Abstract
Although androgen resistance has been characterized in men with a normal chromosome complement and mutations in the androgen-receptor gene, a mutation in the gene encoding estrogen receptor α (ESR1) was previously described only in one man and not, to our knowledge, in a woman. We now describe an 18-year-old woman without breast development and with markedly elevated serum levels of estrogens and bilateral multicystic ovaries. She was found to have a homozygous loss-offunction ESR1 mutation in a completely conserved residue that interferes with estrogen signaling. Her clinical presentation was similar to that in the mouse orthologue knockout. This case shows that disruption of ESR1 causes profound estrogen resistance in women. (Funded by the National Institutes of Health.)
Mutations in the androgen-receptor gene have been shown to cause androgen resistance in 46,XY humans1,2 and in murine models,3 but estrogen-receptor mutations are rare. The action of estrogen in peripheral tissues is mediated through a receptor4 that is predominantly nuclear in location.5 After the cloning of this receptor, termed estrogen receptor α,6,7 in 1985, a second receptor (estrogen receptor β) was identified in 1996.8–10 Nonnuclear estrogen signaling has also been suggested.
The action of estradiol influences breast development, and feedback from estradiol on the hypothalamus and pituitary involves estrogen receptor α.11 In mice, the ablation of both estrogen receptor α12 and estrogen receptor β,13 which are encoded by Esr1 and Esr2, respectively, has been accomplished. Female Esr1 knockout mice (α-ERKO) have hypoplastic uteri and hemorrhagic, multicystic ovaries without corpora lutea. Such mice are infertile, whereas their male counterparts have only reduced fertility.12 In contrast, the ablation of Esr2 in mice results in few signs of reproductive abnormalities beyond moderately reduced folliculogenesis and a decreased litter size.13,14 To our knowledge, only one human ESR1 mutation has been reported in a man,15 whereas none have been reported in a woman. We now report an estrogen-resistant state in a woman with an ESR1 point mutation in a conserved residue.
Case Report
