Abstract
Tissue transglutaminase (tTG) plays an important role in celiac disease pathogenesis and antibodies to tTG are a diagnostic marker of gluten-sensitive enteropathy. The aim of this study was to investigate the localization of tTG in the duodenal mucosa in control tissues and in different histological stages of celiac disease by using a commercial and a novel set of anti-tTG monoclonal antibodies, to see whether this assessment can be useful for diagnostic purpose. The distribution of tTG was firstly evaluated in 18 untreated celiac patients by using a commercial monoclonal antibody (CUB7402) against tissue transglutaminase enzyme and directed against the loop-core region of the enzyme. Thereafter, in further 30 untreated celiac patients we employed three newly characterized anti-tTG monoclonal antibodies produced against recombinant human-tTG. The epitopes recognized are located in three distinct domains of the protein corresponding to the core, C1 and C2 protein structure. Eleven age- and sex-matched patients with chronic duodenitis acted as controls. All subjects underwent upper endoscopy to obtain biopsy samples from the duodenum. Overall, we found that (i) tTG is equally expressed in CD at different stages of disease; (ii) tTG is expressed, at similar level, in CD and controls with duodenitis. Assessment of tTG level in biopsy samples by immunohistochemical methods is not useful in the clinical diagnostic work-up of CD.
Keywords: celiac disease, duodenal mucosa, immunohistochemistry, monoclonal antibodies, tissue transglutaminase, sprue
Introduction
Celiac disease (CD) is a gluten-dependent enteropathy that develops in genetically susceptible individuals, with a prevalence of 1% in the general population [1]. CD is a unique autoimmune disease in which all the factors involved in the autoimmune cascade can be recognized: endogenous risk factors (the HLA-DQ heterodimers A1*0501,B1*0201 (DQ2), A1*0301,B1*0302 (DQ8)); a trigger (the gluten); an autoantigen (type 2 tissue transglutami-nase [tTG]); auto-antibodies (anti-tissue tTG antibodies) [2]. Gluten peptides activated intestinal HLA-DQ2 restricted T cells from CD patients, leading to the release of inflammatory cytokines and T-cell-mediated inflammatory response, have been implicated in the development of intestinal lesions of CD.
Two findings have shed light onto the interaction between the trigger, the auto-antigen and the HLA DQ2. Several gluten pep-tides are glutamyl donor substrate for the tTG, resulting in the formation of deamidated glutamine residues into negatively charged glutamic acid. The gliadin peptides deamidated by tTG bind with higher affinity to HLA DQ2 or DQ8 than their non-deamidated counterparts and can elicit a stronger proliferative response of gliadin-specific CD4+ T-cell clones [3, 4]. Moreover, the tTG catalyses the binding of gliadin peptides to intestinal extracellular matrix proteins, thus demonstrating the role of tTG in creating immunogenic neoepitopes and enhancing antigenic presentation of gliadins [5].
Another inflammatory role of the tTG depends from its ability to mediate T-cell migration across cytokine-activated endothelium into inflamed tissues [6]. Thus, in the development of mucosal atrophy of CD, the tTG is overexpressed, particularly within the basement membrane and the lamina propria, with increased enterocyte expression compared to the controls displaying normal mucosa [7].
The expression of tTG within specific areas suggests that the enzyme could be translocated from the intracellular to the pericellular environment in the lamina propria by some not yet identified cells. Moreover, the tTG deposits along the lamina propria and around the mucosal vessels represent a specific target of the IgA anti-tTG antibodies in revealing early developing CD among symptomatic and asymptomatic patients genetically predisposed to gluten intolerance [8, 9]. Furthermore, it has been recently reported that anti-tTG assay of the culture medium of biopsy specimens can improve the accuracy of CD diagnosis in patients negative for serum antibodies [10].
Despite the interest in the biological properties of the tTG in the pathogenesis of CD, the distribution of this enzyme has been to date only studied by using one monoclonal antibody (CUB7402) developed against guinea-pig type tissue tTG enzyme and directed mainly against the loop-core region of the enzyme [11–13].
