0.05 was considered significant. For Comet assay the differences between means were evaluated by ANOVA, followed by post hoc LSD.
Results
MTT and NR assay
In the MTT assay, rhein in the concentration range of 0.1–10 μg/ml had no significant cytotoxic effect on Caco-2 cells after 24 hrs exposure (control: 100 ± 0.0; rhein 0.1 μg/ml: 94.69 ± 3.45; rhein 1 μg/ml: 95.47 ± 2.49; rhein 10 μg/ml: 93.70 ± 0.42). Deoxycholic acid (DCA, 250 μM), a secondary bile acid used as positive control, significantly (P < 0.001) reduced the cell viability in proliferating Caco-2 cells (control: 100 ± 0.0 and DCA 250 μM: 69.04 ± 2.21). These data have been confirmed by the NR assay (control: 100 ± 0.0; rhein 0.1 μg/ml: 102.5 ± 2.02; rhein 1 μg/ml: 93.26 ± 7.14; rhein 10 μg/ml: 91.21 ± 5.07; DCA 250 μM: 50.5 ± 0.32).
TEER and fluorescein assay
Figures 1 and 2 show the effect of rhein on Caco-2 epithelial monolayer integrity. Differentiated Caco-2 cells exhibit different phenotypes characteristic of villus tip cells or crypt base cells and they are a well-known experimental model to examine the maintenance of junctional integrity according to a procedure previously described [28]. All tested rhein concentrations (0.1–10 μg/ml) had no significant adverse effects on the epithelial monolayer, as indicated by TEER value measurement after 24 and 48 hrs of rhein exposure (Fig. 1A). As expected, the incubation of differentiated Caco-2 cells with DCA (250 μM), resulted in a marked decrease in TEER value (∼30%; P < 0.001), reflecting disruption of enterocytes tight junctions (Fig. 1). The absence of detrimental effects of rhein on tight junction integrity as assessed by TEER, was confirmed by the fluorescein flow assay (Fig. 1B) where no increase in fluorescein flow from the apical to the basolateral compartment of the Caco-2 monolayer occurred.
Fig 1.
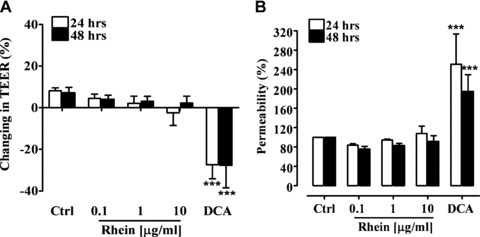
Trans-epithelial electrical resistance (TEER) of polarized Caco-2 monolayer exposed to rhein (0.1–10 μg/ml) and deoxycholic acid (DCA, 250 μM) for 24 and 48 hrs (A). Effect of rhein (0.1–10 μg/ml) and deoxycholic acid (DCA, 250 μM) on the colonic monolayer permeability after 24 and 48 hrs exposure (B). ***P < 0.001 versus control (Ctrl), n= 3, mean ± S.E.M.
Fig 2.
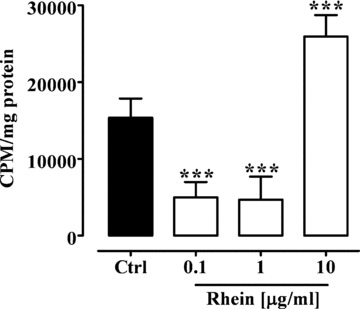
Effect of rhein (0.1–10 μg/ml) on Caco-2 cell proliferation after 24 hrs of incubation. ***P < 0.001 versus control (Ctrl), n= 3, mean ± S.E.M.
