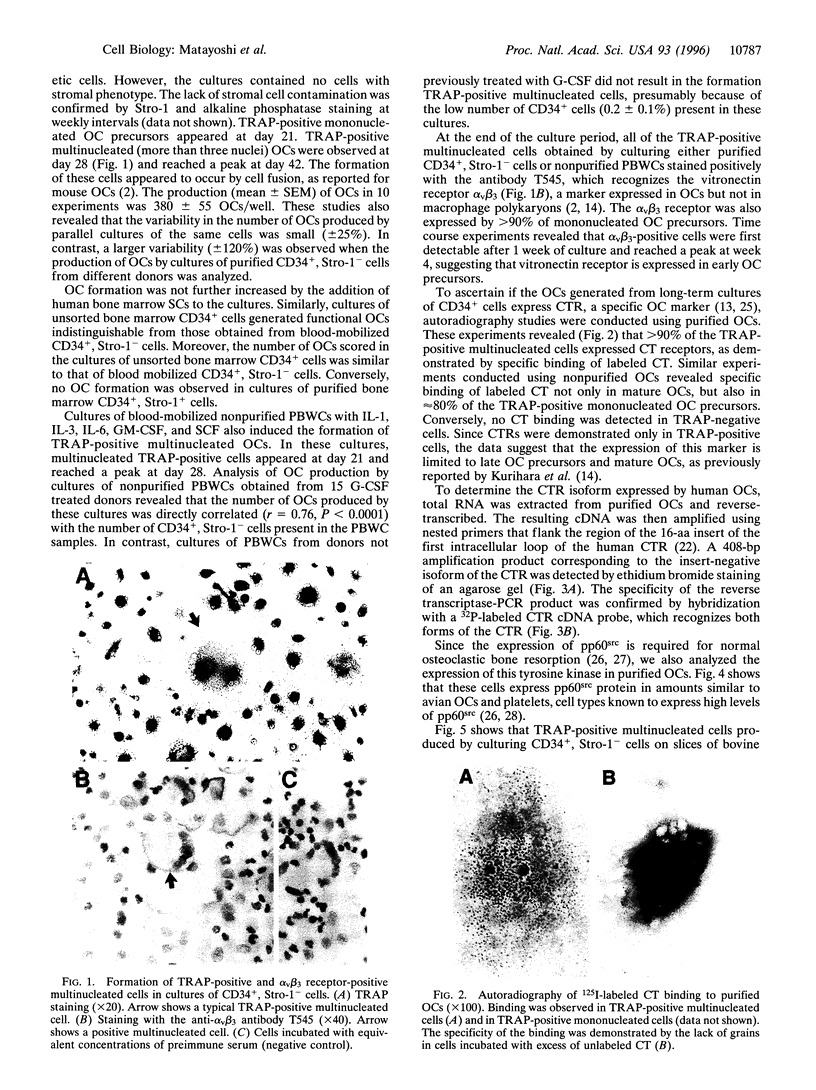
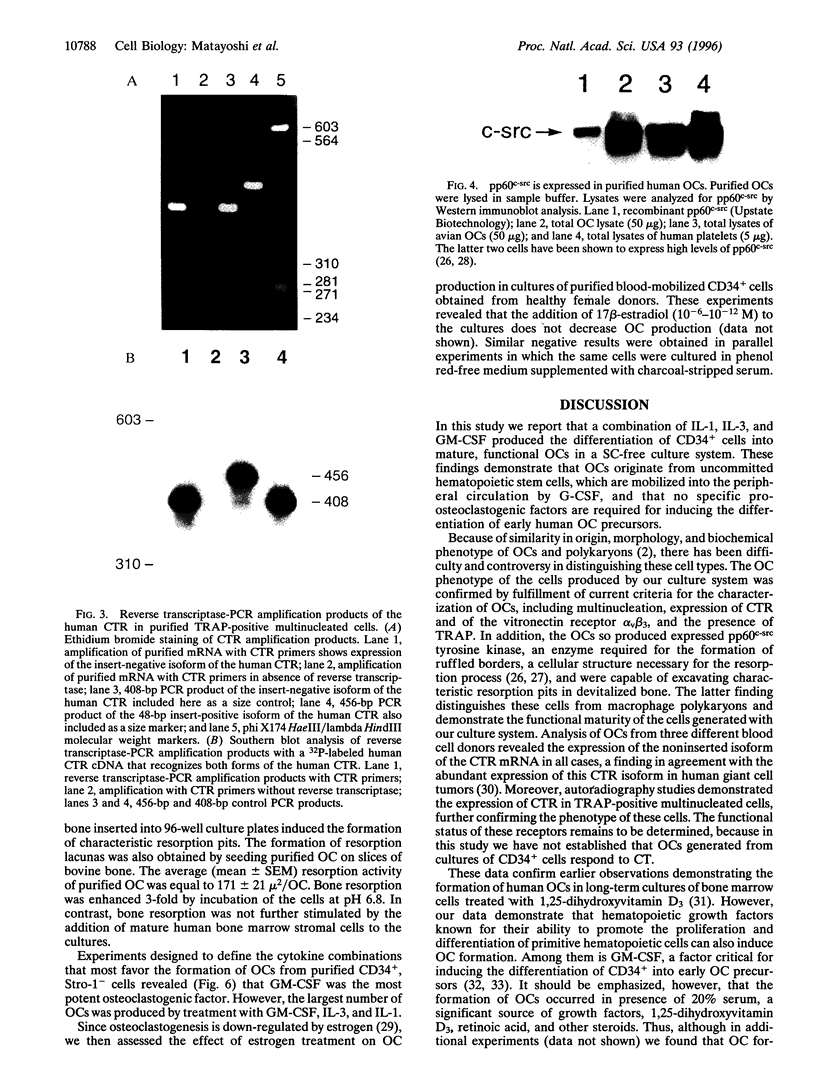
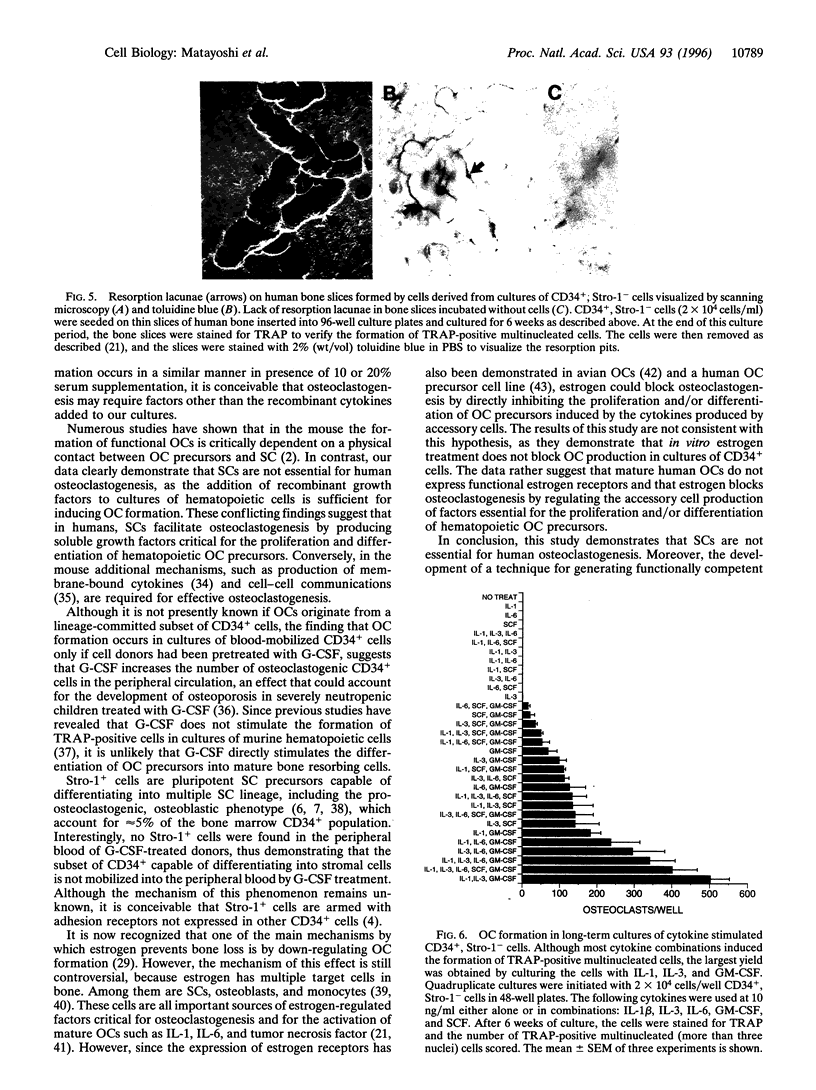
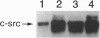Abstract
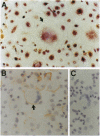
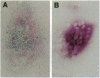
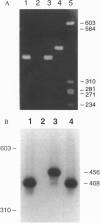
Osteoclastogenesis is a complex process that is facilitated by bone marrow stromal cells (SCs). To determine if SCs are an absolute requirement for the differentiation of human hematopoietic precursors into fully mature, osteoclasts (OCs), CD34+ cells were mobilized into the peripheral circulation with granulocyte colony-stimulating factor, harvested by leukapheresis, and purified by magnetic-activated cell sorting. This procedure yields a population of CD34+ cells that does not contain SC precursors, as assessed by the lack of expression of the SC antigen Stro-1, and that differentiates only into hematopoietic cells. We found that CD34+, Stro-1- cells cultured with a combination of granulocyte/macrophage colony-stimulating factor, interleukin 1, and interleukin 3 generated cells that fulfill current criteria for the characterization of OCs, including multinucleation, presence of tartrate-resistant acid phosphatase, and expression of the calcitonin and vitronectin receptors and of pp60c-src tyrosine kinase. These OCs also expressed mRNA for the noninserted isoform of the calcitonin receptor and excavated characteristic resorption pits in devitalized bone slices. These data demonstrate that accessory SCs are not essential for human osteoclastogenesis and that granulocyte colony-stimulating factor treatment mobilizes OC precursors into the peripheral circulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand R., Wilkinson J. M., Kellie S. Localisation of pp60c-src to the surface membrane of human platelets. Oncogene. 1993 Nov;8(11):3013–3020. [PubMed] [Google Scholar]
- Bensinger W. I., Berenson R. J. Peripheral blood and positive selection of marrow as a source of stem cells for transplantation. Prog Clin Biol Res. 1990;337:93–98. [PubMed] [Google Scholar]
- Bishop N. J., Williams D. M., Compston J. C., Stirling D. M., Prentice A. Osteoporosis in severe congenital neutropenia treated with granulocyte colony-stimulating factor. Br J Haematol. 1995 Apr;89(4):927–928. doi: 10.1111/j.1365-2141.1995.tb08441.x. [DOI] [PubMed] [Google Scholar]