The aim of the present study was to investigate the localization of tTG within the duodenal mucosa in controls and in different histological stages of CD and to identify the cells involved in the synthesis of tTG by using a new set of three anti-tTG monoclonal antibodies. These monoclonal antibodies were produced against recombinant human tTG and their epitopes were shown to be located in three distinct domains of the protein corresponding to the core, C1 and C2 protein structure [14].
Materials and methods
Study groups
For the first step, based on the commercial antibody CUB7402 (see below), 18 untreated CD patients, 9 males and 9 females, age range 15–76 years old, were recruited. These patients were subdivided in: M-O type 1 infiltrative lesions (3 patients); M-O type 2 hyperplastic lesions (6 patients); M-O type 3 destructive lesions (9 patients). The latter patients were classified as: M-O 3C (5 patients), M-O 3B (3 patients), and M-O 3A (1 patient). The clinical suspicion of CD was supported by a positive test for indirect immunofluorescence on sections of monkey esophageal smooth muscle (anti-endomysial antibodies [EMA] IgA) and/or enzyme-linked immunosorbent assay with guinea pig tTG or dot blot test with human recombinant tTG (tTG IgA).
For the second step based on the three new anti-tTG monoclonal antibodies (see below) 30 untreated CD patients, 18 males and 12 females, age range 1–75 years old, were recruited. These patients were classified as: M-O type 1 infiltrative lesions (5 patients); M-O type 2 hyperplastic lesions (9 patients); M-O type 3 destructive lesions (16 patients). The latter patients were classified as M-O 3A (5 patients) M-O 3B (6 patients) and M-O 3C (5 patients).
As control group, 11 age- and sex-matched patients with chronic duodenitis, all negative for serological tests and undergoing endoscopy for chronic aspecific upper gut symptoms were recruited and tested with the immunohistochemical methods described below.
Monoclonal antibody production and purification
Production and characterization of three different anti-tTG monoclonal antibodies was described elsewhere [14]. We identified the epitopes recognized by MAB 1 alpha-TG2, MAB 2 alpha-TG2, MAB 3 alpha-TG2 antibodies as located in the core, C1 and C2 regions of the enzyme, respectively. Ascitic fluid was prepared in BALB/c mice by inoculating them with the hybridoma cells. Then 100–300 μl of ascites were diluted to 1 ml with ddH2O, and the pH of the crude antibody preparation adjusted by adding 1/10 volume of 1.0 M Tris pH 8.0. The antibody solution was passed through a pre-washed protein G bead column, and the beads were washed with 10 column volumes of 100 mM Tris pH 8.0 and 10 column volumes of 10 mM Tris pH 8.0. The column was then eluted with 50 mM glycine pH 3.0. The eluate was collected in tubes containing 1/10 volume of 1M Tris pH 8.0, and immediately each tube was mixed gently to bring the pH back to neutral. Protein concentration was measured using the Bradford reagent (Sigma, Sigma-Aldrich, St Louis, MO, USA).
Immunohistochemistry
Duodenal sampling was carried out after a standard endoscopic procedure, by obtaining at least four samples from different portions of the duodenum, two in the proximal duodenum and two in the distal duodenum, so that some samples could be frozen. Biopsy specimens, correctly oriented on millipore filters, were embedded in paraffin, then 5-μm thick sections were obtained and stained with haematoxylin and eosin. Immunohistochemistry was performed according to a standard technique. In the first step was used the monoclonal CUB7402 (MS-224-P, Neo Markers, Fremont, CA, USA) at dilution 1:1000. In the second step, we employed three new anti-tTG monoclonal antibodies (MAB 1 alpha-TG2, dilution 1:2000; MAB 2 alpha-ETG, dilution 1:4000; MAB 3 alpha-ETG dilution 1:6000). Anti-CD3 monoclonal antibody (Dako, Glostrup, Denmark) was used to better identify intraepithelial lymphocytes (IEL). To test the hypothesis that tTG was produced by endothelial cells, we also used anti-CD31 and anti-CD34 monoclonal antibodies against the vascular and endothelial structures (Dako) and antibodies against IgA (Dako).