The patient is an 18-year-old, adopted white woman who presented at the age of 15 years with absent breast development, primary amenorrhea, and intermittent lower abdominal pain. The patient provided written consent for this study, which was approved by the Georgia Regents University Human Assurance Committee.
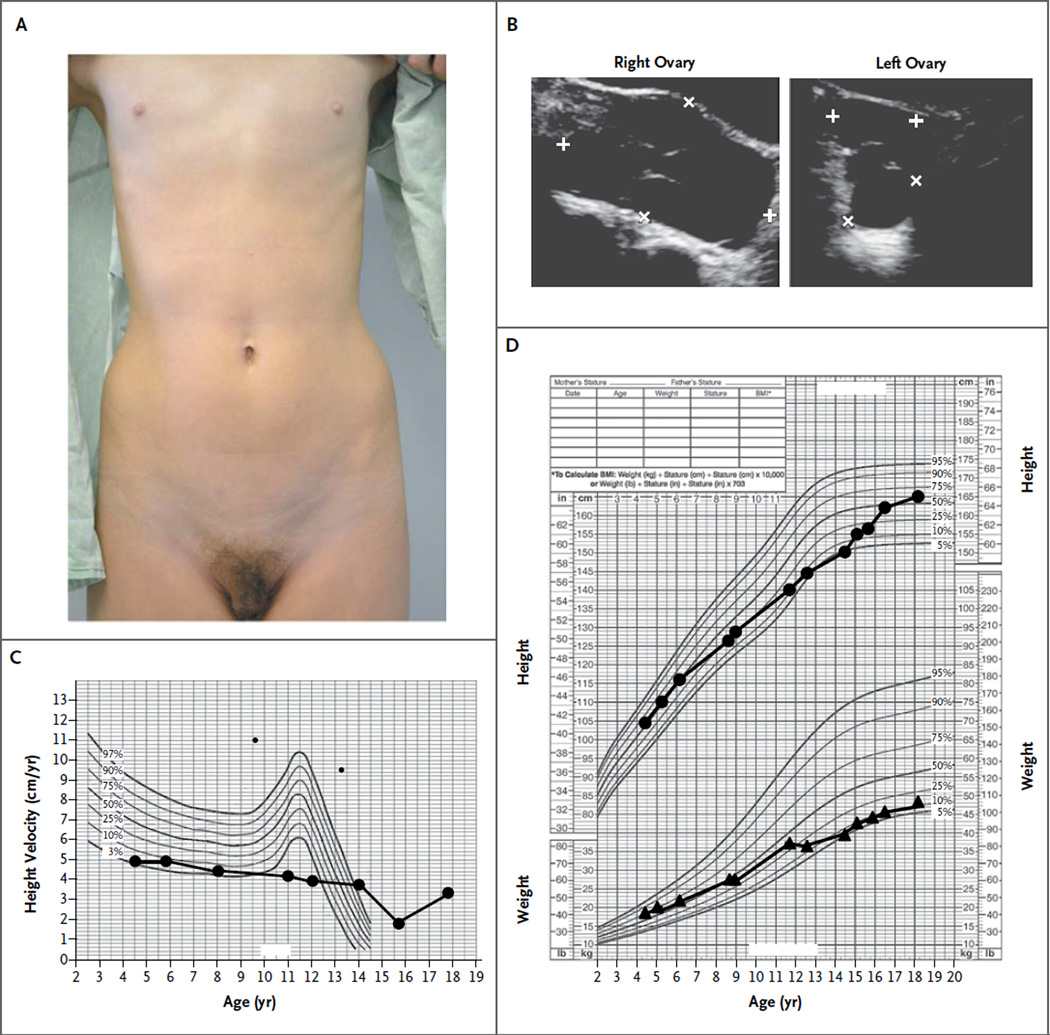
At the age of 17 years 9 months, the patient’s height was 162.6 cm, her weight was 44 kg, and her body-mass index (the weight in kilograms divided by the square of the height in meters) was 16.6. She had Tanner stage 1 breast development and Tanner stage 4 pubic hair (Fig. 1A). She had severe facial acne.
Figure 1. Phenotypic Features of a Female Patient with an ESR1 Mutation.
Panel A shows the absence of breast development (Tanner stage 1) and the presence of pubic hair (Tanner stage 4) when the patient was examined at the age of 17 years 8 months. At the age of 17 years 5 months, when the patient’s weight was 44 kg, total-body dual-energy x-ray absorptiometry indicated that the total body fat was 11,834 g and the total lean mass was 30,459 g. Panel B shows enlarged cystic ovaries, with the right ovary measuring 8.4 by 4.7 by 8.3 cm and the left ovary measuring 2.9 by 2.7 by 3.1 cm at the age of 17 years 9 months. Panel C shows the patient’s growth velocity without the normal steroid-induced growth spurt at the time of puberty. Panel D shows linear percentiles for height (upper curve) and weight (lower curve).
At the time of presentation, laboratory studies revealed a serum estradiol level of 3500 pg per milliliter (normal follicular phase, 11 to 210 [12,848 pmol per liter; normal follicular phase, 40 to 771]), a luteinizing hormone level of 9.6 mIU per milliliter (normal follicular phase, 1.9 to 12.5), a follicle-stimulating hormone level of 6.7 mIU per milliliter (normal follicular phase, 2.5 to 10.2), and a normal female karyotype (46,XX). Her bone age had been 11 to 12 years at a chronologic age of 15 years 5 months. Ultrasonography revealed a small uterus with no clearly identifiable endometrial stripe and markedly enlarged multicystic ovaries (Fig. 1B). This clinical presentation suggested estrogen resistance.
