Abstract
Recently, it was demonstrated that arteriogenesis is enhanced in mice deficient in regulatory T cells (CD4+CD25+FoxP3+ T cell), which can suppress effector T cell responses. The present study investigates the effects of these regulatory T cells on arteriogenesis in more detail by either specific expanding or depleting regulatory T cells. Hind limb ischemia was induced by electro-coagulation of the femoral artery in mice. Regulatory T cells were either expanded by injecting mice with a complex of interleukin (IL)-2 with the IL-2 monoclonal antibody JES6–1, or depleted by anti-CD25 antibody or diphtheria toxin injections in DEREG mice (depletion of regulatory T cells). Blood flow restoration was monitored using laser Doppler perfusion imaging. Collateral arteries were visualized by immunohistochemistry. Regulatory T cell expansion led to a moderate though significant suppression of blood flow restoration after ischemia induction. Surprisingly, depletion of regulatory T cells resulted in minor increase on blood flow recovery. However, collateral and capillary densities in the post-ischemic skeletal muscle were significantly increased in DEREG mice depleted for regulatory T cells. The presence of regulatory T cells after ischemia induction when analysed in non-depleted DEREG mice could be demonstrated by green fluorescent protein staining only in lymph nodes in the ischemic area, and not in the ischemic muscle tissue. The current study demonstrates that, even under conditions of major changes in regulatory T cell content, the contribution of regulatory T cells to the regulation of the arteriogenic response is only moderate.
Keywords: regulatory T cells, arteriogenesis, neovascularization, hind limb ischemia
Introduction
Numerous studies have implicated that the immune system plays a major role in arteriogenesis [1, 2]. A specific cell of the immune system, the regulatory T cell (CD4+CD25+FoxP3+ T cell), is specialized in the suppression of effector T-cell pathogenic immune responses. Regulatory T cells control T-cell homeostasis [3, 4] and are indispensable for balancing immune responses [5, 6]. Accumulating evidence suggests an important role of regulatory T cells in the control of atherosclerotic lesion development and progression [7–9]. Recently, Zouggari et al.[10] assessed for the first time the role of regulatory T cells on blood flow recovery after ischemia induction in mouse models with a reduced regulatory T cell number and function [8, 10–13]. They reported a key role of regulatory T cells in post-ischemic neovascularization. Post-ischemic blood flow recovery was significantly enhanced in these mice up to 70% 21 days after ischemia induction as compared to slow recovery in control mice [10]. Because the mouse models used for depletion of regulatory T cells may also show effect on other T cell subtypes besides the regulatory T cell [14], a more detailed and specific analysis of the role of the regulatory T cell in arteriogenesis is required. The question remains how regulatory T cells regulate arteriogenesis and moreover whether regulatory T cell expansion has effects on arteriogenesis. The effect of expanding regulatory T cell number on arteriogenesis can be studied using the recently described method for in vivo expansion of regulatory T cells by Webster et al.[15] Injecting mice with a complex of interleukin (IL)-2 mixed with JES6–1 IL-2 monoclonal antibody (mAB) led to a marked increase in regulatory T cell number. This approach caused selective expansion of regulatory T cells with little or no change in other cells, making it superior to other attempts [10, 15] to increase regulatory T cells [15].
Next to expansion of regulatory T cell number, depletion of regulatory T cells is highly informative. The standard approach is depletion of CD25+cells. But this approach has its shortcomings because anti-CD25 treatment does not result in a selectively depletion of regulatory T cells only [7, 16], but also affects activated T cells [17], dendritic cells (DCs) [18] and natural killer (NK) cells [19]. Recently, a more powerful approach to fully deplete regulatory T cells was described by Lahl et al.[20]. They generated DEREG mice (depletion of regulatory T cells), expressing a diphtheria toxin (DT) receptor/green fluorescent protein (GFP) fusion protein under the control of the FoxP3 gene locus. DT injections in DEREG mice allowed for highly specific and up to 95–98% depletion of FoxP3+ regulatory T cells at any desired time-point without disease development [20].
In the present study, the contribution of regulatory T cells in the arteriogenic response was studied by major modulations in regulatory T cell number in vivo. Regulatory T cell numbers in mice were either increased by injecting mice with IL-2 mixed with an IL-2 mAB or decreased by anti-CD25 antibody or DT injections in DEREG mice. Furthermore, using the GFP-labelled regulatory T cells in DEREG mice, the presence of regulatory T cells in the ischemic muscle tissue will be analysed in detail.
Material and methods
Experimental animals
Ten-week-old C57Bl6 mice (The Jackson Laboratory, Bar Harbor, ME, USA) were used (10 mice per group). Furthermore, DEREG mice (Leiden University Medical Center, Leiden, The Netherlands), aged 9–16 weeks, were used. Experiments were approved by the committee on animal welfare of our institute.