Data analysis
All slides were coded and analysed in blind by two pathologists (VV and AG). Duodenal histological lesions were classified according to the revised criteria proposed by the United European Gastroenterology [15]. According to recent criteria, the upper normal limit for IEL has been established as 25 IEL/100 epithelial cells [16], thus we adopted this cut-off. A mucosa with a villous/crypt ratio of 3:1 (plus a normal IEL count) was considered as normal, whereas a decrease of the villous/crypt ratio led to the following definition of atrophy: 2:1 ratio = mild atrophy; 1:1 ratio = moderate atrophy; no villi (flat mucosa) = total atrophy.
Results
Localization of tTG within the mucosal duodenal sections with CUB4702
This antibody targets the Core-loop region of the tTG. In the first group of 18 CD patients, a strong staining was shown in epithelial cells and in the lamina propria of the villi under the superficial epithelium; the positivity was similar in frozen and fixed material (Fig. 1). No significant differences in the localization of staining were observed with respect to the grade and severity of villous atrophy. In the control group (11 cases), the distribution of the staining was the same as in CD patients (Fig. 2).
1.
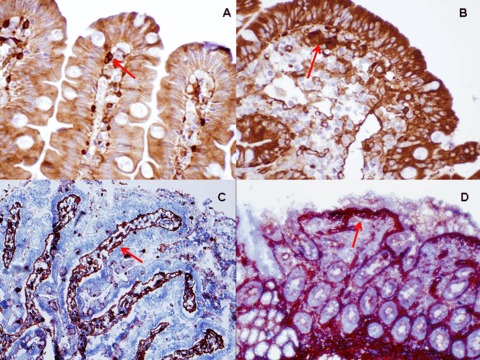
The arrows show positivity for tTG under the superficial epithelium of villi with CUB 7402 in a case of initial lesion (A) and total atrophy (B) (paraffin fixed biopsy, original magnification ×100). (C) (original magnification ×40) and (D) (original magnification ×20) show frozen biopsies.
2.
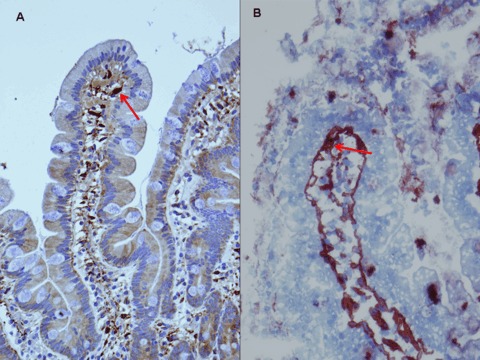
The arrows show positivity for tTG under the superficial epithelium of villi with CUB 7402 on paraffin fixed (A, original magnification ×20) and frozen biopsy (B, original magnification ×100).
Localization of tTG within the mucosal duodenal sections with the three new mAbs
The recently characterized mAbs directed to human tTG display all the determined epitopes corresponding to structural loops exposed on the surface of the protein [14]. These different recognition patterns account for the differences between the antibodies and, from a practical point of view, may be useful for experimental purposes. Thus, we analysed a second set of 30 CD cases with these three different mAbs. As it can be seen in Figure 3, positivity was seen on epithelial cells, more evident for Mab 1 and Mab 3, and under the superficial epithelium in the axis of villi. As for the first set of samples positivity was similar for frozen and fixed material. Interestingly, mAb3 resulted also positive in Paneth cells (Fig. 4). Again, no discernible differences were observed with respect to the grade and severity of villous atrophy.
3.
