Abstract
Recently, a novel type of stromal cell – the telocytes (TC) – was identified in mouse trachea. These cells are known to possess the ultrastructural characteristics, which support their role in intercellular signaling. We found TC in all stromal compartments of the tracheal wall. TC with long prolongations (telopodes, Tp) were lining longitudinally the collagen bundles, and were serially arranged (end-to-end connections of Tp were found). Noteworthy, Tp frequently establish stromal synapses with mast cells (MC). Primary cilia were also identified in TC. In conclusion, tracheal TC could be involved in the tracheal regulation (e.g. secretion, contractility). The tandem TC-MC deserves further investigations.
Keywords: telocytes, telopodes, mast cells, interstitial cells, stromal cells, primary cilium
Telocytes (TC) are stromal cells with extremely long, but slender, prolongations named telopodes (Tp) [1–3]. Characteristically, Tp have thin segments (podomers) and dilations (podoms) [2–7] which house mitochondria, endoplasmic reticulum and caveolae [8, 9]. TC present in mouse trachea were reported very recently by Zheng et al.[8]. It was previously demonstrated that TC are different of fibroblasts and fibroblast-like cells [1–3, 10, 11].
Evidence of nonadrenergic and noncholinergic (NANC) nerves innervating the airways determined the reassessment of the model of airway neural control [12]. One of the principal neurotransmitters of airway NANC neural system is NO [13] and we provided recent evidence for the in vivo neuronal nitric-oxide synthase (nNOS) phenotype of the mast cells (MC) in the tracheal wall layers [12]. However, their cooperation with the airway NANC neural system remains blurred since an intramural ‘scaffold’ has not been proved able to transmit at distance the MC chemical signals. Mast cells activation is thought to mediate the airway hyperresponsiveness (AHR) in asthmatic subjects; however, ablation of MC does not ameliorate AHR, and it was shown that c-kit is required for development of AHR in a MC-independent fashion [14].
TC were identified in mouse trachea by scanning electron microscopy [8] and also in terminal and respiratory bronchioles (human and mice), as well as in alveolar ducts [10]. Telopodes follow a longitudinal course with respect to the long axis of the airways [10].
We report here evidence that indeed TC are present in rat trachea and make structural associations with MC.
Wistar male rats (four) were used (280–300 g., 12 months). After preanaesthesia with ether, the animals were killed by intracardiac injection of 0.2 ml. of T-61 (INTERVET, Kirkland, Quebec, Canada), a veterinary euthanasia drug containing embutramide, mebezonium and tetracaine in dimethylformamide. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and protocols were approved by the ‘Carol Davila’ University of Medicine and Pharmacy Bioethics Committee. The EC Directive 86/609/EEC for animal experiments and the Uniform Requirements for manuscripts submitted to Biomedical journals were followed accordingly.
Tracheal samples from each animal were prepared for transmission electron microscopy (TEM), as previously described [15]. The Formvar coated grids were examined in a Philips electron microscope EM 208S (acceleration voltage of 80 kV) and snapshots were taken using a video camera Veleta and the iTEM Olympus Soft Imaging System.
Stromal cells were identified in the tracheal wall, sending long, slender prolongations (up to 50 micrometers) with a characteristic ‘beads on a string’ appearance (alternation of podoms and podomers) (Fig. 1). As this feature is strictly specific for telopodes (Tp), the respective stromal cells were considered as being TC. Telopodes were longitudinally lining bundles of collagen fibres, were serially arranged (end-to-end connections of Tp were found), and presented, occasionally, primary cilia (Fig. 1). Within the tracheal wall, TC were located in the vicinity of blood vessels and smooth muscle cells (Fig. 1). TC and/or Tp establish close contacts, stromal synapses, with tracheal mast cells, at distances below 100 nm (the macromolecular interaction range) (Figs 24).
Fig 1.