3H-Thymidine incorporation
Thymidine is a precursor of DNA and it is incorporated into new DNA in proliferating cells. For this reason, the 3H-Thymidine incorporation into DNA during 24 hrs has been measured as an indicator of cell proliferation, and thereby DNA synthesis. Rhein, at the concentrations of 0.1 and 1 μg/ml, significantly (P < 0.001) reduced the 3H-Thymidine incorporation in proliferating Caco-2 cells (Fig. 2). By contrast, rhein at the higher concentration (10 μg/ml) significantly (P < 0.001) increased Caco-2 cells proliferation (Fig. 2). Moreover, rhein, at all concentrations used (0.1–10 μg/ml) did not modify the 3H-Thymidine incorporation in proliferating A-431 cells (control: 548.5 ± 60.48 CPM/mg; rhein 0.1 μg/ml: 541.3 ± 56.26 CPM/mg; rhein 1 μg/ml: 612.7 ± 56.63 CPM/mg; rhein 10 μg/ml: 596.2 ± 76.49 CPM/mg) and human fibroblasts (control: 684.0 ± 10.0 CPM/mg; rhein 0.1 μg/ml: 585.5 ± 22.50 CPM/mg; rhein 1 μg/ml: 644.5 ± 29.50 CPM/mg; rhein 10 μg/ml: 670.0 ± 12.0 CPM/mg).
Western blot analysis
The possible molecular mechanism of rhein on cell proliferation was investigated by studying its effect on the MAP kinase (MAPK) signalling pathways. The MAPK pathway involves two closely related kinases, known as ERK 1 (ERK1, p44) and 2 (ERK2, p42) that come from dimerization of total cytosolic ERK. The modulation in ERK1 and ERK2 phosphorylation after 24 hrs rhein exposure was consistent with the observed effect of rhein on cell proliferation. At concentrations of 0.1 and 1 μg/ml, a significant (P < 0.001) reduction in the expression of phosphorylated ERK1 (pERK1) and ERK2 (pERK2) was observed (Fig. 3). By contrast, rhein at the higher concentration (10 μg/ml) significantly (P < 0.001) increased pERK1 and pERK2 expression, resulting in MAP kinase activation (Fig. 3).
Fig 3.
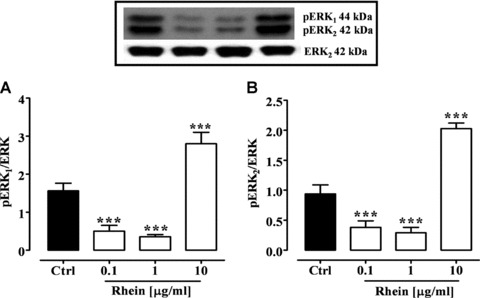
pERK1 (A) and pERK2 (B) expression (MAP-kinase activation) in Caco-2 cells after 24 hrs of rhein (0.1–10 μg/ml) incubation. Insert: representative Western blot analysis. ***P < 0.001 versus control (Ctrl), n= 3, mean ± S.E.M.
Genotoxicity assay
The comet assay is a sensitive and valid method to evaluate the potential genotoxic effect of a substance. Rhein (0.1–10 μg/ml) alone did not significantly affect DNA damage after 24 hrs exposure suggesting the absence of a genotoxic effect. Exposure of Caco-2 cells to hydrogen peroxide (75 μM) produced a significant (P < 0.001) increase in the percentage of DNA in the comet tail, indicating an increase in single-strand breaks (Fig. 4). Pre-treatment Caco-2 cells with rhein for 24 hrs reduced, in a concentration-dependent manner, the H2O2-induced DNA damage. The decrease was significant (P < 0.05) at a rhein concentration of 10 μg/ml (Fig. 4).
Fig 4.
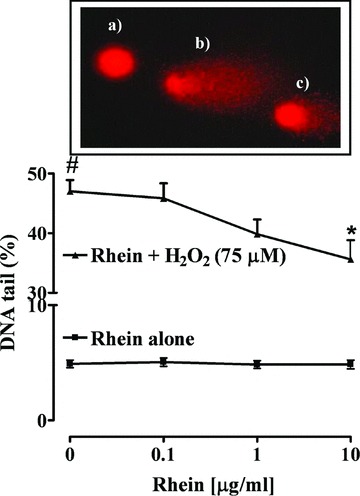
Effect of rhein (0.1–10 μg/ml) on Caco-2 cells exposed (▴) or not (▪) to 75 μM hydrogen peroxide (H2O2). Insert: representative comet images of a control cell (a), a severally damaged cell (b) and a partially genoprotected cell (c). #P < 0.001 versus untreated cells; *P < 0.05 versus H2O2 alone, n= 4, mean ± S.E.M.