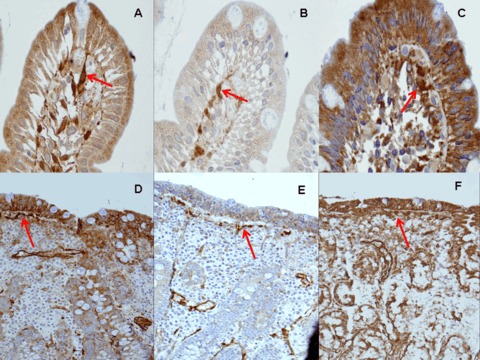
The arrows show the staining patterns of the three new mAbs in paraffin fixed sections. The figures above show initial lesions, the figures below show atrophic lesions. (A) and (D) mAb 1, (B) and (E) mAb 2, (C) and (F) mAb 3 (original magnification ×100).
4.
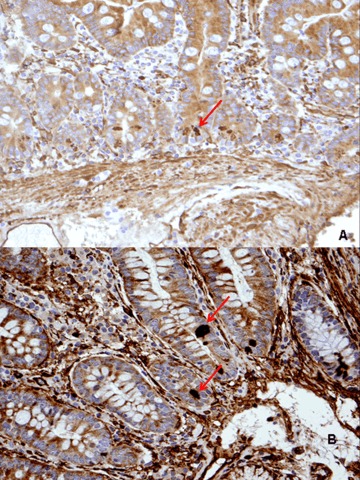
The arrows show positivity for the Paneth cells with mAb 3 (paraffin fixed biopsies, original magnification A ×40, B ×100).
A positive labelling (although of lesser intensity) was also observed in controls. CD31 and CD34 were positive in endothelial cells and vascular channels, with the same localization observed for tTG with CUB 7402 and the three Mabs (Fig. 5). The staining observed for IgA was evident in plasma cells of the basal part of lamina propria (Fig. 6), but not in the lamina propria under the superficial epithelium.
5.
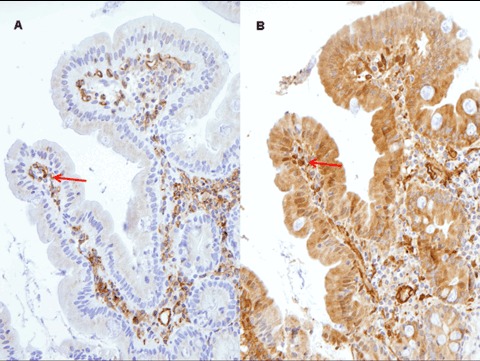
Expression of CD31 (A) and mAb 3 (B) on consecutive sections (original magnification x20).
6.
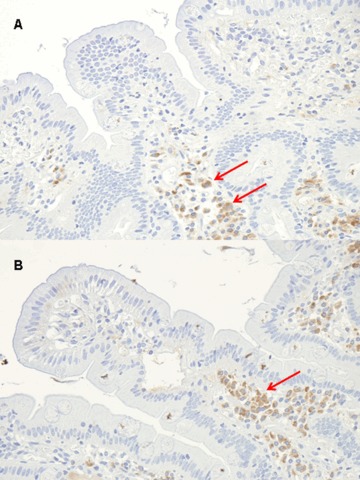
The arrows show positivity for IgA in plasma cells of the basal part of lamina propria (original magnification A ×20, B ×40).
Discussion
To date, there are few doubts that tTG is an important component of CD, for both its pathogenesis and diagnosis [17, 18]. The identification of tTG as the anti-endomysial autoantigen in this condition [19] allowed standardized tests to be introduced in clinical practice that proved tTG to be a reliable marker for CD [20, 21]. In fact, anti-tTG antibodies display high sensitivity (93%) and specificity (98%) for the diagnosis of CD, when villous atrophy is present [22], and values 10 times higher than the cut-off are almost always associated with diagnostic pathological findings [23, 24].
These antibodies are produced in the intestinal mucosa [25] and are deposited along the villous and crypt basement membranes in the mucosa from patients with CD before they can be detected in the circulation and before the mucosal damage is present [26]. Recent evidence in man also suggests that tTG autoantibodies per semay play an important role in the progression and maintenance of the trophic mucosal lesion in CD by interacting with extracellular membrane-bound tTG [27].