Modulation of regulatory T cell levels
Regulatory T cell expansion
To increase regulatory T-cell number, C57Bl6 mice were injected with1 μg IL-2 (PeproTech, Inc., Rocky Hill, NJ, USA) mixed with 5 μg IL-2 mAB (clone JES6–1A12; R&D Systems, Minneapolis, MN, USA) [15]. Mice were injected at days 3, 2 and 1 before ischemia induction and two times a week after the surgical procedure. Expansion of regulatory T cell level was monitored by fluorescence-activated cell sorting (FACS) (mouse regulatory T-cell staining kit; eBioscience Inc., San Diego, CA, USA).
Regulatory T-cell depletion
Depletion of regulatory T cells is accomplished by treating C57Bl6 mice with CD25-depleting PC61 antibody [21]. A total of 100 μg per mice was injected at day 1 before ischemia induction and repeated once per week after surgery.
DEREG mice express a DT receptor/GFP fusion protein under control of the FoxP3 gene locus, leading to selective depletion of FoxP3+ regulatory T cells after injection of 1 μg DT per mouse [22]. Depletion of regulatory T cells was monitored by FACS analyses (mouse regulatory T cell staining kit; eBioscience).
Surgical procedure
In the regulatory T-cell expansion experiments, blood flow was expected to be suppressed. In order to distillate an attenuated blood flow recovery compared to well-known fast recovery of control mice, single electro-coagulation of the femoral artery was performed. Mice depleted for regulatory T cells were expected to have accelerated blood flow recovery compared to controls that are well-known good responders. Therefore, in this situation, double electro-coagulation of both the femoral artery and iliac artery was performed because the double electro-coagulation resulted in a larger therapeutic window [23]. This enables a better analysis of enhancing effects on arteriogenesis.
Before surgery, mice were anesthetized with an intraperitoneal injection of a combination of Midazolam (5 mg/kg; Roche Diagnostics, Almere, The Netherlands), Medetomidine (0.5 mg/kg; Orion Corporation, Espoo, Finland) and Fentanyl (0.05 mg/kg; Janssen Biologics B.V., Leiden, The Netherlands). Unilateral hind limb ischemia was performed as described before [23]. After surgery the skin was closed with 6–0 Ethilon sutures.
Laser Doppler perfusion imaging (LDPI)
Blood flow was measured before ischemia induction, immediately after ischemia induction and 3, 7, 14, 21 and 28 days after surgery in the ischemic and non-ischemic paws, using LDPI (Moor Instruments, Millwey Axminster, Devon, UK). LDPI is performed with a beam from a 2 mW helium laser that sequentially scans tissue surface to a depth of a few hundred micrometres. The penetration depth of the laser beam thus allows measurements of superficial skin perfusion only. Therefore, perfusion in both paws (from the ankle to the base of toes) is obtained at baseline and serially over 4 weeks after hind limb ischemia induction. Paw perfusion functions as a resultant of limb perfusion recovery. Furthermore, to avoid measuring increased perfusion near the wound, it is preferable to exclusively select the paws, more distally, as the region of interest for analyses. Each animal served as its own control. Perfusion was expressed as a ratio of the ischemic to non-ischemic limb, as described previously [23].
Histological analysis
Animals were killed 14 and 28 days after ischemia induction and calf and adductor muscles (from ischemic and non-ischemic paw) were removed and fixed with 4% formaldehyde and paraffin embedded. Serial 5 μm cross-sections were generated. Sections were re-hydrated and endogenous peroxidase activity was blocked for 20 min. in methanol containing 0.3% hydrogen peroxide. Capillaries and collaterals were visualized using antibodies recognizing CD31 on endothelial cells or α-smooth muscle actin (SMA) in smooth muscle cells, respectively.
For CD31 labelling, sections were pre-incubated with trypsin (30 min./37°C), incubated overnight with primary antibody (rat antimouse CD31Ab, dilution 1:200; BD Biosciences B.V., Breda, the Netherlands) followed by a biotin-conjugated secondary antibody (goat anti-rat, dilution 1:300; AbCam, Cambridge, UK). The reaction was enhanced by tyramine amplification and the avidin–biotin–horseradish–peroxidase system (DakoCytomation, Glostrup, Denmark) and visualized by NovaRED (Vector Laboratories Inc., Burlingame, CA, USA).
For SMA labelling, tissue sections were incubated overnight with anti-αSMA (mouse anti-human, dilution 1:800; DAKO, Glostrup, Denmark) without antigen retrieval. Labelling was followed by an HRP-conjugated secondary antibody (rabbit antimouse, dilution 1:300; DAKO).