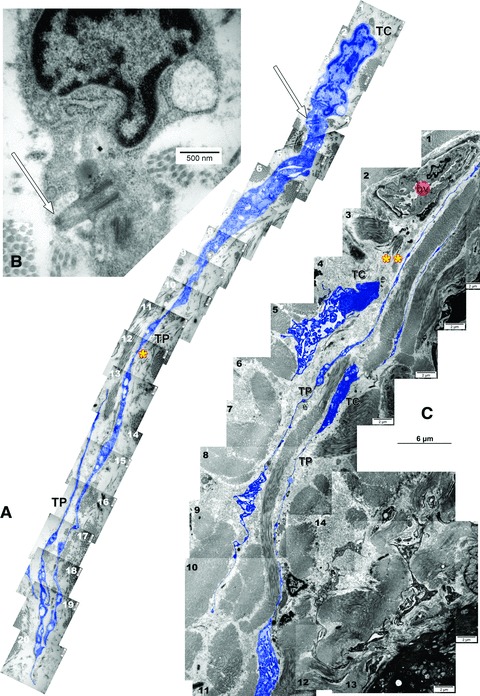
Ultrathin sections of rat trachea. Two-dimensional sequenced concatenation from 20 (A) and 16 (C) serial electron micrographs depicting telocytes (TC) with characteristic long and slender prolongations (TP: telopodes): (A) *= 44 micrometers; (B) **= 45 micrometers. In (B) is detailed a primary cilium (arrow) of the telocyte in (A). bv: blood vessel. Telocytes and telopodes were digitally coloured blue.
Fig 2.

A tracheal mast cell (MC) surrounded by telopodes (Tp: blue). Intercellular distances were measured in four locations: (a) = 29 nm; (b) = 14 nm; (c) = 51 nm; (d) = 72 nm.
Fig 3.
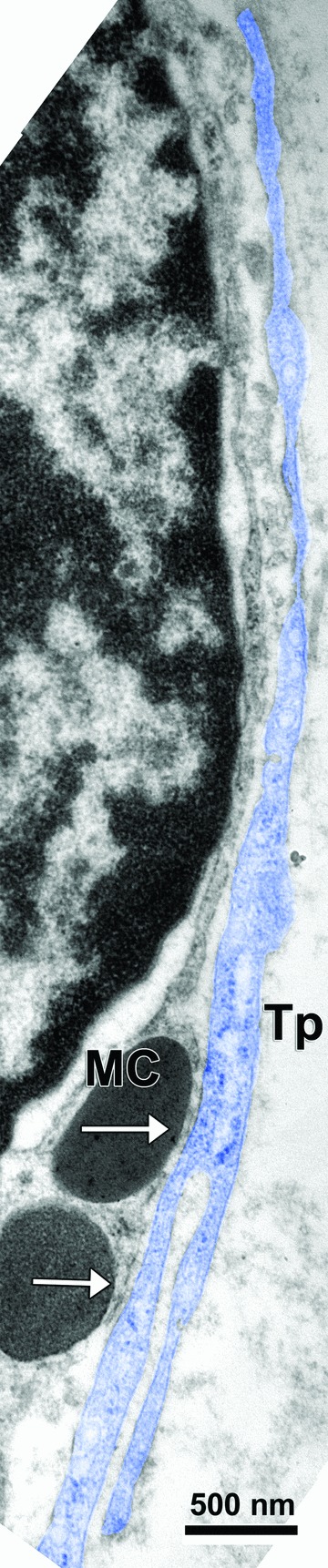
A long and branched telopode (Tp: digitally coloured blue) is closely related to, and is contacting (arrows) a tracheal mast cell (MC).
Fig 4.