We investigated whether the tTG expression by immunohistochemical methods might be useful in the evaluation of CD, since ultrastructural and enzymatic studies showed an increase of tTG in CD compared to controls [28]. To this purpose, we used the commercial mAb (CUB4702) and a new set of three mAbs recently characterized by our group. We decided to use multiple probes as in preliminary results we were able to show that these four monoclonal antibodies provide different staining patterns on histological sections of human umbilical cord. The most likely explanation of this finding is that tTG, the structure of which is highly sensitive to activation by Ca2+ and GTP [29, 30], may assume different functional conformations, thus exposing the epitopes differently in different histological compartments. This interpretation may be of practical relevance, implying that the use of multiple mAbs to tTG, that map different enzyme conformation, could be used for a full characterization of the localization and quantification of enzyme deposits.
The chronic tTG enzyme expression (more than the enzyme quantity) at epithelial sites promotes the formation of high molecular weight complexes containing tTG-gliadin-mucosal proteins cross links, which in turn creates long-term reservoirs of potential autoantigens for the development of glutendependent autoimmune disorders in CD [5]. The endothelial over-expression of the tTG, observed in the present study, confirms the role of the tTG in the course of tissue inflammation. In fact, the tTG on cytokine-activated endothelium actively operates in mediating the recruitment of CD8+ T cells migration across the inflamed endothelium and the infiltration of tissue inflammation [31].
We demonstrated that the tTG enzyme is expressed in the cytoplasm of Paneth cells; there is considerable interest in a possible link between the presence of the tTG in these cells and the development of the gluten-dependent inflammation. It may be that active deamidation of gluten derived peptides by the Paneth's tTG enzyme leads to up-regulation of inflammatory immune response [32].
It has been hypothesized that tTG crosslinks itself to gluten to make macromolecular complexes causing the formation of new antigens which are both self-epitopes and gliadin epitopes, leading to the production of anti-tTG antibodies [33, 34]. The anti-tTG antibodies are synthesized only at the intestinal site [25], and the anti-tTG serum concentration is related to the degree of the intestinal lesion [35]. Furthermore, in the contest of genetically gluten-intolerance and in the absence of morphological or serological signs of CD, patients show gluten-dependent anti-tTG intestinal deposits before the development of the villous atrophy [8, 9]. This means that in the early stage of CD the development of tranglutaminase-dependent immunocomplexes represent specific indicators of the disease and they might be considered a future, reliable marker for the CD diagnosis without villous atrophy.
The overall results showed that: (i) tTG is equally expressed in CD at different stages of disease; (ii) tTG is expressed, almost with the same intensity, in CD, and controls. Our results were at variance with those reported by Sakly and colleagues [13] that found increased tTG expression in CD, but substantially agreed with another study showing that immunohistochemical assessment of tTG is not suitable for diagnosis of CD [36]. Moreover, we were not able to confirm with immunohistochemical methods the findings of Korponay-Szabo and colleagues of IgA positivity in the lamina propria under the superficial epithelium. On the other hand, our results suggest that tTG might be produced by endothelial cells, as hypothesized in animal studies [37].
In conclusion, the assessment of tTG by immunohistochemical methods is not useful in the clinical diagnostic work-up of CD, whereas the detection of anti-tTG levels in patients’ sera represents an important tool for both diagnostic and follow-up purposes [38, 39].
Acknowledgments
This study was supported by research grant RC20/05 from IRCCS ‘Burlo Garofalo’, Trieste, Italy.