For tracing of GFP+ regulatory T cells in DEREG mice, adductor muscle slides were incubated with anti-GFP (rabbit antimouse, dilution 1:4000; Invitrogen, Breda, the Netherlands) without antigen retrieval. After overnight incubation, labelling was followed by a biotin-conjugated secondary antibody (donkey anti-rabbit, dilution 1:300). As a positive control, a slide of GFP+ cardiac muscle tissue was used.
All sections were counterstained with haematoxylin. Isotype control antibodies were used as controls. Quantification of labelled tissue sections was performed with ImageJ (nine sections per mouse were analysed to obtain the mean per animal, 10 animals per group were measured).
Flow cytometry
Cells were labelled with fluorescein isothiocyanate (FITC) anti-CD4 (eBioscience) and Allophycocyanin (APC) anti-CD25. An intracellular FoxP3 staining was performed with PE anti-FoxP3 (eBioscience). Regulatory T cell labelling was analysed by flow cytometry on a LSRII FACS apparatus (Becton Dickinson B.V., Breda, the Netherlands) and analysed with BD FACS DIVA software.
In vitro suppression assay
Peripheral lymph nodes were removed from three DEREG mice after repetitive DT injections during 3 weeks and in three DEREG mice without DT injections. The functional suppression assay was performed as described by Rausch et al.[24].
Statistical analysis
Results are expressed as mean ± S.E.M. Comparisons between means were performed with an independent t-test. P-values <0.05 were considered statistically significant. All calculations were performed in SPSS 16.0.
Results
Expansion of regulatory T cells results in impaired post-ischemic blood flow recovery
The effect of expanding regulatory T cells on arteriogenesis in mice is unknown. Regulatory T cell numbers were expanded in the hind limb ischemia mouse model by injecting mice with IL-2 mixed with an IL-2 mAB. Injections of IL-2-mAB complex led to a 2-fold expansion of regulatory T cells in the peripheral blood (Fig. 1A). Mice treated with injections of IL-2-mAB complex (n= 10) showed significant attenuated blood flow recovery up to 20% at days 7 and 14 after ischemia compared to controls (n= 10), indicating that an increase in regulatory T cell number was responsible for a suppression in blood flow recovery after hind limb ischemia (Fig. 1B). Furthermore, mice treated with IL-2-mAB complex showed a significant lower number of α-SMA-expressing collaterals in the post-ischemic adductor muscle as compared to controls (Fig. 1C).
Fig 1.
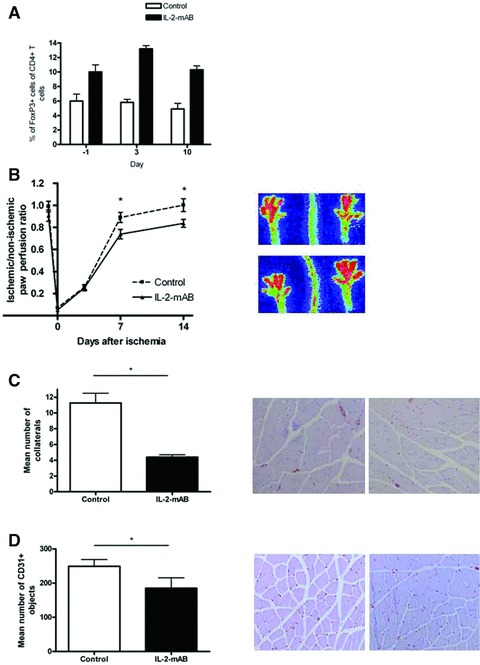
(A) Quantitative flow cytometry analysis of the percentage of CD4+CD25+FoxP3+ regulatory T cells among CD4+ cells in blood of C57Bl6 mice treated with and without IL-2-mAB complex 24 hrs before ischemia induction, 3 and 10 days after the surgical procedure. (B) LDPI graph depicts the mean ± S.E.M. of blood flow recovery in hind limb of C57Bl6 mice injected with phosphate-buffered saline (PBS; n= 10) or IL-2-mAB complex (n= 10) treatment. Blood flow was expressed as ratio between the operated and non-operated limb measured before and directly after surgery and at days 3, 7 and 14. *P < 0.05. Images on the right depict representative LDPI images of paws of mice injected with PBS (upper) or IL-2-mAB complex (lower) at 14 days after induction of hind limb ischemia. (C) Histograms depict mean ± S.E.M. of the number of collaterals in the post-ischemic adductor muscle as quantified by SMA labelling (nine sections per muscle were analysed in five animals/ treatment group). *P < 0.05. Representative photographs of SMA labelling are shown 14 days after hind limb ischemia induction in mice treated with PBS (left) and IL-2 mAB (right). Magnification: 10×. (D) The histogram depicts mean ± S.E.M. of the number of capillaries in the post-ischemic calf muscle as quantified by antimouse-specific CD31 labelling (nine sections per muscle were analysed in five animals/ treatment group). *P < 0.05. Representative photographs of CD31 labelling are shown 14 days after hind limb ischemia induction in mice treated with PBS (left) and IL2 mAB complex (right). Magnification: 10×.