- Boyce B. F., Yoneda T., Lowe C., Soriano P., Mundy G. R. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J Clin Invest. 1992 Oct;90(4):1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civin C. I., Gore S. D. Antigenic analysis of hematopoiesis: a review. J Hematother. 1993 Summer;2(2):137–144. doi: 10.1089/scd.1.1993.2.137. [DOI] [PubMed] [Google Scholar]
- Civin C. I., Strauss L. C., Brovall C., Fackler M. J., Schwartz J. F., Shaper J. H. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984 Jul;133(1):157–165. [PubMed] [Google Scholar]
- Collin-Osdoby P., Oursler M. J., Webber D., Osdoby P. Osteoclast-specific monoclonal antibodies coupled to magnetic beads provide a rapid and efficient method of purifying avian osteoclasts. J Bone Miner Res. 1991 Dec;6(12):1353–1365. doi: 10.1002/jbmr.5650061213. [DOI] [PubMed] [Google Scholar]
- Fiorelli G., Gori F., Petilli M., Tanini A., Benvenuti S., Serio M., Bernabei P., Brandi M. L. Functional estrogen receptors in a human preosteoclastic cell line. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2672–2676. doi: 10.1073/pnas.92.7.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin R. J., Cullison J. W., Avioli L. V., Osdoby P. A. Influence of osteoclasts and osteoclast-like cells on osteoblast alkaline phosphatase activity and collagen synthesis. J Bone Miner Res. 1994 Aug;9(8):1167–1178. doi: 10.1002/jbmr.5650090806. [DOI] [PubMed] [Google Scholar]
- Gorn A. H., Rudolph S. M., Flannery M. R., Morton C. C., Weremowicz S., Wang T. Z., Krane S. M., Goldring S. R. Expression of two human skeletal calcitonin receptor isoforms cloned from a giant cell tumor of bone. The first intracellular domain modulates ligand binding and signal transduction. J Clin Invest. 1995 Jun;95(6):2680–2691. doi: 10.1172/JCI117970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S., Graves S. E., Ohta S., Simmons P. J. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994 Dec 15;84(12):4164–4173. [PubMed] [Google Scholar]