References
- 1.Tommasini A, Not T, Kiren V, Baldas V, Santon D, Trevisiol C, Berti I, Neri E, Gerarduzzi T, Bruno I, Lenhardt A, Zamuner E, Spanò A, Crovella S, Martellossi S, Torre G, Sblattero D, Marzari R, Bradbury A, Tamburlini G, Ventura A. Mass screening for coeliac disease using anti-human transglutaminase antibody assay. Arch Dis Child. 2004;89:512–5. doi: 10.1136/adc.2003.029603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119:234–42. doi: 10.1053/gast.2000.8521. [DOI] [PubMed] [Google Scholar]
- 3.Molberg O, Mcadam SN, Körner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Norén O, Roepstorff P, Lundin KE, Sjöström H, Sollid LM. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gutderived T-cells in celiac disease. Nat Med. 1998;4:713–7. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 4.Van de Wal Y, Kooy Y, van Veelen P, Pena S, Mearin L, Papadopoulos G, Koning F. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T-cell reactivity. J Immunol. 1998;161:1585–8. [PubMed] [Google Scholar]
- 5.Dieterich W, Esslinger B, Trapp D, Hahn E, Huff T, Seilmeier W, Wieser H, Schuppan D. Cross linking to tissue transglutaminase and collagen favours gliadin toxicity in coeliac disease. Gut. 2006;55:478–84. doi: 10.1136/gut.2005.069385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohan K, Pinto D, Issekutz T. Identification of tissue transglutaminase as a novel molecule involved in human CD8+T cell transendothelial migration. J Immunol. 2003;171:3179–86. doi: 10.4049/jimmunol.171.6.3179. [DOI] [PubMed] [Google Scholar]
- 7.Esposito C, Paparo F, Caputo I, Porta R, Salvati V, Mazzarella G, Auricchio S, Troncone R. Expression and enzymatic activity of small intestinal tissue transglutaminase in celiac disease. Am J Gastroenterol. 2003;98:1813–20. doi: 10.1111/j.1572-0241.2003.07582.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaukinen K, Peraaho M, Collin P, Partanen J, Wolley N, Kaartinen T, Nuutinen T, Halttunen T, Maki M, Korponay-Szabo I. Small-bowel mucosal transglutaminase 2-specific IgA deposits in celiac disease without villous atrophy: A prospective and randomized clinical study. Scand J Gastroenterol. 2005;40:564–72. doi: 10.1080/00365520510023422. [DOI] [PubMed] [Google Scholar]
- 9.Salmi T, Collin P, Jarvinen O, Haimila K, Partanen J, Laurila K, Korponai-Szabo I, Hihtala H, Reunala M, Maki M, Kaukinen K. Immunoglobulin A autoantibodies against transglutaminase 2 in the small intestinal mucosa predict forthcoming celiac disease. Alim Pharmacol Ther. 2006;24:541–52. doi: 10.1111/j.1365-2036.2006.02997.x. [DOI] [PubMed] [Google Scholar]
- 10.Carroccio A, Di Prima L, Pirrone G, Scalici C, Florena AM, Gasparin M, Tolazzi G, Gucciardi A, Sciumè C, Iacono G. Antitransglutaminase antibody assay of the culture medium of intestinal biopsy specimens can improve the accuracy of celiac disease diagnosis. Clin Chem. 2006;52:1175–80. doi: 10.1373/clinchem.2005.061366. [DOI] [PubMed] [Google Scholar]
- 11.Sblattero D, Florian F, Azzoni E, Zyla T, Park M, Baldas V, Not T, Ventura A, Bradbury A, Marzari R. The analysis of the fine specificity of celiac disease antibodies using tissue transglutaminase fragments. Eur J Biochem. 2002;269:5175–81. doi: 10.1046/j.1432-1033.2002.03215.x. [DOI] [PubMed] [Google Scholar]