Decline of regulatory T cells using anti-CD25 did not show any effect on blood flow recovery
Next to expansion of regulatory T cells, we were interested in the effects of regulatory T cell depletion too. Originally, regulatory T cells were identified by the expression of CD25 and numerous in vivo experiments studying regulatory T cells have been performed with depleting antibodies directed against CD25 [25, 26]. No effect of anti-CD25 antibody treatment (n= 10) on blood flow recovery was observed as compared to controls (n= 10) after single electro-coagulation of the artery (Fig. 2A). The moderate effects of the depletion on arteriogenesis could be obscured by the rapid response of these mice. Although the therapeutic window was increased after double electro-coagulation of both the femoral artery and iliac artery, CD25 depleting antibody-injections (n= 10) did not result in any alteration of blood flow recovery as compared to controls (n= 10) (Fig. 2B). FACS analyses showed 87% depletion of regulatory T cells directly after the surgical procedure. However, regulatory T cell levels returned to half of normal levels 1 week after ischemia, but at that moment, again anti-CD25 was injected into these mice (Fig. 2C). Thus with anti-CD25 antibody treatment, a minimal CD25 level was present all the time. So, a better approach to deplete regulatory T cells was needed.
Fig 2.
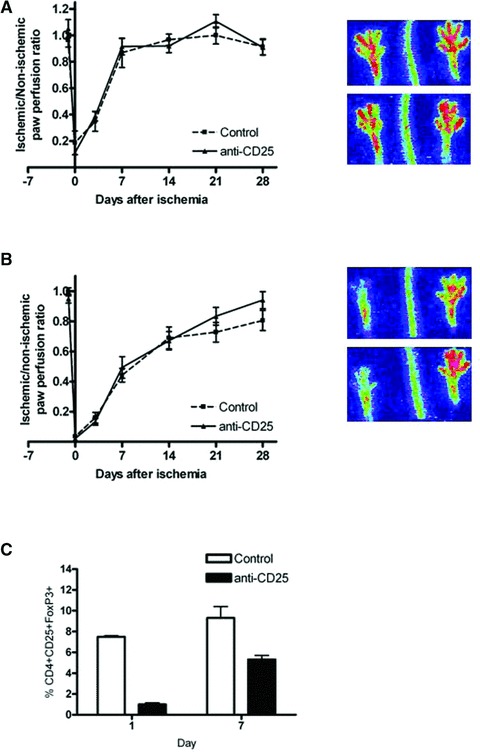
(A) The LDPI graph depicts the mean ± S.E.M. of blood flow recovery after single electro-coagulation of the femoral artery in hind limb of C57Bl6 mice injected with isotype control (n= 10) or anti-CD25 antibody (n= 10). Blood flow was expressed as ratio between the operated and non-operated limb measured before and directly after surgery and at days 3, 7, 14, 21 and 28. Images on the right depict representative LDPI images of paws of mice injected with isotype control (upper) or anti-CD25 antibody (lower) 7 days after induction of hind limb ischemia. (B) The LDPI graph depicts the mean ± S.E.M. of blood flow recovery after double electro-coagulation of the femoral artery and iliac artery in hind limb of C57Bl6 mice injected with isotype control (n= 10) or anti-CD25 antibody (n= 10). Blood flow was expressed as ratio between the operated and non-operated limb measured before and directly after surgery and at days 3, 7, 14, 21 and 28. Images on the right depict representative LDPI images of paws of mice injected with isotype control (upper) or anti-CD25 antibody (lower) 7 days after induction of hind limb ischemia. (C) Quantitative flow cytometry analysis of the percentage of CD4+CD25+FoxP3+ regulatory T cells among CD4+ cells in blood of C57Bl6 mice treated with isotype control or anti-CD25 antibody 24 hrs and 7 days after hind limb ischemia induction.