- Hattersley G., Chambers T. J. Calcitonin receptors as markers for osteoclastic differentiation: correlation between generation of bone-resorptive cells and cells that express calcitonin receptors in mouse bone marrow cultures. Endocrinology. 1989 Sep;125(3):1606–1612. doi: 10.1210/endo-125-3-1606. [DOI] [PubMed] [Google Scholar]
- Horne W. C., Neff L., Chatterjee D., Lomri A., Levy J. B., Baron R. Osteoclasts express high levels of pp60c-src in association with intracellular membranes. J Cell Biol. 1992 Nov;119(4):1003–1013. doi: 10.1083/jcb.119.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M. C. Cytokines and estrogen in bone: anti-osteoporotic effects. Science. 1993 Apr 30;260(5108):626–627. doi: 10.1126/science.8480174. [DOI] [PubMed] [Google Scholar]
- Jilka R. L., Hangoc G., Girasole G., Passeri G., Williams D. C., Abrams J. S., Boyce B., Broxmeyer H., Manolagas S. C. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992 Jul 3;257(5066):88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- Kato K., Radbruch A. Isolation and characterization of CD34+ hematopoietic stem cells from human peripheral blood by high-gradient magnetic cell sorting. Cytometry. 1993;14(4):384–392. doi: 10.1002/cyto.990140407. [DOI] [PubMed] [Google Scholar]
- Kitazawa R., Kimble R. B., Vannice J. L., Kung V. T., Pacifici R. Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J Clin Invest. 1994 Dec;94(6):2397–2406. doi: 10.1172/JCI117606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuestner R. E., Elrod R. D., Grant F. J., Hagen F. S., Kuijper J. L., Matthewes S. L., O'Hara P. J., Sheppard P. O., Stroop S. D., Thompson D. L. Cloning and characterization of an abundant subtype of the human calcitonin receptor. Mol Pharmacol. 1994 Aug;46(2):246–255. [PubMed] [Google Scholar]
- Kurihara N., Chenu C., Miller M., Civin C., Roodman G. D. Identification of committed mononuclear precursors for osteoclast-like cells formed in long term human marrow cultures. Endocrinology. 1990 May;126(5):2733–2741. doi: 10.1210/endo-126-5-2733. [DOI] [PubMed] [Google Scholar]
- Kurihara N., Civin C., Roodman G. D. Osteotropic factor responsiveness of highly purified populations of early and late precursors for human multinucleated cells expressing the osteoclast phenotype. J Bone Miner Res. 1991 Mar;6(3):257–261. doi: 10.1002/jbmr.5650060307. [DOI] [PubMed] [Google Scholar]