- 12.Ciccocioppo R, Di Sabatino A, Ara C, Biagi F, Perilli M, Amicosante G, Cifonesi G, Corazza G. Gliadin and tissue transglutaminase complex in normal and coeliac duodenal mucosa. Clin Exp Immunol. 2003;134:516–24. doi: 10.1111/j.1365-2249.2003.02326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakly W, Sriha B, Ghedira I, Bienvenu F, Aydi A, Tahar Sfar M, Lachaux A, Korbi S, Bienvenu J, Fabien N. Localization of tissue transglutaminase and N (ipsilon)-(gamma)-glutamyl lysine in duodenal mucosal during the development of mucosal atrophy in coeliac disease. Virchows Arch. 2005;446:613–8. doi: 10.1007/s00428-005-1237-z. [DOI] [PubMed] [Google Scholar]
- 14.Di Niro R, Ferrara F, Not T, Bradbury ARM, Chirdo F, Marzari R, Sblattero D. Characterizing monoclonal antibody epi-topes by filtered gene fragment phage display. Biochem J. 2005;388:889–94. doi: 10.1042/BJ20041983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Gastroenterology. When is a coeliac a celiac? Report of a working group of the United European Gastroenterology Week in Amsterdam, 2001. Eur J Gastroenterol Hepatol. 2001;13:1123–8. doi: 10.1097/00042737-200109000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Hayat M, Cairns A, Dixon MF, O’Mahony S. Quantitation of intraepithelial lymphocytes in human duodenum: what is normal? J Clin Pathol. 2002;55:393–5. doi: 10.1136/jcp.55.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin A, Romito G, Pepe I, De Vivo G, Merola MR, Limatola A, Gentile V. Transglutaminase-catalyzed reactions responsible for the pathogenesis of celiac disease and neurodegenerative diseases: from basic biochemistry to clinic. Curr Med Chem. 2006;13:1895–902. doi: 10.2174/092986706777585068. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigo L. Celiac disease. World J Gastroenterol. 2006;12:6585–93. doi: 10.3748/wjg.v12.i41.6585. [DOI] [PubMed] [Google Scholar]
- 19.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 20.Dieterich W, Laag E, Schopper H, Volta U, Ferguson A, Gillett H, Riecken EO, Schuppan D. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology. 1998;115:1317–21. doi: 10.1016/s0016-5085(98)70007-1. [DOI] [PubMed] [Google Scholar]
- 21.Wong RC, Wilson RJ, Steele RH, Radford-Smith G, Adelstein S. A comparison of 13 guinea pig and human anti-tissue transglutaminase antibody ELISA kits. J Clin Pathol. 2002;55:488–94. doi: 10.1136/jcp.55.7.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis NR, Scott BB. Systematic review: the use of serology to exclude or diagnose coeliac disease (a comparison of the endomysial and tissue transglutaminase antibody tests) Aliment Pharmacol Ther. 2006;24:47–54. doi: 10.1111/j.1365-2036.2006.02967.x. [DOI] [PubMed] [Google Scholar]
- 23.Barker CC, Mitton C, Jevon G, Mock T. Can tissue transglutaminase antibody titers replace small-bowel biopsy to diagnose celiac disease in select pediatric populations? Pediatrics. 2005;115:1341–6. doi: 10.1542/peds.2004-1392. [DOI] [PubMed] [Google Scholar]
- 24.Donaldson MR, Firth SD, Wimpee H, Leiferman KM, Zone JJ, Horsley W, O’Gorman MA, Jackson WD, Neuhausen SL, Hull CM, Book LS. Correlation of duodenal histology with tissue transglutaminase and endomysial antibody levels in pediatric celiac disease. Clin Gastroenterol Hepatol. 2007;5:567–73. doi: 10.1016/j.cgh.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Marzari R, Sblattero D, Florian F, Tongiorgi E, Not T, Tommasini A, Ventura A, Bradbury A. Molecular dissection of the tissue transglutaminase autoantibody response in celiac disease. J Immunol. 2001;166:4170–6. doi: 10.4049/jimmunol.166.6.4170. [DOI] [PubMed] [Google Scholar]