Selective depletion of regulatory T cells led to moderate effects on arteriogenesis
Depletion of FoxP3 ± T cells in DEREG mice
DEREG mice allowed depletion of FoxP3+ regulatory T cells upon DT injections. Because two consecutive DT injections led to almost complete [20], but short (<6 days) depletion of regulatory T cells (Fig. 3A), DEREG mice were treated with repetitive DT injections. FACS analyses showed up to 98% depletion of FoxP3+ regulatory T cells for 15 days after repetitive injections with DT (Fig. 3B). Analyses based on the GFP expression of regulatory T cells in DEREG mice, showed outgrowth of GFP– FoxP3 regulatory T cells within week 2 (Fig. 3B) as also was described before [24]. However, these GFP– FoxP3+ cells were non-functional regulatory T cells. To demonstrate this also in the current study, an in vitro suppression assay was performed after 3 weeks of repetitive DT injections in DEREG mice as a functional analysis of these GFP– FoxP3 regulatory T cells. After isolation of the cells from the lymph nodes they were incubated with effector T cells and the effect on effector T cell proliferation was monitored. The GFP– FoxP3+ cells had a marked reduced inhibitory effect on T cell proliferation (Fig. 3C).
Fig 3.

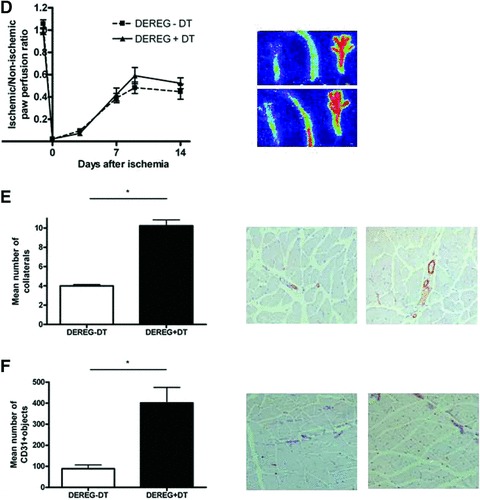
(A) Quantitative flow cytometry analysis of the percentage of CD4+CD25+FoxP3+ regulatory T cells among CD4+ cells in blood of DEREG mice treated without DT injections or DEREG mice with two DT injections at days 2 and 1 before hind limb ischemia induction. (B) Quantitative flow cytometry analysis of the percentage of GFP+CD4+CD25+FoxP3+ regulatory T cells (left) and GFP–CD4+CD25+FoxP3+ regulatory T cells among CD4+ cells in blood during 2 weeks of DEREG mice treated without DT injections, DEREG mice treated with two DT injections (days 2 and 1) and DEREG mice treated with repeated DT injections (4 times a week). (C) In vitro suppression assay after 3 weeks of repetitive DT injections in DEREG mice. After isolation of the regulatory T cells from the lymph nodes they were incubated with effector T cells and the effect on effector T cell proliferation was monitored. Proliferation of effector T cells was significant enhanced in DEREG mice with DT injections indicating that GFP– FoxP3+ cells had a strongly reduced inhibitory effect on T cell proliferation. *P < 0.05. (D) The LDPI graph depicts the mean ± S.E.M. of blood flow recovery after double electro-coagulation of the femoral artery and iliac artery in hind limb of DEREG mice injected without (n= 22) and with repetitive DT injections (n= 20). Blood flow was expressed as ratio between the operated and non-operated limb measured before and directly after surgery and at days 3, 7, 9 and 14. Images on the right depict representative LDPI images of paws of DEREG mice injected without DT (upper) or with DT (lower) 9 days after induction of hind limb ischemia. (E) Histograms depict mean ± S.E.M. of the number of collaterals in the post-ischemic adductor muscle as quantified by SMA labelling (nine sections per muscle were analysed in five animals/ treatment group). *P < 0.05. Representative photographs of SMA labelling are shown 14 days after hind limb ischemia induction in DEREG mice without DT injections (left) and DEREG mice with repetitive DT injections (right). Magnification: 10×. (F) The histogram depicts mean ± S.E.M. of the number of capillaries in the post-ischemic calf muscle as quantified by antimouse-specific CD31 labelling (nine sections per muscle were analysed in five animals/ treatment group). *P < 0.05. Representative photographs of CD31 labelling are shown 14 days after hind limb ischemia induction in DEREG mice without DT injections (left) and DEREG mice with repetitive DT injections (right). Magnification: 10×.
Post-ischemic neovascularization in DEREG mice
Regulatory T cell-depleted DEREG mice (n= 22) showed moderate improvement of blood flow recovery after ischemia induction as compared to control mice (n= 20) (Fig. 3D). Nine days after ischemia induction, DEREG mice depleted for regulatory T cells showed 23% improvement of blood flow recovery. However, probably due to high variance, this was not statistically significant. Repetitive DT injections did not have any effect on post-ischemic flow recovery, because flow recovery between C57Bl6 mice with (n= 10) and without DT injections (n= 10) showed no differences (Fig. S1).
On the tissue level, regulatory T-cell-depleted DEREG mice showed a significant increase of SMA-expressing collateral density in the post-ischemic adductor muscle (Fig. 3E). Furthermore, these mice showed a significantly increase of the CD31+ capillary density of the post-ischemic calf muscles as compared to controls (Fig. 3F). Selective and full depletion of regulatory T cells led to significant increases in capillary and collateral artery formation in the skeletal muscle tissue, but minor improvements of post-ischemic blood flow recovery.