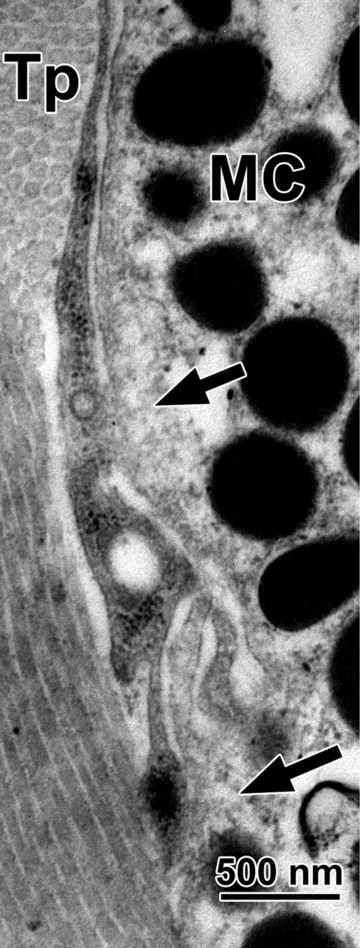
Contacting (arrows) tracheal mast cell (MC) and telopode (Tp).
TC are stromal cells of mesenchymal origin [1–3, 9].
We found tracheal TC provided with primary cilia. Primary cilia of TC were previously identified in various tissues [16–18]. Such primary cilia persist in many differentiated cells; presumably, as semipermanent structures, the cilia function as mechano- or chemosensors and as a cellular global positioning system to detect changes in the surrounding environment, or to initiate cellular replacement after damage [19].
As in the trigeminal ganglion [15], mast cells (MC) establish stromal synapses (connective connections) [20] with TC and/or Tp. This tandem was also found in mucosae [21], prior to the TC definition in 2010 [1], and the cells adjacent to MC were described then only as ‘stromal cells’[21].
It was proven that c-kit is required for the MC-independent development of AHR [14]. On other hand, tracheal TC are c-kit positive cells [8]. These results taken together with our findings suggest that both cell types, the MC and the TC, could be involved in tracheal regulation. Possible implications of TC in pathology were also recently addressed [22, 23].
It is tempting to speculate that the tandem MC-TC may be functionally added to the neural tracheal regulation; this hypothesis is suggested by nNOS positivity of tracheal MC [12]. With this perspective, the NANC system could be regarded as consisting of a neural (canonical) component and a stromal one, MC- and TC-related.
The close structural MC-TC association raises doubts on the use of CD117/c-kit labelling, since both cell types are known as c-kit positive [1–3]. Usually, in light microscopy is considered that a c-kit positive cell with processes could not be a MC (and vice-versa, a c-kit positive cell without processes could not be a TC) [24]. The tandem TC-MC makes this identification unreliable (an apparently c-kit positive cell with processes could be in fact a MC neighboured by Tp).
In conclusion, tracheal TC could be involved in the tracheal regulation (e.g. secretion, contractility). The tandem TC-MC deserves further investigations.
Funding source
This study was supported by the Sectoral Operational Programme Human Resources Development (SOP HRD), financed from the European Social Fund and by the Romanian Government under the contract number POSDRU/89/1.5/S/64153 (author #1).