- 26.Korponay-Szabo IR, Haittunen T, Szalai Z, Laurila K, Kiraly R, Kovacs JB, Fesus L, Maki M. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by celiac autoantibodies. Gut. 2004;53:641–8. doi: 10.1136/gut.2003.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barone MV, Caputo I, Ribecco MT, Maglio M, Marzari R, Sblattero D, Troncone R, Auricchio S, Esposito C. Humoral immune response to tissue transglutaminase is related to epithelial cell proliferation in celiac disease. Gastroenterology. 2007;132:1245–53. doi: 10.1053/j.gastro.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 28.Skovbjerg H, Hansen GH, NIels-Christiansen LL, Anthonsen D, Ascher H, Midhagen G, Hallert C, Norén O, Sjostrom H. Intestinal tissue transglutaminase in coeliac disease of children and adults: ultrastructural localization and variation in expression. Scand J Gastroenterol. 2004;39:1219–27. doi: 10.1080/00365520410003597. [DOI] [PubMed] [Google Scholar]
- 29.Casadio R, Polverini E, Mariani P, Spinozzi F, Carsughi F, Fontana A, Polverino de Laureto P, Matteucci G, Bergamini CM. The structural basis for the regulation of tissue transglutaminase by calcium ions. Eur J Biochem. 1999;262:672–9. doi: 10.1046/j.1432-1327.1999.00437.x. [DOI] [PubMed] [Google Scholar]
- 30.Pinkas DM, Strop P, Brunger AT, Khosla C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007;5:e327. doi: 10.1371/journal.pbio.0050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohan K, Pinto D, Issekutz T. Identification of tissue transglutaminase as a novel molecule involved in human CD8+ T cell transendothelial migration. J Immunol. 2003;171:3179–86. doi: 10.4049/jimmunol.171.6.3179. [DOI] [PubMed] [Google Scholar]
- 32.Elphick D, Mahida Y. Paneth cells: their role in innate immunity and inflammatory disease. Gut. 2005;54:1802–9. doi: 10.1136/gut.2005.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Achyuthan KE, Goodell RJ, Kennedye JR, Lee KN, Henley A, Stiefer JR, Birckbichler PJ. Immunochemicalanalyses of human plasma fibronectin-cytosolic transglutaminase interactions. J Immunol Methods. 1995;180:69–79. doi: 10.1016/0022-1759(94)00300-l. [DOI] [PubMed] [Google Scholar]
- 34.Sollid L, Molberg O, MacAdam S, Lundin K. Autoantibodies in coeliac disease: tissue transglutaminase-guilt by association? Gut. 1997;41:851–2. doi: 10.1136/gut.41.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trevisiol C, Ventura A, Baldas V, Tommasini A, Santon D, Martellossi S, Torre G, Berti I, Spanò A, Crovella S, Amoroso A, Sblattero D, Marzari R, Bradbury A, Not T. A reliable screening procedure for coeliac disease in clinical practice. Scand J Gastroenterol. 2002;37:679–84. doi: 10.1080/00365520212513. [DOI] [PubMed] [Google Scholar]
- 36.Tuncer I, Bayram I, Kaba I, Mercan R, Ugras S. Tissue transglutaminase expression in duodenal mucosa of patients with celiac disease and of normal subjects. Turk J Gastroenterol. 2003;14:185–8. [PubMed] [Google Scholar]
- 37.Haroon ZA, Hettasch JM, Lai TS, Dewhirst MW, Greenberg CS. Tissue transglutaminase is expressed, active, and directly involved in rat dermal wound healing and angiogenesis. FASEB J. 1999;13:1787–95. doi: 10.1096/fasebj.13.13.1787. [DOI] [PubMed] [Google Scholar]
- 38.Leffler DA, Kelly CP. Update on the evaluation and diagnosis of celiac disease. Curr Opin Allergy Clin Immunol. 2006;6:191–6. doi: 10.1097/01.all.0000225159.75521.e4. [DOI] [PubMed] [Google Scholar]
- 39.Craig D, Robins G, Howdle PD. Advances in celiac disease. Curr Opin Gastroenterol. 2007;23:142–8. doi: 10.1097/MOG.0b013e328013ccee. [DOI] [PubMed] [Google Scholar]