Distribution of regulatory T cells to ischemic area
The presence of regulatory T cells in the ischemic hind limb was assessed in the ischemic adductor muscle and draining lymph nodes after 3, 7 and 14 days after surgery. Non-depleted DEREG mice had GFP+ regulatory T cells, which we traced using immunohistochemistry and flow cytometry analyses. Regulatory T cells were not observed in the skeletal muscle tissue at any time-point examined with immunohistochemical stainings (Fig. 4A). Detailed views of vessel structures in the ischemic adductor muscle did not show any regulatory T cells near collaterals. Moreover, regulatory T cells were present in lymph nodes in the ischemic hind limb. Compared to lymph nodes of the non-operated hind limb, significant more regulatory T cells were present in the lymph nodes of the ischemic paw (Fig. 4B).
Fig 4.
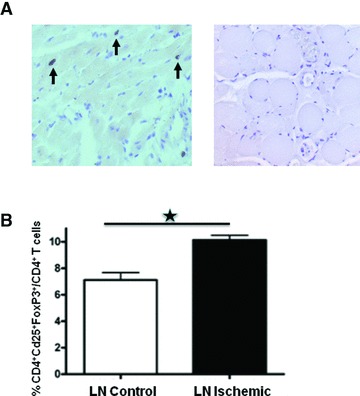
(A) Representative photographs of anti-GFP muscle staining of positive control slide of heart muscle tissue with GFP+ cells (left) and adductor muscle of a non-depleted DEREG mice 7 days after hind limb ischemia induction (right). Arrows indicate GFP+ cells. (B) Quantitative flow cytometry analysis of the percentage of CD4+CD25+FoxP3+ regulatory T cells among CD4+ cells in lymph nodes of ischemic (black box) versus non-ischemic (white box) hind limb 14 days after hind limb ischemia induction. *P < 0.05.
Discussion
In the present study, the contribution of regulatory T cells to the arteriogenic response was studied by analysing the effects of modulation of regulatory T cell levels on arteriogenesis in a mouse hind limb ischemia model. Major changes in regulatory T-cell number resulted in only moderate effects on post-ischemic neovascularization, in contrast to what was reported previously [10]. For modulation of regulatory T cell content, more specific approaches were used for a major expansion or complete depletion of regulatory T cells.
Expansion of regulatory T cells was induced by injecting mice with IL-2 mixed with the JES6–1 IL-2 mAB [15]. Unlike most IL-2 mABs, injections of this IL-2-mAB complex led to selective proliferation of regulatory T cells with little or no change in other inflammatory cells making the IL-2-mAB complex superior to other interventions [15]. In the present study, expansion of regulatory T cells resulted in an attenuated blood flow recovery after ischemia induction. Up to 20% suppression of blood flow at day 14 after ischemia induction was observed. These results are in contrast to the major effects of application of this IL-2-mAB complex in various autoimmune disease mouse models, which resulted in the expansion of regulatory T cells in mice leading to a strong resistance to T cell mediated autoimmune disease [15].
In addition to regulatory T cell expansion, we studied the effects of depletion of regulatory T cells on arteriogenesis. Depletion of CD25+ cells with injections of anti-CD25 antibody did not result in any alteration of post-ischemic blood flow recovery as compared to controls. Zouggari et al.[10] did report significant improved blood flow recovery with the same PC61 CD25 depleting antibody 7 and 14 days after ischemia induction. However, in their control mice a 50% flow recovery after 7 days occurred after a single ligature placement of the femoral artery, whereas our control mice show 90–100% recovery after single electro-coagulation of the femoral artery at that time. Differences in the surgical procedure and differences in anaesthesia may explain the observed differences. In the present study, mice were anesthetized with an intraperitoneal injection of a combination of Midazolam, Medetomidine and Fentanyl, whereas Zouggari et al.[10] anesthetized by isoflurane inhalation, which can easily influence vascular tone and thus paw perfusion results. The final proof for the involvement of regulatory T cells in arteriogenesis has not been provided with this approach because CD25 is not exclusively expressed on regulatory T cells, but also on activated T cells [17], NK cells [19] and myeloid DCs [18]. This is of special interest because CD4+ T cells [27, 28], CD8+ T cells [29] as well as NK cells [27] are known to modulate the arteriogenic response. Along with the fact that there is a CD25– regulatory T cell population [30, 31], a fast return of CD25+ cells and reports about the anti-CD25 antibody shedding the epitope rather than depleting these cells [16], the results of studies using this antibody should be interpreted with caution.