Her growth velocity indicated the lack of an estrogen-induced growth spurt at the time of puberty (Fig. 1C), even though until the age of 6 years, her linear growth and weight were approximately at the 75th and 50th percentiles, respectively; after that, both measures dropped, with the height consistently below the 50th percentile and the weight consistently below the 25th percentile (Fig. 1D). Total-body dual-energy x-ray absorptiometry (DXA) at 17 years 5 months showed a total bone density of 0.862 g per square centimeter (z score, −2.4) and total body fat of 28% (<50th percentile for white adult females, according to the National Health and Nutrition Examination Survey).16 At the age of 17 years 8 months, plain radiography of her left hand showed a bone age of 13.5 years and open radial and ulnar epiphyses (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).
At the age of 18 years 2 months, the level of serum osteocalcin, a marker of osteoblast function, was 65.1 ng per milliliter (normal range, 4.9 to 30.9), bone-specific alkaline phosphatase was 45.1 μg per liter (normal range, 0 to 21.3), and serum C-telopeptide was 1060 pg per milliliter (premenopausal normal range, 112 to 738; postmenopausal normal range, 142 to 1351). Magnetic resonance imaging of the brain and pituitary with and without contrast material was normal at the age of 17 years 10 months.
To determine whether exogenous estrogens would have an effect, for a total of 5 months, the patient took oral estrogen in the form of conjugated equine estrogen (at a dose of 1.25 mg for 3 months) and micronized estradiol (at doses of 3 and 4 mg per day for 1 month each). Her breasts remained at Tanner stage 1. After 5 months of only norethindrone (at a dose of 2.5 to 5 mg per day), her estradiol level dropped to 114 pg per milliliter, and ovarian and cyst sizes diminished. She was also taking 25 μg of levothyroxine for mild hypothyroidism during this time. When norethindrone treatment was discontinued, her ovaries returned to their enlarged pretreatment size and have remained large despite thyroid replacement. At the age of 17 years 8 months, the levothyroxine dose was increased to 50 μg and the results of thyroid-function testing were normal, but levels of estradiol and estrone remained elevated at 1 month and 6 months, respectively, after the increase in the levothyroxine dose (Table 1).
Table 1.
Laboratory Values for the Patient with ESR1 Mutation.*
| Variable | Reference Range |
Age of Patient | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 Yr 1 Mo |
15 Yr 2 Mo |
15 Yr 5 Mo |
15 Yr 6 Mo |
16 Yr 1 Mo |
16 Yr 2 Mo |
17 Yr 4 Mo |
17 Yr 8 Mo† |
17 Yr 8 Mo† |
17 Yr 9 Mo |
18 Yr 2 Mo |
||
| Thyrotropin (μU/ml) | 0.4–5.0 | 5.2 | 4.4 | 1.9 | 3.1 | 3.1 | 4.1 | 4.3 | 9.3 | 0.3, 0.4† | 1.3 | |
| Free thyroxine (ng/dl) | 0.9–1.6 | 0.9 | 1.0 | 0.8 | 1.1 | 1.1 | 1.2 | 1.0 | 2.3, 1.9† | 1.5 | ||
| Thyroxine (μg/dl) | 5.0–12.0 | 6.4 | 7.1 | 7.7 | ||||||||
| Triiodothyronine (ng/dl) | 71–180 | 136 | ||||||||||
| Reverse triiodothyronine (ng/dl) | 13.5–34.2 | 43.6 | 23.8 | |||||||||
| Thyroid-binding globulin (μg/ml) | 15–30 | 24 | ||||||||||
| Corticosteroid-binding globulin (mg/dl) | 2.3–3.9 | 3.3 | ||||||||||
| Sex-hormone–binding globulin (nmol/liter) | 25–122 | 44 | 16 | 46 | 45 | 41 | ||||||
| Estradiol (pg/ml) | 11–120 | 3500 | 765 | 1632 | 1910 | 114 | 712 | 2340‡ | 2360 | 2000‡ | 3050‡ | |