MDA and intracellular ROS measurement
Fenton’s reagent, hydrogen peroxide (1 mM) in the presence of iron (II) ions (1 mM), induced an oxidative stress in Caco-2 cells after 3 hrs exposure, resulting in malondialdehyde production (P < 0.01) (Fig. 5). Pre-incubation of Caco-2 cells for 24 hrs with high concentration of rhein (10 μg/ml) significantly (P < 0.001) reduced the increase of H2O2/Fe2+-induced malondialdehyde cytosolic levels (Fig. 5). Rhein at the concentrations of 0.1 and 1 μg/ml was inactive.
Fig 5.
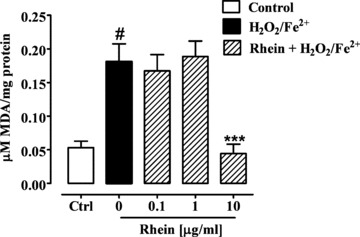
Effect of rhein (0.1–10 μg/ml) on Fenton’s reagent (H2O2/Fe2+ 1 mM)-induced malondialdehyde (MDA) production after 24 hrs exposure in 7 days differentiated Caco-2 cells. #P < 0.01 versus control (Ctrl) and ***P < 0.001 versus H2O2/Fe2+ alone, n= 3, mean ± S.E.M.
The exposure of the Caco-2 cells to H2O2/Fe2+ (2 mM) produced a significant (P < 0.0001) increase in ROS formation (Fig. 6). A pre-treatment for 24 hrs with rhein (10 μg/ml) reduced significantly (P < 0.05) the ROS formation as measured by the inhibition of DCF fluorescence intensity (Fig. 6). Consistent with the results obtained in the MDA assay, rhein at the concentrations of 0.1 and 1 μg/ml was not active (Fig. 6).
Fig 6.
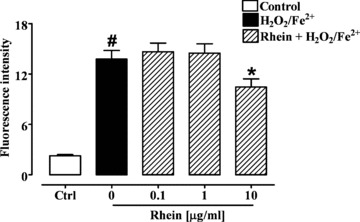
Effect of rhein (0.1–10 μg/ml) on Fenton’s reagent (H2O2/Fe2+ 2 mM)-induced reactive species reagents production after 24 hrs exposure in differentiated Caco-2 cells. #P < 0.001 versus control (Ctrl) and *P < 0.05 versus H2O2/Fe2+ alone, n= 3, mean ± S.E.M.
Discussion
Gastrointestinal tract mucosa shows a rapid cell turnover rate determined by cell proliferation and cell loss (apoptosis) processes [29]. The integrity of the epithelial cell layer is maintained by intercellular junctional complexes composed of tight junctions, adherent junctions and desmosomes. Tight junctions are essential structures for the physiological functions of epithelial and endothelial cells, and have been suggested to have both barrier and defence functions, including the regulation of paracellular pathways. Changes in epithelial cell turnover and alterations of the epithelial monolayer permeability can occur during many gastro-intestinal diseases such as colon cancer. In fact, colon cancer cells exhibit both abnormal cell turnover and impaired tight junction morphology, leading to increase of paracellular permeability [30]. Furthermore, in the latter phases of carcinogenesis, the disruption of cell-cell junctions with concomitant changes in the junctional proteins expression represents a hallmark of cancer cell invasion and metastasis [31].
In the last decade, the use of anthraquinone laxatives, in particular the use of senna, has been associated with a damage of the intestinal epithelial layer and an increased risk to develop colorectal cancer. In this paper, we have demonstrated that rhein, the active metabolite of senna, does not trigger cytotoxic or genotoxic effects in a human colon adenocarcinoma cell line; moreover, rhein shows modulatory effects on cellular proliferation depending on the concentration used and possesses anti-genotoxic activity.