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Popescu LM, Faussone-Pellegrini MS. TELOCYTES – a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popescu LM. The tandem: telocytes – stem cells. Int J Biol Biomed Eng. 2011;5:83–92. [Google Scholar]
- 3.Faussone-Pellegrini M-S, Popescum L. Telocytes. BioMolecular Concepts. 2011 doi: 10.1515/BMC.2011.039. doi: 10.1515/BMC.2011.1039. [DOI] [PubMed] [Google Scholar]
- 4.Suciu L, Nicolescu MI, Popescu LM. Cardiac telocytes: serial dynamic images in cell culture. J Cell Mol Med. 2010;14:2687–92. doi: 10.1111/j.1582-4934.2010.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suciu L, Popescu LM, Gherghiceanu M, et al. Telocytes in human term placenta: morphology and phenotype. Cells Tissues Organs. 2010;192:325–39. doi: 10.1159/000319467. [DOI] [PubMed] [Google Scholar]
- 6.Hinescu ME, Gherghiceanu M, Suciu L, et al. Telocytes in pleura: two- and three-dimensional imaging by transmission electron microscopy. Cell Tissue Res. 2011;343:389–97. doi: 10.1007/s00441-010-1095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rusu MC, Pop F, Hostiuc S, et al. Extrahepatic and intrahepatic human portal interstitial cajal cells. Anat Rec (Hoboken) 2011;294:1382–92. doi: 10.1002/ar.21441. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y, Li H, Manole CG, et al. Telocytes in trachea and lungs. J Cell Mol Med. 2011;15:2262–8. doi: 10.1111/j.1582-4934.2011.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gherghiceanu M, Popescu LM. Cardiomyocyte precursors and telocytes in epicardial stem cell niche: electron microscope images. J Cell Mol Med. 2010;14:871–7. doi: 10.1111/j.1582-4934.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popescu LM, Gherghiceanu M, Suciu LC, et al. Telocytes and putative stem cells in the lungs: electron microscopy, electron tomography and laser scanning microscopy. Cell Tissue Res. 2011;345:391–403. doi: 10.1007/s00441-011-1229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popescu LM, Manole E, Serboiu CS, et al. Identification of telocytes in skeletal muscle interstitium: implication for muscle regeneration. J Cell Mol Med. 2011;15:1379–92. doi: 10.1111/j.1582-4934.2011.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rusu MC, Pop F, Boscu AL, et al. Anatomical and immunohistochemical considerations on the microinnervation of trachea in humans. Ann Anat. 2011;193:13–22. doi: 10.1016/j.aanat.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Rogers DF. Motor control of airway goblet cells and glands. Respir Physiol. 2001;125:129–44. doi: 10.1016/s0034-5687(00)00209-7. [DOI] [PubMed] [Google Scholar]
- 14.Cozzi E, Ackerman KG, Lundequist A, et al. The naive airway hyperresponsiveness of the A/J mouse is Kit-mediated. Proc Natl Acad Sci USA. 2011;108:12787–92. doi: 10.1073/pnas.1106582108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rusu MC, Pop F, Hostiuc S, et al. The human trigeminal ganglion: c-kit positive neurons and interstitial cells. Ann Anat. 2011;193:403–11. doi: 10.1016/j.aanat.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Cantarero I, Luesma MJ, Junquera C. The primary cilium of telocytes in the vasculature: electron microscope imaging. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01312.x. doi: 10.1111/j.1582-4934.2011.01312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinescu ME, Popescu LM, Gherghiceanu M, et al. Interstitial Cajal-like cells in rat mesentery: an ultrastructural and immunohistochemical approach. J Cell Mol Med. 2008;12:260–70. doi: 10.1111/j.1582-4934.2008.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popescu LM, Gherghiceanu M, Manole CG, et al. Cardiac renewing: interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches. J Cell Mol Med. 2009;13:866–86. doi: 10.1111/j.1582-4934.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satir P, Christensen ST. Structure and function of mammalian cilia. Histochem Cell Biol. 2008;129:687–93. doi: 10.1007/s00418-008-0416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popescu LM, Gherghiceanu M, Cretoiu D, et al. The connective connection: interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells. Electron microscope study in situ. J Cell Mol Med. 2005;9:714–30. doi: 10.1111/j.1582-4934.2005.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–79. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 22.Mandache E, Gherghiceanu M, Macarie C, et al. Telocytes in human isolated atrial amyloidosis: ultrastructural remodelling. J Cell Mol Med. 2010;14:2739–47. doi: 10.1111/j.1582-4934.2010.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Y, Bai C, Wang X. Telocyte morphologies and potential roles in diseases. J Cell Physiol. 2011 doi: 10.1002/jcp.23022. doi 10.1002/jcp.23022. [DOI] [PubMed] [Google Scholar]
- 24.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20(Suppl 1):54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]