| Estrone (pg/ml) | 29–105 | 1040‡ | 1283‡ | 1050‡ | ||||||||
| Follicle-stimulating hormone (mIU/ml) | 2.5–10.2 | 6.7 | 14.5 | 9.3 | 8.8 | 19.1 | 12.3 | 12.0 | 13.0 | |||
| Luteinizing hormone (mIU/ml) | 1.9–12.5 | 9.6 | 9.1 | 12.6 | 5.8 | 10.4 | 11.9 | 9.0 | 13.0 | |||
| Prolactin (ng/ml) | 3.6–12.0 | 6.8 | ||||||||||
| Insulin (μU/ml) | 2.6–24.9 | 5.8 | ||||||||||
| Insulin-like growth factor I (ng/ml) | 196–581 | 130 | 368 | |||||||||
| Inhibin (pg/ml) | ||||||||||||
| A§ | 319.0 | 184.4 | ||||||||||
| B¶ | 93.8 | |||||||||||
| DHEAS (μg/dl) | 37–307 | 114 | ||||||||||
| Antimüllerian hormone (ng/ml) | 0.3–11.2 | 2.8 | ||||||||||
| Cortisol in morning (μg/dl) | 6.2–19.4 | 23.0 | ||||||||||
| Testosterone | ||||||||||||
| Total (ng/dl) | 10–50 | 38 | 38 | 13 | 50 | 42‡ | 88‡ | |||||
| Free (pg/ml) | 0.5–3.9 | 0.6 | 4.5 | 3.4 | 5.6 | |||||||
| Progesterone (ng/ml) | <2.0 | 0.7 | 1.6 | 1.3 | 1.3 | 2.0 | ||||||
| Cholesterol (mg/dl) | ||||||||||||
| Total | 100–169 | 149 | 149 | |||||||||
| High-density lipoprotein | >39 | 64 | 52 | |||||||||
| Low-density lipoprotein | 0–99 | 75 | 81 | |||||||||
| Very-low-density lipoprotein | 5–40 | 10 | 16 | |||||||||
| Triglycerides (mg/dl) | 0–149 | 52S | 80 | |||||||||
Test results were negative for thyroid peroxidase and antithyroglobulin antibodies. To convert the values for free thyroxine to picomoles per liter, multiply by 12.87. To convert the values for thyroxine and thyroxine-binding globulin to nanomoles per liter, multiply by 12.87. To convert the values for triiodothyronine to nanomoles per liter, multiply by 0.01536. To convert the values for estradiol to picomoles per liter, multiply by 3.671. To convert the values for estrone to picomoles per liter, multiply by 3.699. To convert the values for insulin to picomoles per liter, multiply by 6.945. To convert the values for morning cortisol to nanomoles per liter, multiply by 27.59. To convert the values for total testosterone to nanomoles per liter, multiply by 0.0347. To convert the values for progesterone to nanomoles per liter, multiply by 3.180. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129.
The patient was evaluated two times in the same month.
Measurement was performed with the use of isotope-dilution liquid chromatography–tandem mass spectrometry.
The reference value for patients with Tanner stage 1 development is less than 7.0 pg per milliliter.
The reference range for patients between the ages of 12 and 18 years is 14 to 362 pg per milliliter (<100 pg per milliliter if Tanner stage 1).
Methods
Serum estradiol was measured by means of immunoassay, but estradiol and estrone were also measured with the use of isotope-dilution highperformance liquid chromatography–mass spectrometry (LC-MS). All samples were measured twice for verification. The estrogen-responsive proteins — corticosteroid-binding globulin, sexhormone– binding globulin, and thyroxine-binding globulin — were measured by means of immunoassay. DNA from peripheral-blood white cells was sequenced with the use of polymerase-chainreaction (PCR) assay for protein-coding exons in ESR1 (GenBank accession number, NM_000125), followed by analysis with the use of Basic Local Alignment Search Tool (BLAST) software and Clustal alignment. The putative mutation was analyzed in 192 samples obtained from white controls.