Caco-2 cells, which undergo differentiation in culture, are widely used as a model for colorectal cancer; particularly to assess effects on epithelial function [32, 33], genotoxicity [34, 35] and cell proliferation [36].
Although there are conflicting data, it has been suggested that the mechanism of the laxative effect of anthranoids is strictly correlated to cell damage. Anthranoids laxatives have been proposed to increase: (i) the number of macrophages in the connective tissue of the colonic mucosa, (ii) the intensity of lysosomal activity and (iii) the number of lysosomes in macrophages, Schwann cells and neurones of the sub-mucosal plexus of the colonic mucosa [37, 38]. In addition, recently, it has been reported that rhein in Caco-2 cells produces changes of membrane fluidity either via a reduction of adenosine-5′-triphosphate (ATP) production or via an intercalation into cell membranes [39]. Using the MTT and NR assays, we demonstrated that rhein did not affect the vitality of proliferating Caco-2 cells; moreover, TEER and the fluorescein flow assays showed that rhein did not alter the integrity of membrane barrier function, thus excluding cell damage in the mechanism of rhein laxative effect. Our results are in disagreement with some previous studies; here, we have used rhein concentrations (0.35 and 3.5 μM) corresponding to concentrations reached in the colonic lumen after therapeutic doses of anthranoid laxatives. Previous studies have reported a cytotoxicity effect of rhein on intestinal mucosal cells using very high concentrations (>50 μM) [11, 40].
It is well known that colorectal cancer is promoted by an uncontrolled proliferation of mucosa cells in the terminal portion of intestine. Conflicting results have been reported on the effect of rhein on cell proliferation. Two pre-clinical in vivo studies reported no effect of rhein on intestinal cell proliferation [41, 42] whereas other authors showed a stimulatory effect [40], and an inhibitory effect [43]. In this paper, for the first time we report a modulatory action of rhein on cell proliferation, depending on the concentration used. In fact, our experiments showed that rhein, at the concentrations of 0.1 and 1 μg/ml (rhein concentrations present in the colon after a human therapeutic dosage of senna) reduced the cell proliferation of human colon adenocarcinoma cells; whereas, rhein at the high concentration (10 μg/ml, concentration corresponding to rhein present in the colon after a dosage of senna ten-fold higher then therapeutic dosage) produced a significant increase of the human colon adenocarcinoma cell proliferation. Interestingly, long-term in vivo studies have shown that senna, used at laxative dosage, reduced the appearance of preneoplastic lesions and tumours, whereas a diarrhogenic dose increases the appearance of tumours induced by a carcinogenic agent [44, 45]. Whether or not the effect of rhein is time dependent has been not determined in this study. Also, it would be interesting to investigate in future studies if the effect of rhein involves c-Jun N-terminal kinase (JNK) or related pathways.
Using the normal human fibroblast cells and an epidermoid carcinoma cell line from human skin (A-431), we have demonstrated that the effect of rhein on cell proliferation is tumour and colon specific, respectively.
One of the most important pathway in cell proliferation is represented by the MAP kinases. MAPKs encompass a group of enzymes recruited in response to cellular stress, including heat and osmotic stress, cytokines and UV irradiation [46, 47]. The accepted role of MAPKs is to produce cellular response to those stresses by integrating multiple stimuli and activating transcription factors. In most cell lines, MAPKs have been implicated in regulating mitogenic responses and in the synthesis of stress response proteins [42]. The three major MAPK families have been recognized as p38 MAPK, ERK1 and ERK2 and stress-activated protein kinase (SAPK/JNK). Although the molecular mechanisms inducing MAPKs pathways activation are not totally clear, it is well known that ERK1,2 phosphorylated proteins stimulate the cell growth and inhibit the apoptotic processes [48, 49]. In this study, we observed that rhein at concentrations of 0.1 and 1 μg/ml reduced ERK1 and ERK2 phosphorylated proteins expression. By contrast, rhein at the concentration of 10 μg/ml determined an increase of ERK1 and ERK2 activation. These findings suggest that rhein modulates cell proliferation via an involvement of the MAPK pathway either inhibiting or stimulating the phosphorylation of ERK proteins. Our results are consistent with a recent study showing that rhein modulates cell proliferation of articular chondrocytes by acting on ERK and JNK-AP-1-dependent pathways [50]. Interestingly, other compounds isolated from wild plants, such as Saxifraga stolonifera and rhubarb have been reported to inhibit the proliferation of cancer cells by apoptosis induction or ERK phosphorylation inhibition [51, 52].