- Kurihara N., Gluck S., Roodman G. D. Sequential expression of phenotype markers for osteoclasts during differentiation of precursors for multinucleated cells formed in long-term human marrow cultures. Endocrinology. 1990 Dec;127(6):3215–3221. doi: 10.1210/endo-127-6-3215. [DOI] [PubMed] [Google Scholar]
- Kurihara N., Suda T., Miura Y., Nakauchi H., Kodama H., Hiura K., Hakeda Y., Kumegawa M. Generation of osteoclasts from isolated hematopoietic progenitor cells. Blood. 1989 Sep;74(4):1295–1302. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Lottsfeldt J. L., Fevold K. L. Identification and characterization of osteoclast progenitors by clonal analysis of hematopoietic cells. Blood. 1992 Oct 1;80(7):1710–1716. [PubMed] [Google Scholar]
- Liapis H., Flath A., Kitazawa S. Integrin alpha V beta 3 expression by bone-residing breast cancer metastases. Diagn Mol Pathol. 1996 Jun;5(2):127–135. doi: 10.1097/00019606-199606000-00008. [DOI] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo J. A., Sousa S. L., Fonseca J. M., Hock J. M., Medlock E. S. Colony-stimulating factors regulate the development of multinucleated osteoclasts from recently replicated cells in vitro. J Clin Invest. 1987 Jul;80(1):160–164. doi: 10.1172/JCI113042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas S. C., Jilka R. L. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995 Feb 2;332(5):305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- Oursler M. J., Osdoby P., Pyfferoen J., Riggs B. L., Spelsberg T. C. Avian osteoclasts as estrogen target cells. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6613–6617. doi: 10.1073/pnas.88.15.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res. 1996 Aug;11(8):1043–1051. doi: 10.1002/jbmr.5650110802. [DOI] [PubMed] [Google Scholar]
- Quinn J. M., McGee J. O., Athanasou N. A. Cellular and hormonal factors influencing monocyte differentiation to osteoclastic bone-resorbing cells. Endocrinology. 1994 Jun;134(6):2416–2423. doi: 10.1210/endo.134.6.8194468. [DOI] [PubMed] [Google Scholar]
- Roodman G. D. Interleukin-6: an osteotropic factor? J Bone Miner Res. 1992 May;7(5):475–478. doi: 10.1002/jbmr.5650070502. [DOI] [PubMed] [Google Scholar]