The Sorting Intolerant from Tolerant (SIFT) algorithm was used to predict whether the missense mutation was deleterious.17 A nonmutated ESR1 clone, pSG5-HEGO-ERα, was used to generate the mutated clone (pSG5-HEGO-ERα-Q375H) by means of site-directed mutagenesis, which was confirmed on DNA sequencing. Transient transfections of mutated and nonmutated constructs were performed in COS-7 cells with a luciferase reporter construct containing two estrogenresponse elements with the use of transfection reagent FuGENE6 (Promega), as described previously. 18 Transfection efficiency was adjusted by cotransfection with CMV-β-gal (a construct containing beta-galactosidase derived from cytomegalovirus). Estradiol dose–response curves (1 fM to 1 μM) were generated in triplicate. Experiments were repeated six times, and results were expressed as the mean percentage and standard deviation of maximal response. Dose–response relationships and values for the half-maximal effective concentration (EC50) were calculated with the use of GraphPad Prism software. Expression levels for both mutated and nonmutated estrogen receptors in transfected cells were assessed with the use of Western blotting with primary antibodies from Millipore (C1355) and Santa Cruz Biotechnology (sc-542). Cellular localization of mutated and nonmutated estrogen receptors was performed in transfected COS-7 cells with the use of C1355 and a Cy3 direct-tagged secondary antibody (Jackson ImmunoResearch Laboratories).
Results
Laboratory Values
Before the initiation of therapy, the patient’s serum estradiol levels on immunoassay were invariably elevated, ranging from 750 to 3500 pg per milliliter (2753 to 12,848 pmol per liter) (Table 1). After isotope dilution and LC-MS, results for serum estradiol (2340 pg per milliliter [8590 pmol per liter]) and estrone (1040 pg per milliliter [3847 pmol per liter]) were 10 times the normal preovulatory levels. Serum levels of corticosteroid-binding globulin, sex-hormone–binding globulin, thyroxine-binding globulin, prolactin, and triglycerides were not increased, despite elevated estrogen levels. Gonadotropins remained mildly elevated. The inhibin B level was normal, but the inhibin A level was elevated, at 319 pg per milliliter and 184 pg per milliliter on measures from two different laboratories, with a premenopausal value of less than 98 pg per milliliter and a postmenopausal value of less than 10 pg per milliliter. The level of antimüllerian hormone was 2.8 ng per milliliter (normal range, 0.3 to 11.2).
The patient’s fasting morning insulin level was 5.8 μU per milliliter (normal range, 2.6 to 24.9 [40 pmol per liter; normal range, 18 to 173]), and the fasting glucose level was 90 mg per deciliter (5 mmol per liter), which resulted in a homeostatic model assessment–insulin resistance (HOMA-IR) value of 0.8 (normal value, <2.5).19 An oral glucose-tolerance test showed glucose levels of 102, 82, and 44 mg per deciliter (5.7, 4.6, and 2.4 mmol per liter) at 0, 1, and 2 hours, respectively, with corresponding insulin levels of 7.3, 27.2, and 23.6 μU per milliliter (51, 189, and 164 pmol per liter). Despite a low 2-hour glucose level, the patient remained asymptomatic. The glycated hemoglobin level was 5.5% (normal range, 4.8 to 5.6).