Studies in human beings and animals implicate oxidative DNA damage as an important factor in mutagenis and carcinogenesis [53–55]. In our experiments, rhein did not induce DNA damage in Caco-2 cells suggesting that it is not a genotoxic agent. In fact, rhein exerted a protective effect on hydrogen peroxide-induced DNA damage in human colon carcinoma cells at the concentration of 10 μg/ml. This is consistent with a previous report showing a rhein-mediated inhibition of DNA damage induced by Trp-P-1 in human lymphocytes [56].
In order to investigate the mechanism responsible for the genoprotective effect of rhein on H2O2-induced DNA damage, we investigated MDA and ROS cytosolic levels in Caco-2 cells. In accordance with other papers, exposure of Caco-2 cells to hydrogen peroxide and ferrous sulphate (Fe2+) resulted in oxidative damage, assessed as increased cytosolic levels of MDA and ROS [57, 58]. Pre-treatment with rhein significantly reduced the Fenton reaction-induced MDA and ROS formation, suggesting a potential antioxidant mechanism in the anti-genotoxic effects of rhein.
In conclusion, this paper provides in vitro evidence that rhein, the active metabolite of senna, does not possess cancer-inducing potential at concentrations corresponding to human therapeutic doses of senna. At such concentrations, rhein was not cytotoxic in both proliferating and differentiated cells, did not disrupt tight junctions and in fact inhibited colon adenocarcinoma cell proliferation. Such results indicate a lack of tumour-promoting activity. The molecular mechanism of the rhein anti-proliferative effect seems to involve directly the MAP kinase pathway. Furthermore, genotoxicity studies have shown that rhein did not induce DNA damage; by contrast, it was able to protect the DNA against the genotoxic action of hydrogen peroxide, probably because of an anti-oxidant action.
Funding
This work was supported by Enrico ed Enrica Sovena Foundation, PRIN and Regione Campania.
Competing interest
None.
References
- 1.Müller-Lissner SA, Kamm MA, Scarpignato C, et al. Myths and misconceptions about chronic constipation. Am J Gastroenterol. 2005;100:232–42. doi: 10.1111/j.1572-0241.2005.40885.x. [DOI] [PubMed] [Google Scholar]
- 2.Wald A. Pathophysiology, diagnosis and current management of chronic constipation. Nat Clin Pract Gastroenterol Hepatol. 2006;3:90–100. doi: 10.1038/ncpgasthep0406. [DOI] [PubMed] [Google Scholar]
- 3.Capasso F, Gaginella TS. Laxative. A practical guide. Milan: Springer-Verlag; 1997. [Google Scholar]
- 4.Lemli J, Lemmens L. Metabolism of sennosides and rhein in the rat. Pharmacol. 1980;20:50–7. doi: 10.1159/000137398. [DOI] [PubMed] [Google Scholar]
- 5.Capasso F, Gaginella TS, Grandolini G, et al. Phytotherapy: a quick reference to herbal medicine. New York: Springer-Verlag; 2003. [Google Scholar]
- 6.Leng-Peschlow E. Dual effect of orally administered sennosides on large intestine transit and fluid absorption in the rat. J Pharm Pharmacol. 1986;38:606–10. doi: 10.1111/j.2042-7158.1986.tb03089.x. [DOI] [PubMed] [Google Scholar]
- 7.Riecken EO, Zeitz M, Emde C, et al. The effect of an anthraquinone laxative on colonic nerve tissue: a controlled trial in constipated women. Z Gastroenterol. 1990;28:660–4. [PubMed] [Google Scholar]
- 8.Joo JS, Ehrenpreis ED, Gonzalez L, et al. Alterations in colonic anatomy induced by chronic stimulant laxatives: the cathartic colon revisited. J Clin Gastroenterol. 1998;26:283–6. doi: 10.1097/00004836-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Villanacci V, Bassotti G, Cathomas G, et al. Is pseudomelanosis coli a marker of colonic neuropathy in severely constipated patients. Histopathology. 2006;49:132–7. doi: 10.1111/j.1365-2559.2006.02481.x. [DOI] [PubMed] [Google Scholar]