- Russell J. A., Luider J., Weaver M., Brown C., Selinger S., Railton C., Karlsson L., Klassen J. Collection of progenitor cells for allogeneic transplantation from peripheral blood of normal donors. Bone Marrow Transplant. 1995 Jan;15(1):111–115. [PubMed] [Google Scholar]
- Sato T., Abe E., Jin C. H., Hong M. H., Katagiri T., Kinoshita T., Amizuka N., Ozawa H., Suda T. The biological roles of the third component of complement in osteoclast formation. Endocrinology. 1993 Jul;133(1):397–404. doi: 10.1210/endo.133.1.8319587. [DOI] [PubMed] [Google Scholar]
- Scheven B. A., Visser J. W., Nijweide P. J. In vitro osteoclast generation from different bone marrow fractions, including a highly enriched haematopoietic stem cell population. Nature. 1986 May 1;321(6065):79–81. doi: 10.1038/321079a0. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Simmons P. J., Torok-Storb B. CD34 expression by stromal precursors in normal human adult bone marrow. Blood. 1991 Dec 1;78(11):2848–2853. [PubMed] [Google Scholar]
- Simmons P. J., Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991 Jul 1;78(1):55–62. [PubMed] [Google Scholar]
- Stella C. C., Cazzola M., De Fabritiis P., De Vincentiis A., Gianni A. M., Lanza F., Lauria F., Lemoli R. M., Tarella C., Zanon P. CD34-positive cells: biology and clinical relevance. Haematologica. 1995 Jul-Aug;80(4):367–387. [PubMed] [Google Scholar]
- Suda T., Takahashi N., Martin T. J. Modulation of osteoclast differentiation. Endocr Rev. 1992 Feb;13(1):66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Kukita T., MacDonald B. R., Bird A., Mundy G. R., McManus L. M., Miller M., Boyde A., Jones S. J., Roodman G. D. Osteoclast-like cells form in long-term human bone marrow but not in peripheral blood cultures. J Clin Invest. 1989 Feb;83(2):543–550. doi: 10.1172/JCI113916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Takahashi N., Udagawa N., Tamura T., Akatsu T., Stanley E. R., Kurokawa T., Suda T. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest. 1993 Jan;91(1):257–263. doi: 10.1172/JCI116179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa N., Takahashi N., Akatsu T., Tanaka H., Sasaki T., Nishihara T., Koga T., Martin T. J., Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]