DNA Sequencing of ESR1
DNA sequencing of ESR1 revealed a homozygous mutation, c.1125G→T in exon 5, in p.Gln375His (Fig. 2A), which was absent in the single-nucleotide polymorphism database (dbSNP) and in 192 samples provided by white controls. Within the ligand-binding domain, the neutral polar glutamine 375 was changed to a basic, polar histidine, which was highly conserved in all 21 species examined (Fig. 2B) and was predicted to be deleterious with the use of SIFT (P<0.001).17 Transactivation of an estrogen-response-element (ERE)– dependent luciferase reporter gene in transfected COS-7 cells revealed greatly reduced activity in the mutated estrogen receptor (Fig. 2C). The EC50 for the nonmutated gene (20.51 pM) was lower by a factor of 240 than that in the mutated gene (5 nM). Expression levels for both the mutated and nonmutated estrogen receptors were similar in transfected COS-7 whole-cell lysates and localized to the nucleus on Western blotting (Fig. S2 in the Supplementary Appendix) and immunofluorescence (Fig. S3 in the Supplementary Appendix). Since the patient had been adopted, parental samples were not available. However, a high-density microarray with 750,000 SNPs and 1.9 million copy-number probes revealed that the patient had a region of homozygosity of approximately 11%, suggesting that her biologic parents were second-degree relatives and heterozygous carriers of the mutation (Table S1 in the Supplementary Appendix).20
Figure 2. Analysis of the ESRI Mutation in the Patient and Resulting Reduced Estrogen-Receptor Activity.
A representative electropherogram obtained on DNA sequencing of ESR1 shows the location of the mutation found in the patient (Panel A). Alignment of the affected Gln (Q) 375 residue with other species indicates complete conservation in all 21 species (Panel B). Transactivation of an ERE-LUC (estrogen-response element upstream of luciferase) construct in transfected COS-7 cells revealed greatly reduced activity in the mutated estrogen receptor, as compared with the nonmutated estrogen receptor (Panel C).
Discussion
In this report, we describe an 18-year-old woman who had presented with persistently elevated serum estrogen levels, mildly elevated gonadotropin levels, and intermittent pelvic pain secondary to multicystic ovaries. She had had no breast development after 5 months of receiving oral estrogen — 3 months at standard doses and 2 months with an increased dose of micronized estradiol. We subsequently identified a homozygous ESR1 missense mutation in a highly conserved Gln residue within the ligand-binding domain of estrogen receptor α. In vitro analysis indicated that the mutant ESR1 did not affect nuclear localization but markedly impaired estrogen signaling except during the administration of very high doses of estradiol, when estrogen signaling was minimal.
Untreated, the patient had a plasma estradiol level that was 10 times the normal value, similar to that of the α-ERKO mouse. Her normal serum levels of sex-hormone–binding globulin, corticosteroid-binding globulin, thyroxine-binding globulin, prolactin, and triglycerides, which are known to be increased by estrogen, provide further evidence of estrogen resistance. In the male patient with an ESR1 mutation who was reported previously, 3 normal levels of corticosteroid-binding globulin and thyroxine-binding globulin were also found. In contrast, the male patient had an elevated baseline level of sex-hormone–binding globulin, which did not rise after the administration of exogenous estrogen.
Estrogen resistance has been associated with hyperinsulinemia, which was diagnosed in the male patient with the ESR1 mutation15 but not in our female patient. The male patient’s hyperinsulinemia may have been related to obesity, since he had a body-mass index of 30.5, as compared with 16.6 in our patient. However, both sexes of α-ERKO mice have hyperinsulinemia after the administration of oral glucose, as well as increased fat.21 Our patient had normal levels of fasting glucose, insulin, and glycated hemoglobin and a normal HOMA-IR, indicating no impaired glucose tolerance. However, since her glucose dropped during an oral glucose-tolerance test, she will require follow-up for insulin resistance. Her body weight and fat were normal, but it is unknown whether she will be at increased risk for obesity or diabetes in the future.