- 10.Van Gorkom BA, Karrenbeld A, Koudstaal J, et al. Effects of sennosides on colonic epithelial cell proliferation and bcl-2 gene expression: possible mechanisms for carcinogenic effects abstract. Gastroenterology. 1996;110:A608. [Google Scholar]
- 11.Van Gorkom BA, Karrenbeld A, Van Der Sluis T, et al. Influence of a highly purified senna extract on colonic epithelium. Digestion. 2000;61:113–20. doi: 10.1159/000007743. [DOI] [PubMed] [Google Scholar]
- 12.Van Gorkom BA, Karrenbeld A, Van Der Sluis T, et al. Apoptosis induction by sennoside laxatives in man; escape from a protective mechanism during chronic sennoside use. J Pathol. 2001;194:493–9. doi: 10.1002/path.914. [DOI] [PubMed] [Google Scholar]
- 13.Kune GA. Laxative use not a risk for colorectal cancer: data from the Melbourne Colorectal Cancer Study. Z Gastroenterol. 1993;31:140–3. [PubMed] [Google Scholar]
- 14.Nusko G, Schneider B, Muller G, et al. Retrospective study on laxative use and melanosis coli as risk factors for colorectal neoplasma. Pharmacology. 1993;47:234–41. doi: 10.1159/000139863. [DOI] [PubMed] [Google Scholar]
- 15.Nusko G, Schneider B, Schneider I, et al. Anthranoid laxative use is not a risk factor for colorectal neoplasia: results of a prospective case control study. Gut. 2000;46:651–5. doi: 10.1136/gut.46.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nascimbeni R, Donato F, Ghirardi M, et al. Constipation, anthranoid laxatives, melanosis coli, and colon cancer: a risk assessment using aberrant crypt foci. Cancer Epidemiol Biomarkers Prev. 2002;11:753–7. [PubMed] [Google Scholar]
- 17.Roberts MC, Millikan RC, Galanko JA, et al. Constipation, laxative use, and colon cancer in a North Carolina population. Am J Gastroenterol. 2003;98:857–64. doi: 10.1111/j.1572-0241.2003.07386.x. [DOI] [PubMed] [Google Scholar]
- 18.Siegers CP, Von Hertzberg-Lottin E, Otte M, et al. Anthranoid laxative abuse-a risk for colorectal cancer. Gut. 1993;34:1099–101. doi: 10.1136/gut.34.8.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel PM, Selby PJ, Deacon J, et al. Anthraquinone laxatives and human cancer: an association in one case. Postgrad Med J. 1989;65:216–7. doi: 10.1136/pgmj.65.762.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleibeuker JH, Cats A, Zwart N, et al. Excessively high cell proliferation in sigmoid colon after an oral purge with anthraquinone glycosides. J Natl Cancer Inst. 1995;87:452–3. doi: 10.1093/jnci/87.6.452. [DOI] [PubMed] [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Repetto G, Del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;3:1125–31. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- 23.Leonard M, Creed E, Brayden D, et al. Evaluation of the Caco-2 monolayer as a model epithelium for ionophoretic transport. Pharm Res. 2002;17:1181–8. doi: 10.1023/a:1026454427621. [DOI] [PubMed] [Google Scholar]
- 24.Leone V, Di Palma A, Ricchi P, et al. PGE2 inhibits apoptosis in human adenocarcinoma Caco-2 cell line through Ras-PI3K association and cAMP- dependent kinase A activation. Am J Physiol Gastrointest Liver Physiol. 2007;293:G673–81. doi: 10.1152/ajpgi.00584.2006. [DOI] [PubMed] [Google Scholar]
- 25.Gill C, Boyd A, McDermott E, et al. Potential anti-cancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int J Cancer. 2005;117:1–7. doi: 10.1002/ijc.21083. [DOI] [PubMed] [Google Scholar]