Thus, a new approach to fully deplete specifically regulatory T cells within mice is crucial to bypass imperfections of anti-CD25 antibody treatment. A relatively new mouse model, achieving the ablation of cells by genetic introduction of primate DT receptor targeted to specific tissue by the use of a specific promoter, was described in 2001 [32]. Cells of these mice are 103–105 times more susceptible to DT induced cell death due to their low-affinity DT receptor. Lahl et al.[22] generated DEREG mice, which allowed direct depletion of FoxP3+ cells up to 98% upon DT injections. Importantly, this depletion is not sufficient enough for disease development, as in contrast to FoxP3 knockout mice. FoxP3 knockout mice succumb at young age to lymphoproliverative disease characterized by multiorgan lymphocytic infiltration [33]. The present data showed full depletion of regulatory T cells in DEREG mice, but only moderate effects on neovascularization. Blood flow improved 23%, 9 days after ischemia in regulatory T cell-depleted DEREG mice as compared to control DEREG mice, but this difference was not statistically significant. In addition to LDPI monitoring, immunohistochemical analysis was used to examine neovascularization at tissue level. In line with LDPI data, anti-SMA and anti-CD31 staining of post-ischemic muscles showed an increase in collateral and capillary density in regulatory T cell-depleted DEREG mice. Zouggari et al.[10] observed significant improved blood flow recovery up to 70% in CD28–/– mice and B7–1/2–/– mice, which had a reduced regulatory T cell number and function [8, 10–13]. However, CD28 is the major B7-binding costimulatory receptor on T cells and is critical for T cell responses in vivo[14]. CD4+ T-cell and CD8+ T cell responses seemed to be disturbed in these mice too. The interpretation that the observed effects in CD28–/– mice and B7–1/2–/– mice are due to regulatory T cell modulation should be challenged.
To disentangle the mechanism of how regulatory T cells influence the arteriogenic response, the presence of regulatory T cells in the ischemic tissue was studied. No GFP+ regulatory T cells could be detected in post-ischemic adductor muscle in non-depleted DEREG mice with anti-GFP immunohistochemical staining 3, 7 and 14 days after surgery. The absence of regulatory T cells in the ischemic tissue can be related to the only moderate effects of regulatory T cells in arteriogenesis, especially because regulatory T cells are present in the lymph nodes in the ischemic hind limb area. These results suggest that regulatory T cells contribute to the arteriogenic response not directly by local induction of collateral artery formation, but rather in a paracrine manner by affecting other inflammatory cells in the ischemic muscle tissue from a distance.
An interesting observation in the current study is that even full depletion of regulatory T cells showed only moderate effects on arteriogenesis. This is in contrast to other studies on vascular remodelling as in atherosclerosis. There, the involvement of regulatory T cells in atherosclerotic lesion development was convincingly demonstrated using only selective depletion. Ait-Oufella et al.[8] reported significant effects on atherosclerosis already with minor modulations in regulatory T cell content. Moreover, vaccination of mice with FoxP3 transfected DCs resulted in a 27–30% decrease in regulatory T cells in blood and organs [7]. Although depletion is limited and far from complete, mice vaccinated against FoxP3 showed a significant 34% increase in plaque size as compared to controls [7].
In conclusion, the present study demonstrates that regulatory T cells do contribute to the regulation of post-ischemic neovascularization. However, only moderate effects on post-ischemic neovasculzarization were observed after major modulations of regulatory T cell number.
Acknowledgments
The authors thank H.A.B. Peters for technical assistance and A.C. Foks and J. Kuiper for helping with the expansion of regulatory T cells with IL-2-mAB complex. The authors gratefully acknowledge the financial support of the Translational Excellence in Regenerative Medicine (TeRM) Smart Mix Program of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture and Science.
Conflict of interest
The authors declare that they have no competing financial interests.
Supporting Information
(A) The LDPI graph depicts the mean± S.E.M. of blood flow recovery after doubleelectro-coagulation of the femoral artery and iliac artery in hindlimb of C57Bl6 mice injected without (n = 10) and withrepetitive DT injections (n = 10). Blood flow was expressed as ratio between the operated and non-operated limb measured before and directly after surgery and at days 3, 7, 9 and 14. Images on the right depict representative LDPI images of paws of C57Bl6 mice injected without DT (upper) or with DT (lower) 7 days after induction of hind limb ischemia.