Current evidence indicates that in women, both negative and positive feedback in the hypothalamus and pituitary are mediated by estrogen receptor α rather than by estrogen receptor β.11 The male patient with estrogen resistance had a mildly increased serum estradiol level, but levels of both follicle-stimulating hormone and luteinizing hormone were markedly elevated, at approximately 30 mIU per milliliter (normal range, <15 and <20, respectively). This finding was similar to those in male α-ERKO mice.12 In contrast, our patient had only mildly elevated gonadotropin levels. Female α-ERKO mice have a luteinizing hormone level that is eight times the normal value and normal serum levels of follicle-stimulating hormone; the latter is probably due to an increase in inhibin A levels by a factor of 5 in such mice.12 Inhibin A levels in our patient were markedly elevated, which probably contributed to the mild elevation in the level of follicle-stimulating hormone. Since men do not produce inhibin A, follicle-stimulating hormone levels were not suppressed in the estrogen-resistant male patient. Serum testosterone levels in our patient ranged from 38 to 88 ng per deciliter, which is only slightly above the normal range; however, the levels were higher than those in women with complete estrogen deficiency caused by mutations in follicle-stimulating hormone beta (FSHB).22 We speculate that testosterone may have partially suppressed the patient’s luteinizing hormone levels and contributed to facial acne, since this androgen effect was unopposed by estrogen in our patient. In addition, late follicular or early luteal levels of progesterone could have suppressed luteinizing hormone in our patient, but actions associated with estrogen receptor β or other atypical actions cannot be excluded.
Our patient’s presenting symptom was intermittent bilateral pelvic pain caused by hemorrhagic ovarian cysts, a condition that was probably caused by mildly elevated levels of gonadotropins (follicle-stimulating hormone, 6.7 to 19.1 mIU per milliliter; luteinizing hormone, 5.8 to 13.2 mIU per milliliter). With impaired estrogen-mediated negative feedback, the persistent, mildly elevated gonadotropin levels could stimulate and maintain multiple ovarian cysts producing estradiol and progesterone. In addition, the administration of norethindrone was temporally associated with reductions in ovarian volume and number of cysts, which suggests that negative feedback mediated by progesterone receptor was intact. During this treatment, there were slight reductions in serum gonadotropin levels and a substantial reduction in the serum estradiol level. Although levothyroxine administration may decrease the size of ovarian cysts,23 the patient’s cysts persisted, despite adequate thyroid replacement, which argues against hypothyroidism as a contributory factor. Women with a deficiency in aromatase, which converts androgens to estrogens, show sexual ambiguity at birth, but ovarian cysts, clitoromegaly, and hypergonadotropic hypogonadism develop at puberty.24 However, in such patients, the response to estrogen is normal.
Estrogen resistance would be predicted to affect bone metabolism. Our patient had a lowerthan-expected bone mass for her age on the basis of DXA scanning. Levels of both osteocalcin and C-telopeptide were elevated, suggesting increased bone turnover. However, she had not yet achieved peak bone age and was still growing (on the basis of open epiphyses); thus, high markers of bone turnover could simply reflect ongoing bone growth. Long-term consequences of estrogen resistance could include osteoporosis,25 but this will require follow-up.
In conclusion, our patient with an ESR1 mutation showed clinical signs of complete estrogen insensitivity, the counterpart to complete androgen insensitivity in 46,XY males.1–3 However, we cannot exclude the possibility that some residual estrogen sensitivity could be present in some tissues. The prevalence of ESR1 mutations in humans is unknown but they are probably rare. It remains to be seen whether milder ESR1 mutations could cause an incomplete estrogen-deficient state similar to the partial androgen resistance caused by mild mutations in the gene encoding androgen receptor.2 The presence of biallelic mutations suggested that the patient’s estrogen resistance was autosomal recessive, especially since microarray analysis revealed a region of homozygosity consistent with a parental history of second-degree relation.20 Nineteen years after the description of an estrogen-resistant state in a man, we provide evidence that ESR1 mutations in women are not lethal but confer a profoundly estrogen-resistant state.
Supplementary Material
Acknowledgments
Supported by a grant from the National Institutes of Health (HD33004, to Dr. Layman).