- 26.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–21. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 27.Yokomizo A, Moriwaki M. Effects of uptake of flavonoids on oxidative stress induced by hydrogen peroxide in human intestinal Caco-2 cells. Biosci Biotechnol Biochem. 2006;70:1317–24. doi: 10.1271/bbb.50604. [DOI] [PubMed] [Google Scholar]
- 28.Commane DM, Shortt CT, Silvi S, et al. Effects of fermentation products of pro- and prebiotics on trans-epithelial electrical resistance in an in vitro model of the colon. Nutr Cancer. 2005;51:102–9. doi: 10.1207/s15327914nc5101_14. [DOI] [PubMed] [Google Scholar]
- 29.Lipkin M. Proliferation and differentiation of normal and diseased gastrointestinal cells. In: Johnson LR, editor. Physiology of the gastrointestinal tract. New York: Raven; 1987. pp. 255–84. [Google Scholar]
- 30.Martinez-Palamo A. Ultrastructural modifications of intercellular junctions between tumor cells. In Vitro. 1970;6:15–20. doi: 10.1007/BF02616130. [DOI] [PubMed] [Google Scholar]
- 31.Dhawan P, Singh AB, Deane NG, et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115:1765–76. doi: 10.1172/JCI24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viswanathan VK, Koutsouris A, Lukic S, et al. Comparative analysis of EspF from enteropathogenic and enterohemorrhagic Escherichia coli in alteration of epithelial barrier function. Infect. Immun. 2004;72:3218–27. doi: 10.1128/IAI.72.6.3218-3227.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morita H, Katsuno T, Hoshimoto A, et al. Connexin 26-mediated gap junctional intercellular communication suppresses paracellular permeability of human intetsinal epithelial cell monolayers. Exp Cell Res. 2004;1:1–8. doi: 10.1016/j.yexcr.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 34.Fässler C, Gill CI, Arrigoni E, et al. Fermentation of resistant starches: influence of in vitro models on colon carcinogenesis. Nutr Cancer. 2007;58:85–92. doi: 10.1080/01635580701308232. [DOI] [PubMed] [Google Scholar]
- 35.Bellion P, Hofmann T, Pool-Zobel BL, et al. Antioxidant effectiveness of phenolic apple juice extracts and their gut fermentation products in the human colon carcinoma cell line caco-2. J Agric Food Chem. 2008;56:6310–7. doi: 10.1021/jf8005068. [DOI] [PubMed] [Google Scholar]
- 36.Renis M, Calandra L, Scifo C, et al. Response of cell cycle/stress-related protein expression and DNA damage upon treatment of CaCo2 cells with anthocyanins. Br J Nutr. 2008;100:27–35. doi: 10.1017/S0007114507876239. [DOI] [PubMed] [Google Scholar]
- 37.Steer HW, Colin-Jones DG. Melanosis coli: studies of the toxic effects of irritant purgatives. J Pathol. 1975;115:199–205. doi: 10.1002/path.1711150403. [DOI] [PubMed] [Google Scholar]
- 38.Yagi T, Miyawaki Y, Yamauchi K, et al. Involvement of prostaglandin E-like material in the propulsive action oh rhein anthrone, the intraluminal active metabolite of Sennosides A and B in mice. J Pharm Pharmacol. 1988;40:27–30. doi: 10.1111/j.2042-7158.1988.tb05144.x. [DOI] [PubMed] [Google Scholar]
- 39.Laitinen L, Takala E, Vuorela H, et al. Anthranoid laxatives influence the absorption of poorly permeable drugs in human intestinal cell culture model (Caco-2) Eur J Pharm Biopharm. 2007;66:135–45. doi: 10.1016/j.ejpb.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Schörkhuber M, Richter M, Dutter A, et al. Effect of anthraquinone-laxatives on the proliferation and urokinase secretion of normal, premalignant and malignant colonic epithelial cells. Eur J Cancer. 1998;34:1091–8. doi: 10.1016/s0959-8049(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 41.Geboes K, Nijs G, Mengs U, et al. Effects of ‘contact laxatives’ on intestinal and colonic epithelial cell proliferation. Pharmacology. 1993;47:187–95. doi: 10.1159/000139858. [DOI] [PubMed] [Google Scholar]