References
- 1.van Weel V, van Tongeren RB, van Hinsbergh VW, et al. Vascular growth in ischemic limbs: a review of mechanisms and possible therapeutic stimulation. Ann Vasc Surg. 2008;22:582–97. doi: 10.1016/j.avsg.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 2.van Oostrom MC, van Oostrom O, Quax PH, et al. Insights into mechanisms behind arteriogenesis: what does the future hold. J Leukoc Biol. 2008;84:1379–91. doi: 10.1189/jlb.0508281. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 4.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–44. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 6.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–60. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 7.van Es T, van Puijvelde GH, Foks AC, et al. Vaccination against Foxp3(+) regulatory T cells aggravates atherosclerosis. Atherosclerosis. 2010;209:74–80. doi: 10.1016/j.atherosclerosis.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 8.Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–80. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 9.Mallat Z, Ait-Oufella H, Tedgui A. Regulatory T-cell immunity in atherosclerosis. Trends Cardiovasc Med. 2007;17:113–8. doi: 10.1016/j.tcm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Zouggari Y, Ait-Oufella H, Waeckel L, et al. Regulatory T cells modulate postischemic neovascularization. Circulation. 2009;120:1415–25. doi: 10.1161/CIRCULATIONAHA.109.875583. [DOI] [PubMed] [Google Scholar]
- 11.Tang Q, Henriksen KJ, Boden EK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–52. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 12.Tang Q, Smith JA, Szot GL, et al. CD28/B7 regulation of anti-CD3-mediated immunosuppression in vivo. J Immunol. 2003;170:1510–6. doi: 10.4049/jimmunol.170.3.1510. [DOI] [PubMed] [Google Scholar]
- 13.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 14.Mak TW. In: The gene knockout factsbook: A-H. Mak TakW., editor. London: Academic Press; 1998. pp. 161–4. [Google Scholar]
- 15.Webster KE, Walters S, Kohler RE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–60. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohm AP, McMahon JS, Podojil JR, et al. Cutting edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006;176:3301–5. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 17.Shibata K, Yamada H, Nakamura R, et al. Identification of CD25+ gamma delta T cells as foetal thymus-derived naturally occurring IL-17 producers. J Immunol. 2008;181:5940–7. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 18.Driesen J, Popov A, Schultze JL. CD25 as an immune regulatory molecule expressed on myeloid dendritic cells. Immunobiology. 2008;213:849–58. doi: 10.1016/j.imbio.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. 2005;86:209–39. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- 20.Lahl K, Loddenkemper C, Drouin C, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan ME, Sutmuller RP, Witteveen HJ, et al. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–60. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 22.Lahl K, Loddenkemper C, Drouin C, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellingman AA, Bastiaansen AJ, de Vries MR, et al. Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. Eur J Vasc Endovasc Surg. 2010 doi: 10.1016/j.ejvs.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Rausch S, Huehn J, Loddenkemper C, et al. Establishment of nematode infection despite increased Th2 responses and immunopathology after selective depletion of Foxp3+ cells. Eur J Immunol. 2009;39:3066–77. doi: 10.1002/eji.200939644. [DOI] [PubMed] [Google Scholar]
- 25.Taguchi O, Takahashi T. Mouse models of autoimmune disease suggest that self-tolerance is maintained by unresponsive autoreactive T cells. Immunology. 1996;89:13–9. doi: 10.1046/j.1365-2567.1996.d01-712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McHugh RS, Shevach EM. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol. 2002;168:5979–83. doi: 10.4049/jimmunol.168.12.5979. [DOI] [PubMed] [Google Scholar]
- 27.van Weel V, Toes RE, Seghers L, et al. Natural killer cells and CD4+ T-cells modulate collateral artery development. Arterioscler Thromb Vasc Biol. 2007;27:2310–8. doi: 10.1161/ATVBAHA.107.151407. [DOI] [PubMed] [Google Scholar]
- 28.Stabile E, Burnett MS, Watkins C, et al. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation. 2003;108:205–10. doi: 10.1161/01.CIR.0000079225.50817.71. [DOI] [PubMed] [Google Scholar]
- 29.Stabile E, Kinnaird T, la SA, et al. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation. 2006;113:118–24. doi: 10.1161/CIRCULATIONAHA.105.576702. [DOI] [PubMed] [Google Scholar]
- 30.Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and. J Immunol. 2000;165:3105–10. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- 31.Lehmann J, Huehn J, de la Rosa M, et al. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as. Proc Natl Acad Sci USA. 2002;99:13031–6. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito M, Iwawaki T, Taya C, et al. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19:746–50. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 33.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The LDPI graph depicts the mean± S.E.M. of blood flow recovery after doubleelectro-coagulation of the femoral artery and iliac artery in hindlimb of C57Bl6 mice injected without (n = 10) and withrepetitive DT injections (n = 10). Blood flow was expressed as ratio between the operated and non-operated limb measured before and directly after surgery and at days 3, 7, 9 and 14. Images on the right depict representative LDPI images of paws of C57Bl6 mice injected without DT (upper) or with DT (lower) 7 days after induction of hind limb ischemia.