We thank Geoffrey Greene of the University of Chicago for providing the ESR1 clone used in this study.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Brown TR, Lubahn DB, Wilson EM, Joseph DR, French FS, Migeon CJ. Deletion of the steroid-binding domain of the human androgen receptor gene in one family with complete androgen insensitivity syndrome: evidence for further genetic heterogeneity in this syndrome. Proc Natl Acad Sci U S A. 1988;85:8151–8155. doi: 10.1073/pnas.85.21.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson MN, Hughes IA, Gottlieb B, Pinsky L. The androgen receptor gene mutations database. Nucleic Acids Res. 1994;22:3560–3562. [PMC free article] [PubMed] [Google Scholar]
- 3.Charest NJ, Zhou ZX, Lubahn DB, Olsen KL, Wilson EM, French FS. A frame-shift mutation destabilizes androgen receptor messenger RNA in the Tfm mouse. Mol Endocrinol. 1991;5:573–581. doi: 10.1210/mend-5-4-573. [DOI] [PubMed] [Google Scholar]
- 4.Toft D, Gorski J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci U S A. 1966;55:1574–1581. doi: 10.1073/pnas.55.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King WJ, Greene GL. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984;307:745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- 6.Green S, Walter P, Greene G, et al. Cloning of the human oestrogen receptor cDNA. J Steroid Biochem. 1986;24:77–83. doi: 10.1016/0022-4731(86)90035-x. [DOI] [PubMed] [Google Scholar]
- 7.Walter P, Green S, Greene G, et al. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985;82:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tremblay GB, Tremblay A, Copeland NG, et al. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol. 1997;11:353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- 10.Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 11.Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM. Classical estrogen receptor alpha signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology. 2008;149:5328–5334. doi: 10.1210/en.2008-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krege JH, Hodgin JB, Couse JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris HA. Estrogen receptor-beta: recent lessons from in vivo studies. Mol Endocrinol. 2007;21:1–13. doi: 10.1210/me.2005-0459. [DOI] [PubMed] [Google Scholar]
- 15.Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [Erratum, N Engl J Med 1995; 332:131.] [DOI] [PubMed] [Google Scholar]
- 16.Kelly TL, Wilson KE, Heymsfield SB. Dual energy x-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4(9):e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreihofer DA. Transcriptional regulation by phytoestrogens in neuronal cell lines. Mol Cell Endocrinol. 2005;231:13–22. doi: 10.1016/j.mce.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Katsuki A, Sumida Y, Gabazza EC, et al. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care. 2001;24:362–365. doi: 10.2337/diacare.24.2.362. [DOI] [PubMed] [Google Scholar]
- 20.Sund KL, Zimmerman SL, Thomas C, et al. Regions of homozygosity identified by SNP microarray analysis aid in the diagnosis of autosomal recessive disease and incidentally detect parental blood relationships. Genet Med. 2013;15:70–78. doi: 10.1038/gim.2012.94. [DOI] [PubMed] [Google Scholar]
- 21.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Layman LC, Lee EJ, Peak DB, et al. Delayed puberty and hypogonadism caused by mutations in the follicle-stimulating hormone β-subunit gene. N Engl J Med. 1997;337:607–611. doi: 10.1056/NEJM199708283370905. [DOI] [PubMed] [Google Scholar]
- 23.Durbin KL, Diaz-Montes T, Loveless MB. Van Wyk and Grumbach syndrome: an unusual case and review of the literature. J Pediatr Adolesc Gynecol. 2011;24(4):e93–e96. doi: 10.1016/j.jpag.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Ito Y, Fisher CR, Conte FA, Grumbach MM, Simpson ER. Molecular basis of aromatase deficiency in an adult female with sexual infantilism and polycystic ovaries. Proc Natl Acad Sci U S A. 1993;90:11673–11677. doi: 10.1073/pnas.90.24.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith EP, Specker B, Bachrach BE, et al. Impact on bone of an estrogen receptor-alpha gene loss of function mutation. J Clin Endocrinol Metab. 2008;93:3088–3096. doi: 10.1210/jc.2007-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.