- 42.Geboes K. Laxatives and intestinal epithelial cells: a morphological study of epithelial cell damage and proliferation. Verh K Acad Geneeskd Belg. 1995;57:51–74. [PubMed] [Google Scholar]
- 43.Cichewicz RH, Seeram NP, Zhang Y, et al. Anti-tumor effects of novel anthraquinones from daylilies against human cancer cell lines. Life Sci. 2004;74:1791–9. doi: 10.1016/j.lfs.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 44.Mascolo N, Mereto E, Borrelli F, et al. Does senna extract promote growth of aberrant crypt foci and malignant tumors in rat colon. Dig Dis Sci. 1999;44:2226–30. doi: 10.1023/a:1026696402212. [DOI] [PubMed] [Google Scholar]
- 45.Borrelli F, Capasso R, Aviello G, et al. Senna and the formation of aberrant crypt foci and tumors in rats treated with azoxymethane. Phytomed. 2005;12:501–5. doi: 10.1016/j.phymed.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Pearson G, Robinson F, Beers Gibson T, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 47.Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–65. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- 48.Chambard JC, Lefloch R, Pouysségur J, et al. ERK implication in cell cycle regulation. Biochim Biophys Acta. 2007;1773:1299–310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 49.Meloche S, Pouysségur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–39. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 50.Legendre F, Bogdanowicz P, Martin G, et al. Rhein, a diacerhein-derived metabolite, modulates the expression of matrix degrading enzymes and the cell proliferation of articular chondrocytes by inhibiting ERK and JNK-AP-1 dependent pathways. Clin Exp Rheumatol. 2007;25:546–55. [PubMed] [Google Scholar]
- 51.Chen Z, Liu YM, Yang S, et al. Studies on the chemical constituents and anticancer activity of Saxifraga stolonifera (L) Meeb. Bioorg Med Chem. 2008;16:1337–44. doi: 10.1016/j.bmc.2007.10.072. [DOI] [PubMed] [Google Scholar]
- 52.Zhou X, Song B, Jin L, Hu D, Diao C, Xu G, Zou Z, Yang S. Isolation and inhibitory activity against ERK phosphorylation of hydroxyanthraquinones from rhubarb. Bioorg Med Chem Lett. 2006;16:563–8. doi: 10.1016/j.bmcl.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 53.Loft S, Poulsen HE. Cancer risk and oxidative DNA damage in man. J Mol Med. 1996;74:297–312. doi: 10.1007/BF00207507. [DOI] [PubMed] [Google Scholar]
- 54.Wargovich MJ, Goldberg MT, Newmarck HL, et al. Nuclear aberrations as a short-term test for genotoxicity to the colon: evaluation of nineteen agents in mice. J Natl Cancer Inst. 1983;71:133–7. [PubMed] [Google Scholar]
- 55.Ronen A, Heddle JA. Site-specific induction of nuclear anomalies (apoptotic bodies and micronuclei) by carcinogens in mice. Cancer Res. 1984;44:1536–40. [PubMed] [Google Scholar]
- 56.Wu CH, Yen GC. Antigenotoxic properties of Cassia tea (Cassia tora L.): mechanism of action and the influence of roasting process. Life Sci. 2004;76:85–101. doi: 10.1016/j.lfs.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 57.Nakabeppu Y, Sakumi K, Sakamoto K, et al. Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids. Biol Chem. 2006;387:373–9. doi: 10.1515/BC.2006.050. [DOI] [PubMed] [Google Scholar]
- 58.Karihtala P, Soini Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. APMIS. 2007;115:81–103. doi: 10.1111/j.1600-0463.2007.apm_514.x. [DOI] [PubMed] [Google Scholar]


